Abstract
In this study, the authors used an enzyme called papain sourced from the Carica Papaya to improve the comfort and water-absorbing properties of a fabric made from a blend of polyester and cotton (65/35). The experiment was designed using the Box Behnken method to determine the most important variable and the best levels of parameters. The focus was on testing the wettability, moisture regain, and surface characteristics of the material. The results showed that all the comfort properties of the fabric improved after treatment with papain enzyme. After testing different parameters, the best conditions for treating the fabric with papain enzyme were determined to be a temperature of 30 °C, a papain concentration of 14%, and a treatment time of 50 min. Under these optimized conditions, the moisture regain and wettability of the polyester/cotton blend fabric treated with papain enzyme improved to 1.9 ± 0.02% and 6 cm capillary rise (measured with a 2-s drop test and 2-s sinking time) within just 3 min of wicking time. The Polyester/cotton blend fabrics treated with papain enzyme exhibited several noteworthy characteristics, including a significantly reduced susceptibility to fabric pilling (4–5), a limited capacity to attract oily impurities, and a high oil-soil-release capability with a stain removal index of 85%. Additionally, the fabrics showed a one-order-of-magnitude decrease in surface resistivity under normal conditions, with a half-life decay time of 513 s. Observations of the treated fabrics revealed the presence of cracks, grooves, nanostructures, and a high degree of roughness on the surfaces that were treated with papain enzyme. To further evaluate the effects of the lipase enzyme treatment on the fabric properties, several tests were conducted, including Fourier Trasform Infrared spectroscopy (FT-IR), Thermogravimetric Analysis (TGA), Differential scanning calorimetry (DSC), Moisture Regain, Tensile Strength, Stain Repellency, pilling resistance, and Anti-static charge generation.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Polyester/cotton (P/C) blends make up a significant portion of the textile industry, accounting for 58.45% of the world's market share [1, 2]. Polyester/cotton (P/C) blend is a widely used fabric in various applications such as apparel, home furnishing, and household products. This is primarily due to its desirable properties, including user-friendliness, functional performance, aesthetic value, printability, and affordability. These characteristics are more challenging to achieve in either polyester (polyethylene terephthalate) or cotton fabric alone, which is why P/C blend is a popular choice in the textile industry [2, 3]. The presence of hydroxyl groups in the cellulose structure of cotton fibers enables them to provide wear comfort to the blend fabric. These groups make the fibers hydrophilic, allowing for higher moisture absorption and wettability. On the other hand, polyester fibers contribute to the blend fabric by offering high thermal stability, good strength, dimensional stability, good chemical resistance, and acceptable easy-care properties [4, 5].
Despite the excellent properties of P/C blend fabric mentioned earlier, the hydrophobic surface of its polyester portion can negatively impact its comfort properties when used for apparel purposes. Specifically, the thermal comfort of the blend fabric depends on its ability to transmit heat and moisture vapour and absorb body sweat. The hydrophobic nature of polyester fibers can hinder these processes, making the fabric less comfortable to wear [6]. When wearing a garment made from P/C blend fabric with a higher proportion of polyester, the fabric can trap perspiration between the skin and the cloth due to the lower water vapour absorbability of polyester compared to cotton. This can result in discomfort and irritation for the wearer [7, 8]. Therefore, surface modification of P/C blend fabric is necessary due to the presence of polyester fibers.
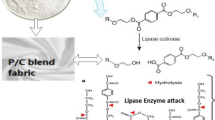
To address the issue of the hydrophobic surface of P/C blend fabric, various surface modification techniques have been employed to enhance its Hydrophilicity. These methods include alkaline hydrolysis [9], aminolysis [10], natural biopolymer application [11], gas treatment [12], silanization [13], UV treatment [14], flame treatment [15], and tethering molecules in bio-conjugation [16]. However, wet chemical processes for surface modification are non-specific, which can change the bulk properties of the fabric, require large amounts of water and energy, and release hazardous effluent into the environment [9, 17, 18]. Flame and UV treatment can also affect the optical properties of the polymer [19]. To overcome these issues, papain enzyme surface modification is an ideal option.
