Abstract
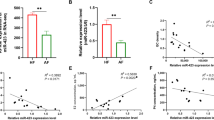
Our study aimed to elucidate the effect of miR-34a mimics and miR-34a inhibitor and their combination on basic functions of ovarian cells cultured with and without FSH, and effect of FSH on expression of endogenous miR-34a. Viability, proliferation, proportion of proliferative active cells, apoptosis, proportion of DNA fragmented cells, accumulation of FSHR, steroid hormones, IGF-I, oxytocin, and prostaglandin E2 release, and expression levels of miR-34a were analysed. MiR-34a mimics decreased proliferation, apoptosis, testosterone, and estradiol output, stimulated release of progesterone, IGF-I, oxytocin, and occurrence of FSHR. MiR-34a inhibitor had an opposite effect and prevented effects of miR-34a mimics. FSH promoted expression of miR-34a, viability, proliferation, steroid hormones, IGF-I, oxytocin, and prostaglandin E2 output, and reduced apoptosis. Furthermore, miR-34a mimics supported effect of FSH on viability, apoptosis, progesterone, and IGF-I and inverted FSH action on proliferation, testosterone, and estradiol output. Our observations suggest that miR-34a can be involved in control of basic ovarian functions and that miR-34a and FSH are synergists in their actions on ovarian cell functions. Ability of FSH to promote miR-34a expression and ability of miR-34a mimics to increase occurrence of FSHR and to modify FSH effects suggest the existence of self-stimulating FSH-miR-34a axis, and that miR-34a can mediate actions of FSH on ovarian cells.
Graphical Abstract














Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Code Availability
Not applicable.
References
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402. https://doi.org/10.3389/fendo.2018.00402.
Salas-Huetos A, James ER, Aston KI, Jenkins TG, Carrell DT, Yeste M. The expression of miRNAs in human ovaries, oocytes, extracellular vesicles, and early embryos: a systematic review. Cells. 2019;8(12):1564. https://doi.org/10.3390/cells8121564.
Tu J, Cheung AH, Chan CL, Chan WY. The role of microRNAs in ovarian granulosa cells in health and disease. Front Endocrinol. 2019;10:174. https://doi.org/10.3389/fendo.2019.00174.
Alshamrani AA. Roles of microRNAs in ovarian cancer tumorigenesis: two decades later, what have we learned? Front Oncol. 2020;10:1084. https://doi.org/10.3389/fonc.2020.01084.
Reza A, Choi YJ, Han SG, Song H, Park C, Hong K, Kim JH. Roles of microRNAs in mammalian reproduction: from the commitment of germ cells to peri-implantation embryos. Biol Rev Camb Philos Soc. 2019;94(2):415–38. https://doi.org/10.1111/brv.12459.
Salilew-Wondim D, Gebremedhn S, Hoelker M, Tholen E, Hailay T, Tesfaye D. The role of microRNAs in mammalian fertility: from gametogenesis to embryo implantation. Int J Mol Sci. 2020;21(2):585. https://doi.org/10.3390/ijms21020585.
Maalouf SW, Liu WS, Pate JL. MicroRNA in ovarian function. Cell Tissue Res. 2016;363(1):7–18. https://doi.org/10.1007/s00441-015-2307-4.
Gecaj RM, Schanzenbach CI, Kirchner B, Pfaffl MW, Riedmaier I, Tweedie-Cullen RY, Berisha B. The dynamics of microRNA transcriptome in bovine corpus luteum during its formation, function, and regression. Front Genet. 2017;8:213. https://doi.org/10.3389/fgene.2017.00213.
Toms D, Pan B, Li J. Endocrine regulation in the ovary by microRNA during the estrous cycle. Front Endocrinol. 2018;8:378. https://doi.org/10.3389/fendo.2017.00378.
Azhar S, Dong D, Shen WJ, Hu Z, Kraemer FB. The role of miRNAs in regulating adrenal and gonadal steroidogenesis. J Molecul Endocrinol. 2020;64(1):R21–43. https://doi.org/10.1530/JME-19-0105.
Zhang L, Liao Y, Tang L. MicroRNA-34 family: a potential tumor suppressor and therapeutic candidate in cancer. J Experiment Clin Cancer Res: CR. 2019;38(1):53. https://doi.org/10.1186/s13046-019-1059-5.
Li R, Shi X, Ling F, Wang C, Liu J, Wang W, Li M. MiR-34a suppresses ovarian cancer proliferation and motility by targeting AXL. Tumour Biol: J Int Soc Oncod Biol Med. 2015;36(9):7277–83. https://doi.org/10.1007/s13277-015-3445-8.
