Abstract
Purpose
Mango is a well-known and widely consumed fruit for its savoury taste and nutritional benefits. However, a lack of efficient postharvest handling prior to its storage could gradually lead to undesirable changes that cause postharvest losses. Dehydration techniques such as hot air drying have shown to minimize the water activity thereby preserving fruit shelf-life. Pretreatment prior drying has the advantage of shortening the drying times, consuming less energy, substituting chemical use, and maintaining the quality attributes of agricultural products. Therefore, the main purpose of this research is to assess the application of cold plasma (CP) as a pretreatment step before drying ‘Tropica’ and ‘Keitt’ mango slices.
Methods
The effect of low-pressure cold plasma pretreatment duration (5 and 10 min) and mango cultivar differences was investigated on drying properties, quality attributes, and microbial load. Thin layer mathematical models fitted were fitted to the data collected to describe the drying behaviour.
Results
Mango cultivars behaved differently during drying as ‘Keitt’ samples had a shorter drying time (10 h) compared to ‘Tropica’ samples (12 h). Logarithmic model best predicted the drying behaviour with a determination coefficient R2 of 0.99 and RMSE of 0.0664. Change in bioactive compounds, antioxidant capacity, and microbial load of ‘Tropica’ and ‘Keitt’ mango slices were significantly affected by CP pretreatment and drying (p < 0.05).
Conclusion
The findings of this study showed that cold plasma improved the drying rate of dried mango slices. Total phenolic and antioxidant activity were improved with cold plasma treatment of 10 min. In summary, cold plasma improves drying kinetics and the quality attributes of mango fruit.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Known as one of the most significant and well-liked climacteric fruits, mango is widely farmed, consumed worldwide, and has a high nutritional content (Mwamba & Mputu, 2022). Due to its attractive colour, flavour, and distinctive fragrant attributes, mango is quite popular and highly sought-after in the culinary sector (Dereje & Abera, 2020). In addition, many nutrients of the fruit are ever more valued and include carotenoids, antioxidants, polyphenols, and flavonoids.
The postharvest losses for mango fruit occur mainly in the production area, which is in subtropical and tropical agro-climatic regions, and mostly in developing countries where postharvest handling infrastructures and management technologies are critically limited (Ntsoane et al., 2020). Postharvest loss of mango fruit could range from 25 to 30% due to inefficient postharvest handling practices and facilities (Patel, 2022) and up 50% depending on the mango varieties (Le et al., 2022). In addition, mango fruit being climacteric ripen even without ethylene and rapidly to senescence and decay under high ethylene and above optimum storage conditions. Fresh mango fruit has a high moisture content, which makes it very sensitive to deterioration with a limited storage life (Yanclo et al., 2023).
Drying is an old technique that has been used to reduce spoilage of fresh produce and extend shelf-life of agricultural crops (Onwude et al., 2018). Drying consists of a simultaneous transfer of heat and mass during which moisture is removed to an acceptable level in fresh produce (Onwude et al., 2018). Drying reduces the moisture content of fresh produce, thereby, minimizing biochemical/enzymatic reactions accountable for the deterioration, retarding the growth of spoilage microorganisms and improve shelf life (Pushparaja et al., 2023). Numerous organic and synthetic chemical and physical pretreatments have been shown to have minimal impact on reducing the drying time (Bao et al., 2021). Furthermore, the application of a pretreatment prior to the drying process has proven to be beneficial in preventing fruit browning, and to minimize the negative impact of the drying process like nutrient degradation, biochemical activity, and maintaining overall quality (Yanclo et al., 2023).
Thermal pretreatments (such as hot water and steam blanching), alkaline solutions, and sulphating, have been used to enhance drying time of fruits (Deng et al., 2019). For instance, hot water and steam blanching are primarily used to inactivate enzymes present in the fruits, which helps to preserve the natural colour during drying process (Xiao et al., 2017). Pretreatment in alkaline solutions such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) prior to drying could enhance the texture (via degradation of pectin and cellulose in the cell wall) and colour attributes of the fruit (Bassey et al., 2021). The disadvantage of these techniques includes the possibility of negatively altering the flavour and taste due to changes in the natural acidity of the fruits. Similarly, sulphating as a pretreatment prior to drying fruits has been extensively used by the industry, due to the ability to inhibit enzymatic browning and maintain natural fruit colour during the drying process. However, the use possesses potential for adverse health effects and allergic reactions. Sulphur-based compounds are known to cause respiratory issues, particularly in individuals with asthma or sulphite sensitivity (Yanclo et al., 2023). As a result, several alternative pretreatment methods have been explored (alone or in combination) to address the potential challenges associated with conventional pretreatment methods. This includes the application of ultrasonication (Memis et al., 2023; Sun et al., 2023; Wu et al., 2020), microwave (Önal et al., 2021), and pulsed electric field (Nowacka et al., 2019). These pretreatments have shown great potential to reduce drying time and better preserve quality. However, the need for pretreatment optimization suitable industrial scale application and temperature control remains a limitation. An effective pretreatment for drying could be achieved by using a non-thermal, environmentally friendly method such as cold plasma as an alternative to chemical pretreatments.
Cold plasma (CP) consists of ionized gases including ions, electrons, and reactive neutral species (Pathare et al., 2023). Lately, CP pretreatment of food has been proven to effectively block enzyme processes, enhance the product’s quality and drying kinetics, and guarantee its safety (Deng et al., 2020; Karim et al., 2021). According to the available literature, it has been shown that CP pretreatment with 29 kV and 6 kHz during 30, 50, 70, and 90 s dried at 50 °C can speed up the drying time of mushroom (Agaricus bisporus) and improve the bioactive compounds (Ashtiani et al., 2023). Furthermore, tomato (Solanum lycopersicum L.) slices pretreated with cold plasma operated at 50 V, 1.0 kHz and 1.5 m/s for 6 min, and dried at 55 °C, improved lycopene content, enhanced the drying duration, and increased ascorbic acids and polyphenols contents (Obajemihi et al., 2023).
Based on these findings and available literature, there are no comparative studies evaluating the impact of low-pressure atmospheric cold plasma pretreatment prior to drying on different cultivars of mangoes. Therefore, the aim of this study is to investigate and elucidate the influence of mango fruit cultivar and the use of low-pressure cold plasma as a pretreatment on the drying characteristics, physical-biochemical, phytonutrients, and microbial quality of ‘Tropica’ and ‘Keitt’ mangoes.
Materials and Methods
Raw Materials
Two cultivars of mango fruit, namely ‘Tropica’ and ‘Keitt’ were obtained in the Western Cape Province of South Africa. Raw mangoes were harvested from Tamarak Mango Estate adjacent the Clanwilliam Dam at the foot of the Cederberg mountains (− 32°24ʹ85.03ʺS, 18°94ʹ07.71ʺE). Selected, undamaged fruits were uniform in shape, size, and colour and transported to the laboratory (Agricultural Research Council, Infruitec-Nietvoorbij, Agro-Processing Pilot Plant, Stellenbosch, South Africa).
Pretreatments and Hot Air Drying
Whole fresh ‘Tropica’ and ‘Keitt’ mango fruit were pretreated with a low-pressure atmospheric cold plasma equipment (Diener, Zepto Model 2, Germany) generated with oxygen prior to drying. Inside the plasma system, was contained a vacuum chamber made of borosilicate glass. The diameter has a width of 105 mm and a length of 200 mm. For this experiment, a working voltage of 90 kV and 1.4 mbar of pressure were applied to the equipment. The optimization of cold plasma treatment time was selected according to studies previously conducted by Phan et al. (2017) and Chatha et al. (2020). Factors such as the range of treatment/exposure times were determined at this stage. Initial screening was conducted where a range of treatment times were evaluated, and samples were treated for 5, 10, 15, and 20 min. Based on the preliminary results, the treatment times (5 and 10 min) that yielded the most favourable outcomes were selected hence were used.
The different treatments were applied as follows: The first set of whole fresh mango fruit were individually placed inside the vacuum chamber, where cold plasma was applied for a duration of 5 and 10 min. The second set of samples were the untreated ones which served as control while the third set of fruit were dipped for 2 min in 1% (10 g/kg) sodium metabisulphite (SMB) to simulate the industry practice.
After pretreatment was completed, the batches were minimally processed by carefully peeling and slicing the fruit using a sharp knife. The slicing was about 8-mm thick and placed on tray designed with steel with a parallel airflow pattern. To ensure steady-state temperature, the dehydrator tunnel (designed and built in-house) was turned on 1 h before the experiment and set at 60 °C and 35% relative humidity (RH) with airflow rate of 49.50 Hz. Prior to conducting the trials, the dryer was heated to the proper temperature. A thermocouple included within the apparatus was used to gauge the temperature inside the dehydrator. At 3-h intervals, a Labotech Precision Toploader analytical electronic balance (precision 0.01 g) was used to measure sample weight loss during drying until the samples attained a consistent weight. All the experiments were carried out six times.
Drying Kinetics
The moisture ratio and drying rate were used to assess the hot air-drying properties of mango slices. Drying was maintained until samples had the appropriate ultimate moisture content of 10.0% (w.b.), which is thought to be a secure level for mango preservation over an extended period. The following Eqs. (1) and (2) respectively by Xiao et al. (2017) were used to compute the mango slices’ moisture content (Mc) and moisture ratio (Mr) during thin layer drying experiments:
- Mf:
-
mass of fresh mango at initial instant t
- Md:
-
mass of fresh mango after drying
- M:
-
moisture at time t
- Mo:
-
moisture content at time zero
- Me:
-
equilibrium moisture content negligible equal to zero
The following formula by Wang et al. (2017a) expressed below was used to calculate the drying rate:
- Mo:
-
initial moisture content (g water gdb−1)
- Mt:
-
moisture content at a specific time (g water gdb−1)
Mathematical Models of Drying Data
With the aim to predict how fresh produce behaves during the drying process, several drying kinetics models were applied. These models were chosen because they could be used for a wide range of agrifood commodities, and they forecast how a horticultural commodity would behave during drying. These models include theoretical, semi-theoretical, and empirical. In our study, seven different models proposing various formulas were used to explain/fit the experimental data. Three of the models considered as semi-theoretical models analyzing both heat and mass transfer and include logarithmic, Henderson and Pabis, and Midilli Kucuk derived from Fick’s second law of diffusion were selected. They are described by Eqs. (4) (Wang et al., 2007), (5) (Henderson & Pabis, 1961), and (6) (Midilli et al., 2002):
The fourth model is considered as an empirical model and uses regression analysis to explain the relationship between the drying time and the moisture content represented in Eq. (7) (Wang & Singh, 1978). Two more significant models which describe the dynamics of the drying process represented in Eqs. (8) (Verma et al., 1985) and (9) (Aghbashlo & Samimi-Akhijahani, 2008), respectively, were used also. These equations are represented below:
In the equations above, t = the drying time and Mr = moisture ratio, and a, b, c, k, k1, k2, n, and g characterize the model drying constant and were determined using non-linear regression analysis.
