Abstract
Prey species develop anti-predatory strategies as a response to minimising the risk of being predated. However, how the European rabbit (Oryctolagus cuniculus) adapts to different predator pressure is not fully known. Here, we studied the adaptive anti-predatory responses of European rabbits exposed to different terrestrial predation pressure. To do this, we took advantage of a rabbit translocation programme in the Sierra Norte Natural Park of Sevilla (SW Spain), where rabbits from the same donor population were translocated in plots with and without terrestrial predator exclusion fences (aerial predation was not excluded in any of the plots). This presented an ideal opportunity to observe whether the behaviour of individuals from the same population adapts to situations with different predator pressure; thus, their behaviour was evaluated through direct observations. Although most rabbits were observed close to cover, differences in distance to cover, group size and behaviour were observed between fenced and unfenced plots. Overall, both adult and juvenile rabbits moved further from cover in the unfenced plot than in the fenced plot. Most of the observations in the unfenced plot corresponded to rabbits in pairs or alone; whereas in the fenced plot, rabbits were primarily in pairs or in larger groups. Our findings suggest that in the unfenced plot, rabbits that moved further from cover were often part of larger groups (≥ 4 rabbits); whereas in the fenced plot, it was rabbits in smaller groups (< 4 rabbits). Rabbits in the unfenced plot were alert and running more frequently than rabbits in the fenced one; in the latter, these rabbits were mostly feeding. Other relaxed behaviours such us grooming or resting were more frequent close to cover. In summary, our results highlight rabbits' capacity to promptly adjust behaviour in response to predation risk, exhibiting adaptive anti-predatory responses tailored to different predation pressures. These insights contribute to understanding the nuanced dynamics of prey species' responses to diverse predation scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prey species have developed a variety of strategies to reduce the strong selective pressures caused by predators (Barnard 1983; Futuyma and Moreno 1988). Physiological, morphological and also behavioural adaptations serve as mechanisms to enhance survival in response to predation risk (Nilsson et al. 1995; Teplitsky et al. 2005; Rouco et al. 2011a; Tobajas et al. 2023). These adaptations can manifest as distinct anti-predatory strategies depending on the type of predation pressure experienced by the prey (e.g. terrestrial vs. aerial predators) (Curio 1975; Hanson and Coss 1997; Taraborelli et al. 2008). However, anti-predatory strategies are costly, including energetic investments in defensive structures and mechanisms (e.g. flight, autotomy), or by potential reductions in mating success (Preisser et al. 2005; Ferretti et al. 2019; Savvides et al. 2019). Therefore, several factors may influence predation risk, such as predator diversity and abundance, availability of alternative prey, habitat characteristics or the perceived risk of predation by the prey. This perception can depend on various factors, including age, group size, predator recognition or habitat characteristics (Bolles 1970; Lima 1995; Villafuerte and Moreno 1997; Blanchard et al. 2016; Savvides et al. 2019).
One of the mammalian prey species that has received significant attention in terms of its anti-predatory responses is the wild European rabbit (Oryctolagus cuniculus). It is native from the Iberian Peninsula, where it is a key prey species for more than 30 predators and where it is one of the main small game species (Delibes-Mateos et al. 2008). Consequently, the European rabbit has been used as a model to test several ecological hypotheses regarding predation risk in mammal prey. This is not only for purely scientific interest (e.g. Villafuerte and Moreno 1997; Monterroso et al. 2013; Descalzo et al. 2021), but also holds significance for conservation efforts, such as bolstering rabbit populations for endangered predators (Ferreira and Delibes-Mateos 2010) and/or game management, including predator control interventions to increase rabbit populations (Calvete and Estrada 2004; Tobajas et al. 2021a, b). Previous studies have unveiled diverse anti-predatory responses in rabbits, including behavioural and physiological mechanisms (Monclús et al. 2005, 2006a, b; Villafuerte and Moreno 1997; Monterroso et al. 2013; Rocha et al. 2022), in addition to naïve short-term adaptive responses to predators (Rouco et al. 2011a; Descalzo et al. 2021). These anti-predatory strategies may be modulated by the hunting strategy of the predator (Jaksic and Soriguer 1981; Moreno et al. 1996). Nevertheless, there is a knowledge gap regarding the rapidity of this adaptation to the presence of predators and whether rabbits will adjust differently to distinct predation pressures.