Ding N, Wu H, Tao T, Peng E. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. Onco Targets Ther. 2017;10:4905–15. https://doi.org/10.2147/OTT.S142446.
Slabáková E, Culig Z, Remšík J, Souček K. Alternative mechanisms of miR-34a regulation in cancer. Cell Death Dis. 2017;8(10): e3100. https://doi.org/10.1038/cddis.2017.495.
Lv T, Song K, Zhang L, Li W, Chen Y, Diao Y, Yao Q, Liu P. miRNA-34a decreases ovarian cancer cell proliferation and chemoresistance by targeting HDAC1. Biochem Cell Biol. 2018;96(5):663–671
Welponer H, Tsibulak I, Wieser V, Degasper C, Shivalingaiah G, Wenzel S, Sprung S, Marth C, Hackl H, Fiegl H, Zeimet AG. The miR-34 family and its clinical significance in ovarian cancer. J Cancer. 2020;11(6):1446–56. https://doi.org/10.7150/jca.33831.
Tu F, Pan ZX, Yao Y, Liu HL, Liu SR, Xie Z, Li QF. miR-34a targets the inhibin beta B gene, promoting granulosa cell apoptosis in the porcine ovary. Gen Mol Res: GMR. 2014;13(2):2504–12. https://doi.org/10.4238/2014.January.14.6.
Sirotkin AV, Lauková M, Ovcharenko D, Brenaut P, Mlyncek M. Identification of microRNAs controlling human ovarian cell proliferation and apoptosis. J Cell Physiol. 2010;223(1):49–56. https://doi.org/10.1002/jcp.21999.
Sirotkin AV, Ovcharenko D, Grossmann R, Lauková M, Mlyncek M. Identification of microRNAs controlling human ovarian cell steroidogenesis via a genome-scale screen. J Cell Physiol. 2009;219(2):415–20. https://doi.org/10.1002/jcp.21689.
Sirotkin AV. Regulators of ovarian functions. 2014;New York: Nova Publishers Inc.
Yin M, Wang X, Yao G, Lü M, Liang M, Sun Y, Sun F. Transactivation of micrornA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J Biol Chem. 2014;289(26):18239–57. https://doi.org/10.1074/jbc.M113.546044.
Zhang L, Zhang X, Zhang X, Lu Y, Li L, Cui S. MiRNA-143 mediates the proliferative signaling pathway of FSH and regulates estradiol production. J Endocrinol. 2017;234(1):1–14. https://doi.org/10.1530/JOE-16-0488.
Shukla A, Dahiya S, Onteru SK, Singh D. Differentially expressed miRNA-210 during follicular-luteal transition regulates pre-ovulatory granulosa cell function targeting HRas and EFNA3. J Cell Biochem. 2018;119(10):7934–43. https://doi.org/10.1002/jcb.26508.
Yao N, Yang BQ, Liu Y, Tan XY, Lu CL, Yuan XH, Ma X. Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine. 2010;38(2):158–66. https://doi.org/10.1007/s12020-010-9345-1.
Noferesti SS, Sohel MM, Hoelker M, Salilew-Wondim D, Tholen E, Looft C, Rings F, Neuhoff C, Schellander K, Tesfaye D. Controlled ovarian hyperstimulation induced changes in the expression of circulatory miRNA in bovine follicular fluid and blood plasma. J Ovar Res. 2015;8:81. https://doi.org/10.1186/s13048-015-0208-5.
Yuan H, Lu J, Xiao SY, Han XY, Song XT, Qi MY, Liu GS, Yang CX, Yao YC. miRNA expression analysis of the sheep follicle during the prerecruitment, dominant, and mature stages of development under FSH stimulation. Theriogenology. 2022;181:161–9. https://doi.org/10.1016/j.theriogenology.2022.01.001.
Jiajie T, Yanzhou Y, Hoi-Hung AC, Zi-Jiang C, Wai-Yee C. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci Rep. 2017;7:41304. https://doi.org/10.1038/srep41304.
Du X, Li Q, Pan Z, Li Q. Androgen receptor and miRNA-126* axis controls follicle-stimulating hormone receptor expression in porcine ovarian granulosa cells. Reproduction. 2016;152(2):161–9. https://doi.org/10.1530/REP-15-0517.
Du X, Zhang L, Li X, Pan Z, Liu H, Li Q. TGF-β signaling controls FSHR signaling-reduced ovarian granulosa cell apoptosis through the SMAD4/miR-143 axis. Cell Death Dis. 2016;7(11):e2476. https://doi.org/10.1038/cddis.2016.379.
