Abstract
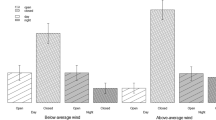
Predation is an important selective force on prey species, but avoiding predators can be costly. Efficient decisions on who to avoid (predator recognition) and when (situations with different predation risk) will determine the chances of prey survival. In coevolved predator-prey systems, detection of predator odours generally induces a response in potential prey to reduce predation risk. However, prey may not necessarily respond to odours of predators with which they have no previous experience, as predicted by the prey naïveté hypothesis. In turn, perceived predation risk may also modulate predator recognition and the intensity of antipredator responses. In this study, we investigate the responses of an introduced prey species, the European rabbit (Oryctolagus cuniculus), to odours from coevolved and non-coevolved, sympatric and allopatric predators in situations of high and low numbers of predators in Australia. We used pellet counts to quantify rabbits’ use of space on experimental plots treated with predator odours. Rabbit avoidance of plots scented with coevolved predator odours was strongest, but they also responded to non-coevolved, marsupial predators. Predator abundance did not affect predator recognition in rabbits, although responses were more intense in situations where perceived predation risk was low and the intensity of responses decreased over time. Our results suggest that rabbits may have learned to recognize novel marsupial predators after 150 years of exposure in their introduced range.


Similar content being viewed by others
References
Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS (2005) The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144
Banks PB (2000) Can foxes regulate rabbit populations? J Wildlife Manag 64:401–406
Banks PB, Dickman CR (2007) Alien predation and the effects of multiple levels of prey naivete. Trends Ecol Evol 22:229–230
Banks PB, Dickman CR, Newsome AE (1998) Ecological costs of feral predator control: foxes and rabbits. J Wildlife Manag 62:766–772
Banks PB, Hume ID, Crowe O (1999) Behavioural, morphological and dietary response of rabbits to predation risk from foxes. Oikos 85:247–256
Banks PB, Bytheway J, Carthey AJ, Hughes NK, Price CJ (2014) Olfaction and predator-prey interactions amongst mammals in Australia. In: Glen A, Dickman CR (eds) Carnivores of Australia: past, present and future. CSIRO Publishing, Sydney, pp 389–412
Barrio IC, Bueno CG, Banks PB, Tortosa FS (2010) Prey naiveté in an introduced prey species: the wild rabbit in Australia. Behav Ecol 21:986–991
Belche CA (1995) Diet of the tiger quoll (Dasyurus maculatus). Wildlife Res 22:341–357
Benton MJ (1990) Phylogeny of the major tetrapod groups: morphological data and divergence dates. J Mol Evol 30:409–424
Berger J (1998) Future prey: some consequences of the loss and restoration of large carnivores. Behav Ecol Conserv Biol 12:80–100
Bininda-Emonds ORP, Decker-Flum DM, Gittleman JL (2001) The utility of chemical signals as phylogenetic characters: an example from the Felidae. Biol J Linn Soc 72:1–15
Blackhall S (1980) Diet of the eastern native-cat, Dasyurus viverrinus (Shaw), in Southern Tasmania. Aust Wildlife Res 7:191–197
Blumstein DT, Mari M, Daniel JC, Ardron JG, Griffin AS, Evans CS (2002) Olfactory recognition: wallabies may have to learn to be wary. Anim Conserv 5:87–93
Boag B, Mlotkiewicz JA (1994) Effect of odor derived from lion faeces on behavior of wild rabbits. J Chem Ecol 20:631–637
Brown RE, MacDonald DW (1985) Social odours in mammals. Clarendon, Oxford
Burwash MD, Tobin ME, Woolhouse AD, Sullivan TP (1998) Field testing synthetic predator odors for roof rats (Rattus rattus) in Hawaiian macadamia nut orchards. J Chem Ecol 24:603–630
Bytheway JP, Carthey AJ, Banks PB (2013) Risk vs. reward: how predators and prey respond to aging olfactory cues. Behav Ecol Sociobiol 67:715–725
Carthey AJR (2013) Chemical composition of mammalian predator odours. Naïveté, novelty and native status: mismatched ecological interactions in the Australian environment. PhD Thesis, The University of Sydney, NSW Australia
Carthey AJR, Banks PB (2012) When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term alien predator. PLoS ONE 7:e31804
Carthey AJR, Banks PB (2014) Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biol Rev 89:932–949
Chivers DP, McCormick ML, Mitchell MD, Ramasamy RA, Ferrari MCO (2014) Background level of risk determines how prey categorize predators and non-predators. Proc R Soc B 281:20140355
Cox JG, Lima SL (2006) Naïveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends Ecol Evol 21:674–680
Cox TE, Murray PJ, Hall GP, Li X (2010) Pest responses to odors from predators fed a diet of target species conspecifics and heterospecifics. J Wildife Manag 74:1737–1744
Dickman CR (1992) Predation and habitat shift in the house mouse, Mus-domesticus. Ecology 73:313–322
Epple G, Mason JR, Nolte DL, Campbell D (1993) Effects of predator odors on feeding in the mountain beaver (Aplodontia rufa). J Mammal 74:715–722
Fey K, Banks PB, Korpimäki E (2006) Different microhabitat preferences of field and bank voles under manipulated predation risk from an alien predator. Ann Zool Fenn 43:9–16
Glen AS, Dickman CR (2006) Diet of the spotted-tailed quoll (Dasyurus maculatus) in eastern Australia: effects of season, sex and size. J Zool 269:241–248
Griffin AS, Blumstein DT, Evans CS (2000) Training captive-bred or translocated animals to avoid predators. Conserv Biol 14:1317–1326
Guiler ER (1970) Observations on the Tasmanian devil, Sarcophilus harrisii (Marsupialia : Dasyuridae) I. numbers, home, range, movements and food in two populations. Aust J Zool 18:49–62
Hamer R, Lemckert F, Banks PB (2011) Adult frogs are sensitive to the predation risks of olfactory communication. Biol Lett 7:361–363
Hayes RA, Nahrung HF, Wilson JC (2006) The response of native Australian rodents to predator odours varies seasonally: a by-product of life history variation? Anim Behav 71:1307–1314
Hughes NK, Kelley JL, Banks PB (2009) Receiving behaviour is sensitive to risks from eavesdropping predators. Oecologia 160:609–617
Hughes NK, Korpimäki E, Banks PB (2010) The predation risks of interspecific eavesdropping: weasel-vole interactions. Oikos 119:1210–1216
Hughes NK, Kelley J, Banks PB (2012) Dangerous liaisons: the predation risks of receiving social signals. Ecol Lett 15:1326–1339
Jaksic FM, Soriguer RC (1981) Predation upon the European rabbit (Oryctolagus cuniculus) in Mediterranean habitats of Chile and Spain: a comparative analysis. J Anim Ecol 50:269–281
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kavaliers M (1990) Responsiveness of deer mice to a predator, the short-tailed weasel: population differences and neuromodulatory mechanisms. Physiol Zool 63:388–407
Kloppers EL, St Clair CC, Hurd TE (2005) Predator-resembling aversive conditioning for managing habituated wildlife. Ecol Soc 10:31
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Madison DM, Maerz JC, Sullivan AM (2005) Decline in avoidance of predator chemical cues: habituation or biorhythm shift? In: Mason RT, LeMaster MP, Muller-Schwarze D (eds) Chemical signals in vertebrates. Springer, Corvallis
Mirza RS, Mathis A, Chivers DP (2006) Does temporal variation in predation risk influence the intensity of antipredator responses? A test of the risk allocation hypothesis. Ethology 112:44–51
Monclús R, Rödel HG, von Holst D, De Miguel J (2005) Behavioural and physiological responses of naïve European rabbits to predator odour. Anim Behav 70:753–761
Monclús R, Rödel HG, Palme R, von Holst D, De Miguel J (2006a) Noninvasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16:25–29
Monclús R, Rödel HG, von Holst D (2006b) Fox odour increases vigilance in European rabbits: a study under semi-natural conditions. Ethology 112:1186–1193
Murray D, Jenkins CL (1999) Perceived predation risk as a function of predator dietary cues in terrestrial salamanders. Anim Behav 57:33–39
Myers K, Parer I, Wood D, Cooke BD (1994) The rabbit in Australia. In: Thompson HV, King CM (eds) The European rabbit: the history and biology of a successful colonizer. Oxford Science, Oxford, pp 108–147
Newsome A, Pech R, Smyth R, Banks PB, Dickman CR (1997) Potential impacts on Australian native fauna of rabbit calicivirus disease. Biodiversity Group, Environment Australia [internal report]
Nolte DL, Mason JR, Epple G, Aronov E, Campbell DL (1994) Why are predator urines aversive to prey. J Chem Ecol 20:1505–1516
Pillay N, Alexander GJ, Lazenby SL (2003) Responses of striped mice, Rhabdomys pumilio, to faeces of a predatory snake. Behaviour 140:125–135
Rödel HG, Monclús R, von Holst D (2006) Behavioral styles in European rabbits: social interactions and responses to experimental stressors. Physiol Behav 89:180–188
Rolls EC (1969) They all ran wild: the story of pests on the land in Australia. Angus and Robertson, Sydney
Rouco C, Villafuerte R, Castro F, Ferreras P (2011) Responses of naïve and experienced European rabbits to predator odour. Eur J Wildlife Res 57:395–398
Russell BG (2005) The role of odour in Australian mammalian predator/prey interactions. PhD thesis, University of New South Wales, Sydney
Sullivan TP, Crump DR (1986) Feeding responses of snowshoe hares (Lepus americanus) to volatile constituents of red fox (Vulpes vulpes) urine. J Chem Ecol 12:729–739
Wyatt TD (2003) Pheromones and animal behaviour: communication by smell and taste. Cambridge University Press, Cambridge
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We want to thank Phil Barnes and Will for their help and logistic support to conduct the experiments in their estates and James Dawson (Office of Environment and Heritage, NSW) for his help in selecting field sites and providing tiger quoll video recording. Thanks to people in the Banks’ Lab at the University of Sydney for their valuable comments on earlier drafts.
Compliance with ethical standards
ᅟ
Funding
FST was funded by a Grant of the Spanish Research Council (Programa Salvador de Madariaga PR2010-0556).
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The experimental treatment had ethics approval number 09/99B by the University of Sydney, and all applicable, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. I. Schulte-Hostedde
Rights and permissions
About this article
Cite this article
Tortosa, F.S., Barrio, I.C., Carthey, A.J.R. et al. No longer naïve? Generalized responses of rabbits to marsupial predators in Australia. Behav Ecol Sociobiol 69, 1649–1655 (2015). https://doi.org/10.1007/s00265-015-1976-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-1976-z