In 2009, Monclús et al. (2009) tested the threat-sensitive predator avoidance hypothesis in mammals for the first time. This investigation focused on a European rabbit population in Doñana National Park (southwest Spain). This hypothesis states that animals modulate their anti-predatory responses based on the perceived risk of predation (Helfman 1989; Horat and Semlitsch 1994). That study suggested that rabbits exposed to higher predation pressure showed higher levels of faecal corticosterone metabolites, indicating heightened physiological stress (Monclús et al. 2009). The rabbit populations studied by Monclús et al. (2009) in Doñana National Park (i.e. one with high and another with low predation pressure) had inhabited the study areas for decades before the experiment. This extended exposure allowed for long-term adaptation to the different predation pressure conditions. However, the study did not assess potential changes in rabbit behaviour, leaving the short-term behavioural adaptations of rabbits unexplored. In a subsequent investigation, Descalzo et al. (2021) demonstrated that rabbits can adjust their daily activity patterns to reduce predation risk depending on the pressure exerted by different mammalian predator species. However, it is unclear whether this adaptation to the presence of predators could go beyond the activity pattern (Monterroso et al. 2013; Tobajas et al. 2023).
Previous investigations have established that both distance from cover and group size can serve as proxies for perceived predation risk and can affect European rabbit behaviour (Moreno et al. 1996; Caro 2005; Blanchard et al. 2016; but see Monclús and Rödel 2008). Specifically, an increase in distance from cover correlates with heightened perceived predation risk. Consequently, rabbits are expected to venture shorter distances from cover when predation pressure is higher (Jaksic and Soriguer 1981; Villafuerte and Moreno 1997). Regarding rabbit group size, their response may vary depending on the type of predator. Large groups may attract terrestrial predators due to an increase in prey odour (Roberts 1996). Nevertheless, these large groups may also act as a deterrent to raptors, as the absence of a clear individual target makes it challenging for the raptors to single out prey (Villafuerte and Moreno 1997).
In this study, we leveraged a large-scale recovery programme for European rabbits that was implemented in the Sierra Norte Natural Park of Sevilla (SW Spain) to assess the anti-predatory responses of European rabbits to aerial and terrestrial predation. For this purpose, we translocated rabbits to two distinct plots: an unfenced plot with higher predation pressure (exposed to both aerial and terrestrial predation) and another fenced plot with lower predation pressure (terrestrial predation excluded but exposed to aerial predation). In line with the threat-sensitive predator avoidance hypothesis, our hypothesis posited that rabbits in the area with lower predation pressure (without terrestrial predators) would exhibit larger group sizes, move to larger distances from cover and exhibit behaviours that are typically associated with a low level of risk perception (e.g. feeding, apparent inactivity), while decreasing those associated with a higher level of risk (e.g. running, alertness).
Materials and methods
Study area
The experiment was conducted in the area of Los Melonares (Sierra Norte Natural Park of Sevilla, SW Spain). This region has two main biotopes: Mediterranean grassland (70%) and scrubland (30%). The rabbit population in the study area prior to conducting the experiment was virtually non-existent (see below), but both mammalian (9 species) and raptor (19 species) predators that prey upon rabbits were recorded (complete list in Rouco 2008). In particular, the most common species of terrestrial carnivores recorded at our study area were red fox (Vulpes vulpes), Egyptian mongoose (Herpestes ichneumon), and to a lesser extent stone marten (Martes foina) and least weasel (Mustela nivalis) (Rouco et al. 2008). The bird of prey community was mainly composed by the short-toed snake eagle (Circaetus gallicus), Bonelli's eagle (Aquila fasciata), golden eagle (Aquila chrysaetos) and to a lesser extent black kite (Milvus migrans), booted eagle (Hieraaetus pennatus), Spanish imperial eagle (Aquila adalberti) and the Eurasian eagle owl (Bubo bubo) (Rouco 2008; Tobajas et al. 2021a, b).
Experimental design
The study population originates from a rabbit translocation programme carried out by a governmental entity (i.e. Confederacion Hidrográfica del Guadalquivir) as a compensatory measure for the construction of a reservoir in the Sierra Norte Natural Park of Sevilla. During autumn 2002 (i.e. October–November), rabbits from a high-density source population located at a hunting estate ~ 300 km from our study site (Cádiz province, southern Spain) were translocated into two experimental plots 1 km apart (for details regarding housing conditions of the rabbit population, see Ferreira et al. 2009; Rouco et al. 2008, 2011b). Rabbits corresponded to the subspecies O. c. algirus, which is predominant in southern Spain (Ferreira et al. 2015). Prior to the translocation, rabbit abundance in the plots was negligible. Each of the two plots consisted of a grassland field approximately 4 ha in size. To reduce the effect of confinement in rabbit movements and behaviour, this size is much larger than a rabbit average home range in high-density natural populations (e.g. ~ 0.7–1.2 ha, Lombardi et al. 2007; Devillard et al. 2008). To completely exclude predation risk due to terrestrial carnivores, one of the plots had a fence (1.0 m below ground, 2.5 m above ground) with an electrified wire on top (hereinafter fenced plot; Rouco et al. 2008). The mesh size was small enough to prevent the passage of any predator, such as the weasel. The other plot had the same characteristics excepting the presence of the fence (hereinafter unfenced plot). Birds of prey were not excluded from either plot. Each plot contained 18 artificial rabbit warrens built above ground, consisting of piles of stumps and rocks covered with loam and branches (Rouco et al. 2011b). Since no vegetation existed in the plots, warrens were the only refuge available against predators within the plots. Water and food suppliers were situated close to each warren (~ 4 m), and water and food were available ad libitum. Fresh alfalfa was additionally provided once a week and placed close to warren entrances (~ 1 m). Throughout the experiment, the plots were regularly inspected for depredated rabbits or predator scats. In the fenced plots, no rabbits depredated by carnivores or their scats were detected; however, pellets from raptors like the Eurasian eagle owl were found in both fenced and unfenced plots.
Distance to cover, rabbit group size and rabbit behaviour
Rabbit’s response to predation pressure was assessed by direct observations of rabbits in the two plots. Observations were carried out between February and March 2003 during fine evenings at dusk, starting three hours before sunset for a total of 12 non-consecutive days (6 days on each plot) and finishing observations just after sunset (i.e. an average of 3-h observation per day; total of 18-h observation per plot). The same experimental observer (CR) conducted rabbit focal observations from a fixed position approximately 100 m away from the plots, using a field telescope with a 25–60 power lens from a hideout. Camouflage clothing was consistently worn to blend with the surroundings, and the observation point was strategically placed behind shrubs or bushes for maximum inconspicuousness. No effect on the behaviour of the rabbits was observed due to the presence of the observer. Poles were strategically positioned at various known distances on the ground within each plot, serving as reference points to ensure precise distance estimations during observational assessments.
The methodology consisted of scanning the whole plot starting from one fixed point and continuing until the whole plot has been scanned. The same procedure was repeated successively until the end of all plots each day. Every time a rabbit was sighted, the observer focused on the animal to estimate its age based on body size (i.e. adult or juvenile, but only really obvious juveniles based on previous studies; Rouco et al. 2008), and to estimate distance to nearest cover. To calculate the rabbit distances, previously placed poles at known distances were used. The observer also estimated the distance to the nearest rabbit. Rabbits were considered paired or in a group when the distance between them did not exceed 5 m (Villafuerte and Moreno 1997). If any animal was further than 5 m away from another rabbit when first sighted it was considered as solitary. Thus, rabbits were assigned to four group sizes: solitary, pairs, groups of three rabbits or groups of four rabbits and larger (Villafuerte and Moreno 1997). The rabbits were assigned to each group regardless of their age, as long as they were within 5 m. Distance of a group to cover was estimated as the mean distance of all the individuals in the group to cover. Finally, the observer classified all rabbit’s behaviour in one of the following categories: alert, feeding, running or other (the latter included grooming, sniffing and/or resting).
Statistical analysis
To assess the statistical differences in the proportions of observations of rabbits across different group sizes in fenced versus unfenced plots, standard contingency table tests (chi-square) were employed. General linear models (GLM) with a Poisson distribution and a log link function using R package ‘lmtest’ (Zeileis and Hothorn 2002) were fit to the data to test whether distance of rabbits to cover was affected by rabbit group size, age and type of predation pressure (unfenced and fenced) and their interactions. To assess the effect of factors on each rabbit behaviour, GLMs with a binomial distribution and a logit link function were applied. The response variable was the presence of the behaviour in each rabbit observation, and the factors included predation pressure (fenced, unfenced), age, group size and distance from cover. If significant differences were found in GLMs, a pairwise post hoc comparison between factor levels was performed using Tukey’s test with the package ‘emmeans’. Collinearity between predictors was checked (all predictors r < 0.5), as well as violations to all modelling assumptions through analyses of residuals (Zuur et al. 2009). All analyses were carried out using the R statistical computing environment (version 4.0.2, R Core Team 2020).
Results
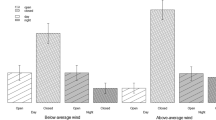
A total of 504 rabbit observations were recorded, 207 in the unfenced plot (116 adults and 91 juveniles) and 297 in the fenced plot (136 adults and 161 juveniles). Most of the observations in the unfenced plot corresponded to rabbits in pairs or solitary, whereas in the fenced plot rabbits were mostly in pairs or in larger groups, and these differences were statistically significant (χ2 = 95.59, df = 3, P < 0.001, Fig. 1). In the later, only 10% of the observations were solitary animals. The GLM results revealed that rabbits moved further from cover in the unfenced plot than in the fenced plot (χ2 = 15.34, df = 1, P < 0.001) (Fig. 2). No significant differences were found in group size (χ2 = 5.43, df = 3, P = 0.14) and age (χ2 = 0.56, df = 1, P = 0.46), but there was a significant effect in the interaction between group size and plot (χ2 = 27.9, df = 3, P < 0.001). However, post hoc analyses showed that these differences (P < 0.05) were observed in larger groups (≥ 4 rabbits) that were in the unfenced plot, whereas rabbits in the fenced plot that moved further from cover were solitary rabbits or smaller groups rather than larger groups (Fig. 2). Finally, a significant interaction between group and age was found (χ2 = 8.21, df = 3, P = 0.042), showing differences in the distance from cover depending on age, especially in the solitary rabbits ventured further than adults.
Percentage of direct observations of adult (A) and juvenile (B) rabbits according to group size in unfenced and fenced plots. Rabbits were considered paired or in a group when the distance between them did not exceed 5 m. Rabbits further than 5 m away from another rabbit were considered as solitary. The rabbits were assigned to each group regardless of their age
Mean distance (+ 95% confidence intervals) from cover of rabbit groups in unfenced and fenced plots. Rabbits were considered paired or in a group when the distance between them did not exceed 5 m. Rabbits further than 5 m away from another rabbit were considered as solitary. *Indicates significant (P < 0.05) differences between plots
Overall, direct observations revealed that both adult and juvenile rabbits in the fenced plot behaved different to those in the unfenced plot (Fig. 3). In particular, rabbits in the unfenced plot were alert and running more frequently than in the fenced plot; the latter were mostly feeding and other relaxed behaviours (Fig. 3). The GLM showed a significant effect of plot for running (χ2 = 12.17, df = 1, P < 0.001), feeding (χ2 = 7.38, df = 1, P = 0.006) and slightly effect for alert (χ2 = 2.71, df = 1, P = 0.09). No other significant effect of factors was found for these behaviours. Interestingly, only a significant negative relationship was found in other behaviours (related with relaxing activity such us grooming or resting) with distance from cover (χ2 = 4.86, df = 1, P = 0.027), spending the rabbits less time in this behaviour far from cover.
Discussion
The results show that, regardless of the presence of carnivores, both adult and juvenile rabbits exhibited limited movement away from cover owing to the risk of predation. In Iberian Mediterranean habitats there are various diurnal and visible predator species (mainly birds of prey), contributing to a persistent predation risk for rabbits but diminishing during last hours of daylight (Moreno et al. 1996; Penteriani et al. 2006), coinciding with the observation period in our study. In our study area we observed a high diversity of raptor species (detailed in study area section, Rouco 2008), which may explain that rabbits preferred to feed closer to cover during the day (i.e. it is safer). In addition, rabbits tend to not move far from cover in open grasslands if few shelters are available (Palomares and Delibes 1997), as occurred in our study site. In contrast to our expectations, rabbits in the unfenced plot, where they were less abundant (see Rouco et al. 2011b) and terrestrial carnivores access was unrestricted (see Rouco et al. 2008), moved further than those in the fenced plot. This is in disagreement with other findings. For example, Banks et al. (1999) found that rabbits moved further from cover in an area where red foxes had been removed than in other areas where this predator was present, which was attributed by these authors to a perceived reduction in predation risk. The fact that rabbits moved further from cover in the area with a higher predation risk in our study could be due to several reasons. First, rabbits tend to have larger home ranges (i.e. move further) in areas of lower density (Devillard et al. 2008), a pattern observed in social species (Efford et al. 2016). Second, the increased distance from cover in the unfenced plot could be driven by nutritional needs, as rabbits were observed feeding at greater distances where fresh pasture, potentially of higher quality than supplementary food, was available. While we did not quantify food availability, the unfenced plot had more pasture due to lower rabbit abundance and, consequently, reduced grazing pressure. Therefore, rabbits could have reached better quality food (e.g. fresh pasture) found at further distances from the warren (Crowell et al. 2016), than the weekly supplementary food. Thirdly, it is possible that due to the higher rabbit abundance found in the fenced plot, rabbits were closer to their warren for territorial defence. In larger groups, higher density is usually related to increased aggression rates and social instability (Monclús et al. 2009). Nonetheless, most adults and juveniles in the fenced plot were feeding at close distance (< 5 m) to cover.
In this study, we assessed experimentally how a reduction in terrestrial predation pressure affected the behaviour of European rabbit populations. Prey behaviour in any given situation depends on the proportion of time that prey species spend in high-risk versus low-risk situations (Lima and Bednekoff 1999). When high-risk situations are scarce rabbits devote most time to feeding and maintaining a moderate level of vigilance (Sih and Ziemba 2000), as observed in the fenced plot (Fig. 3). Conversely, it is expected that in a high predation risk situation rabbits would spend more time alert or engaging in evasive running behaviours. However, because safe periods are infrequent, prey must forage intensely and exhibit minimal or no vigilance during these periods (Sih and Ziemba 2000), which agrees with our results in the unfenced plot (Fig. 3).
Interestingly, we predominantly observed solitary or pairs of rabbit in the area with higher terrestrial predation pressure (i.e. unfenced plot), and the few larger groups were only observed at larger distances from cover (Fig. 2), where rabbits spent most of the time feeding. In contrast, pairs and larger groups were more common in situations where terrestrial predation was excluded (Fig. 2), and the rabbits that ventured further from cover usually did so in smaller groups. These contrasting responses can be attributed to a common principle, namely a cooperative vigilance among rabbits (Roberts 1988, 1996). In scenarios where terrestrial predators posed the primary predation pressure (i.e. unfenced plot where foxes accounted for more than twice the mortality caused by raptors, Rouco 2008), rabbits tended to be in smaller groups, perhaps to reduce predator attraction by reducing group prey odour. Larger groups were only observed when rabbits needed to feed at a greater distance from cover, likely because the vigilance of group-mates increases the probability of detecting a predator (Roberts 1996). In the fenced plot, the presence of larger groups could be an anti-predatory strategy against birds of prey (Villafuerte and Moreno 1997), the only predators that could access those rabbits. Individuals in larger groups can enjoy the same or improved predator detection rate while scanning less frequently and having more time to feed (e.g. Roberts 1996).
In general, our results suggest that European rabbits seem to adjust their behavioural responses according to the type of perceived predation risk, in accordance with previous studies (Monterroso et al. 2013; Descalzo et al. 2021). These results resemble those obtained by Monclús et al. (2009), and therefore tend to agree with the threat-sensitive predator avoidance hypothesis. Notably, our study additionally suggests that behavioural adaptations to reduce the predation risk can be adopted by rabbits in a short period of time (i.e. ~ 3 months). Complementarily, this study also reveals that these adaptations extend beyond changes in activity patterns previously observed (Monterroso et al. 2013; Martín-Díaz et al. 2018; Descalzo et al. 2021), encompassing alterations in spatial utilisation and cooperative vigilance behaviours. Finally, our results suggest that these adaptations depend on the type of predator, the rabbits adjusting their response as a function of whether they are being predated from the air or from the ground. It is remarkable that in this experiment, rabbits were translocated, whose adaptation is presumed more difficult than for rabbits born in the study area. However, our results show the high plasticity of this species to adapt to environmental conditions significantly different from those of its place of origin (natural population). These types of studies help wildlife managers to implement conservation measures based on translocations, since they show that prey species can have the ability to quickly adapt to new environments, including different predation pressures (Descalzo et al. 2021). These results offer new insights into the behavioural ecology of the European rabbit, aiding the development of conservation strategies for this threatened species (Villafuerte and Delibes-Mateos 2019).
Data availability
The data used in the article will be available on the first author’s personal and/or Researchgate website, and additional information may be requested from the corresponding authors upon reasonable request.
References
Banks PB, Hume ID, Crowe O (1999) Behavioural, morphological and dietary response of rabbits to predation risk from foxes. Oikos 85(2):247–256
Barnard CJ (1983) Animal behaviour: ecology and evolution. Croom Helm, London
Blanchard P, Lauzeral C, Chamaillé-Jammes S, Yoccoz NG, Pontier D (2016) Analyzing the proximity to cover in a landscape of fear: a new approach applied to fine-scale habitat use by rabbits facing feral cat predation on Kerguelen archipelago. PeerJ 4:e1769
Bolles RC (1970) Species-specific defense reactions and avoidance learning. Psychol Rev 77(1):32–48
Calvete C, Estrada R (2004) Short-term survival and dispersal of translocated European wild rabbits. Improving the Release Protocol. Biol Conserv 120:507–516
Caro T (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Crowell MM, Shipley LA, Camp MJ, Rachlow JL, Forbey JS, Johnson TR (2016) Selection of food patches by sympatric herbivores in response to concealment and distance from a refuge. Ecol Evol 6(9):2865–2876
Curio E (1975) The functional organization of anti-predator behavior in the Pied Flycatcher. A study of avian visual perception. Anim Behav 23:1–115
Delibes-Mateos M, Delibes M, Ferreras P, Villafuerte R (2008) The key role of European rabbits in the conservation of the western Mediterranean basin hotspot. Conserv Biol 22:1106–1117
Descalzo E, Tobajas J, Villafuerte R, Mateo R, Ferreras P (2021) Plasticity in daily activity patterns of a key prey species in the Iberian Peninsula to reduce predation risk. Wildl Res 48:481–490
Devillard S, Aubineau J, Berger F, Léonard Y, Roobrouck A, Marchandeau S (2008) Home range of the European rabbit (Oryctolagus cuniculus) in three contrasting French populations. Mamm Biol 73:128–137
Efford MG, Dawson DK, Jhala YV, Qureshi Q (2016) Density-dependent home-range size revealed by spatially explicit capture-recapture. Ecography 39:676–688
Ferreira C, Delibes-Mateos M (2010) Wild rabbit management in the Iberian Peninsula: state of the art and future perspectives for Iberian lynx conservation. Wildl Biol Pract 6(3):48–66
Ferreira C, Ramírez E, Castro F, Ferreras P, Alves PC, Redpath S, Villafuerte R (2009) Field experimental vaccination campaigns against myxomatosis and their effectiveness in the wild. Vaccine 27:6998–7002
Ferreira C, Castro F, Piorno V, Barrio I, Delibes-Mateos M, Rouco C, Mínguez LE, Aparicio F, Blanco-Aguiar JA, Ramírez E, Iriarte C, Ríos-Saldaña CA, Cañadilla J, Arias de Reyna L, Ferreras P, Alves PC, Villafuerte R (2015) Biometric differences stand out between European rabbit subspecies in their native range. Biol J Linn Soc 116(1):106–116
Ferretti A, Rattenborg NC, Ruf T, McWilliams SR, Cardinale M, Fusani L (2019) Sleeping unsafely tucked in to conserve energy in a nocturnal migratory songbird. Curr Biol 29(16):2766–2772
Futuyma DJ, Moreno G (1988) The evolution of ecological specialization. Annu Rev Ecol Syst 19:207–233
Hanson MT, Coss RG (1997) Age differences in the response of California ground squirrels (Spermophilus beecheyi) to avian and mammalian predators. J Comp Psychol 111(2):174–184
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Horat P, Semlitsch RD (1994) Effects of predation risk and hunger on the behaviour of two species of tadpoles. Behav Ecol Sociobiol 34:393–401
Jaksic FM, Soriguer RC (1981) Predation upon the European rabbit (Oryctolagus cuniculus) in Mediterranean habitats of Chile and Spain: a comparative analysis. J Anim Ecol 50:269–281
Lima SL (1995) Back to the basics of anti-predatory vigilance: the group-size effect. Anim Behav 49(1):11–20
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lombardi L, Fernández N, Moreno S (2007) Habitat use and spatial behaviour in the European rabbit in three Mediterranean environments. Basic Appl Ecol 8:453–463
Martín-Díaz P, Gil-Sánchez JM, Ballesteros-Duperón E, Barea-Azcón JM, Virgós E, Pardavila X, Moleón M (2018) Integrating space and time in predator-prey studies: the case of wildcats and rabbits in SE Spain. Mamm Biol 88:114–122
Monclús R, Rödel HG (2008) Different forms of vigilance in response to the presence of predators and conspecifics in a group-living mammal, the European Rabbit. Ethology 114(3):287–297
Monclús R, Rödel HG, von Holst D, de Miguel J (2005) Behavioural and physiological responses of naive European rabbits to predator odour. Anim Behav 70:753–761
Monclús R, Rödel HG, Palme R, von Holst D, de Miguel J (2006a) Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16:25–29
Monclús R, Rödel HG, von Holst D (2006b) Fox odour increases vigilance in European rabbits: a study under semi-natural conditions. Ethology 112:1186–1193
Monclús R, Palomares F, Tablado Z, Martínez-Fontúrbel A, Palme R (2009) Testing the threat-sensitive predator avoidance hypothesis: physiological responses and predator pressure in wild rabbits. Oecologia 158:615–623
Monterroso P, Alves PC, Ferreras P (2013) Catch me if you can: diel activity patterns of mammalian prey and predators. Ethology 119(12):1044–1056
Moreno S, Villafuerte R, Delibes M (1996) Cover is safe during the day but dangerous at night: the use of vegetation by European wild rabbits. Can J Zool 74:1656–1660
Nilsson PA, Brönmark C, Pettersson LB (1995) Benefits of a predator-induced morphology in crucian carp. Oecologia 104:291–296
Palomares F, Delibes M (1997) Predation upon European rabbits and their use of open and closed patches in Mediterranean habitats. Oikos 80:407–410
Penteriani V, Fortuna MA, Melián JC, Otalora F, Ferrer M (2006) Can prey behaviour induce spatially synchronic aggregation of solitary predators? Oikos 113:497–505
Preisser EL, Bolnick DI, Benard MF (2005) Scared to death? The effects of intimidation in predator-prey interactions. Ecology 86:501–509
R Core Team (2020) R: a language and environment for statistical computing. Version 4.0.2. Vienna, Austria, R Foundation for Statistical Computing. ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 20 Apr 2023
Roberts SC (1988) Social influences on vigilance in rabbits. Anim Behav 36:905–913
Roberts G (1996) Why individual vigilance declines as group size increases. Anim Behav 51(5):1077–1086
Rocha M, Serronha A, Rodrigues M, Alves PC, Monterroso P (2022) Comfort over safety: thermoregulation overshadows predation risk effects in the activity of a keystone prey. J Zool 316(3):209–222
Rouco C (2008) Restauración de las poblaciones de conejo de monte y mejora de la gestión para su conservación. PhD thesis, University of Castilla-La Mancha, Spain.
Rouco C, Ferreras P, Castro F, Villafuerte R (2008) The effect of exclusion of terrestrial predators on short-term survival of translocated European wild rabbits. Wildl Res 35:625–632
Rouco C, Villafuerte R, Castro F, Ferreras P (2011a) Responses of naïve and experienced European rabbits to predator odour. Eur J Wildl Res 57:395–398
Rouco C, Villafuerte R, Castro F, Ferreras P (2011b) Effect of artificial warren size on a restocked European wild rabbit population. Anim Conserv 14(2):117–123
Savvides P, Poliviou V, Stavrou M, Sfenthourakis S, Pafilis P (2019) Insights into how predator diversity, population density and habitat type may affect defensive behaviour in a Mediterranean lizard. Ethol Ecol Evol 31(1):12–27
Sih A, Ziemba R (2000) New insights on how temporal variation in predation risk shapes prey behavior. Trends Ecol Evol 15(1):3–4
Taraborelli PA, Moreno P, Srur A, Sandobal AJ, Martínez MG, Giannoni SM (2008) Different antipredator responses by Microcavia australis (Rodentia, Hystricognate, Caviidae) under predation risk. Behaviour 145(6):829–842
Teplitsky C, Plénet S, Joly P (2005) Costs and limits of dosage response to predation risk: to what extent can tadpoles invest in anti-predator morphology? Oecologia 145:364–370
Tobajas J, Descalzo E, Villafuerte R, Jimenez J, Mateo R, Ferreras P (2021a) Conditioned odor aversion as a tool for reducing post-release predation during animal translocations. Anim Conserv 24(3):373–385
Tobajas J, Rouco C, Fernandez-de-Simon J, Díaz-Ruiz F, Castro F, Villafuerte R, Ferreras P (2021b) Does prey abundance affect prey size selection by the Eagle Owl (Bubo bubo)? J Ornithol 162(3):699–708
Tobajas J, Ramos-López B, Piqué J, Sanchez-Rojas G (2023) Predation risk in tree squirrels: implications of the presence of free-ranging dogs. J Zool 319(4):308–318
Villafuerte R, Moreno S (1997) Predation risk, cover type, and group size in European rabbits in Doñana (SW Spain). Acta Theriol 42(2):225–230
Villafuerte R, Delibes-Mateos M (2019) Oryctolagus cuniculus. The IUCN red list of threatened species. https://www.iucnredlist.org/species/41291/45189779. Accessed 20 Apr 2023
Zeileis A, Hothorn T (2002) Diagnostic checking in regression relationships. R News 2(3):7–10
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank E. Grosso and M. A. Puerta from Confederación Hidrográfica del Guadalquivir (M.M.A.) and P. González. Special thanks go to C. Calvete, G. Calabuig, C. Iriarte, J. Castillo, R. Estrada, A. Finque, I. Rouco, A. Linares, S. Luna, L. E. Mínguez, O. Rodriguez, M. Reglero and J. Retamar for their help during the field work.
Funding
Funding for open access publishing: Universidad de Córdoba/CBUA. Jorge Tobajas benefitted from a postdoctoral contract funded by the University of Cordoba and the Consejería de Transformación Económica, Industria, Conocimiento y Universidades of Junta de Andalucía through the grants programme “Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020)”. C. Rouco Zufiaurre was supported by the Plan Propio grant funded by University of Córdoba. The study was partially funded by the project PID2020114724RB-I00 (Spanish Ministerio de Ciencia Innovación y Universidades).
Author information
Authors and Affiliations
Contributions
RV and CRZ did the conceptualization. JT, CCF, MDM, RV and CRZ did the field work and investigation, JT and CRZ did the data analyses, JT and CRZ wrote the main manuscript text and prepared figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare no conflicts of interests. One of the authors of this article, C. Rouco Zufiaurre, is a member of the editorial board of Mammalian Biology.
Ethical statement
Since the present study was a strictly observational research, no manipulations were carried out on any animals. The rabbit translocation programme, upon which the present study is based, was approved by the Ethical Committee for Animal Experimentation of the University of Castilla-La Mancha and is in accordance with Spanish and European regulations (Law 32/2007, R.D. 1201/2005, and Council Directive 2010/63/EU).
Additional information
Handling editor: Raquel Monclús.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tobajas, J., Ferreira, C.C., Delibes-Mateos, M. et al. Adaptive anti-predatory responses of European rabbits exposed to different predation pressure. Mamm Biol 104, 185–192 (2024). https://doi.org/10.1007/s42991-024-00398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42991-024-00398-3