Carica Papaya-derived papain enzymes are protein systems that are organic and fully biodegradable. They operate via a four-step mechanism, starting with the attachment of the substrate to the active serine, which produces a tetrahedral intermediate. Next, the alcohol molecule is released, leading to the formation of an acyl-enzyme complex [20, 21]. The papain enzyme derived from Carica Papaya exhibits a high reaction rate, can operate at low temperatures, has no significant environmental impact, and causes minimal damage to the fibers [22].
There has been no recent research conducted on using papain enzymatic surface modification to improve the comfort qualities of P/C blend fabric. When P/C blend fabric is modified using papain enzyme, it leads to the formation of hydroxyl and carboxyl groups on the surface of the polyester fibres. The presence of these hydrophilic groups (-OH, -COOH) changes the surface's character from hydrophobic to hydrophilic, offering an opportunity to modify various undesirable characteristics of fibres and fabrics.
This study focused on investigating the impact of papain enzyme concentration, temperature, and reaction time on the comfort properties of P/C blend fabric, specifically moisture regain, wickability, and weight loss. The experimental design and data analysis were conducted using the Box Behnken design expert software approach. Additionally, surface characterization techniques were utilized to study the surface morphology and chemistry of the blend fabric.
2 Materials and method
2.1 Materials
A fabric made of a blend of polyester and cotton in a ratio of 65:35, which has been partially bleached, was obtained from EITEX textile chemistry laboratory. The fabric has a specification of 90 picks per inch, 152 ends per inch, and is a 2/1 twill warp face. The warp count is 32tex, the weft count is 36tex, and it has a weight of 200 GSM. To activate papain enzymes, sodium acetate (CH3COONa, 98% reagent grade) as a buffering agent; calcium chloride (CaCl2, Reagent grade, 99%); and Sodium-Sulfite- Anhydrous 99% (Na2SO3) were used as buffering and activating agents. The chemicals were purchased from Sachem chemicals plc. Addis Abeba, Ethiopia, and used without additional purification.
2.2 Methods
2.2.1 Papain enzyme surface modification
To carry out enzymatic processing on polyester/cotton blend materials, a solution containing 4 g per liter of calcium chloride and sodium acetate buffer in a liquid ratio of 1:40 was utilized. The treatment involved applying papain enzyme to the blend materials under specific conditions, including a pH of 7.5, a temperature range of 30–60 degrees Celsius, papain concentration of 10–20% own weight of the fabric(o.w.f), and a treatment time of 50–70 min [23].
The papain enzyme was applied to cotton/polyester blend fabrics using an Auto wash machine (BS-20, Jeio Tech) at 50 rpm. Following the treatment, the samples were thoroughly rinsed with water at a liquor ratio of 1:50 and air-dried. The Box-Behnken design of experiment software was employed to conduct the papain enzyme surface modification of the p/c blend in 17 separate runs.
2.2.2 Surface wettability test
The impact of the treatment on the fabric's wettability was assessed by measuring its water absorption, capillary rise, and sinking time. The AATCC standard test method 79–1992 was used to determine the water absorbency of the polyester/cotton blend fabric, which involves measuring the time it takes for a drop of water placed on the fabric surface to be absorbed [24].
The capillary rise method involved submerging one end of the fabric sample vertically into a water reservoir, allowing water to be drawn into the sample through capillary action. After 5 min, the height the water had risen to within the sample was measured as the "wicking height." To make the process visible, each specimen was cut into a 20 × 2 cm strip, which was then hung lengthwise and submerged in water containing an acid dye [25].
To assess sinking time, each fabric sample was introduced carefully into a glass beaker filled with water, with each piece tested separately. The time it took for the sample to reach the bottom of the beaker was recorded. In this case, each specimen was cut to a standard size of 2.5 cm by 2.5 cm [23].
2.2.3 Measurement of moisture regain
The moisture regains of the surface-treated P/c blend fabrics were determined using the ASTM standard test method 2654. Prior to testing, the modified blended fabrics were conditioned for 24 h in a standard testing environment of 20 °C and 65% relative humidity, as specified in Eq. 1
where: % a = proportion of fibre 'a', %; and %b = proportion of fibre 'b', %
2.2.4 Measurement of weight loss
To assess the impact of papain surface modification on the damage of p/c blend fabrics, weight loss analysis was conducted. The weights of the samples before and after pre-treatment were measured using an electronic balance (Sartorius-GD 503) to determine the percentage weight reduction of the treated fabrics. The samples were then dried in a drying oven at 105 °C for 90 min and weighed in a closed weighing bottle after cooling. Equation 2 was utilized to compute the percentage weight reduction.
where w1 and w2 are the dry weights of the fabrics before and after papain enzyme treatment, respectively,
2.3 Characterization of papain treated P/C blend fabric
2.3.1 Fourier Transform Infrared spectroscopy (FTIR) analysis
To assess the surface chemistries of control and enzymatically modified p/c blend fabrics, FTIR (Fourier Transform Infrared Spectroscopy) was conducted using a Perkin Elmer FTIR instrument, following ASTM 7575 test standard procedures. The data was recorded in the 4000–400 cm-1 frequency band, with 20 scans used for each sample. A 500 ml lab beaker (Model No. P230, 115-V) was utilized for all of the papain surface modification processes on the polyester fabric.
2.3.2 Thermo Gravimetrical Analysis (TGA)
Thermal analysis was conducted using a TGA (Thermo gravimetric Analysis) system manufactured by Perkin Elmer to compare the thermal stability of p/c blend fabrics before and after papain treatment (Model No. TGA4000). The measurements were taken from ambient temperature to 500 °C at a heating rate of 20 °C/min in a nitrogen atmosphere. The denaturation characteristics of the modified and control samples of polyester/cotton blends were examined based on the results obtained from the TGA test.
2.3.3 Differential scanning calorimetry (DSC)
Crystallinity tests were conducted on a Perkin Elmer Pyris Series-DSC 7 using ordinary aluminum pans. The papain-treated and control samples (10 mg for films and 20.70 mg for GPET) were subjected to heat at a rate of 20 °C/min from 0 to 300 °C. The melt and cold crystallization peak regions of the first heating run were examined to calculate crystallinity using Eq. 3.
where ∆Hm, ∆Hc, and ∆H0m are, the temperatures of melting, crystallization, and melting of 100% crystalline PET (140.1 J/g) respectively.
2.3.4 Tensile strength measurement
To assess the tensile strength of papain-treated polyester/cotton blend fabric before and after treatment, the ASTM D5034-textile grab method test was conducted using the Tensolab 100 (Mesdan Lab) apparatus. The test specimen was clamped in the tensile testing apparatus, and a force was applied until the specimen broke. The breaking force and elongation were recorded during the test.
2.3.5 Pilling resistance measurement
The susceptibility of unmodified and modified polyester/cotton blend fabrics to pilling was examined using the ICI pillbox method. The fabric was wrapped around rubber tubes, with the face outermost, and placed inside rotating cork-lined boxes for a set period of time. The resulting "pills" were then compared to photographic standards on a scale of 1 to 5, where 5 indicated no pilling and 1 indicated severe pilling. Pilling was measured according to ASTM D4970 Method.
2.3.6 Electric properties—surface resistivity
The anti-static properties of both papain-treated and untreated polyester/cotton blend fabric samples in both warp and weft directions were determined using a STATIC voltmeter R-1020 and resistance measurement according to the ISO 18080–1:2015(en) standard test method. The fabric samples were charged up, and the elapsed time required for discharging half of the charge present in the fabric samples was measured and expressed as half-life decay time. The shorter the half-life decay time, the better the anti-static property of the fabric. The papain-treated polyester/cotton blend fabric was conditioned at 65 ± 2% relative humidity and 21 ± 1 °C for at least 24 h prior to further testing.
2.3.7 Stain repellent finishes
To assess the oil removability of the fabric, a colour testing instrument (DATACOLOR 600) was utilized. The colour of the fabric at the blank and oil-contaminated positions was measured using the colour testing instrument. The stain removal index was calculated using Eq. 4.
Were \(\Delta \) K/S is the difference between K/S values of the oil-contaminated position and the blank position of each fabric.
3 Results and discussion
3.1 Optimization of lipase enzyme surface modification
In this study, the papain enzyme was utilized for the surface modification of polyester/cotton blend fabric, and various parameters were considered during the process, including papain concentration, reaction time, and temperature, as shown in Table 1. Calcium chloride was used as a catalyst to speed up the reaction and activate the papain enzyme. The responses to weight loss and wettability were used to evaluate the degree of Hydrophilicity of the polyester/cotton blend fabric by hydrolysing ester linkages. An experimental design was employed to investigate the relationship between the independent variables (weight loss and wettability) and the dependent variables (lipase concentration, reaction time, and temperature). The effects of temperature, reaction time, and papain concentration on weight loss and wettability were analysed using Analysis of Variance (ANOVA).
Based on the results of analyzing all the factors (papain enzyme concentration, reaction time, and temperature) and their corresponding P-values (< 0.0001, 0.0005, and < 0.0001, respectively), which are all less than the significance level of 0.05, it can be concluded that all the factors have a significant impact on the response variable of wettability.
Furthermore, the analysis revealed that there is no significant interaction effect between factors AB, AC, and BC. The lack of fit test also yielded a P-value of 0.3544, which is greater than the significance level of 0.05. This indicates that the model based on the wettability values can be considered a reliable predictor of the response variable. Overall, the results suggest that the chosen model fits the data well, as depicted in Table 2.
According to Table 3, the P-values of 0.0003 and F-values of 21.70 indicate that the model terms are statistically significant. This means that the factors of papain concentration (A), reaction time (B), and temperature (C) have a significant and measurable impact on the response variable of weight loss. On the other hand, the interaction effects of factors AB and BC were found to be non-significant, as evidenced by their P-values of 0.6772 and 0.5433, respectively, which are both greater than the significance level of 0.05.
The analysis indicates that the interaction effects of papain concentration and reaction time, as well as reaction time and temperature, on weight loss are not statistically significant. Additionally, the fit-value deficiency of 1.06 suggests that there is a 45% chance that the F-value deficiency is due to noise. However, despite this, the weight loss values model can still be considered a reliable predictor of the response variable of percentage weight loss.
The general regression equation after eliminating insignificant factors is, Y = BO + X1 + X2 + X3 + X22 + X32, Wettability = 6.02 + 0.8875 X1 + 0.5625 X2 + 0.9250 X3 + 0.4275 X22 + 0.3525 X3.2
The coefficient value for temperature is + 0.9250, which is higher than the coefficient values for both reaction time and papain.
Enzyme concentration, which are 0.8875 and 0.5625, respectively. This suggests that temperature has a stronger positive relationship with the response variable than the other two factors. In other words, an increase in temperature is more likely to result in an increase in the response variable (e.g., weight loss) than an increase in either reaction time or papain enzyme concentration.
The regression equation for the model after eliminating insignificant factors is, Y = BO + X1 + X2 + X3 + X1 X3 + X22 + X32, Weight loss = 0.0916 + 0.1959X1 + 0.0692X2 + 0.2618 X3 + 0.1496X1 X3 + 0.1422 X22 + 0.1430X32,
According to the analysis, temperature has a higher coefficient value of + 0.2618 compared to the coefficient values of papain enzyme concentration and reaction time, which are 0.1959 and 0.0692, respectively. This indicates that temperature has a stronger positive effect on the response variable compared to the other two independent variables. In other words, an increase in temperature is more likely to result in an increase in the response variable (e.g. product yield) than an increase in either lipase enzyme concentration or reaction time.
3.1.1 Effects of papain concentration on weight loss
Based on the data presented in Fig. 1a, it appears that increasing the concentration of papain enzyme leads to an increase in weight loss. Further analysis indicates that this is due to an increase in the extent of hydrolysing ester linkage, resulting in a higher percentage of weight loss. The maximum weight reduction of 0.8 was achieved when the papain concentration was increased from 10 to 20%. This increase in weight loss can be attributed to the formation of an obvious ridge and uneven rough trench structure, as well as the removal of low molecular weight segments and short chains from the fibres. This results in an increase in the contact areas between the fibre and papain enzyme, ultimately leading to an improvement in the adhesive property [26, 27].
3.1.2 Effects of papain concentration on wettability
Water drop absorbency, sinking time, and capillary rise methods were used to measure the wettability of the fabric. According to the results presented in Fig. 1b, increasing the concentration of papain enzyme leads to an increase in the fabric's water absorbency capacity. This is because hydrolysis creates pits, voids, or an increase in surface porosity on the hydrolysed fabric, enabling it to absorb more water. As shown in Table 1, the highest capillary rise of 8.5 cm was achieved when the amount of lipase enzyme was increased from 10 to 20%. Hydrolysis has the potential to produce hydrophilic groups such as -OH and –COOH, which are responsible for the increase in wettability of the fabric [28].
3.1.3 Effects of reaction time on weight loss
The weight loss value of the papain enzymatic modified material is highly influenced by the reaction time. Figure 2a shows the relationship between reaction time and weight loss, indicating that as the reaction time increases, the percentage of weight loss also increases. The maximum weight loss of 0.8 percent was achieved when the reaction time was increased from 50 to 70 min. This increase can be attributed to the higher release of degradation products, short chains, and low molecular segments that occur as the reaction time increases.
3.1.4 Effects of Reaction Time on Wettability
Figure 2b illustrates the impact of reaction time on the wettability of polyester and cotton fabrics in the 50 -70 min range. The results show that the maximum capillary rise of 8.5 cm was achieved when the reaction time was increased from 50 to 70 min. This is due to the increase in nucleophile attack to the nucleoside of the carbonyl atom along the fabric chain. As the treatment period was increased to 70 min, the sinking time and water absorption time decreased rapidly.
3.1.5 Effects of temperature on weight loss
Figure 3a illustrates the weight loss of polyester/cotton blend materials treated with papain at different temperatures. The papain enzyme was applied to the fabric at temperatures ranging from 30 to 60 °C. The weight loss was approximately four times higher under the 60 °C treatment compared to the 30 °C treatment. This increase in weight loss is attributed to the papain enzyme's ability to hydrolyse the ester linkages in the fabric, which results in an increase in the amount of carboxylic groups [29]. However, it is important to note that the hydrolytic activity of papain decreases at temperatures above 60 °C due to its temperature sensitivity.
3.1.6 Effect of temperature on wettability
Figure 3b demonstrates the impact of temperature on the wettability of polyester/cotton blend fabrics treated with papain. The highest wettability value was observed in PET fabrics at 60 °C. In addition, the sinking time and water absorption time decreased as the temperature increased to 60 °C. The enhancement in wettability of the polyester/cotton blend fabrics after papain treatment is attributed to the hydrolytic activity of the papain enzyme [30].
3.1.7 Fourier Transform Infrared Spectroscopy (FTIR) analysis
The presence of strengthened absorption peaks indicates that the polyester/cotton blend fabrics were modified with additional oxygen and polar groups were added to the fabric surface. Figure 4 illustrates the FT-IR spectra of both untreated and papain-treated P/C blend fabrics. The spectra of both fabrics exhibit absorption peaks at 3346, 2927, 2854, 1708, 1337, 1244, 1107, 1057, and 724 cm-1. However, these absorption peaks were stronger in the papain-treated fabric compared to the untreated sample. The peak at 3346 cm-1 corresponds to O–H stretching, which indicates a broad and strong absorption and the stretching vibration of aliphatic O–H connected to the glycosidic ring. The peak at 2854 cm-1 can be assigned to the O–H stretching, which is a very broad signal. The peak at 2109 cm-1 is attributed to the O-C = O stretching vibration of -COOH. The peaks at 1708, 1337, and 1244 cm-1 correspond to the coupling frequency of -C-O- and O–H. The peak at 1057 cm-1 can be assigned to the C-O trans-vibration, while the peak at 724 cm-1 represents the C-H (CH2) out-of-plane bending vibration (Fig. 5).
3.1.8 Thermo Gravimetrical Analysis (TGA)
The thermogravimetric analysis of papain-treated polyester/cotton blend fabric before and after treatment was comparable to the lipase-treated sample, as shown in Fig. 4.15. This analysis involved monitoring the mass of a substance as a function of temperature, and the resulting curves were divided into three sections. In the temperature range of 100–150 ºC, there was no significant thermal decomposition observed. A minor thermal decomposition appeared in the range of 250–300 ºC. The major decompositions for both control and treated samples were found in the range of 310–510 ºC and 326–530 ºC, respectively. The maximum thermal decomposition for the control and lipase-treated polyester/cotton blend fabrics was 615 °C and 618.32 °C, respectively .
3.1.9 Differential Scanning Calorimetry analysis (DSC)
Figure 6 illustrates the thermal curves of the papain-treated and control samples. This analysis is based on the distinct values of the heats of fusion for crystalline and non-crystalline forms of the polymer. The degree of crystallinity of the lipase-treated polyester/cotton blend fabric was determined by integrating the peak area of a differential scanning calorimetry curve. The results show that the heat of fusion and glass transition temperature decreased due to papain enzyme treatment. The heat of fusion and TGA (thermogravimetric) analysis are proportional to the % crystallinity of the fabric. The percent crystallinity of the papain-treated sample is approximately 32%. The heat of fusion decreases after treatment of the fabric with papain enzyme, indicating a loss of the compact structure of the polyester/cotton blend fabric (Table 4).
3.2 Moisture regain of modified P/C blend
The moisture regains of papain enzyme treated and controlled p/c blend samples were determined by Eq. 2 [31]. Figure 7 demonstrates that the moisture regain was the highest (1.9 ± 0.02%) with the optimized values of lipase concentration of 14%, a reaction time of 50 min, and temperature of 30 °C, which is almost a 2.25-fold improvement over that of untreated p/c blend fabrics. This is owing to the likelihood that hydrolysis may result in the development of hydrophilic groups like the formation of hydrophilic groups like -COOH and OH on the surface of the lipase-treated fabric, which would enhance wettability, moisture regain, and comfort properties [32].
3.2.1 Tensile strength of the papain-treated sample
As shown in Table 5, the tensile strength of papain treated polyester/cotton blend fabrics is almost similar to the untreated fabric due to low weight loss.
3.2.2 Stain repellency
According to Table 6, the papain enzyme treatment of polyester/cotton blend fabric resulted in an increase in oil removability from 78 to 85%. This improvement is attributed to the enhanced hydrophilicity of the fabric caused by the papain treatment, which could weaken the hydrophobic interactions between fibres and stains and greatly improve the fabric's stain removability. The increased hydrophilicity also led to a lower affinity of oil to the fabric surface, making it easier to remove oily stains as shown in Fig. 8.
3.2.3 Susceptibility to pilling
Table 7 shows that the papain enzyme treatment was effective in improving the pilling resistance property of polyester/cotton blend fabric, resulting in a significant reduction in the fabric's susceptibility to pilling (degree 4–5). This improvement is attributed to the removal of short chains and low molecular weight segments from the surface of the papain-treated fabric.
3.2.4 Anti-Static property
According to Table 8, the optimized papain-treated polyester/cotton blend fabric showed a significant improvement in half-life decay time, which is expressed in seconds. Specifically, the half-life decay time was reduced from 1480 to 513 s after the papain enzyme treatment. This improvement is attributed to the increased wettability of the fabric, which in turn improved its anti-static property.
The improved wettability of the papain-treated polyester/cotton blend fabric has the effect of dissipating localized static charges more easily, resulting in a better anti-static property for the fabric. This is because the increase in wettability reduces the half-life decay time, thereby improving the fabric's ability to resist static charges.
4 Conclusion
This study focused on investigating how surface modification with papain enzyme affects the comfort properties of polyester/cotton blend fabric. The optimization of the papain enzyme surface modification process was carried out using the Box Behnken design method, analyzing the weight loss, moisture regain, and wettability of the treated fabrics under different treatment conditions, such as enzyme concentration, treatment time, and temperature. The experimental results showed that the optimal conditions for the papain enzyme surface modification process were an enzyme concentration of 14%, a reaction time of 50 min, and a temperature of 30 °C, resulting in a weight loss of 0.6% and a wettability of 6 cm (2 s. drop test, 2 s. sinking time). Under these conditions, the moisture regain of the papain-treated fabric was 2.25 times better than that of untreated polyester/cotton blend fabric. The papain -treated fabric also exhibited a reduced susceptibility to pilling (4–5), decreased ability to retain oily impurities, high oil-soil-release capability (stain removal index of 85), and reduced surface resistivity under normal conditions (513 s of half-life decay time). The papain enzyme treatment significantly improved the comfort properties of the polyester/cotton blend fabric, with the treated fabric surface showing many cracks and voids compared to the untreated fabric surface. These cracks and voids enhanced the wettability and moisture recapture of the papain-treated fabric, but had little impact on the fabric's handling and tensile strength due to the negligible weight loss. This surface modification method has significant potential for industrial application as it is relatively safe, environmentally friendly, and requires less energy, water, and time.
Availability of data and materials
The datasets used and/or analysed during the current study are available from all authors upon reasonable request.
References
Yoon H, Buckley A. Improved comfort polyester: part I: transport properties and thermal comfort of polyester/cotton blend fabrics. Text Res J. 1984;54(5):289–98.
Tegegne W, et al. One-bath one-dye class dyeing of polyester/cotton blend fabric with disperse dye after esterification of cotton fabric. Discov Mater. 2022;2(1):14.
Yoon H, Sawyer L, Buckley A. Improved comfort polyester: part II: mechanical and surface properties. Text Res J. 1984;54(6):357–65.
Varshney R, Kothari VK, Dhamija S. A study on thermophysiological comfort properties of fabrics in relation to constituent fibre fineness and cross-sectional shapes. J Text Inst. 2010;101(6):495–505.
Schneider AM, Hoschke B, Goldsmid H. Heat transfer through moist fabrics. Text Res J. 1992;62(2):61–6.
Prahsarn C, Barker RL, Gupta B. Moisture vapor transport behavior of polyester knit fabrics. Text Res J. 2005;75(4):346–51.
Kothari V. Thermo-physiological comfort characteristics and blended yarn woven fabrics. 2006.
Adler MM, Walsh WK. Mechanisms of transient moisture transport between fabrics. Text Res J. 1984;54(5):334–43.
Koh J. Alkali-hydrolysis kinetics of alkali-clearable azo disperse dyes containing a fluorosulphonyl group and their fastness properties on PET/cotton blends. Dyes Pigm. 2005;64(1):17–23.
Yilma BB, Luebben JF, Tadesse MG. Effect of plasma surface modification on comfort properties of polyester/cotton blend fabric. Mater Res. 2021. https://doi.org/10.1590/1980-5373-mr-2021-0021.
Sampath UGT, et al. Fabrication of porous materials from natural/synthetic biopolymers and their composites. Materials. 2016;9(12):991.
Kale K, Palaskar S. Atmospheric pressure glow discharge of helium-oxygen plasma treatment on polyester/cotton blended fabric. 2011.
Patiño-Herrera R, et al. Intraradicular dentine silanization by a new silicon-based endodontic sealer. Int J Adhes Adhes. 2016;69:115–24.
Kathirvelu S, D’souza L, Dhurai B. UV protection finishing of textiles using ZnO nanoparticles. 2009.
Al-Balakocy NG, El-Badry K, Hassan TM. Multi-finishing of polyester and polyester cotton blend fabrics activated by enzymatic treatment and loaded with zinc oxide nanoparticles. In: Pascual AR, Martin MEE, editors. Cellulose. London: IntechOpen; 2019. p. 105.
Zhang BG, et al. Bioactive coatings for orthopaedic implants—recent trends in development of implant coatings. Int J Mol Sci. 2014;15(7):11878–921.
Rajwin JA, Tarefder RA, Prakash C. A study on the effect of plasma treatment on thermal comfort properties of cotton fabric. West Conshohocken: ASTM International; 2018.
Jeyakodi Moses J, Pitchai S. A study on the dyeing of sodium hydroxide treated polyester/cotton blend fabrics. Int J Sci Technol Soc. 2015;3(1):1–9.
Reddy N, et al. Reducing environmental pollution of the textile industry using keratin as alternative sizing agent to poly (vinyl alcohol). J Clean Prod. 2014;65:561–7.
Kumar JA, Kumar MS. Lipase hydrolysis of polyester cotton blends-Part-II. Man-Made Text India. 2019;47(8):262.
Kumar JA, Senthil Kumar M. A study on improving dyeability of polyester fabric using lipase enzyme. Autex Res J. 2020;20(3):243–9.
Hsieh YL et al. Enzyme treatment to enhance wettability and absorbancy of textiles. 2002, Google Patents.
Hsieh YL et al. Enzyme treatment to enhance wettability and absorbency of textiles. 2000, Google Patents.
Toprak T, Aniş P. Investigation of the effects of enzymatic finishing processes on the dyeing properties of cotton-polyester and polyester fabrics. In book of abstracts.
El-Shemy N, El-Hawary N, El-Sayed H. Basic and reactive-dyeable polyester fabrics using lipase enzymes. J Chem Eng Process Technol. 2016;7(1):1000271.
Yoon M-Y, Kellis J, Poulose A. Enzymatic modification of polyester. AATCC Rev. 2002;2(6):312.
Alisch-Mark M, Herrmann A, Zimmermann W. Increase of the hydrophilicity of polyethylene terephthalate fibres by hydrolases from Thermomonospora fusca and Fusarium solani f. sp. pisi. Biotechnol Lett. 2006;28(10):681–5.
Araújo R, et al. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6, 6 fibers. J Biotechnol. 2007;128(4):849–57.
Kim HR, Song WS. Optimization of papain treatment for improving the hydrophilicity of polyester fabrics. Fibers Polym. 2010;11(1):67–71.
Cavaco-Paulo A, Gubitz G. Textile processing with enzymes. Vol. 29. Sawston: Woodhead publishing; 2003.
Khurshid MF, et al. Assessment of eco-friendly natural antimicrobial textile finish extracted from aloe vera and neem plants. Fibres Text East Eur. 2015;23(6(114)):120–3.
Lee SH, Song WS. Surface modification of polyester fabrics by enzyme treatment. Fibers Polym. 2010;11(1):54–9.
Acknowledgements
The authors would like to acknowledge the Ethiopian Institute of Textile and Fashion Technology (EiTEX) finance office.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
In this research work all authors participated in documentation, collection of information, funding, and data inputting. All authors have agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tegegne, W., Haile, A. Effect of papain enzyme surface modification on hydrophilic and comfort properties of polyester/cotton blend fabric. Discov Mater 4, 2 (2024). https://doi.org/10.1007/s43939-023-00071-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43939-023-00071-5