In this study, two factors including the root mean square error (RMSE) and the correlation coefficient (R2) were used to determine the best model presented in Table 1 to predict the variations observed in the moisture of the sliced mango. The best fit that characterized the drying of thin-sliced mango is represented with the maximum R2 calculated using Eq. (10) and the lower RMSE (11) (Yanclo et al., 2022) below:
Mrexp,i is the moisture ratio value from experiment, Mrpre,i is the moisture ratio value predicted, N is the observations, and Z is the drying constants.
Colour Change
Six mango slices were measured for colour both before and after drying using a calibrated Chroma meter Minolta CR-400 (Minolta Corp., Osaka, Japan). The relevant colour parameters measured include the lightness (L*), yellowness (b*), and chroma (C*) representing the intensity and hue angle (h°) (Xiao et al., 2014). The total colour difference (ΔE) of the fresh and dried mango slices, as well as the chroma and hue angle, was evaluated using the following equations:
L*f, a*f, and b*f symbolize the fresh cut mango slices; L*, a*, and b* symbolize the dried mango slices.
Changes in Quality Attributes
Biochemical Attributes
Total soluble solids (TSS) values of fresh mango juice, which serve as baseline, were evaluated before and after drying using a refractometer (Atago N1, Tokyo, Japan). The dried mango slices obtained after performing the drying experiments were allowed to cool before packaging and storage in a dark cabinet for 2 days. Dried sample (6 g) was milled into powder using a blender (Model RSH – 080475, China) and this was mixed with distilled water (60 mL). After fully mixing, one drop of the solution was placed on an Abbe refractometer to measure the TSS. The measurement was recorded and represented as °Brix. The titratable acidity (TA) expressed as percentage citric acid (%, CA) was determined mixing mango powder with distilled water and using 60 mL to titrate against standardized 0.33 N of NaOH solution. When pH 8.2 was attained as the endpoint, TA measurements were conducted using a CRISON titrosampler (Crison Instruments, S.A. E-08328 ALELLA-Barcelona) (Nsumpi et al., 2020). Digital pH meter (Crison Model 00924 basic 20 + , South Africa) was used to measured pH of fresh mango juice and dried samples.
Bioactive Compounds
Total Polyphenol Content (TPC)
Mango powder (10 mg) was homogenized with distilled water (2000 μL). The solution was mixed with vortex (30 s) and further placed in the Hermle Z206A compact centrifuge (Wehingen, Germany) for 5 min at 2951 × g. The bioactive compounds were assessed using the supernatant obtained and all analyses were repeated in triplicate. The Folin-Ciocalteu technique was used to calculate TPC in accordance with the methods outlined by Phan et al. (2018). Firstly, 50 μL of the mixture of 200 μL of mango extract and 1800 μL of distilled water was poured into a clear plate well. The vials are filled in the second stage with 0.5 mL of Folin-Ciocalteu’s reagent, 1 mL of saturated 7.5% Na2CO3, and 1 mL of distilled water. The mixture was plated in a volume of 50 μL, and the reaction plate was incubated for 2 h at 20°C in a darkened atmosphere. Using a spectrophotometer model Fluostar Omega, BMG Labtech, Offenburg, Germany, the absorbance was measured at 750 nm and the results were reported using concentrations varying from 180 to 220 mg/L of gallic acid standard curve equation Y = 0.0093X + 0.0529 with R2 = 0.9995 was used and reported in mg gallic acid equivalents GAE/L.
Total Flavonols and Flavanol Content
A modified version of the technique described Pavun et al. (2018) was adjusted to determine the total flavonol content. In this experiment, quercetin served as standard. A 5 mL of mango juice was placed in the polytron and was further homogenized for a duration for 30 s. The supernatant obtained after homogenization was subjected to an extraction by firstly being rotated on a tube rotator for 15 min, and secondly being centrifuged for 3 min at 4000 rpm, covered from light, and kept at room temperature in the dark. 12.5 mL of quercetin, 12.5 μL of 0.1% hydrochloric acid in 95% ethanol, and 225 μL 2% HCl were pipetted into each well plate and the mixture was incubated for 30 min at room temperature. After reading, the results obtained were interpreted using values between 27 and 33 mg/L, calibration curve (Y = 0.0024X + 0.0089) with R2 = 0.9933, and reported as mg quercetin equivalent (QE)/g.
The method described by Wang et al. (2015) was modified to evaluate total flavanol content. After extraction of 25 μL of supernatant, the juice underwent sonication and was further centrifuged at 4000 rpm for 5 min. The reaction was started by adding 1 mL of p-DMACA solution (0.1% in 1 M HCl in MeOH) to the supernatant and kept for 10 min at room temperature. Using a constructed blank, the absorbance was calculated at 640 nm. Catechin served as the reference, and TFAC was computed using a calibration curve with concentrations ranging from 5.94 to 4.86 mg/L, the curve equation (Y = 0.0372X + 0.0036) with R2 = 0.9996. Total flavanol was reported in mg catechin equivalents (CE)/g.
Trolox Equivalent Antioxidant Capacity (TEAC)
Duda-Chodak et al. (2011) method was modified to conduct the Trolox equivalent antioxidant capacity (TEAC) experiment. Trolox was used as standard and a volume of 25 μL was pipetted into each well plate where 25 μL of the supernatant was previously added. A mixture of 20 mL EtOH and 1 mL ABTS were prepared, and a multichannel pipette was used to pipette 300 L of ABTS into each well, and the plate was kept at room temperature for 30 min followed by readings. Trolox concentrations ranging from 190 to 210 μM were used for TEAC extrapolations with standard curve equation Y = 0.0043X + 0.0065 and R2 value of 0.9983, with the results expressed as μM Trolox/mg.
Ferric-Reducing Antioxidant Power (FRAP) Assay
To measure the ferric-reducing antioxidant power (FRAP), reagent was prepared using 6.6 mL of distilled water, 30 mL of acetate buffer, 3 mL of ferric 2,4,6-tripyridyl-s-triazine (TPTZ) solution, and 3 mL of FeCl3 solution. The standard used was ascorbic acid and a volume of 10 μL was poured into the allocated wells on a clear well plate. With a multichannel pipette, 300 μL of the FRAP reagent was poured to each well and the plate was stored for 30 min at 37°C in the incubating oven. FRAP concentrations that ranged from 360 to 440 μM were used to obtain the concentration curve Y = 0.0072X + 0.001 at R2 value of 0.9998, and the results expressed as μM vitamin C/mg.
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
DPPH solution of 0.1 mM was combined with 95% ethanol and 180 µL of the volume was added to 20 µL of mango juice extract and kept at ambient temperature for half hour out of light. Each microplate well received 275 μL of DPPH reagent, while the Trolox standard wells and control well each received 25 μL of standard. The mixture was determined at 593 nm with a spectrophotometer after incubation at room temperature for half hour using concentrations ranging from 190 to 210 M. The curve equation used to calculate DPPH activity was Y = 0.0125X + 0.2312 with R2 value 0.9829. Results were stated as μM Trolox/mg.
Microbial Analysis
The total aerobic mesophilic bacteria, yeasts, and moulds found in mango slices were measured using the total plate count technique published by Nyamende et al. (2022). This was done to evaluate the low-pressure cold plasma treatment’s antimicrobial effectiveness. The fresh whole fruit and dried mango slices were sterilized by placing them in physiological saline solution and gently vortexed for 60 min. Thereafter, 1 mL from each diluent was then mixed with 9 mL of sterile physiological saline solution to create a threefold serial dilution. Enumeration of microbial load for bacteria and for yeast and mould was achieved by taking 1 mL from each dilution and pouring onto the plate count agar (PCA) and potato dextrose agar (PDA), with an incubation of 48 h for the PCA plates at 37°C and 3 to 5 days for the PDA plates at 25°C. The colony-forming units (CFU) were tallied within the range of 25 to 250 after incubation expressed as log CFU/cm2. The experiment was conducted in triplicate per dilution, with a total of nine replicates (n = 9).
Statistical Analysis
The General Linear Models Procedure (PROC GLM) in SAS software (Version 9.4; SAS Institute Inc, Cary, USA) was used to analyze the data and the results were expressed as mean (n = 6) ± standard error. Factors (main effects) and factor interactions were considered using a factorial analysis of variance (ANOVA). The Fisher’s least significant difference (LSD) test was used to compare the means of the measured variables, and means were separated at a significance level of 95% confidence.
Results and Discussion
Moisture Ratio (MR)
During the drying process of sliced ‘Tropica’ and ‘Keitt’ mango samples, a decline in MR was observed as the drying time progressed (Fig. 1). The increase in drying temperature (60°C) from the ambient condition facilitated the migration of water vapour from mango slices to the surrounding air. At the early stage of drying, it was observed that ‘Tropica’ mango slices showed a rapid decline in moisture content after 3 h of drying (data not shown) followed by a continuous decline until 9 h of drying. Predrying treatment using SMB prior to drying had no distinct effect on the moisture ratio compared to CP treatment (Fig. 1A). A similar trend was observed for ‘Keitt’ mango with a continuous decline in MR as the drying process progressed (Fig. 1B). Similar results related to the changes in moisture ratio were observed by preceding studies on goldenberry by Ashtiani et al. (2022), on jujube slices by Bao et al. (2021), and on chili pepper by Zhang et al. (2019). The higher MC removal after CP treatment during the drying compared to both untreated slices and SMB pretreated in both cultivars could be attributed to the ability of the pretreatment to modify the internal structure of the mango slices, facilitating the movement of moisture towards the surface. This increased permeability allows for faster moisture diffusion within the mango slices, enabling more efficient moisture removal during drying. CP improves the efficiency of moisture removal during drying and promotes faster evaporation of moisture from the outer surface and interior of the product, leading to reduced drying times (Du et al., 2022). This can be particularly beneficial for large-scale drying operations, as it increases productivity and reduces energy consumption (Ranjbar Nedamani & Hashemi, 2022). By accelerating the drying process, it minimizes the exposure of the product to high temperatures, reducing the risk of heat-induced degradation (Shishir et al., 2020). Additionally, the CP pretreatment has antimicrobial benefit, which can enhance the safety of dried products (Liao et al., 2020). This contributes to longer shelf life and reduces microbial risks associated with dried products. CP pretreatment can improve energy efficiency in the drying process; by reducing drying times, it decreases the energy required for drying operations (Du et al., 2022). This not only helps to reduce operational costs but also has environmental benefits by lowering energy consumption and associated carbon emissions compared to traditional methods.
However, this study showed a distinct effect of cultivar difference on the moisture ratio and the attained constant MC. The ‘Keitt’ and ‘Tropica’ mango slices reached MC equilibrium after 10 h and 12 h, respectively (Fig. 1). The variations in cultivars may be connected to the variability in the initial moisture content seen in the mango samples examined. During the drying process, the MC decline could be affected either by decreasing or increasing the number of pores and pore size (depending on the cultivar and maturity stage), as well as by the changes in tissue microstructures of the fruit. High porosity of mango fruit tissue and the difficulty of surface moisture to rapidly evaporate because of changes in structure and difference in cultivar could influence the internal capillary forces and affect mass flow (Yi et al., 2017).
Furthermore, the results of our study show that the application of cold plasma reduced the drying time of the mango slices, and the desired MCs were reached faster after 9 h and 10 h for ‘Keitt’ and ‘Tropica’, respectively, compared to the other samples. Sarangapani et al. (2015) explained that active species produced during cold plasma could dissolve the chemical bonds on the surface of rice grains. This phenomenon shortened the soaking period and boosted water absorption while causing fissures and surface disturbance. According to Tabibian et al. (2020), the formation of pores on the surface of fruit samples after application of cold plasma could be the source of the fast decrease in MR. Consequently, free radicals can pierce more deeply than active species like electrons, ions, and atomic oxygen, which have shorter lifetimes. Talens et al. (2017) explained that heat created during drying is transferred from the product’s surrounding air to the inside, which causes a slower dispersion of heat transmission.
Drying Rates
This study demonstrated that the internal moisture diffusion efficiency and the physical characteristics of mango fruit contributed to the drying rate not remaining consistent throughout the drying duration. The cultivars behaved differently as the longest drying time (12 h) was observed for untreated fruit and SMB-treated ‘Tropica’ samples, while all ‘Keitt’ samples had a shorter drying time of 10 h (Fig. 1). In addition, notably sliced mangoes pretreated with cold plasma had significantly reduced drying time and the highest drying rate (Fig. 1). CP pretreated ‘Keitt’ mango achieved a faster drying time (≈9 h) compared to ‘Tropica’ (≈ 10 h). This can be related to the impact of low-pressure and charged particles or ions produced by cold plasma leading to the formation of microscopic channels in the fruit and modifications in the fruit cell structure (Zhang et al., 2019). Tabibian et al. (2020) and Bao et al. (2021) also agreed that cold plasma pretreatment enhanced the drying properties of saffron and of jujube slices, respectively. More characteristics include the nature of the produce, the cultivar variety, and the water content in the produce (Ranjbar Nedamani & Hashemi, 2022). Overall, CP pretreatment improved the drying rate of ‘Keitt’ and ‘Tropica’ mangoes dried at 60 °C and efficiently reduced the drying time. The cold plasma treatment involves exposing the fruit to a high-energy plasma under low pressure, which can alter the surface chemistry and structure via etching through the reactive species which are generated (Won et al., 2017). This treatment can lead to changes in the water-holding capacity of the fruit and affect the rate at which moisture is lost during drying. It is suggested that the low-pressure vacuum chamber, cold plasma discharged, and the thin fruit peel with the presence of pores influenced the fruit tissue and the drying rate by affecting the moisture transport characteristics.
For both cultivars, the drying began with the rapid removal of moisture from the surface majorly via evaporation and the time taken for this first drying phase was about 2 h (Fig. 2). Thereafter, drying occurred in the falling rate period due to a departure of moisture from the surface of the fruit in both cultivars at the initial stage. The falling-rate period is controlled by the mechanism of diffusion from inside the fruit. Torki-Harchegani et al. (2016) corroborate these findings by explaining that during the drying process of agriproducts, diffusion is the prevailing mechanism for water removal. In this case, the molecule diffusion controls water migration from the inside of the product to the outer surface of the fruit. A subsequent decrease in drying rate was observed at the second stage, where a slow decrease was observed. This phenomenon could be explained by the shrinkage of the mango slices, leading to a further slowing down of the drying process due to an increase in the resistance to water flow. A similar increase in drying rate at the early stage of drying process was reported by Workneh and Oke (2012). Sadin et al. (2013) concluded that the increase in drying rate within the first hour of drying could be explained by the increase in the internal temperature of the product.
Thin Layer Drying Model
To match the thin layer drying models, the moisture content data from the drying experiment was translated to the moisture ratio. The graphical description of the model fits is summarized in supplementary Figs. 1, 2, 3, and 4 (S-Figs. 1, 2, 3, and 4). The thin layer drying models were fitted, parameter constants generated, coefficients for goodness of fit, and RMSE values are summarized in Table 1. Out of the seven models, the logarithmic model had the desired high R2 (0.99) combined with the lowest RMSE (0.0664–0.1528) values across all the dried samples followed by Henderson and Pabis model. Overall, the logarithmic model can be selected as the best to describe the thin layer drying behaviour for ‘Tropica’ and ‘Keitt’ mango slices. In addition, the existing conformity between R2 and RMSE wherein the higher the R2, the lower the RMSE, the better the fitting of the model.
Our findings corroborate with the reports of Yanclo et al. (2023) and Perea-Flores et al. (2012) who found that the logarithmic and Henderson and Pabis models best described the drying curves of mango slices. Similarly, Ampah et al. (2022) reported that logarithmic models had good fit and described best the drying behaviour of ‘Keitt’ mango. Doymaz (2004) also observed that logarithmic model had the good fit predicting the drying behaviour of ‘Hacihaliloglu’ apricots pretreated with potassium metabisulphite and alkaline ethyl oleate. The consistency of the model and relationship between the coefficients and drying variables was evident with the R2.
Colour Changes
Changes in visual colour confirm the effects of pretreated and hot air drying on the colour attributes for ‘Keitt’ and ‘Tropical’ mango sliced samples (Fig. 3). Lightness attribute is the first quality trait consumers evaluate when determining whether to purchase a dried product. It is therefore considered as a critical quality metric (Salehi & Kashaninejad, 2018). In this study, the L* values of fresh-cut mango samples decreased significantly (p < 0.05) after drying and were further influenced by cultivar and pretreatment in comparison to the control group for both ‘Tropica’ and ‘Keitt’ mango fruit (Table 2). The decline in lightness has been linked to the concentration effect because of water evaporation loss, surface deformation (shrinkable), and the production of brown pigments. These pigments can develop through several processes, including enzymatic browning involving phenolic compounds and polyphenol oxidases, and non-enzymatic (Maillard reaction and/or auto-oxidation) browning (Marquez et al., 2013).
According to research, a correlation was found between higher food browning and lower L* readings (Dea et al., 2010; Lüle & Koyuncu, 2015). After the drying process, the SMB treated samples maintained the highest L* values compared to the CP-treated samples (p < 0.05) for both cultivars. Similar findings were observed in a study by Dereje and Abera (2020) on ‘Keitt’ mangoes, where dried slices revealed a reduction in lightness compared to their fresh counterparts. It is evident that SMB due to their anti-browning effects have a beneficial effect on the L* value of dried mango slices compared to CP treatment (Sra et al., 2014). The inhibition of enzymatic and non-enzymatic browning by sulphites has been documented by Gulzar et al. (2018). Kim et al. (2017) reported that the colour parameters are barely affected by cold plasma. These results align with the observations made by Nyangena et al. (2019) on dried mango slices. The authors attributed the decline in L* values to water evaporation during the drying process, surface deformation of the dried slices, and the formation of brown pigments. Additionally, the prolonged drying hours and the presence of oxygen were considered factors contributing to these effects (Ali et al., 2016).
The predominant colour in mango is yellow, making the Hunter colour b* (yellowness) the most appropriate representation to determine the colour during drying process as affected by different pretreatment. In our study, the b* value of mango slices was influenced by pretreatment and cultivar (p < 0.05). Comparing the b* values for fresh samples, ‘Tropica’ was significantly higher than ‘Keitt’, demonstrating the uniqueness of each cultivar (Table 2). After drying, however, b* value increased for samples under SMB treatment and exhibited the highest values of 58.6 ± 2.36 and 64.9 ± 2.36, for ‘Tropica’ and ‘Keitt’, respectively. Changes were cultivar dependent and increase in b* value was observed across the ‘Keitt’ samples, while CP treatments resulted in a decline in b* value for ‘Tropica’ sample. According to Salehi and Kashaninejad (2018), an increase in b* parameter leads to yellowish products, which is often recommended for dried mangoes. Dereje and Abera (2020) reported a decrease in the yellowness of dried mango samples compared to the fresh ones. Additionally, Zou et al. (2013) reported that the drying process could reduce the b* value in samples. The b* value is also indicative of the presence and better conservation of carotenoid contents in dried products, which are typically responsible for the yellowish colour of mangoes as highlighted by Nyangena et al. (2019).
The result of our study indicates that ‘Tropica’ cultivars exhibited reduced yellowness values, while ‘Keitt’ cultivars showed increased yellowness values in cold plasma treated batch, as compared to the baseline measurements. Changes in b* values could be attributed to the varietal differences. Lacombe et al. (2015) suggested that cold plasma could easily influence materials without a thick protective layer. Mangoes are known to have relatively large pores on their peel surface depending on the cultivar, and the size and density of lenticels can vary among different fruit varieties and even within the same fruit (Everett et al., 2008). The presence of large pores can have implications for various processes, including gas exchange, water loss, and potential uptake of external substances (Everett et al., 2008) such as reactive species generated during cold plasma treatment in this study. For example, ‘Keitt’ mangoes are large-sized mangoes, which may have larger and more pronounced lenticels compared to ‘Tropica’. Therefore, the increase in yellowness observed in ‘Keitt’ mangoes could be attributed to the increase is possible direct impact of reactive species generated during CP treatment, followed by hot air drying, and the subsequent decrease in moisture content. Furthermore, the reactive species can interact with the surface of the mango peel, via etching causing surface modification such as the alteration of the peel topography and chemistry (Bao et al., 2021). Furthermore, the reactive species generated during CP treatment could contribute to reducing activity of enzymes involved in browning (Dantas et al., 2021) and delaying discolouration.
Higher chroma (C*) value trend was observed in dried ‘Keitt’ mango slices (53.1 to 65.3) compared to ‘Tropic’ (48.6 to 60.5), with ‘Keitt’ samples SMB-pretreated retaining the highest value (65.3 ± 1.25) followed by untreated control (p < 0.05), as shown in Table 2. A higher C* value for ‘Keitt’ could suggest that the cultivar has a better colour retention than ‘Tropic’ mango (Chaethong & Pongsawatmanit, 2015). Furthermore, h° values follow similar trends and observation as C* value (Table 2). The hue angle of 90° represents pure yellow, and the observed changes and decline in this study reflect an intensification of orange colour and increased browning index (Pandiselvam et al., 2023). Notably, dried ‘Keitt’ mango slices better retained yellowness. This study demonstrates that the industry SMB pretreatment was effective retaining dried fruit colour attributes compared to treatments compared to cold plasma pretreatment. Consistent with the h° and C* values, the highest total colour difference (∆E) was found in CP5-pretreated ‘Tropica’ slice mangoes (15.0 ± 1.90) compared to CP10 (13.2 ± 2.19), with SMB (13.5 ± 3.12) followed by the control which showed lower values (12.21 ± 1.57). Concerning ‘Keitt’ cultivar, SMB-treated samples presented the highest ∆E values (13.9 ± 1.46) compared to other samples (Table 2). A higher ΔE signifies a greater distance or change between colour attributes from fresh to dried fruit sample (Wang et al., 2019). Similarly, a decrease in ΔE in the dried mango slices was explained by the inactivation of enzymes associated with browning in fruit samples pretreated (Wang et al., 2019). The decrease in ΔE could be attributed to the reduced decomposition of polyphenols and other bioactive compounds in the CP-pretreated samples with shorter drying times, as suggested by Guiné and Barroca (2012). It is worth noting that colour plays a crucial role in consumers’ choices, and the changes in colour depend on pretreatment methods, drying techniques, and temperatures, as well as the chemical composition of the fresh fruit (Coklar et al., 2018).
Biochemical Attributes
A comparison of pH values for the freshly harvested mango cultivars showed that ‘Tropica’ had significantly (p < 0.05) lower pH (4.9) compared to ‘Keitt’ (3.4) as shown in Table 3. Following the drying process, a gradual but significant increase in pH levels was observed across pretreated and control samples (p < 0.05). For the ‘Tropica’ cultivar, control samples exhibited increasing pH values (5.03 ± 0.02), followed by CP pretreated samples maintained relatively higher pH level of ≈ 4.98, and the samples pretreated with SMB (4.95 ± 0.02) at the end of the drying process. A similar trend was observed with ‘Keitt’ samples where dried slices had the highest pH values. Pretreated samples had higher pH values compared to the control. The rise in pH from the fresh-cut state to the dried product is likely attributed to the loss of water experienced by the mango samples during the drying process. This effect is consistent with findings in a study conducted by Yanclo et al. (2022) on ‘Sunectwentyone’ dried nectarines at 50 °C, where a similar increase in pH was observed.
Like the pH results, the TA value of the fresh mangoes differed slightly but statistically significantly as detailed in Table 3 for both cultivars. The TA concentration for ‘Tropica’ and ‘Keitt’ was noticeably lower in the fresh samples (0.14 ± 0.03% and 0.08 ± 0.003%, respectively) than in the dried samples (p < 0.005). However, there was not a substantial difference in TA among all the pretreated samples compared to the untreated control in both ‘Tropica’ and ‘Keitt’ dried mango slices. Additionally, Verma and Joshi (2000) explained that the reduction in TA during postharvest storage of fresh-cut samples could be due to acid metabolism during fruit ripening, leading to the conversion of starch and acid into sugar. On the other hand, the increase in TA as anhydrous citric acid found in dried mango slices was linked to the formation of acids like propionic, lactic, formic, pyruvic, acetic, and citric acid, which arose from non-enzymatic reactions when the drying process was carried out (Rufián-Henares et al., 2006).
The TSS for fresh cut ‘Tropica’ mango was significantly higher than ‘Keitt’ with 18.7 ± 0.03°Brix and 14.0 ± 0.0.58°Brix, respectively (Table 3). However, TSS did not change significantly for dried ‘Tropica’ samples but exhibited slightly lower values compared to the fresh-cut baseline fruit. In contrast, dried ‘Keitt’ samples showed higher values compared to the fresh cut control slices with CP10 (17.7 ± 0.06°Brix) and CP5 (17.44 ± 0.21°Brix) showing the highest TSS level (p > 0.05). This observed increase in TSS for ‘Keitt’ could be attributed to the drying process (removal of moisture), which resulted in the formation a crusted top surface layer for the mango slices. Moisture loss resulted in retention and increase concentration of the soluble solids within the fruit tissue. Similar findings were stated by Bao et al. (2021), and their work linked the increase in TSS for cold plasma pretreated fruit samples to the possible formation of a protective layer on the surface after moisture evaporated.
However, the lack of significant difference in TSS content for ‘Tropica’ could be attributed to the cultivar difference and the maturity stage of fruit tissue during the drying process (Mukhtar et al., 2020). Ripening is closely associated with the change in textural profile of mango fruit tissues and accumulation of soluble solids (Jha et al., 2013; Liu et al., 2022; Zhang et al., 2022). The softening of fruit tissue is marked by the degradation of pectin polysaccharides, cellulose, and hemicellulose in the cell wall, which is accompanied primarily by the hydrolysis of starch, accumulation of non-reducing sugars, and degradation of non-reducing sugars to reducing sugars (Liu et al., 2019, 2022). This results in the accumulation of soluble sugars to enhance sweetness (Liu et al., 2022). Therefore, under these conditions of increase ripeness, low firmness, and accumulated soluble sugars, the drying process did not have a significant effect on TSS for ‘Tropica’ mango fruit, due to higher TSS in the centre of the fruit (Padda et al., 2011). However, further studies are needed to explore the impact of the CP pretreatment and drying process on the individual sugars of the mango slices to obtain a comprehensive understanding of these effects.
Bioactive Compounds
Total Polyphenols, Flavanols, and Flavonols
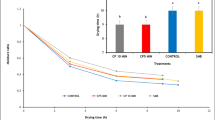
Both treatments and cultivar selection had a significant impact on the initial total polyphenol content (TPC) in the fresh ‘Tropica’ and ‘Keitt’ mango sample before processing and drying at 60°C was approximately 564.2 ± 51.4 mg GAE/100 g and 404.3 ± 8.1 mg GAE/100 g, respectively, which were significantly lower (p < 0.05) than the TPC in the final dried control and pretreated samples (Fig. 4A). The highest TPC were retained by CP5 (1992.0 ± 61.8 mg GAE/100 g) and CP10 (1999.2 ± 61.8 mg GAE/100 g) pretreated dried ‘Keitt’ mango slices (p < 0.05). For the total flavonol contents, the interaction of pretreatments and cultivar played a significant role (p = 0.001). Results suggest that the CP and SMB pretreatment could help in preserving the flavonol content in dried ‘Tropica’ and ‘Keitt’ mango slices. A gradual but significant decline was noted from fresh cut to dried mango slices across all treatments (Fig. 4B). In contrast, a drastic and significant decline (p < 0.0000) was observed in the total flavanol content of both cultivars from fresh sample to after drying (Fig. 4C). Sodium metabisulphite (SMB)-treated samples showed a higher content of total flavanols compared to the untreated control and CP10 (Fig. 4C).
Impacts of cold plasma treatment for 5 min, 10 min, sodium metabisulphite, and untreated (control) on changes in total polyphenol content (A), total flavonols (B), and total flavanols (C) for freshly harvest and dehydrated slices at 60°C, for ‘Tropica’ and ‘Keitt’ mangoes. Error bars represent standard deviation (SD) of mean (n = 6) values of treatments. Different lower-case letters indicate significant difference in mean values (p < 0.05). *Coloured graphs only available online
According to Chang et al. (2006), it could be presumed that minimal processing of fresh produce would induce or enhance the release of more bound phenolic compounds from the disrupted plant or fruit tissue. This initial tissue disruption of the fruit could have activated the release of oxidative and hydrolytic enzymes that could degrade the antioxidants in fruits. However, high drying temperature might neutralize these enzymes and minimize the loss of phenolic content. Studies on apples and pears have demonstrated that total phenolic content decreases as drying temperature rises (Santos et al., 2014; Vega-Gálvez et al., 2012). Similarly, an investigation on dried tomatoes, raisins, and dried apricots showed that using a high drying temperature is particularly successful for raising the overall phenolic content in agricultural commodities (Carranza-Concha et al., 2012; Chang et al., 2006; Sultana et al., 2012). In contrast, Zanoelo et al. (2006) and Sultana et al. (2012) reported that fresh produce that are thermally processed showed a decline in their overall phenolic content, while no variations in phenolic compounds were reported by Dewanto et al. (2002). Wang et al., (2017a, 2017b) reported that another factor that tends to weaken cell walls is thermal processing, which causes a significant loss of bioactive substances. Based on the variation in these results, it is notable that fruit cultivar differences, types of pretreatments applied, and drying procedures may not have the same impact on phenolic compounds.
Antioxidant Activity
The changes and/or variations in antioxidant capacity of ‘Tropica and Keitt’ mango slices as affected by pretreatment and drying are presented in Fig. 5. The interaction of treatments and cultivar had a significant impact on the TEAC of fresh and dried mango slice samples (p = 0.008). Whole fresh ‘Tropica’ TEAC (1507 ± 51.04 mg TE 100/g) was found to be higher than ‘Keitt’ (970 ± 102.6 mg TE 100/g). In the fresh ‘Tropica’, a slight increase in TEAC was observed after CP treatment; however, after drying treatments, this value significantly declined for untreated (control) and CP5. However, the samples treated with CP5 had the lowest TEAC compared to both the fresh-cut and dried control samples (Fig. 5). Similarly, the interaction of treatments and cultivar had a significant impact on the FRAP activity (p = 0.0004). Notably, FRAP increased significantly in dried samples compared to fresh cuts. Cold plasma-treated samples had the highest FRAP activity for ‘Tropica’ cultivar (Fig. 5B). A different trend to FRAP was observed for DPPH activity, where the highest values were recorded in the fresh-cut samples prior to drying (Fig. 5C). Overall, cold plasma-treated samples maintained relatively high antioxidant activity.
Impacts of cold plasma treatment for 5 min, 10 min, sodium metabisulphite, and untreated (control) on the changes in Trolox equivalent antioxidant capacity (TEAC) (A), ferric-reducing antioxidant power (FRAP) (B), and 1,1-diphenyl-2-picryl-hydrazyl (DPPH) (C) of ‘Tropica’ and ‘Keitt’ mangoes dehydrated at 60 °C. Error bars represent standard deviation (SD) of mean (n = 6) values of treatments. Different lower-case letters indicate significant difference in mean values (p < 0.05). *Coloured graphs only available online.
A possible explanation for the results obtained could be attributed to the impact of discharged particles and low pressure, which could have caused etching on the fruit surface. This phenomenon could have induced and made it easier for antioxidants to be released during the pretreatment and hot air drying (Won et al., 2017). The increase in the reaction power of active species after cold plasma application, such as the hydroxyl radical at higher drying times, could be linked to a decline in antioxidant activity. In addition, the variation observed between the cultivars combined with treatments is consistent with literature. Factors, such as the cultivar and structure of the fruit treated, the type of cold plasma used, and the input voltage, time, working gas, and power applied, could induce the variation observed in the bioactive compounds (Pathare et al., 2023). For example, Albanese et al. (2013) attributed the rise in antioxidant capacity after drying process to the production of novel antioxidant molecules in the food items. Studies on hot air-drying experiments using temperatures from 60 to 80°C by İzlİ (2016) have resulted in a reduction of antioxidant compounds of dried products which was due to the increased temperature used. Another research by Di Scala et al. (2011) suggested that the complex chemical interactions, degradation of polyphenols, and oxidation of these bioactive compounds could be responsible for the decline in antioxidant capacity. The present study suggests that the pretreatment used might not effectively preserve the bioactive components, but it does not critically degrade these compounds.
Microbial Analysis
The interaction of treatments and cultivar played a significant role in the observed decline in both aerobic mesophilic bacteria (AMB) and yeast and mould (Y&M) load on the fresh whole fruit treated and dried mango slices (p < 0.000001) as presented in Fig. 6. For the fresh untreated whole fruit, ‘Tropica’ had significantly higher AMB (4.1 ± 0.35 log CFU/cm2) and Y&M (4.0 ± 0.071 log CFU/cm2) in comparison to ‘Keitt’ with 2.9 ± 0.28 and 2.9 ± 0.14 log CFU/cm2, respectively (Fig. 6). The impact of CP10 treatment was observed to be consistent for ‘Tropica’ variety resulting in the highest reduction of AMB and Y&M in the treated whole fruit (p < 0.05). However, for ‘Keitt’, no statistically significant difference in microbial count was found between CP5 and CP10 treatments. These observations support the established application theory that prolonged exposure to cold plasma treatment could be efficient in inhibiting microbial growth, but also indicated that cultivar variation could influence the efficiency of low-pressure cold plasma treatment irrespective of the treatment duration (Pathare et al., 2023). From our study, it can be deduced that the efficacy of cold plasma treatment in reducing microbial load in mango fruit depends on various factors such as treatment time, temperature, relative humidity, type of gas, voltage, power, frequency, and material thickness (Liao et al., 2018; Yadav et al., 2019).
Impacts of cold plasma treatment for 5 min, 10 min, sodium metabisulphite, and untreated (control) on the total aerobic mesophilic bacteria (AMB) and yeast and mould count on the surface of fresh whole (WF) and dried (DF) ‘Tropica’ and ‘Keitt’ mangoes dehydrated at 60°C. Error bars represent standard deviation (SD) of mean (n = 9) values of treatments. Different lower-case letters indicate significant difference in mean values (p < 0.05). *Coloured graphs only available online
The decrease in aerobic mesophilic bacterial occurrence observed in the WF-CP-treated group can be attributed to the effectiveness of CP to inhibit the growth of these bacteria (Liao et al., 2019). CP treatment discharges ionized gas rich in charged particulates and reactive species, such as reactive oxygen species and free radicals, which can damage cell membrane, leading to their inactivation or death (Cheng et al., 2020). On the other hand, the increase in the aerobic mesophilic bacterial noted within the DF-CP groups for ‘Tropica’ samples could be a function of the initial microbial load on the fresh whole fruit (Fig. 6), as well as the selective effect of plasma discharge on different types of bacterial species (Mai-Prochnow et al., 2014). For instance, according to Stoffels et al. (2008), it is widely believed that inactivation through erosion is more easily accomplished in Gram-negative bacteria compared to Gram-positive species, primarily due to the vulnerability of the cell wall in Gram-negative bacteria and the thicker membrane structure found in Gram-positive species. It is important to note that factors, such as the initial microbial load, sample composition, and environmental conditions, could influence the outcomes of CP treatments (Pathare et al., 2023). Thus, further research focused on the identification and characterization of changes in aerobic mesophilic bacteria would be required to fully understand the underlying microbial community dynamics.
CP-pretreatment combined with additional drying step further significantly reduced the microbial load on mango slices compared to untreated (control) sample. Overall, pretreated CP10 and SMB treatment maintained the lowest microbial load compared to untreated and CP5 (p < 0.05). For Y&M, the microbial counts were below detection. The decline may be a result of the effect of the increase in drying temperature and lower moisture content during dehydration process (Dereje & Abera, 2020). It is worth noting that the final AMB and Y&M counts were below the permissible limit established by the International Commission for Microbiological Specifications for Foods and South African legislation (FCDA, Act 54, 1979). These results demonstrate that the combination of low-pressure CP pretreatment and drying at 60°C further reduces the microbial load on dried mango slices. The lack of significant differences in antimicrobial efficacy between the low-pressure CP-treated and SMB-treated groups could be due to several factors such as the treatment efficacy. Low-pressure CP has been reportedly used for “flash-sterilization” of products surface. It does not achieve complete sterilization and/or decontamination (Fiebrandt et al., 2018), but it is effective in reducing microbial load (Braný et al., 2020).
Cold plasma treatment has been shown to have antimicrobial effects on various fruits and vegetables, including mangoes (Lee et al., 2015; Yarabbi et al., 2023). Specific reduction in microbial activity can vary depending on factors such as the plasma parameters (e.g. power, treatment time) and the specific microbial strains present (Chen et al., 2020). Different mango cultivars may naturally possess varying initial microbial loads, and this initial microbial load can impact the effectiveness of the CP treatments. In this study, ‘Tropica’ had significantly higher initial microbial count compared to ‘Keitt’. In addition, the composition of these mango cultivars differs, including variations in pH, sugar content, moisture content, and organic matter. These compositional differences can influence the natural micro flora on the fruit surface. For instance, the ‘Tropica’ had significantly (p < 0.05) lower pH (4.9) compared to ‘Keitt’ (3.4). Hertwig et al. (2015) investigated the impact of the air-plasma technique on the microbial flora of different vegetable powders. The treatment resulted in a logarithmic decrease of around 4.0. Only crushed oregano samples had a lower reduction rate than other samples. The authors attributed the differences to the original native microbial load on the samples. According to Lin et al. (2011), genetic variations in fruits can influence the expression of defence mechanisms, antimicrobial compound production, or susceptibility to treatments, resulting in differences in antimicrobial effects. It is essential to consider these factors when evaluating the antimicrobial effects of pretreatments on different cultivars. Understanding the inherent characteristics and attributes of ‘Keitt’ and ‘Tropica’ cultivars, as well as their interactions with the treatments, will help to identify the reasons behind observed variations in antimicrobial effects and guide future research and optimization efforts.
Cold plasma treatment has been shown to effectively inactivate a wide range of microorganisms, including bacteria, yeast, and moulds. Plasma generates reactive species, free radicals, UV radiation, and electric fields, which collectively contribute to the destruction of microbial cells (Bourke et al., 2017). CP leaves no chemical residues on the treated food products. This is particularly important for organic or minimally processed ready to eat fresh produce, where consumers prefer additive-free products (Bourke et al., 2018). In contrast, SMB primarily acts as a reducing agent that inhibits microbial growth but may not eliminate pathogens or spoilage organisms (Ahmadi et al., 2018). However, this treatment leaves behind residues that can affect the taste, odour, and quality of the treated food (Bhavadharini et al., 2022). In addition, CP is considered non-toxic and environmentally friendly. It does not rely on the use of chemical additives or preservatives, making it a desirable option for clean-label products (Yepez et al., 2022). In contrast, sodium metabisulphite may have adverse effects on individuals with sulphite sensitivity, and its excessive use or improper disposal can have negative environmental implications (Bhavadharini et al., 2022).
Conclusion
The impact of low-pressure cold plasma and cultivar differences on the drying characteristics, kinetics, and quality attributes of ‘Tropica’ and ‘Keitt’ mango slices was investigated. This study showed that low-pressure cold plasma used as pretreatment is effective in enhancing the drying rate and maintaining the quality attributes of dried ‘Tropica’ and ‘Keitt’ mango slices. Drying time for CP pretreated mango slices was significantly reduced in both cultivars, but ‘Keitt’ mango slices attained desired moisture content earlier. The result obtained showed that sodium metabisulphite still better retained colour attributes followed by CP pretreatment for 10 min. The highest total phenolic content retention was achieved in both cultivars pretreated with cold plasma for 10 min. Furthermore, low-pressure cold plasma pretreatment enhanced microbial inactivation especially yeast and moulds in the samples. These findings effectively demonstrate the potential for low-pressure cold plasma to balance antimicrobial efficacy and preservation of bioactive compounds. Overall, the logarithmic thin layer model applied adequately described the drying behaviour of ‘Tropica and Keitt’ mango slices. Furthermore, a study characterizing the changes in the tissue microstructures (using scanning electron microscopy or X-ray micro-computed tomography) of both mango cultivars would be required to better understand their response to drying. Similarly, investigation into the shift in microbial profile dynamics via high throughput molecular techniques (e.g. new generation sequencing) is needed.
Data Availability
The authors declare that the data supporting the findings of this study are available from the corresponding author upon request.
References
Aghbashlo, M., & Samimi-Akhijahani, H. (2008). Influence of drying conditions on the effective moisture diffusivity, energy of activation and energy consumption during the thin-layer drying of berberis fruit (Berberidaceae). Energy Conversion and Management, 49(10), 2865–2871. https://doi.org/10.1016/j.enconman.2008.03.009
Ahmadi, F., Lee, Y. H., Lee, W. H., Oh, Y. K., Park, K. K., & Kwak, W. S. (2018). Preservation of fruit and vegetable discards with sodium metabisulfite. Journal of Environmental Management, 224, 113–121. https://doi.org/10.1016/j.jenvman.2018.07.044
Albanese, D., Cinquanta, L., Cuccurullo, G., & Di Matteo, M. (2013). Effects of microwave and hot-air drying methods on colour β-carotene and radical scavenging activity of apricots. International Journal of Food Science & Technology, 48(6), 1327–1333. https://doi.org/10.1111/ijfs.12095
Ali, M. A., Yusof, Y. A., Chin, N. L., & Ibrahim, M. N. (2016). Effect of different drying treatments on colour quality and ascorbic acid concentration of guava fruit. International Food Research Journal, 23, S155–S161. http://psasir.upm.edu.my/id/eprint/50532
Ampah, J., Dzisi, K. A., Addo, A., & Bart-Plange, A. (2022). Drying kinetics and chemical properties of mango. International Journal of Food Science, 2022, 6243228. https://doi.org/10.1155/2022/6243228
Ashtiani, S. H. M., Rafiee, M., Mohebi Morad, M., & Martynenko, A. (2022). Cold plasma pretreatment improves the quality and nutritional value of ultrasound-assisted convective drying: The case of goldenberry. Drying Technology, 40(8), 1639–1657. https://doi.org/10.1080/07373937.2022.2050255
Ashtiani, S. H. M., Aghkhani, M. H., Feizy, J., & Martynenko, A. (2023). Effect of cold plasma pretreatment coupled with osmotic dehydration on drying kinetics and quality of mushroom (Agaricus bisporus). Food and Bioprocess Technology, 16, 2854–2876. https://doi.org/10.1007/s11947-023-03096-z
Bao, T., Hao, X., Shishir, M. R. I., Karim, N., & Chen, W. (2021). Cold plasma: An emerging pretreatment technology for the drying of jujube slices. Food Chemistry, 337, 127783. https://doi.org/10.1016/j.foodchem.2020.127783
Bassey, E. J., Cheng, J. H., & Sun, D. W. (2021). Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of fruits and vegetables. Trends in Food Science & Technology, 112, 137–148. https://doi.org/10.1016/j.tifs.2021.03.045
Bhavadharini, B., Kavimughil, M., Malini, B., Vallath, A., Prajapati, H. K., & Sunil, C. K. (2022). Recent advances in biosensors for detection of chemical contaminants in food—A review. Food Analytical Methods, 15(6), 1545–1564. https://doi.org/10.1007/s12161-021-02213-y
Bourke, P., Ziuzina, D., Han, L., Cullen, P. J., & Gilmore, B. F. (2017). Microbiological interactions with cold plasma. Journal of Applied Microbiology, 123(2), 308–324. https://doi.org/10.1111/jam.13429
Bourke, P., Ziuzina, D., Boehm, D., Cullen, P. J., & Keener, K. (2018). The potential of cold plasma for safe and sustainable food production. Trends in Biotechnology, 36(6), 615–626. https://doi.org/10.1016/j.tibtech.2017.11.001
Braný, D., Dvorská, D., Halašová, E., & Škovierová, H. (2020). Cold atmospheric plasma: A powerful tool for modern medicine. International Journal of Molecular Science, 21(8), 2932. https://doi.org/10.3390/ijms21082932
Carranza-Concha, J., Benlloch, M., Camacho, M. M., & Martínez-Navarrete, N. (2012). Effects of drying and pretreatment on the nutritional and functional quality of raisins. Food and Bioproducts Processing, 90(2), 243–248. https://doi.org/10.1016/j.fbp.2011.04.002
Chaethong, K., & Pongsawatmanit, R. (2015). Influence of sodium metabisulfite and citric acid in soaking process after blanching on quality and storage stability of dried chili. Journal of Food Processing and Preservation, 39(6), 2161–2170. https://doi.org/10.1111/jfpp.12460
Chang, C. H., Lin, H. Y., Chang, C. Y., & Liu, Y. C. (2006). Comparisons on the antioxidant properties of fresh freeze-dried and hot-air-dried tomatoes. Journal of Food Engineering, 77(3), 478–485. https://doi.org/10.1016/j.jfoodeng.2005.06.061
Chatha, Z. A., Ahmad, A., Faiyaz, F., & Ayub, H. (2020). Comparative effects of gamma irradiation, UV-C and hot water treatments on sensory attributes of mango fruit (Mangifera indica L.) cv. white and black chaunsa. Pakistan Journal of Agricultural Sciences, 57(2), 499–504. https://doi.org/10.21162/PAKJAS/19.9284
Chen, Y. Q., Cheng, J. H., & Sun, D. W. (2020). Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Critical Reviews in Food Science and Nutrition, 60(16), 2676–2690. https://doi.org/10.1080/10408398.2019.1654429
Cheng, J. H., Lv, X., Pan, Y., & Sun, D. W. (2020). Foodborne bacterial stress responses to exogenous reactive oxygen species (ROS) induced by cold plasma treatments. Trends in Food Science & Technology, 103, 239–247. https://doi.org/10.1016/j.tifs.2020.07.022
Coklar, H., Akbulut, M., Kilinc, S., Yildirim, A., & Alhassan, I. (2018). Effect of freeze, oven and microwave pretreated oven drying on colour, browning index, phenolic compounds, and antioxidant activity of hawthorn (Crataegus orientalis) fruit. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 46(2), 449–456. https://doi.org/10.15835/nbha46211027
Dantas, A. M., Batista, J. D. F., dos Santos Lima, M., Fernandes, F. A., Rodrigues, S., Magnani, M., & Borges, G. D. S. C. (2021). Effect of cold plasma on açai pulp: enzymatic activity, color and bioaccessibility of phenolic compounds. LWT - Food Science Technology, 149, 111883.
Dea, S., Brecht, J. K., Nunes, M. C. N., & Baldwin, E. A. (2010). Quality of fresh-cut “Kent” mango slices prepared from hot water or non-hot water-treated fruit. Postharvest Biology and Technology, 56(2), 171–180. https://doi.org/10.1016/j.postharvbio.2010.01.007
Deng, L. Z., Tao, Y., Mujumdar, A. S., Pan, Z., Chen, C., Yang, X. H., & Xiao, H. W. (2020). Recent advances in non-thermal decontamination technologies for microorganisms and mycotoxins in low-moisture foods. Trends in Food Science & Technology, 106, 104–112. https://doi.org/10.1016/j.tifs.2020.10.012
Deng, L. Z., Mujumdar, A. S., Zhang, Q., Yang, X. H., Wang, J., Zheng, Z. A., ... & Xiao, H. W. (2019). Chemical and physical pretreatments of fruits and vegetables: Effects on drying characteristics and quality attributes–A comprehensive review. Critical Reviews in Food Science and Nutrition, 59(9), 1408–1432. https://doi.org/10.1080/10408398.2017.1409192
Dereje, B., & Abera, S. (2020). Effect of pretreatments and drying methods on the quality of dried mango (Mangifera Indica L.) slices. Cogent Food & Agriculture, 6(1), 1747961. https://doi.org/10.1080/23311932.2020.1747961
Dewanto, V., Wu, X., Adom, K. K., & Liu, R. H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50(10), 3010–3014. https://doi.org/10.1021/jf0115589
Di Scala, K., Vega-Gálvez, A., Uribe, E., Oyanadel, R., Miranda, M., Veragara, J., & Quispe, I. (2011). Changes of quality characteristics of pepino fruit (Solanum muricatum Ait) during convective drying. International Journal of Food Science & Technology, 46(4), 746–753. https://doi.org/10.1111/j.1365-2621.2011.02555.x
Doymaz, I. (2004). Effect of pre-treatments using potassium metabisulphide and alkaline ethyl oleate on the drying kinetics of apricots. Biosystems Engineering, 89(3), 281–287. https://doi.org/10.1016/j.biosystemseng.2004.07.009
Du, Y., Yang, F., Yu, H., Xie, Y., & Yao, W. (2022). Improving food drying performance by cold plasma pretreatment: A systematic review. Comprehensive Reviews in Food Science and Food Safety, 21(5), 4402–4421. https://doi.org/10.1111/1541-4337.13027
Duda-Chodak, A., Tarko, T., & Tuszyński, T. (2011). Antioxidant activity of apples – An impact of maturity stage and fruit part. Acta Scientiarum Polonorum, Technologia Alimentaria, 10, 443–454. https://www.food.actapol.net/volume10/issue4/3_4_2011.pdf
Everett, K. R., Hallett, I. C., Rees-George, J., Chynoweth, R. W., & Pak, H. A. (2008). Avocado lenticel damage: The cause and the effect on fruit quality. Postharvest Biology and Technology, 48(3), 383–390. https://doi.org/10.1016/j.postharvbio.2007.09.008
Fiebrandt, M., Lackmann, J. W., & Stapelmann, K. (2018). From patent to product? 50 years of low-pressure plasma sterilization. Plasma Processes and Polymers, 15, 1800139. https://doi.org/10.1002/ppap.201800139
Guiné, R. P., & Barroca, M. J. (2012). Effect of drying treatments on texture and colour of vegetables (pumpkin and green pepper). Food and Bioproducts Processing, 90(1), 58–63. https://doi.org/10.1016/j.fbp.2011.01.003
Gulzar, A., Ahmed, M., Qadir, M. A., Shafiq, M. A., Ali, S., Ahmad, I., & Mukhtar, M. F. (2018). Effect of blanching techniques and treatments on nutritional quality of dried mango slices during storage. Polish Journal of Food and Nutrition Sciences, 68(1), 5–13. https://doi.org/10.1515/pjfns-2017-0012
Henderson, S. M., & Pabis, S. (1961). Grain drying theory (I) temperature effect on drying coefficient. Journal of Agricultural Engineering Research, 6(3), 169–174. https://doi.org/10.5555/19621700719
Hertwig, C., Reineke, K., Ehlbeck, J., Erdoğdu, B., Rauh, C., & Schlüter, O. (2015). Impact of remote plasma treatment on natural microbial load and quality parameters of selected herbs and spices. Journal of Food Engineering, 167, 12–17. https://doi.org/10.1016/j.jfoodeng.2014.12.017
İzli, G. (2016). Total phenolics, antioxidant capacity, colour, and drying characteristics of date fruit dried with different methods. Food Science and Technology, 37, 139–147. https://doi.org/10.1590/1678-457X.14516
Jha, S. N., Jaiswal, P., Narsaiah, K., Kaur, P. P., Singh, A. K., & Kumar, R. (2013). Textural properties of mango cultivars during ripening. Journal of Food Science and Technology, 50(6), 1047–1057. https://doi.org/10.1007/s13197-011-0431-z
Karim, N., Shishir, M. R. I., Bao, T., & Chen, W. (2021). Effect of cold plasma pretreated hot air drying on the physicochemical characteristics, nutritional values and antioxidant activity of shiitake mushroom. Journal of the Science of Food and Agriculture, 101(15), 6271–6280. https://doi.org/10.1002/jsfa.11296
Kim, J. E., Oh, Y. J., Won, M. Y., Lee, K. S., & Min, S. C. (2017). Microbial decontamination of onion powder using microwave-powered cold plasma treatments. Food Microbiology, 62, 112–123. https://doi.org/10.1016/j.fm.2016.10.006
Lacombe, A., Niemira, B. A., Gurtler, J. B., Fan, X., Sites, J., Boyd, G., & Chen, H. (2015). Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiology, 46, 479–484. https://doi.org/10.1016/j.fm.2014.09.010
Le, T. D., Viet Nguyen, T., Muoi, N. V., Toan, H. T., Lan, N. M., & Pham, T. N. (2022). Supply chain management of mango (Mangifera indica L.) fruit: A review with a focus on product quality during postharvest. Fronier in Sustainable Food Systems, 5, 799431. https://doi.org/10.3389/fsufs.2021.799431
Lee, H., Kim, J. E., Chung, M. S., & Min, S. C. (2015). Cold plasma treatment for the microbiological safety of cabbage, lettuce, and dried figs. Food Microbiology, 51, 74–80. https://doi.org/10.1016/j.fm.2015.05.004
Liao, X., Li, J., Muhammad, A. I., Suo, Y., Chen, S., Ye, X., & Ding, T. (2018). Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for Escherichia coli inactivation in apple juice. Journal of Food Science, 83(2), 401–408. https://doi.org/10.1111/1750-3841.14045
Liao, X., Muhammad, A. I., Chen, S., Hu, Y., Ye, X., Liu, D., & Ding, T. (2019). Bacterial spore inactivation induced by cold plasma. Critical Reviews in Food Science and Nutrition, 59(16), 2562–2572. https://doi.org/10.1080/10408398.2018.1460797
Liao, X., Cullen, P. J., Muhammad, A. I., Jiang, Z., Ye, X., Liu, D., & Ding, T. (2020). Cold plasma–based hurdle interventions: New strategies for improving food safety. Food Engineering Reviews, 12, 321–332. https://doi.org/10.1007/s12393-020-09222-3
Lin, J., Gong, D., Zhu, S., Zhang, L., & Zhang, L. (2011). Expression of PPO and POD genes and contents of polyphenolic compounds in harvested mango fruits in relation to Benzothiadiazole-induced defense against anthracnose. Scientia Horticulturae, 130(1), 85–89. https://doi.org/10.1016/j.scienta.2011.06.014
Liu, B., Jiao, W., Wang, B., Shen, J., Zhao, H., & Jiang, W. (2019). Near freezing point storage compared with conventional low temperature storage on apricot fruit flavor quality (volatile, sugar, organic acid) promotion during storage and related shelf life. Scientia Horticulturae, 249, 100–109. https://doi.org/10.1016/j.scienta.2019.01.048
Liu, B., Xin, Q., Zhang, M., Chen, J., Lu, Q., Zhou, X., Li, X., Zhang, W., Feng, W., Pei, H., & Sun, J. (2022). Research progress on mango post-harvest ripening physiology and the regulatory technologies. Foods, 12(1), 173. https://doi.org/10.3390/foods12010173
Lüle, F., & Koyuncu, T. (2015). Convective and microwave drying characteristics of sorbus fruits (Sorbus domestica L.). Procedia Social and Behavioural Sciences, 195, 2634–2643. https://doi.org/10.1016/j.sbspro.2015.06.467
Mai-Prochnow, A., Murphy, A. B., McLean, K. M., Kong, M. G., & Ostrikov, K. K. (2014). Atmospheric pressure plasmas: Infection control and bacterial responses. International Journal of Antimicrobial Agents, 43(6), 508–517. https://doi.org/10.1016/j.ijantimicag.2014.01.025
Marquez, A., Serratosa, M. P., & Merida, J. (2013). Anthocyanin evolution and colour changes in red grapes during their chamber drying. Journal of Agricultural and Food Chemistry, 61, 9908–9914. https://doi.org/10.1021/jf402263f
Memis, H., Bekar, F., Guler, C., Kamiloğlu, A., & Kutlu, N. (2023). Optimization of ultrasonic-assisted osmotic dehydration as a pretreatment for microwave drying of beetroot (Beta vulgaris). Food Science and Technology International. https://doi.org/10.1177/10820132231153501
Midilli, A. D. N. A. N., Kucuk, H. A. Y. D. A. R., & Yapar, Z. İY. A. (2002). A new model for single-layer drying. Drying Technology, 20(7), 1503–1513. https://doi.org/10.1081/DRT-120005864
Mukhtar, A., Latif, S., & Mueller, J. (2020). Effect of heat exposure on activity degradation of enzymes in mango varieties sindri, sb chaunsa, and Tommy Atkins during drying. Molecules, 25(22), 5396. https://doi.org/10.3390/molecules25225396
Mwamba, I., & Mputu, J. N. (2022). Contribution to the physicochemical and microbiological study of dried mango. Comparison of two drying methods (oven and solar drying). Current Overview Science Technology Research, 1, 93–102. https://doi.org/10.9734/bpi/costr/v1/16879D
Nowacka, M., Wiktor, A., Anuszewska, A., Dadan, M., Rybak, K., & Witrowa-Rajchert, D. (2019). The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrasonic Sonochemistry, 56, 1–13. https://doi.org/10.1016/j.ultsonch.2019.03.023
Nsumpi, A. N., Belay, Z. A., & Caleb, O. J. (2020). Good intentions, bad outcomes: Impact of mixed-fruit loading on banana fruit protein expression, physiological responses, and quality. Food Packaging and Shelf Life, 26, 100594. https://doi.org/10.1016/j.fpsl.2020.100594
Ntsoane, M. L., Sivakumar, D., & Mahajan, P. V. (2020). Optimisation of O2 and CO2 concentrations to retain quality and prolong shelf life of ‘shelly’ mango fruit using a simplex lattice mixture design. Biosystem Engineering, 192, 14–23. https://doi.org/10.1016/j.biosystemseng.2020.01.009
Nyamende, N. E., Belay, Z. A., Keyser, Z., Oyenihi, A., & Caleb, O. J. (2022). Impacts of alkaline electrolyzed water treatment on physicochemical, phytochemical, antioxidant properties and natural microbial load on ‘Granny Smith’ apples during storage. International Journal of Food Science and Technology, 57, 447–456. https://doi.org/10.1111/ijfs.15426
Nyangena, I., Owino, W., Ambuko, J., & Imathiu, S. (2019). Effect of selected pretreatments prior to drying on physical quality attributes of dried mango chips. Journal of Food Science and Technology, 56, 3854–3863. https://doi.org/10.1007/s13197-019-03857-9
Obajemihi, O. I., Esua, O. J., Cheng, J. H., & Sun, D. W. (2023). Effects of pretreatments using plasma functionalized water, Osmo-dehydration and their combination on hot air-drying efficiency and quality of tomato (Solanum lycopersicum L.) slices. Food Chemistry, 406, 134995. https://doi.org/10.1016/j.foodchem.2022.134995
Önal, B., Adiletta, G., Di Matteo, M., Russo, P., Ramos, I. N., & Silva, C. L. M. (2021). Microwave and ultrasound pre-treatments for drying of the “Rocha” Pear: Impact on phytochemical parameters, colour changes and drying kinetics. Foods, 10(4), 853. https://doi.org/10.3390/foods10040853
Onwude, D. I., Hashim, N., Abdan, K., Janius, R., Chen, G., & Kumar, C. (2018). Modelling of coupled heat and mass transfer for combined infrared and hot-air drying of sweet potato. Journal of Food Engineering, 228, 12–24. https://doi.org/10.1016/j.jfoodeng.2018.02.006
Padda, M. S., do Amarante, C. V. T., Garcia, R. M., Slaughter, D. C., & Mitcham, E. J. (2011). Methods to analyze physico-chemical changes during mango ripening: A multivariate approach. Postharvest Biology and Technology, 62(3), 267–274. https://doi.org/10.1016/j.postharvbio.2011.06.002
Pandiselvam, R., Mitharwal, S., Rani, P., Anjaly Shanker, M., Kumar, A., Aslam, R., et al. (2023). The influence of non-thermal technologies on colour pigments of food materials: An updated review. Current Research in Food Science, 6, 100529. https://doi.org/10.1016/j.crfs.2023.100529
Patel, K. K. (2022). Recent developments in application of precooling of mangoes—An overview. Journal of Biosystem Engineering, 47, 318–329. https://doi.org/10.1007/s42853-022-00149-7
Pathare, P. B., Caleb, O. J., Prasath, V. R., & Garud, S. R. (2023). Application of cold plasma for fresh produce quality and shelf-life extension. In B. P. Singh, S. Agnihotri, G. Singh, & V. K. Gupta (Eds.), Postharvest management of fresh produce (pp. 165–194). Elsevier Inc. Academic Press. https://doi.org/10.1016/B978-0-323-91132-0.00009-5
Pavun, L., Uskoković-Marković, S., Jelikić-Stankov, M., Dikanović, D., & Durdević, P. (2018). Determination of flavonoids and total polyphenol contents in commercial apple juices. Czech Journal of Food Sciences, 36, 233–238. https://doi.org/10.17221/211/2017-CJFS
Perea-Flores, M. J., Garibay-Febles, V., Chanona-Perez, J. J., Calderon-Dominguez, G., Mendez-Mendez, J. V., Palacios-González, E., & Gutierrez-Lopez, G. F. (2012). Mathematical modelling of castor oil seeds (Ricinus communis) drying kinetics in fluidized bed at high temperatures. Industrial Crops and Products, 38, 64–71. https://doi.org/10.1016/j.indcrop.2012.01.008
Phan, K. T. K., Phan, H. T., Uthaichana, K., & Phimolsiripol, Y. (2017). Effect of non-thermal plasma on physicochemical properties of ‘Nam Dok Mai’ mango. International Journal on Advanced Science, Engineering and Information Technology, 7(1), 263–268. https://doi.org/10.18517/ijaseit.7.1.1620
Phan, K. T. K., Phan, H. T., Boonyawan, D., Intipunya, P., Brennan, C. S., Regenstein, J. M., & Phimolsiripol, Y. (2018). Non-thermal plasma for elimination of pesticide residues in mango. Innovative Food Science & Emerging Technologies, 48, 164–171. https://doi.org/10.1016/j.ifset.2018.06.009
Pushparaja, V., Vasantharuba, S., & Nadarajah, K. (2023). Effect of cabinet drying on nutritional quality and drying kinetics of brinjal (Solanum melongena L) and bitter gourd (Momordica charantia). Journal of Biosystem Engineering, 48, 257–268. https://doi.org/10.1007/s42853-023-00189-7
Ranjbar Nedamani, A., & Hashemi, S. J. (2022). Energy consumption computing of cold plasma-assisted drying of apple slices (Yellow Delicious) by numerical simulation. Journal of Food Process Engineering, 45(5), e14019. https://doi.org/10.1111/jfpe.14019
Rufián-Henares, J. A., Delgado-Andrade, C., & Morales, F. J. (2006). Occurrence of acetic acid and formic acid in breakfast cereals. Journal of the Science of Food and Agriculture, 86(9), 1321–1327. https://doi.org/10.1002/jsfa.2510
Sadin, R., Chegini, G.-R., & Sadin, H. (2013). The effect of temperature and slice thickness on drying kinetics tomato in the infrared dryer. Heat and Mass Transfer, 50(4), 501–507. https://doi.org/10.1007/s00231-013-1255-3
Salehi, F., & Kashaninejad, M. (2018). Modelling of moisture loss kinetics and colour changes in the surface of lemon slice during the combined infrared-vacuum drying. Information Processing in Agriculture, 5(4), 516–523. https://doi.org/10.1016/j.inpa.2018.05.006
Santos, S. C., Guiné, R. P., & Barros, A. (2014). Effect of drying temperatures on the phenolic composition and antioxidant activity of pears of Rocha variety (Pyrus communis L.). Journal of Food Measurement and Characterization, 8(2), 105–112. https://doi.org/10.1007/s11694-014-9170-y
Sarangapani, C., Devi, Y., Thirundas, R., Annapure, U. S., & Deshmukh, R. R. (2015). Effect of low-pressure plasma on physico-chemical properties of parboiled rice. LWT-Food Science and Technology, 63(1), 452–460. https://doi.org/10.1016/j.lwt.2015.03.026
Shishir, M. R. I., Karim, N., Bao, T., Gowd, V., Ding, T., Sun, C., & Chen, W. (2020). Cold plasma pretreatment–A novel approach to improve the hot air drying characteristics, kinetic parameters, and nutritional attributes of shiitake mushroom. Drying Technology, 38(16), 2134–2150. https://doi.org/10.1080/07373937.2019.1683860
Sra, S. K., Sandhu, K. S., & Ahluwalia, P. (2014). Effect of treatments and packaging on the quality of dried carrot slices during storage. Journal of Food Science and Technology, 51, 645–654. https://doi.org/10.1007/s13197-011-0575-x
Stoffels, E., Sakiyama, Y., & Graves, D. B. (2008). Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Transactions on Plasma Science, 36(4), 1441–1457. https://doi.org/10.1109/TPS.2008.2001084
Sultana, B., Anwar, F., Ashraf, M., & Saari, N. (2012). Effect of drying techniques on the total phenolic contents and antioxidant activity of selected fruits. Journal of Medicinal Plants Research, 6(1), 161–167. https://doi.org/10.5897/JMPR11.916
Sun, M., Xu, Y., Ding, Y., Gu, Y., Zhuang, Y., & Fan, X. (2023). Effect of ultrasound pretreatment on the moisture migration and quality of Cantharellus cibarius following hot air drying. Foods, 12(14), 2705. https://doi.org/10.3390/foods12142705
Tabibian, S. A., Labbafi, M., Askari, G. H., Rezaeinezhad, A. R., & Ghomi, H. (2020). Effect of gliding arc discharge plasma pretreatment on drying kinetic, energy consumption and physico-chemical properties of saffron (Crocus sativus L.). Journal of Food Engineering, 270, 109766. https://doi.org/10.1016/j.jfoodeng.2019.109766
Talens, C., Arboleya, J. C., Castro-Giraldez, M., & Fito, P. J. (2017). Effect of microwave power coupled with hot air drying on process efficiency and physico-chemical properties of a new dietary fibre ingredient obtained from orange peel. LWT-Food Science and Technology, 77, 110–118. https://doi.org/10.1016/j.lwt.2016.11.036
Torki-Harchegani, M., Ghasemi-Varnamkhasti, M., Ghanbarian, D., Sadeghi, M., & Tohidi, M. (2016). Dehydration characteristics and mathematical modelling of lemon slices drying undergoing oven treatment. Heat and Mass Transfer, 52, 281–289. https://doi.org/10.1007/s00231-015-1546-y
Vega-Gálvez, A., Ah-Hen, K., Chacana, M., Vergara, J., Martínez-Monzó, J., García-Segovia, P., Lemus-Mondaca, R., & Di Scala, K. (2012). Effect of temperature and air velocity on drying kinetics antioxidant capacity total phenolic content colour texture and microstructure of apple (var. Granny Smith) slices. Food Chemistry, 132(1), 51–59. https://doi.org/10.1016/j.foodchem.2011.10.029
Verma, L. R., & Joshi, V. K. (2000). Postharvest technology of fruits and vegetables: Handling, processing, fermentation, and waste management. Volume 2: Technology. Indus Publishing Company. https://doi.org/10.5555/20013059033
Verma, L. R., Bucklin, R. A., Endan, J. B., & Wratten, F. T. (1985). Effects of drying air parameters on rice drying models. Transactions of the ASABE, 28(1), 296–0301. https://doi.org/10.13031/2013.32245
Wang, C. Y., & Singh, R. P. (1978). A single layer drying equation for rough rice. ASAE.
Wang, Z., Sun, J., Liao, X., Chen, F., Zhao, G., Wu, J., & Hu, X. (2007). Mathematical modelling on hot air drying of thin layer apple pomace. Food Research International, 40(1), 39–46. https://doi.org/10.1016/j.foodres.2006.07.017
Wang, X., Li, C., Liang, D., Zou, Y., Li, P., & Ma, F. (2015). Phenolic compounds and antioxidant activity in red-fleshed apples. Journal of Functional Foods, 18, 1086–1094. https://doi.org/10.1016/j.jff.2014.06.013
Wang, J., Fang, X. M., Mujumdar, A. S., Qian, J. Y., Zhang, Q., Yang, X. H., Liu, Y. H., Gao, Z. J., & Xiao, H. W. (2017a). