Abstract
Purpose of Review
3D bioprinting of cardiovascular tissues for in vitro and in vivo applications is currently investigated as a potential solution to better mimic the microenvironment typical of the human heart. However, optimal cell viability and tissue vascularization remain two of the main challenges in this regard. Silk fibroin (SF) as a natural biomaterial with unique features supports cell survival and tissue vascularization. This review aims to evaluate the potential of hydrogels containing SF in 3D bioprinting of cardiac tissue that better recapitulate the native cardiac microenvironment.
Recent Findings
SF hydrogels spontaneously develop nanocrystals, which limit their use for 3D bioprinting applications. Nevertheless, the printability of SF is improved in hybrid hydrogels by mixing it with other natural polymers (such as alginate and gelatin). This is achieved by adding SF with other polymers or by crosslinking it by peroxidase catalysis (i.e., with alginate). Compared to only SF-based hydrogels, hybrid hydrogels provide a durable bioprinted construct with improved mechanical stability and biological properties. To date, studies using cardiac cells in bioprinted SF constructs are yet to be performed.
Summary
Mixing SF with other polymers in bioprinted hybrid hydrogels improves the printability and durability of 3D bioprinted tissues. Studies using these hydrogels with cardiac cells will be required to evaluate the biocompatibility of SF hybrid hydrogels and to establish their potential use for cardiovascular applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The myocardium of adult hearts does not present regenerative capacity following an ischemic event, such as a heart attack (or myocardial infarction, MI). This phenomenon together with other cardiovascular complications, such as heart failure and diabetes, are the leading causes of global mortality [1,2,3].
Even if some recent studies suggest cardiac regeneration may be preserved in an adult heart, the adult human heart tends to lose this capacity [3, 4]. In fact, following an injury, such as a prolonged myocardial infarction, cardiac cells die and most of them are replaced by scar tissue, comprising collagen fibers deposited by activated fibroblasts. This irreversible damage in the muscle wall, which results in cardiomyocyte depletion, leads to a failing heart [3, 5, 6].
A heart transplant is the gold-standard treatment for heart failure patients [7, 8]. Although the majority of heart transplant patients survive for years, the procedure comes with major risks for the patient, including transplant rejection and other complications, such as vasculopathies, dysfunctions, and infections [7,8,9]. Another issue linked to heart transplantation is the lack of availability of donor hearts. Altogether, these complications lead to the search for new alternatives for these patients [8].
Given the poor regenerative capacity of an adult heart after injuries, researchers are investing new strategies, by stimulating endogenous or by transplanting cells to deliver a new viable and functional tissue within the injured heart [5, 6].
In recent years, stem cells have become a promising cell source as potential novel therapeutics in humans and for tissue regeneration because of their ability to self-renew and differentiate into new specialized cells, allowing the replacement of damaged tissues with new ones [10,11,12].
In order to better target the clinical need for personalized human heart tissues and therefore prevent the risk of cardiac tissue rejection, patient-specific stem cell-derived cardiac cells represent promising tools to treat heart failure patients [10, 13]. Moreover, due to the limited source of primary cardiomyocytes, the requirement of stem cell-derived cardiac cells has become the starting point for studying heart disease and modeling the human heart in vitro [14].
However, because of their limited survival following their injection in a failing heart, many studies have focused on new alternatives to culture stem cell-derived cardiac cells in a more physiological 3D microenvironment to promote proper viability and functionality of the transplanted cells [13,14,15]. Compared to monolayer cell cultures, 3D cardiac cultures show prolonged viability and improved cellular physiological functions, like contractile properties, and they tend to behave in a way that is more characteristic of the in vivo tissue microenvironment [13, 14]. Moreover, they provide the maintenance of the gene expression profile and cells interactions, and together with the extracellular matrix (ECM) regulate and improve the tissue microenvironment homeostasis [16].
To build these 3D microenvironments, the field of tissue engineering developed novel and advanced strategies, such as 3D bioprinting technology, to include several cues typical of the human heart [10, 13].
Instead of replacing the damaged tissue, the 3D bioprinting technology aims at biofabricating tissue substitutes, based on natural or synthetic biomaterials, which integrate within the tissue after being transplanted, to increase cell viability and maintain or improve tissue function [17, 18].
In order to create a complete vascular network, the 3D bioprinting technology aims to combine different cell types and biomaterials and leads an enhanced cell repopulation within a 3D structure. By the layer-by-layer deposition of cells and biomaterial, this strategy enables the mimicry of the native tissue architecture [10, 19••].
Recently, a range of new biomaterials showed good features with the potential to be used in cardiovascular tissue engineering; this includes silk fibroin (SF), a natural polymer that possesses many properties and provides physical cues that support new blood vessels formation in vitro and in vivo, which will be the focus of this manuscript [20, 21•].
Cardiovascular Microenvironment or Niche
Heart Tissue: Structure, Cells Types, Cell Ratio, 3D Models
Developmentally, the heart is the first organ to form in the embryo and it plays a vital role in supplying oxygen and nutrients to the body [8, 12]. It is composed of four chambers: two atria, which collect the non-oxygenated blood, and two ventricles, which pump oxygenated blood to all organs and tissues [12, 22]. The inner layer of these chambers is characterized by the endocardium, which comprises a layer of endothelium and connective tissue. The myocardium (or cardiac muscle) defines the heart wall and controls the contraction of the heart. Superficially, the heart is covered by the epicardium, composed of connective tissue and mesothelium, and a dense fibrous layer [22].
The key cell types found in the cardiac tissue are cardiomyocytes (CMs), smooth muscle cells (SMCs), endothelial cells (ECs), and fibroblasts. Neurons and immune cells also characterize the microenvironment and together with the other cell types, they play a key role in tissue homeostasis and immune response during an adverse situation or tissue damage [23,24,25]. The cardiac tissue is supported by a dense vascular network essential in delivering oxygen and nutrients to this highly metabolically active tissue [12, 22, 26••].
Due to its position, the endothelial monolayer composing blood vessels provides a barrier between the blood vessels inner wall and the circulating blood, but it also regulates the vascular homeostasis, blood pressure and maintains the SMCs physiological phenotype [23, 25]. Furthermore, fibroblasts and ECM define the forming vasculature and they play a key role in tissue growth and immune response during tissue damage [23].
In order to investigate and mimic the in vivo biology and function of the human cardiovascular tissue, novel strategies based on the use of biomaterials or cells are utilized to engineer 3D structures, to lead cell migration and repopulation of the damaged tissue [14, 16].
Stem cell-derived cardiomyocytes or cardiac progenitor cells are used to generate engineered heart tissues and include the co-culturing of different cell types present in the heart tissue [13, 14]. One of the latest cell-based systems for cardiac tissue engineering refers to cardiac spheroids [14, 27]. The hanging drop co-culture of primary cardiomyocytes or cardiomyocytes derived from iPSCs (iCMs), cardiac fibroblasts (CFs), or iPSC-derived cardiac fibroblasts (iCFs) and endothelial cells, in ratios replicating the ones present in vivo, allows cardiac spheroid formation (2:1:1) [13, 14].
Extracellular Matrix
The ECM makes up the non-cellular component of the cardiovascular system and it has an important function in maintaining the architecture and regulating tissue function and homeostasis [28, 29•]. The ECM microenvironment also confers important mechanical and biochemical cues to guide the morphogenesis of the tissue [28].
Cardiovascular ECM includes several structural and non-structural proteins [28]. In particular, perimysial and endomysial collagen (Col) and Col IV are typical of the myocardium; Col I, II, and IV are found in heart valves; and Col IV characterizes blood vessels. Elastins, glycoproteins such as fibronectin and laminin; glycosaminoglycans such as hyaluronan; and many proteoglycans like syndecan, versican, and perlecan, are also key components of the cardiac and vascular ECM [28, 29•]. Through interactions between these molecules and proteins, it establishes a network that confers dynamicity and mechanical support to the ECM [23, 28, 29•]. Moreover, a variety of aqueous pores characterizes the ECM morphology and they bring the matrix toward acting as a semi-permeable barrier, regulating the biochemical signaling and molecules diffusion [23, 29•].
The vascular ECM of the human heart is located between the endothelium and the SMCs, and thanks to its unique elastic properties, it plays a key role in regulating the forces during cardiac contraction and maintaining a proper vessel wall pressure [29•, 30].
During the development of the cardiovascular system, ECs and SMCs secrete ECM and its remodeling process is regulated by the metalloproteinases (MMPs), enzymes that degrade ECM [28, 30]. Metalloproteinases activate to remodel the tissue also after injury, highlighting their function in wound healing and tissue repair through the maturation of a scar [28, 31].
Following a MI, a huge loss of CMs occurs and they face apoptosis. Given the limited regenerative capacity of the human heart, the heart muscle repairs the damaged tissue through the maturation of a fibrous scar that changes the ratio of ECM molecules compared to a healthy muscle wall [31].
Vascularization
The human vasculature comprises pericytes, SMCs, and ECs, which constitute a selective barrier for circulating immune cells and molecules between the inner lumen and the outer layer of all vessels, and a basement membrane [26••, 29•]. It aims to perfuse blood from the heart to all tissues through a complex network of large and small vessels, ensuring the oxygenation and blood draining of all the tissues, but at the same time allowing the delivery of cells, nutrients, and hormones and the exchange of solutes and waste within cells and tissues [12, 22, 25, 26••].
The heart works as a pump and a concerted contractile activity due to the working CMs is transmitted as an impulse through gap junctions to the whole heart muscle, which ensures the blood diffusion to the tissues [12]. SMCs have an important role in blood diffusion; because of their ability to contract and relax, they allow the blood propulsion through the vascular network [26••].
The blood supply to the heart is crucial; even if the four heart chambers are filled with blood, the heart walls are too thick to absorb the blood through its diffusion. The heart is a highly metabolic tissue and is nourished by the coronary arterial system, which arises from the base of the aorta and runs along the entire wall of the cardiac tissue. At the same time, all the waste substances are drained through a venous system [22]. Following myocardial ischemia, the blood supply is reduced due to the partial occlusion of the coronary artery caused by the formation of a clot and CMs tend to lose their contractile capacity. A persistent blockage of the coronary artery prevents oxygen delivery to the heart and leads to a MI and the death of CMs [12, 31]. One way to address such cardiovascular disorders is via re-vascularization, including the promotion of the angiogenesis, with either bypass or reconstructive surgery [26••].
During the embryonic development, the vascular network growth can occur via the de novo formation of primitive vascular structures by endothelial progenitor cells, known as vasculogenesis, or through the endothelial sprouting and microvascular growth from pre-existing vessels, known as angiogenesis [29•, 32, 33]. A remodeling process then follows angiogenesis; quiescent endothelial cells start to migrate and interact with interstitial ECM components and proteins, forming a tree of arteries, arterioles, veins, venules, and capillaries that allow the blood circulation in remote avascular regions [26••, 29•, 32, 33].
The angiogenetic and vascularization processes of the cardiovascular tissue involve the secretion of several growth factors (GFs) that are essential for cells proliferation and migration into a new tissue [32]. The main angiogenetic factors are the fibroblast growth factor (FGF), the vascular endothelial growth factor (VEGF), the transforming growth factor-β (TGF-β), and the platelet-derived growth factor (PDGF) [26••, 32]. During the angiogenic remodeling, FGF and VEGF stimulate endothelial cells, which produce enzymes and other factors to degrade the vessel basement membrane and migrate in the surrounding matrix [29•, 32]. ECs proliferate, deposit novel basement membrane, and secrete other GFs to allow the formation of new vessels into a new lumen [32].
3D Bioprinting of Tissues Using Hybrid Hydrogels for Cardiovascular Applications
Bioprinting of Cardiovascular Tissues
3D bioprinting has emerged as an advanced and novel technology with the potential to revolutionize tissue engineering and regenerative medicine fields by allowing a better recapitulation of tissue architecture in a high throughput manner compared to other biofabrication approaches [34,35,36,37]. It combines cells and biomaterials, producing 3D functional and biomimetic substitutes, which can mimic the native tissue architecture [35, 36, 38••]. In addition, it allows a homogeneous cell and molecules distribution within the material together with good cell growth and proliferation [39•, 40].
By developing customized and complex 3D models of human tissue, the bioprinting approach has the potential to enable reduction in animal testing, overcoming ethical concerns associated with animal studies and allowing testing of therapeutic interventions on engineered human tissues, potentially improving patient treatments [35, 36, 38••].
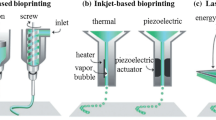
Bioprinters work following a number of different strategies and the main ones adapted to 3D bioprinting cardiovascular tissues are extrusion-based, inkjet-based, and light-assisted printing (Table 1) [19••, 38••, 44, 45].
Extrusion-based bioprinting allows the layer-by-layer deposition of bioinks through a nozzle, using pneumatic or mechanical forces (Fig. 1) [41••, 46•, 47]. This has the ability to print many types of cells encapsulated in different biomaterials [19••, 41••, 46•]. Moreover, a bioprinter can extrude biomaterials of different viscosities in several shapes that guarantee the structure maintenance following printing [19••, 38••, 41••, 42••, 44].
Bioprinting of tissues using SF-hydrogels and extrusion bioprinters. Schematic representation of the extrusion-based bioprinting process of a cell-embedding SF-based hydrogel (bioink) using a 6-well-plate as a printing surface. It shows an example of bioprinted SF-based network in the form of a 3D patch with an inner grid structure, represented on the right. Above the grid, there is the single well with the 3D patch in the center
Inkjet-based bioprinting is based on the use of a droplet-based bioprinting strategy and includes drop-on-demand, continuous, or electrodynamic inkjet bioprinting [41••]. Droplet extrusion from the nozzle occurs through different energy sources, including thermal forces, Rayleigh instability, and electric field [19••, 41••, 44, 46•]. Compared to extrusion-based bioprinting, the inkjet method deposits bioinks with high speed, but it only allows printing of droplets or spheroids [19••, 38••, 41••, 42••]. This strategy enables the printing of low viscosity biomaterials and creates precise architectures with high resolution [19••, 38••, 41••, 42••, 46•].
Light-assisted bioprinting comprises laser-assisted bioprinting and stereolithography [38••]. The laser-assisted method allows printing of both solid and liquid biomaterials, which possess a relatively low viscosity [19••, 42••]. This strategy does not release the bioink through a nozzle; a laser beam hits a donor layer, creating a bubble with high pressure. This bubble adds pressure on the bioink layer underneath and pulses the bioink on a substrate [19••, 41••, 44]. Stereolithography, on the other hand, enables the printing of photo-polymerizable biomaterials without viscosity limitations [19••, 38••, 41••, 46•]. Moreover, bioinks are printed with high speed and maintain a high architectural complexity and flexibility [19••, 38••, 41••, 42••]. However, bioinks often require a chemical modification with a functional group to aid photo-crosslinking [44].
Hybrid Hydrogels as Bioinks
The term “bioink” refers to any biomaterial, natural-, or synthetic-derived polymers, containing living cells that are loaded to a bioprinter [39•, 41••, 48•]. To ensure all the functional and mechanical properties that are fundamental to fabricate a biomimetic 3D construct, a biomaterial may be functionalized with additional components or molecules or it can be crosslinked to other polymers [39•, 42••, 49•].
The addition of tissue-tailored biomaterials to bioinks aims at improving the microenvironment for improved cellular viability and function in bioengineered tissues [48•, 50]. Optimal printability is one of the desired features of a bioink for the maintenance of the structural integrity of a 3D bioprinted construct [48•]. Other biomimetic features include biocompatibility, biodegradability, and suitable elasticity and rheological characteristics [48•, 51]. In addition, bioink viscosity is fundamental in the 3D bioprinting process; viscosity is determined by polymer chains crosslinking and this confers resistance to the applied pressure during the extrusion-based bioprinting process [42••]. Moreover, it enables ductility during the extrusion of the material and preserves the 3D model structure after printing [52]. Another key factor is the surface tension, which results from the cohesive forces between the components in the hydrogel and regulates the droplet formation [53••, 54]. During the bioink extrusion, the surface tension must be able to prevent the droplets generating on the nozzle tip, in order to guarantee the material flow from the nozzle [53••]. In contrast, in the inkjet-based bioprinting, the bioink surface charges are not as strong as the surface tension and this leads to droplet production [54]. The main bioinks used in 3D bioprinting are represented by hydrogels, microcarriers, cell aggregates, and decellularized matrices [41••].
Hydrogels are polymeric networks characterized by hydrophilic chains that confer them specific features to mimic the native tissue microenvironment [55•, 56]. Given their molecular interactions between functional groups present in the hydrophilic chains, they can swell and hold a high water content, becoming highly hydrated polymer networks [54,55,56]. This characteristic convenes a level of plasticity to the network that is comparable to the native tissue one [57]. Due to their mechanical and chemical properties that can support cell adhesion and proliferation and promote vascularization of the 3D structure, hydrogels are commonly used for cardiovascular applications [56]. Moreover, they must be biocompatible, biodegradable, and non-immunogenic to match the characteristics of the native cardiovascular tissue [18].
For cardiovascular tissue engineering applications, both natural and synthetic biomaterials have been used [56]. Hydrogels derived from natural polymer sources are normally considered promising candidates for the regeneration of the damaged tissue, as they exhibit low toxicity and good biocompatibility [54, 55•]. However, they are less stable and reproducible than the synthetic-based ones. Synthetic polymer-based hydrogels can be easily produced and modified to meet the required features of a biomaterial to be used in a particular application [18]. Nevertheless, they include some disadvantages, such as risk of infection, potential reduced biocompatibility, and bioactivity. Therefore, to develop hydrogels able to control the mechanical and biochemical properties without compromise the biocompatibility and degradability of the materials, both natural and synthetic polymers are often combined to produce hybrid and/or composite hydrogels [18, 58]. While composite hydrogels possess organic or inorganic components encapsulated within the polymeric network, the hybrid ones are composed by several polymers or phases, which are chemically or physically crosslinked [59]. Hybrid hydrogels can also include bioactive proteins, peptides, or micro/nanostructures in their heterogenic architecture, which can ameliorate the construct mechanical properties and cellular interactions [60].
To date, more hybrid hydrogel formulations using either natural- and synthetic-based polymers have been used for cardiovascular tissue engineering applications, like poly-lactic-co-glycolic acid (PLGA) crosslinked with alginate or gelatin, poly-lactic-co-glycolic acid nanoparticles encapsulated into hyaluronic acid and chitosan hydrogels, poly-ε-caprolactone (PCL) bound to a gelatin-chitosan hydrogel, chitosan crosslinked with poly-ethilene-glycol (PEG) and fibrin, and silk fibroin (SF) crosslinked with alginate or glycidyl methacrylate (GMA) (Table 2) [59,60,61, 64••, 65••, 66••].
Silk Fibroin as a Natural Hydrogel for Cardiovascular Applications
Source and Structure of Silk Fibroin
Silk fibroin (SF) is a natural fibrous protein produced by the Bombyx mori silkworm, which is a major source of silk globally [72••, 92•]. Silk proteins are principally produced by silkworms, like mulberry (B. mori), tussah, muga, and eri silkworms, but they can also be produced by other animals, such as mussels, bees, scorpions, or spiders [20, 92•].
Silkworm SF is stored in the lumen of salivary larvae glands, after its production by epithelial cells, and during the metamorphosis, it spins into a continuous thread that wraps itself forming the different layers of the cocoon structure [20, 92•]. SF from silkworms is produced mixed with another gum-like protein, sericin, and this characterizes the outer coating of the silk thread [21•, 92•]. The inner core consists of two SF fibers, each one composed of a multitude of fibrils [72••]. The fibrils present heavy and light chains linked by disulfide bonds and a glycoprotein binds non-covalently to the chains conferring integrity to the complex [20, 72••]. The heavy chains are mainly composed of hydrophobic repetitive domains that form the anti-parallel crystalline β-sheets linked by hydrogen bonds, whereas the minor part of the chain is characterized by non-repetitive hydrophilic motifs, which connect the hydrophobic domains and form the amorphous part [20, 21•, 72••]. The light chains are hydrophilic with non-repetitive sequences and it is relatively elastic (Fig. 2a) [20, 72••]. The elasticity to the fibers is given by the α-helices, while the β-sheets confer strength and toughness to the silk fiber [21•, 72••]. β-sheets are also fundamental structures, as they act as crosslinking points, and they can affect the properties of SF when used as a natural biomaterial for tissue engineering [20, 74••].
a SF structure and composition. The image on the left represents the structure of the Bombyx mori silkworm thread. The outer structure is characterized by the sericin, which coats two fibroin fibers (on the left). A fibrils bundle composes each fibroin fiber and their inner structure possesses specific aminoacidic sequences (on the right). The fibrils heavy chain is composed by a repetition of glycine, alanine, serine, and tyrosine aminoacids, forming the hydrophobic anti-parallel SF β-sheets. Each β-sheet is linked by hydrogen bonds, represented by the dashed lines. They are also connected by hydrophilic non-repetitive domains, which form the amorphous part of SF structure. SF motifs possess also hydrophilic non-repetitive α-helices that compose the light chain of the fibrils. b SF denaturation and extraction process. A Bombyx mori silkworm cocoons are cut with scissors and B delaminated, in order to obtain multiple thin layers. C Representation of the SF fibers after the elimination of the sericin coating (degumming process) through the treatment with sodium carbonate (Na2CO3). D SF is denaturated through a lithium bromide (LiBr) solution and the fibers are dissolved to obtain a SF aqueous solution. E The dialysis process eliminates all the debris present in the SF solution. F The sonication is one of the methods used to crosslink SF and G shows the polymeric SF. H SF is lyophilized with liquid nitrogen to obtain a solid structure, analyzed with the scanning electron microscope (SEM)
Given the different sequences and motifs that characterize the structure of the protein, the biological and mechanical features can be affected [74••]. For example, the heavy chains confer most of the mechanical and physical properties, giving an optimal balance between modulus, strength, and elongation [20, 93]. Moreover, the crystalline and semi-crystalline structures rule the thermal features, because of the transition from α-helices to β-sheets induced by heating [93]. SF exhibits outstanding and controllable mechanical properties that make it a good biomaterial to be utilized for biomedical applications. Additionally, compared to other natural polymers, SF is abundantly available in nature and possess accessible chemical groups, making it very malleable and modifiable [20, 74••, 93, 94]. Silk biomaterials also present a good structure to be functionalized on their surface with specific factors or molecules that guide and enhance cellular responses [87•, 88]. In addition, SF is water-processable, possesses controllable degradation rates, and is easy to load with bioactive agents, without losing function [53••, 74••, 94].
Because of its main hydrophobic composition and the polarity of sericin, SF is insoluble in water and many organic solvents [93]. The regenerated SF is obtained by a three-step process [72••]. During the degumming, sericin is removed from the fiber by steeping the delaminated cocoons in a sodium carbonate solution at high temperatures. The degummed fibers are then dissolved in a salt solution, like lithium bromide. The salt breaks the hydrogen bonds between the β-sheets and it is eliminated during a dialysis process (Fig. 2b) [72••, 73••, 93].
From the Bench to the Bedside: Clinical Potential of Silk Fibroin
Initially, SF fibers were used in the textile industry and material for surgical sutures, but it was further involved in many biomedical applications, such as tissue engineering and the delivery of therapeutic agents [74••, 77]. In the field of tissue engineering, SF is used either in its natural morphology, after the degumming process, and its regenerated form, obtained following its degumming and dissolution [20, 74••]. As regenerated protein, SF can be assembled in different forms. For example, most of the commonly SF-based structures used are fibroin films, sponges, fiber mats, particles, hydrogels, and 3D scaffolds [20, 77]. These forms have revealed to be useful as carriers for drug release and delivery for pharmacological therapies, but they were also used as scaffolds for tissue engineering toward tissue replacement/regeneration and in vitro tissue models (Table 2) [77,78,79].
SF-based scaffolds or hydrogels show low immunogenicity and good cell compatibility in vivo [94, 95•]. The tailorable architecture of SF-based biomaterials allows specific molecular elements to bind on their surface [87•, 88]. For example, SF-based structures functionalized with the fifth domain of human perlecan, which exhibit an important role during vasculogenesis, angiogenesis, and wound healing (Table 2) [88]. Bioactive molecules can also be bound to their architectures, in order to modulate the immune response and improve cell viability within the structure [94, 95•]. It has been demonstrated that modified SF specific amino acidic motifs by adding particular chemical groups or peptides, such as sulfonic acid group, zwitterionic phosphobetaine, or hirudin, showed less thrombogenicity and platelets adhesion and aggregation compared to SF alone, improving the anticoagulation features of SF. Particular amino acidic sequences can be also modified without changing the SF conformational characteristics, which control the polymer properties [95•]. Moreover, amino acid fragments characterized the SF degradation and they did not lead to adverse in vivo immune responses [94].
SF-based biomaterials have been employed for several tissues, such as cartilage bone, skin, neural, cardiac, and vascular tissues [20]. Cartilage and bone defects were the most studied cases, but in the last decades, the attention also shifted to soft-engineered tissues, such as skin, muscle, and adipose tissues (Table 2) [75, 76]. Given the tunable properties and the suitable biocompatibility features and low immunogenic rates, SF has been chosen as promising scaffold to fabricate new tissues and modulate cell behavior [94]. In its different structural formats, SF exhibits prominent features to support stem cells adhesion, proliferation, and differentiation, leading the regeneration process to yield the new tissue [95•]. Moreover, it shows adequate porosity characteristics, allowing oxygen and nutrient diffusion that are fundamental for cell growth and viability [94].
In order to address the cell proliferation and differentiation, SF materials are modified with polysaccharides, such as chitosan and/or hyaluronic acid [69•, 96]. In addition, the crosslinking between SF with calcium phosphates improves the conductivity and bioactivity of the structure, building a favorable environment for tissue regeneration, and supporting the release of fundamental GFs for tissue vascularization, like VEGF (Table 2) [75]. The conductivity of the 3D structure is another critical factor to be considered for optimal cardiovascular tissue engineering. In order to allow proper tissue organization and improve the contractile signal, SF-based structures have been modified with sulfonic acid groups to enable the internalization of the poly-pyrrole element, which possesses electro-conductive features (Table 2) [82•].
Novel technologies using SF as a natural biomaterial showed good integration with the neighboring tissue and support cell growth and viability after in vivo implantation when hollow micro-channels are engineered within the 3D structure [84•, 85•]. After pre-seeding with endothelial cells and implantation of the construct, the infiltration by the host vessels after implantation, but also promotes the tissue ingrowth within the 3D structure and tissue regeneration followed by stem cell transplantation (Table 2) [83].
Other studies demonstrate either in vitro or in vivo a fast extent of endothelial cells and revascularization of SF-based small-diameter vascular grafts, showing elasticity and resistance to coagulation and fibrin network formation (Table 2) [86•].
To promote wounds healing, SF-based electrospuns revealed optimal characteristics to be repopulated by cells and lead the process. Moreover, these kind of silk scaffolds are recently used for bone or ligament replacements and for vascular implants (Table 2) [74••].
Cardiac tissue engineering also includes the optimization SF hydrogels with components directly derived from the native tissue. For instance, silk-based hydrogels including cardiac tissue derived ECM show an enhanced cell infiltration and ingrowth and improve the interactions between cells and ECM within the hydrogel (Table 2) [89].
Silk Fibroin as a Bioink for Cardiovascular Tissues
Silk Fibroin Used as Bioink and/or Hydrogel Formulation to 3D Bioprint Blood Vessels and Cardiac Tissue
Given its exceptional structural and biological properties, SF is a promising biomaterial to be used in many tissue engineering approaches [66••, 97•]. Recently, it has been employed as a bioink to reproduce the native morphology and characteristics of the tissue environment through via 3D bioprinting [91••, 98].
Although SF has been already used in cardiovascular tissue engineering applications, such as vascular implants, only limited SF-based bioink formulations to 3D bioprint blood vessels and cardiac tissues have been investigated [64••, 74••]. However, the bioprinting of SF-based constructs has been used to engineer skin, cartilage, bone, and neural tissues (Table 2) [65••, 67, 68••]. Nevertheless, due to unique features of SF and its relative ease of modification, SF has the potential to be used as bioink to 3D bioprint cardiovascular tissue models [74••, 94, 95•].
The most common technique used to 3D bioprint SF-based cardiovascular tissues is based on the extrusion of the biomaterial [42••, 91••]. This methodology leads the building of hollow and complex networks, which enable cell ingrowth and survival after the bioink layer-by-layer deposition (Fig. 1) [42••]. However, SF-based cardiovascular bioinks can also be bioprinted through the inkjet technology by depositing spheroidal structures [65••, 66••].
Silk chemical composition shows excellent function when formulated as a hydrogel [74••]. Due to the hydrophilic motifs present in its inner architecture, SF-based hydrogels are able to trap many water molecules that lead the swelling of the structure [54, 55•, 74••]. This feature is important to make the construct hydrated enough to support cell viability for a long period [53••]. Moreover, SF hydrogel porosity enables cell encapsulation and the diffusion of oxygen and nutrients inside 3D network, leading a fine cell functioning and attachment within the bioink formulation [53••, 70••, 89]. Given the porosity and hydrophilic properties, SF hydrogel formulations also demonstrate a very efficient drug and GF delivery [74••].
SF hydrogel formulations present a combination of different polymers. Typical SF combinations with natural polymers used in 3D bioprinting technology are SF-alginate, SF-gelatin, SF-collagen, and SF-chitosan, while SF and synthetic polymer formulations are SF-polyethylene glycol (PEG), SF-glycidyl methacrylate (GMA). Normally, they are involved in bone and cartilage tissue engineering, but bioinks like SF-collagen are utilized in neural tissue engineering too [53••, 64••, 65••, 67,68,69,70,71]. SF-alginate bioinks were found to be optimal for soft tissue engineering, like cartilage, ligament, and skin tissues, but they also displayed promising characteristics to be utilized in vascular tissue engineering [65••, 70••]. An example of 3D bioprinted vascular structures is given by the inkjet bioprinting of SF-based bioink using alginate as a sacrificial hydrogel [66••]. The SF-alginate gelation process faces a two-step crosslinking process using the horseradish peroxide (HRP) and hydrogen peroxide. Another example of SF hydrogel formulation used as bioink to bioprint cardiovascular structures is SF crosslinked with GMA (Table 2) [64••, 65••]. GMA is covalently bound to SF through the methacrylation reaction and the digital light processing bioprinting technology allowed to 3D print both cardiac and vascular structures [64••]. To enhance the fabrication of blood vessels, the PEG-tetra acrylate is tuned by embedding melanin nanoparticles into SF hydrogel [65••].
Challenges to Overcome with the Use of Silk Fibroin in Bioinks
The selection of a bioink is a key factor in the success of the bioprinting of a 3D construct. It has been demonstrated that bioinks formed by a single hydrogel component can lack fundamental elements for the bioprinting process, like a good printability and cell function [42••]. Moreover, given their weak mechanical properties, many hydrogels are not capable of maintaining the desired shape after being bioprinted [41••, 42••].
Though SF displays relevant features to be used as hydrogel or bioink, such as high hydrophilicity, good porosity, and cytocompatibility, recent studies proved that SF is difficult to print alone, because of its slow gelation and degradation rates [53••, 66••, 68••, 70••, 72••]. The conformation transition from α-motifs to β-sheets induced by shear-thinning forces during the printing process causes SF slow degradation and gelation processes [53••, 70••]. Moreover, β-sheets formation can cause the obstruction of the nozzle during the extrusion-based bioprinting process [42••, 53••]. Thus, due to its printability limits, SF is mixed with other polymers in order to create hybrid hydrogels [42••, 53••, 70••]. Furthermore, the combination of different biomaterials involves a major structural integrity of the construct after being bioprinted and results in a more mechanical stability and improved biological properties (Table 2) [42••, 49•]. For instance, the crosslinking of SF with natural biomaterials, like alginate, chitosan, collagen, gelatin, and hyaluronic acid, enhances the rheological and mechanical properties of the hydrogel and supports a better cell adhesion and growth [65••].
In addition, the crosslinking of SF with synthetic polymers, such as PEG, helps to control the β-sheets generation and augments the flow of the hydrogel through the tip and nozzle of the bioprinter [42••]. To improve the printability of SF-based bioinks, SF is also crosslinked to natural polymers, like alginate or gelatin (Table 2). The binding to gelatin polymer occurs through weak interactions, such as hydrogen bonding and Van der Waals forces, which are reversible and guarantee the transition between the aqueous solution and gel formation. However, they require additional crosslinking, because they can result in uncontrolled degradation processes [53••].
In order to promote quick gelation during the bioprinting process, SF is mixed with alginate, which allows the first part of the gelation process (Table 2). Alginate has the property to gelate? fast in presence of bivalent cations, like calcium ions [66••, 70••]. Following the HRP catalysis in the presence of hydrogen peroxide, HRP covalently binds two SF tyrosine residues, resulting in a complete alginate and SF biomaterials crosslinking [65••, 66••].
The gelation process through enzymes has been proved to support high cell viability within the bioink. Using enzymes, such tyrosinase, SF is crosslinked with gelatin via oxidation of some SF tyrosine residues either on heavy and light chains and some residues on gelatin. These oxidized residues bind to free SF and/or gelatin amine groups and build a positive crosslinking between both biomaterials, revealing good cell compatibility features (Table 2) [53••].
The SF crosslinking process could also be enabled by the chemical modification of the protein. The methacrylation process of SF by linking GMA to its amino groups (NH2) led to the fabrication of a methacrylated SF-based hydrogel (Sil-MA) (Fig. 3 a and b). This kind of modified SF formulation showed improved mechanical features, such as strength and resistance after printing, and good cytocompatibility in vitro (Table 2) [64••, 65••].
a SF methacrylation with glycidyl methacrylate (GMA). SF molecular structure is tuned with GMA, which forms a covalent linkage with the SF amino groups (NH2), to fabricate a methacrylated SF (Sil-MA). Reproduced with permission from Springer Nature, April 2018, under Creative Commons CC BY 4.0 license [64••]. b SF polymerization using Lithium phenyl(2,4,6-trimethylbenzoyl) phosphinate (LAP). Sil-MA solution is modified by adding LAP photoinitiator that starts the polymerization process. Because GMA is a donor of vinyl double bond (−CH=CH2) as a UV-crosslinking site, in the presence of LAP, GMA reacts between or intra-chain and crosslinks with SF chains. The green chains indicate SF α-helices and the purple ones represent SF β-sheets. Reproduced with permission from Springer Nature, April 2018, under Creative Commons CC BY 4.0 license [64••]
A novel rapid and efficient approach to crosslink SF is a light-mediated reaction. This method permits a high-density cell embedding and great cell survival within the 3D network, which exhibits more stable mechanical features without undertaking structural transitions, typical during enzymatic crosslinking processes (Table 2) [73••].
Discussion
In this manuscript, the importance of the surrounding microenvironment post injury to heart tissue is described. In particular, it focuses on advanced technologies, which enable the fabrication of 3D networks, based on polymeric materials, reflecting the native tissue features. The current advancements in framing 3D constructs focus on biomaterials, which can recapitulate in vivo ECM features in in vitro cardiac cultures. The prime interest of introducing such biomaterials to 3D cultures is to provide the cells with a suitable platform to migrate and proliferate and in case of an injury to help promoting repair of damaged tissue [48•, 55•, 56].
It has been shown in various different studies that the 3D cultures either bioprinted or cells encapsulated within biomaterials better modulate the artificial ECM that recreates the native tissue microenvironment and promote cell attachment within the cultures. Moreover, these biomaterials enhance the viability of cells after injecting them in the damaged site [16, 55•].
In this context, SF as a natural polymer possesses unique properties making it a potential biomaterial to be utilized for biofabrication of cardiovascular tissues and to promote revascularization [21•, 74••].
Biomaterials formulated as hydrogels display relevant features, such as biocompatibility and biodegradability, to be used in many tissue engineering applications, which are fundamental for in vivo testing, but emerged also promising formulations to be utilized as cell-laden bioinks in the 3D bioprinting technology [41••, 42••].
SF-based hydrogels exhibit good stiffness and elasticity typical of cardiac tissues: they are ductile, resistant, and versatile, and due to their porosity and viscosity, they show good potential to be used as bioinks in the 3D bioprinting technology [53••, 74••]. This high-throughput technology offers many advantages compared to the manual procedure to fabricate of 3D cultures; for instance, it enables a good cell delivery within the hydrogel and guarantees a more homogeneous environment for cell growth [39•, 40].
However, even if SF hydrogels possess tunable properties, they do not satisfy all the requirements to be used in 3D printing procedures. Like most single hydrogel bioinks, it does not meet the all features required to bioprint a 3D construct, which are bioprintability and biofunctionality. For example, during the bioprinting process, SF bioinks face shear-thinning forces that result in conformational modifications. These include the transition from α-helices to β-sheets motifs, which causes slower gelation and degradation rates [42••, 53••, 70••, 72••].
To overcome this silk behavior and promote an ameliorated printability and biocompatibility, silk is often crosslinked with other biomaterials, building hybrid hydrogels (Table 2). Specifically, the use of natural polymer-based hybrid hydrogels maintains the low immunogenicity of the 3D network, but also increases the fluidity of SF formulations when applied to the bioprinter, avoiding the obstruction of the nozzle. Moreover, this kind of hydrogels optimizes their printability, showing better strength, preserving their shape and making them more durable and stable after the bioprinting process [42••, 53••, 65••, 70••]. An efficient mechanism of combining SF with other polymers, like gelatin, was shown by the crosslinking via an enzymatic reaction. This resulted in an improved resistance of the 3D construct with good cell viability. All these features can be obtained by changing the SF formulation too. Binding an ester (GMA) to the SF structure inducing a methacrylation reaction, the SF network shows increased strength and good cytocompatibility [53••, 64••].
All these considerations lead to the conclusion that the use of more than two biomaterials in the development of bioinks to bioprint cardiovascular tissues can be an effective solution to ensure a better printability of the sample, but also good cell survival. Nevertheless, natural polymers have already been used to print hybrid cardiovascular tissues are for example alginate and gelatin, showing very promising results in terms of cell viability and tissue revascularization. Given the excellent stability and cytocompatibility characteristics of alginate and the rheological properties of gelatin, in combination with those of SF, new bioink formulations can be obtained to fabricate 3D vascularized tissue capable of integrating with the patient’s tissue [44, 47, 65••].
Conclusions
The emerging and novel field of 3D bioprinting technology represents a big opportunity in the world of biomedicine. Through the blend of biomaterials and stem cells, 3D bioprinters guide the building of 3D cellular and extracellular networks that act as the native tissue environment to promote cell attachment, proliferation, and regeneration.
This new method is able to produce 3D models to study and modulate the growth of the novel tissues and represents a promising approach to replace organ or tissue transplants, avoiding the risks of rejection present in allografts.
Moreover, thanks to the favorable properties of SF for cardiovascular tissue engineering, this natural biomaterial presents great potential to be used in hydrogel formulations to 3D bioprint cardiovascular tissues. The combination of SF and other biomaterials represents an optimal approach to overcome many issues occurring during the printing process, such as cell viability and vascular network formation.
Therefore, we propose hybrid SF-based bioprinted constructs as promising cardiovascular 3D model to better recapitulate the in vivo human heart features compared to other biomaterials.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: a review of global methodologies of mortality measurement. Circulation. 2013;127:749–56. https://doi.org/10.1161/circulationaha.112.128413.
Stoppel WL, Hu D, Domian IJ, Kaplan DL, Black LD 3rd. Anisotropic silk biomaterials containing cardiac extracellular matrix for cardiac tissue engineering. Biomed Mater. 2015;10:034105. https://doi.org/10.1088/1748-6041/10/3/034105.
Uygur A, Lee RT. Mechanisms of cardiac regeneration. Dev Cell. 2016;36:362–74. https://doi.org/10.1016/j.devcel.2016.01.018.
Zhu W, Zhang E, Zhao M, Chong Z, Fan C, Tang Y, et al. Regenerative potential of neonatal porcine hearts. Circulation. 2018;138:2809–16. https://doi.org/10.1161/circulationaha.118.034886.
Kikuchi K, Poss KD. Cardiac regenerative capacity and mechanisms. Annu Rev Cell Dev Biol. 2012;28:719–41. https://doi.org/10.1146/annurev-cellbio-101011-155739.
Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6:239rv1. https://doi.org/10.1126/scitranslmed.3006681.
Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014;6:1120–8. https://doi.org/10.3978/j.issn.2072-1439.2014.06.44.
Tehzeeb J, Manzoor A, Ahmed MM. Is stem cell therapy an answer to heart failure: a literature search. Cureus. 2019;11:e5959. https://doi.org/10.7759/cureus.5959.
Vega E, Schroder J, Nicoara A. Postoperative management of heart transplantation patients. Best Pract Res Clin Anaesthesiol. 2017;31:201–13. https://doi.org/10.1016/j.bpa.2017.06.002.
Campbell M, Surija L, Peceros K, Sharma P, Figtree G, Gentile C. Stem cell spheroids. Encyclopedia of Tissue Engineering and Regenerative Medicine: Elsevier; 2019. p. 387–93.
Mawad D, Figtree G, Gentile C. Current technologies based on the knowledge of the stem cells microenvironments. Adv Exp Med Biol. 2017;1041:245–62. https://doi.org/10.1007/978-3-319-69194-7_13.
Woodcock EA, Matkovich SJ. Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol. 2005;37:1746–51. https://doi.org/10.1016/j.biocel.2005.04.011.
Campbell M, Chabria M, Figtree GA, Polonchuk L, Gentile C. Stem cell-derived cardiac spheroids as 3D in vitro models of the human heart microenvironment. Methods Mol Biol. 2019;2002:51–9. https://doi.org/10.1007/7651_2018_187.
Polonchuk L, Chabria M, Badi L, Hoflack JC, Figtree G, Davies MJ, et al. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci Rep. 2017;7:7005. https://doi.org/10.1038/s41598-017-06385-8.
Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, et al. A hyaluronan-based injectable hydrogel improves the survival and integration of stem cell progeny following transplantation. Stem Cell Rep. 2015;4:1031–45. https://doi.org/10.1016/j.stemcr.2015.04.008.
Günter J, Wolint P, Bopp A, Steiger J, Cambria E, Hoerstrup SP, et al. Microtissues in cardiovascular medicine: regenerative potential based on a 3D microenvironment. Stem Cells Int. 2016;2016:9098523–0. https://doi.org/10.1155/2016/9098523.
O'Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14:88–95. https://doi.org/10.1016/S1369-7021(11)70058-X.
Peña B, Laughter M, Jett S, Rowland TJ, Taylor MRG, Mestroni L, et al. Injectable hydrogels for cardiac tissue engineering. Macromol Biosci. 2018;18:e1800079. https://doi.org/10.1002/mabi.201800079.
•• Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3:144–56. https://doi.org/10.1016/j.bioactmat.2017.11.008. It provides a detailed description about the different 3D novel bioprinting technologies, the biomaterial characteristics, and their applications.
Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65:457–70. https://doi.org/10.1016/j.addr.2012.09.043.
• Wang D, Liu H, Fan Y. Silk fibroin for vascular regeneration. Microsc Res Tech. 2017;80:280–90. https://doi.org/10.1002/jemt.22532. It provides information about SF conformation and its main features.
Weinhaus AJ, Roberts KP. Anatomy of the human heart. Handbook of cardiac anatomy, physiology, and devices: Springer; 2005.
Li J, Zhang K, Huang N. Engineering cardiovascular implant surfaces to create a vascular endothelial growth microenvironment. Biotechnol J. 2017;12. https://doi.org/10.1002/biot.201600401.
Quagliariello V, Piscopo G, Maurea N. Cardiac and vascular microenvironment: biological and clinical implications in cardiology. Biomed J Sci Tech Res. 2018;7:5980–2. https://doi.org/10.26717/BJSTR.2018.07.001523.
Reinhart-King CA, Fujiwara K, Berk BC. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol. 2008;443:25–44. https://doi.org/10.1016/s0076-6879(08)02002-8.
•• Fleischer S, Tavakol DN, Vunjak-Novakovic G. From arteries to capillaries: approaches to engineering human vasculature. Adv Funct Mater. 2020;1910811. https://doi.org/10.1002/adfm.201910811. It provides a detailed description of the blood vessel tree, its structure, and its importance during the vascularization process.
Figtree GA, Bubb KJ, Tang O, Kizana E, Gentile C. Vascularized cardiac spheroids as novel 3D in vitro models to study cardiac fibrosis. Cells Tissues Organs. 2017;204:191–8. https://doi.org/10.1159/000477436.
Daley MC, Fenn SL, Black LD 3rd. Applications of cardiac extracellular matrix in tissue engineering and regenerative medicine. Adv Exp Med Biol. 2018;1098:59–83. https://doi.org/10.1007/978-3-319-97421-7_4.
• Del Monte-Nieto G, Fischer JW, Gorski DJ, Harvey RP, Kovacic JC. Basic biology of extracellular matrix in the cardiovascular system, part 1/4: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:2169–88. https://doi.org/10.1016/j.jacc.2020.03.024. It gives a good description of the cardiovascular microenvironment.
Barallobre-Barreiro J, Loeys B, Mayr M, Rienks M, Verstraeten A, Kovacic JC. Extracellular matrix in vascular disease, part 2/4: JACC focus seminar. J Am Coll Cardiol 2020:75:2189–2203. https://doi.org/10.1016/j.jacc.2020.03.018.
Frangogiannis NG, Kovacic JC. Extracellular matrix in ischemic heart disease, part 4/4: JACC focus seminar. J Am Coll Cardiol 2020:75:2219–2235. https://doi.org/10.1016/j.jacc.2020.03.020.
Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci 2001:22:201–207. https://doi.org/10.1016/s0165-6147(00)01676-x.
Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neuro-Oncol. 2000;50:1–15. https://doi.org/10.1023/a:1006493130855.
ROKIT H. ROKIT Invivo. 2016 [Available from: https://rokithealthcare.com/invivo/.
REGEMAT3D. REGEMAT 3D. 2011 [Available from: https://www.regemat3d.com/en/.
INVENTIA LS. RASTRUM. 2013 [Available from: https://inventia.life/.
CELLINK LS BIO X 2020 [Available from: https://www.cellink.com/product/cellink-bio-x/.
•• Zhang B, Gao L, Ma L, Luo Y, Yang H, Cui Z. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering. 2019;5:777–94. https://doi.org/10.1016/j.eng.2019.03.009. It provides a detailed description about the different 3D novel bioprinting technologies, the biomaterials characteristics, and their applications.
• Ashammakhi N, Ahadian S, Xu C, Montazerian H, Ko H, Nasiri R, et al. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater Today Bio. 2019;1:100008. https://doi.org/10.1016/j.mtbio.2019.100008. It provides information about the characteristics, properties, and requirements of a bioink during the printing process.
Wei L, Wu S, Kuss M, Jiang X, Sun R, Reid P, et al. 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering. Bioact Mater. 2019;4:256–60. https://doi.org/10.1016/j.bioactmat.2019.09.001.
•• Donderwinkel I, Van Hest JCM, Cameron NR. Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem. 2017;8:4451–71. https://doi.org/10.1039/C7PY00826K. It provides a detailed description about different 3D bioprinting techniques, biomaterials characteristics, and their applications.
•• Cui X, Li J, Hartanto Y, Durham M, Tang J, Zhang H, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Adv Healthc Mater. 2020:e1901648. https://doi.org/10.1002/adhm.201901648. It provides a detailed description about the different 3D bioprinting technniques, biomaterials characteristics, and their applications. It is one of the latest studies on the use and applications of SF in the field of tissue engineering, including SF hydrogel formulations.
Zhang X, Zhang Y. Tissue engineering applications of three-dimensional bioprinting. Cell Biochem Biophys. 2015;72:777–82. https://doi.org/10.1007/s12013-015-0531-x.
Tomasina C, Bodet T, Mota C, Moroni L, Camarero-Espinosa S. Bioprinting vasculature: materials, cells and emergent techniques. Materials (Basel, Switzerland). 2019;12. https://doi.org/10.3390/ma12172701.
Zhang YS, Oklu R, Dokmeci MR, Khademhosseini A. Three-dimensional bioprinting strategies for tissue engineering. Cold Spring Harbor Perspect Med. 2018;8. https://doi.org/10.1101/cshperspect.a025718.
• Cui H, Miao S, Esworthy T, Zhou X, Lee SJ, Liu C, et al. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev. 2018;132:252–69. https://doi.org/10.1016/j.addr.2018.07.014. It provides information about several 3D bioprinting characteristics and techniques, especially on how to 3D bioprint cardiovascular tissues.
Roche CD, Brereton RJL, Ashton AW, Jackson C, Gentile C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur J Cardiothorac Surg. 2020;58:500–10. https://doi.org/10.1093/ejcts/ezaa093.
• Jang J. 3D bioprinting and in vitro cardiovascular tissue modeling. Bioengineering (Basel, Switzerland). 2017;4. https://doi.org/10.3390/bioengineering4030071. It provides information about properties and requirements of a bioink during the printing process.
• Buitrago JO, Patel KD, El-Fiqi A, Lee JH, Kundu B, Lee HH, et al. Silk fibroin/collagen protein hybrid cell-encapsulating hydrogels with tunable gelation and improved physical and biological properties. Acta Biomater. 2018;69:218–33. https://doi.org/10.1016/j.actbio.2017.12.026. It includes studies to overcome some issues in the generation of optimal SF-based hydrogels.
Hassan ME, Bai J, Dou DQ. Biopolymers; definition, classification and applications. Egypt J Chem. 2019;62:1725–37. https://doi.org/10.21608/ejchem.2019.6967.1580.
Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR. Bioinks for 3D bioprinting: an overview. Biomater Sci. 2018;6:915–46. https://doi.org/10.1039/c7bm00765e.
Zhang YS, Yue K, Aleman J, Moghaddam KM, Bakht SM, Yang J, et al. 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng. 2017;45:148–63. https://doi.org/10.1007/s10439-016-1612-8.
•• Chawla S, Midha S, Sharma A, Ghosh S. Silk-based bioinks for 3D bioprinting. Adv Healthc Mater. 2018;7:e1701204. https://doi.org/10.1002/adhm.201701204. It provides a list of SF formulations and their applications for tissue engineering. It is one of the latest studies on the use and applications of SF in the field of tissue engineering, including SF hydrogel formulations.
Hölzl K, Lin S, Tytgat L, Van Vlierberghe S, Gu L, Ovsianikov A. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8:032002. https://doi.org/10.1088/1758-5090/8/3/032002.
• Alagarsamy KN, Yan W, Srivastava A, Desiderio V, Dhingra S. Application of injectable hydrogels for cardiac stem cell therapy and tissue engineering. Rev Cardiovasc Med. 2019;20:221–30. https://doi.org/10.31083/j.rcm.2019.04.534. It provides insights about the biomaterials characteristics and their cardiac tissue engineering utilize.
Camci-Unal G, Annabi N, Dokmeci MR, Liao R, Khademhosseini A. Hydrogels for cardiac tissue engineering. NPG Asia Mater. 2014;6:e99-e. https://doi.org/10.1038/am.2014.19.
Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21. https://doi.org/10.1016/j.jare.2013.07.006.
Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2015;2:1500122. https://doi.org/10.1002/advs.201500122.
Vasile C, Pamfil D, Stoleru E, Baican M. New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules (Basel, Switzerland). 2020;25. https://doi.org/10.3390/molecules25071539.
Palmese LL, Thapa RK, Sullivan MO, Kiick KL. Hybrid hydrogels for biomedical applications. Current opinion in chemical engineering. 2019;24:143–57. https://doi.org/10.1016/j.coche.2019.02.010.
Lau HK, Kiick KL. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules. 2015;16:28–42. https://doi.org/10.1021/bm501361c.
McGann CL, Levenson EA, Kiick KL. Resilin-based hybrid hydrogels for cardiovascular tissue engineering. Macromolecules. 2013;214:203–13. https://doi.org/10.1002/macp.201200412.
Dong Q, Zhong X, Zhang Y, Bao B, Liu L, Bao H, et al. Hyaluronic acid-based antibacterial hydrogels constructed by a hybrid crosslinking strategy for pacemaker pocket infection prevention. Carbohydr Polym. 2020;245:116525. https://doi.org/10.1016/j.carbpol.2020.116525.
•• Kim SH, Yeon YK, Lee JM, Chao JR, Lee YJ, Seo YB, et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat Commun. 2018;9:1620. https://doi.org/10.1038/s41467-018-03759-y. It one of the latest studies addressing how to overcome some issues in bioprinting SF-based formulations and obtain better features of the 3D bioprinted structures.
•• Wang Q, Han G, Yan S, Zhang Q. 3D printing of silk fibroin for biomedical applications. Materials (Basel, Switzerland). 2019;12. https://doi.org/10.3390/ma12030504. It is one of the latest studies to overcome some issues in SF-based formulations.
•• Compaan AM, Christensen K, Huang Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater Sci Eng. 2017;3:1519–26. https://doi.org/10.1021/acsbiomaterials.6b00432. It represents one of the latest studies to overcome some issues in bioprinting SF-based formulations.
Jiang JP, Liu XY, Zhao F, Zhu X, Li XY, Niu XG, et al. Three-dimensional bioprinting collagen/silk fibroin scaffold combined with neural stem cells promotes nerve regeneration after spinal cord injury. Neural Regen Res. 2020;15:959–68. https://doi.org/10.4103/1673-5374.268974.
•• Singh YP, Bandyopadhyay A, Mandal BB. 3D bioprinting using cross-linker-free silk-gelatin bioink for cartilage tissue engineering. ACS Appl Mater Interfaces. 2019;11:33684–96. https://doi.org/10.1021/acsami.9b11644. It is one of the latest studies in overcoming some issues in bioprinting SF hydrogel formulations.
• Li DW, Lei X, He FL, He J, Liu YL, Ye YJ, et al. Silk fibroin/chitosan scaffold with tunable properties and low inflammatory response assists the differentiation of bone marrow mesenchymal stem cells. Int J Biol Macromol. 2017;105:584–97. https://doi.org/10.1016/j.ijbiomac.2017.07.080. It provides information about SF hemocompatibility and regenerative properties.
•• Nguyễn TT, Ratanavaraporn J, Yodmuang S, editors. Alginate-silk fibroin bioink: a printable hydrogel for tissue engineering. 2019 12th Biomedical Engineering International Conference (BMEiCON); 2019: IEEE. It is one of the latest studies in overcoming some issues in bioprinting SF hydrogel formulations.
Das S, Pati F, Choi YJ, Rijal G, Shim JH, Kim SW, et al. Bioprintable, Cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233.
•• Huang W, Ling S, Li C, Omenetto FG, Kaplan DL. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem Soc Rev. 2018;47:6486–504. https://doi.org/10.1039/c8cs00187a. It provides information about SF conformation and its main features, including its denaturation and extraction processes.
•• Cui X, Soliman BG, Alcala-Orozco CR, Li J, Vis MAM, Santos M, et al. Rapid photocrosslinking of silk hydrogels with high cell density and enhanced shape fidelity. Adv Healthc Mater. 2020;9:e1901667. https://doi.org/10.1002/adhm.201901667. It is one of the latest studies to overcome some issues in bioprinting SF-based formulations. It provides information about SF conformation and its main features, including the denaturation and extraction processes.
•• Holland C, Numata K, Rnjak-Kovacina J, Seib FP. The biomedical use of silk: past, present, future. Adv Healthc Mater. 2019;8:e1800465. https://doi.org/10.1002/adhm.201800465. It provides a list of SF regenerated forms and applications in the field of tissue engineering.
Ribeiro VP, Pina S, Costa JB, Cengiz IF, García-Fernández L, Fernández-Gutiérrez MDM, et al. Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration. ACS Appl Mater Interfaces. 2019;11:3781–99. https://doi.org/10.1021/acsami.8b21259.
Rnjak-Kovacina J, Wray LS, Burke KA, Torregrosa T, Golinski JM, Huang W, et al. Lyophilized silk sponges: a versatile biomaterial platform for soft tissue engineering. ACS Biomater Sci Eng. 2015;1:260–70. https://doi.org/10.1021/ab500149p.
Koh L-D, Cheng Y, Teng C-P, Khin Y-W, Loh X-J, Tee S-Y, et al. Structures, mechanical properties and applications of silk fibroin materials. Prog Polym Sci. 2015;46:86–110. https://doi.org/10.1016/j.progpolymsci.2015.02.001.
Rajkhowa R, Wang X. Silk powder for regenerative medicine. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. Elsevier; 2014.
Talukdar S, Kundu SC. Silk scaffolds for three-dimensional (3D) tumor modeling. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. Elsevier; 2014.
Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials. 2015;71:145–57. https://doi.org/10.1016/j.biomaterials.2015.08.039.
Ling S, Zhang Q, Kaplan DL, Omenetto F, Buehler MJ, Qin Z. Printing of stretchable silk membranes for strain measurements. Lab Chip. 2016;16:2459–66. https://doi.org/10.1039/c6lc00519e.
• Tsui JH, Ostrovsky-Snider NA, Yama DMP, Donohue JD, Choi JS, Chavanachat R, et al. Conductive silk-polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. J Mater Chem B. 2018;6:7185–96. https://doi.org/10.1039/c8tb01116h. It is one of the latest studies to overcome some issues in SF-based formulations.
Zhang W, Wray LS, Rnjak-Kovacina J, Xu L, Zou D, Wang S, Zhang M, Dong J, Li G, Kaplan DL, Jiang X Vascularization of hollow channel-modified porous silk scaffolds with endothelial cells for tissue regeneration. Biomaterials 2015:56:68–77. https://doi.org/10.1016/j.biomaterials.2015.03.053.
• Rnjak-Kovacina J, Gerrand YW, Wray LS, Tan B, Joukhdar H, Kaplan DL, et al. Vascular pedicle and microchannels: simple methods toward effective in vivo vascularization of 3D scaffolds. Adv Healthc Mater. 2019;8:e1901106. https://doi.org/10.1002/adhm.201901106. It is one of the latest studies on the use and applications of SF in the field of vascular tissue engineering.
• Tang F, Manz XD, Bongers A, Odell RA, Joukhdar H, Whitelock JM, et al. Microchannels are an architectural cue that promotes integration and vascularization of silk biomaterials in vivo. ACS Biomater Sci Eng. 2020;6:1476–86. https://doi.org/10.1021/acsbiomaterials.9b01624. It is one of the latest studies on SF applications in the field of cardiovascular tissue engineering.
• Filipe EC, Santos M, Hung J, Lee BSL, Yang N, Chan AHP, et al. Rapid endothelialization of off-the-shelf small diameter silk vascular grafts. JACC Basic Transl Sci. 2018;3:38–53. https://doi.org/10.1016/j.jacbts.2017.12.003. It is one of the latest studies on SF applications in the field of cardiovascular tissue engineering.
• Kondyurin A, Lau K, Tang F, Akhavan B, Chrzanowski W, Lord MS, et al. Plasma ion implantation of silk biomaterials enabling direct covalent immobilization of bioactive agents for enhanced cellular responses. ACS Appl Mater Interfaces. 2018;10:17605–16. https://doi.org/10.1021/acsami.8b03182. It is one of the latest studies on the use and applications of SF in the field of cardiovascular tissue engineering.
Rnjak-Kovacina J, Tang F, Whitelock JM, Lord MS. Silk biomaterials functionalized with recombinant domain V of human perlecan modulate endothelial cell and platelet interactions for vascular applications. Colloids Surf B: Biointerfaces. 2016;148:130–8. https://doi.org/10.1016/j.colsurfb.2016.08.039.
Stoppel WL, Gao AE, Greaney AM, Partlow BP, Bretherton RC, Kaplan DL, et al. Elastic, silk-cardiac extracellular matrix hydrogels exhibit time-dependent stiffening that modulates cardiac fibroblast response. J Biomed Mater Res A. 2016;104:3058–72. https://doi.org/10.1002/jbm.a.35850.
• Feng J, Wu Y, Chen W, Li J, Wang X, Chen Y, et al. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J Mater Chem B. 2020;8:308–15. https://doi.org/10.1039/c9tb01971e. It is one of the latest studies in overcoming some issues in SF-based formulations.
•• Włodarczyk-Biegun MK, Del Campo A. 3D bioprinting of structural proteins. Biomaterials. 2017;134:180–201. https://doi.org/10.1016/j.biomaterials.2017.04.019. It shows some studies and applications of SF in the field of tissue engineering.
• Karthik T, Rathinamoorthy R. Sustainable silk production. Sustain Fibres Text. Elsevier. 2017; It provides information about SF conformation and its main features.
Murphy AR, Romero IS. Biochemical and biophysical properties of native Bombyx mori silk for tissue engineering applications. Silk Biomaterials for Tissue Engineering and Regenerative Medicine. Elsevier. 2014
Wray LS, Rnjak-Kovacina J, Mandal BB, Schmidt DF, Gil ES, Kaplan DL. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials. 2012;33:9214–24. https://doi.org/10.1016/j.biomaterials.2012.09.017.
• Mulinti P, Brooks JE, Lervick B, Pullan JE, Brooks AE. Strategies to improve the hemocompatibility of biodegradable biomaterials. Hemocompatibility Biomater Clin Appl. Elsevier. 2018; It provides information about SF hemocompatibility and regenerative properties.
Yang MC, Wang SS, Chou NK, Chi NH, Huang YY, Chang YL, et al. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials. 2009;30:3757–65. https://doi.org/10.1016/j.biomaterials.2009.03.057.
• Chan AHP, Filipe EC, Tan RP, Santos M, Yang N, Hung J, et al. Altered processing enhances the efficacy of small-diameter silk fibroin vascular grafts. Sci Rep. 2019;9:17461. https://doi.org/10.1038/s41598-019-53972-y. It is one of the latest studies in overcoming some issues in bioprinting SF-based formulations.
Sommer MR, Schaffner M, Carnelli D, Studart AR. 3D printing of hierarchical silk fibroin structures. ACS Appl Mater Interfaces. 2016;8:34677–85. https://doi.org/10.1021/acsami.6b11440.
Funding
LV is supported by a University of Technology Sydney (UTS) International Research Scholarship (2020-2023) and a UTS President’s Scholarship (2020-2023). CG is supported by a University of Sydney Kick-Start Grant, CDIP Grant, Cardiothoracic Surgery Research Grant, UTS Seed Funding, and Catholic Archdiocese of Sydney Grant for Adult Stem Cell Research. JR-K was partially supported by the Heart Foundation of Australia Future Leader Fellowship (101896).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomimetic Approaches in Regenerative Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vettori, L., Sharma, P., Rnjak-Kovacina, J. et al. 3D Bioprinting of Cardiovascular Tissues for In Vivo and In Vitro Applications Using Hybrid Hydrogels Containing Silk Fibroin: State of the Art and Challenges. Curr. Tissue Microenviron. Rep. 1, 261–276 (2020). https://doi.org/10.1007/s43152-020-00026-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43152-020-00026-5