Gao S, Zhao J, Xu Q, Guo Y, Liu M, Zhang C, Schinckel AP, Zhou B. MiR-31 targets HSD17B14 and FSHR, and miR-20b targets HSD17B14 to affect apoptosis and steroid hormone metabolism of porcine ovarian granulosa cells. Theriogenology. 2022;180:94–102. https://doi.org/10.1016/j.theriogenology.2021.12.014.
Fabová Z, Loncová B, Mlynček M, Sirotkin AV. Kisspeptin as autocrine/paracrine regulator of human ovarian cell functions: possible interrelationships with FSH and its receptor. Reprod Biol. 2021;22(1):100580. https://doi.org/10.1016/j.repbio.2021.100580.
Sirotkin AV, Pelleova B, Fabova Z, Makovicky P, Alwasel S, Halim Harrath A. Rutin directly affects stimulatory action of FSH on the ovarian cell. PharmaNutrition. 2021;15:100247. https://doi.org/10.1016/j.phanu.2020.100247.
Strober W. Trypan blue exclusion test of cell viability. Current Prot Immunol. 2001;Appendix 3. https://doi.org/10.1002/0471142735.ima03bs21
Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annual Rev. 2005;11:127–52. https://doi.org/10.1016/S1387-2656(05)11004-7.
Gaumer S, Guénal I, Brun S, Théodore L, Mignotte B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Different. 2000;7(9):804–14. https://doi.org/10.1038/sj.cdd.4400714.
Osborn M, Brandfass S. Immunocytochemistry of frozen and of paraffin tissue sections. In Celis JE (ed), Cell Biology. 2006;563–569. https://doi.org/10.1016/B978-012164730-8/50069-1
Nygard AB, Jørgensen CB, Cirera S, Fredholm M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Molecular Biol. 2007;8:67. https://doi.org/10.1186/1471-2199-8-67.
Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. https://doi.org/10.1093/nar/29.9.e45.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, Calif.). 2001;25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Rooda I, Hensen K, Kaselt B, Kasvandik S, Pook M, Kurg A, Salumets A, Velthut-Meikas A. Target prediction and validation of microRNAs expressed from FSHR and aromatase genes in human ovarian granulosa cells. Sci Rep. 2020;10(1):2300. https://doi.org/10.1038/s41598-020-59186-x.
Shiomi Y, Nishitani H. Control of genome integrity by RFC complexes; conductors of PCNA loading onto and unloading from chromatin during DNA replication. Genes. 2017;8(2):52. https://doi.org/10.3390/genes8020052.
Nakayama Y, Yamaguchi N. Role of cyclin B1 levels in DNA damage and DNA damage-induced senescence. Int Rev Cell Mol Biol. 2013;305:303–37. https://doi.org/10.1016/B978-0-12-407695-2.00007-X.
Lin X, Guan H, Huang Z, Liu J, Li H, Wei G, Cao X, Li Y. Downregulation of Bcl-2 expression by miR-34a mediates palmitate-induced Min6 cells apoptosis. J Diabetes Res. 2014;2014:258695. https://doi.org/10.1155/2014/258695.
Peña-Blanco A, García-Sáez AJ. Bax, Bak and beyond—mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–31. https://doi.org/10.1111/febs.14186.
An LS, Yuan XH, Hu Y, Shi ZY, Liu XQ, Qin L, Wu GQ, Han W, Wang YQ, Ma X. Progesterone production requires activation of caspase-3 in preovulatory granulosa cells in a serum starvation model. Steroids. 2012;77(13):1477–82. https://doi.org/10.1016/j.steroids.2012.07.011.
Mirzayans R, Murray D. Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int J Mol Sci. 2020;21(23):9090. https://doi.org/10.3390/ijms21239090.
Hockenbery DM. Targeting mitochondria for cancer therapy. Environ Molecul Mutagen. 2010;51(5):476–89. https://doi.org/10.1002/em.20552.
Das N, Kumar TR. Molecular regulation of follicle-stimulating hormone synthesis, secretion and action. J Molecul Endocrinol. 2018;60(3):R131–55. https://doi.org/10.1530/JME-17-0308.
Cohen A, Burgos-Aceves MA, Smith Y. Global microRNA downregulation: all roads lead to estrogen. J Xiangya Med. 2017;2(7). https://jxym.amegroups.com/article/view/4049.
Funding
This research was financially supported by the Slovak Research and Development Agency (APVV), the project APVV-15-0296 and the Scientific Grant Agency of the Ministry of Education, Science, and Sport of Slovak Republic (VEGA), the projects VEGA 13-ENV1321-02 and 1/0680/22.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fabová, Z., Loncová, B., Bauer, M. et al. Interrelationships Between miR-34a and FSH in the Control of Porcine Ovarian Cell Functions. Reprod. Sci. 30, 1789–1807 (2023). https://doi.org/10.1007/s43032-022-01127-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-022-01127-2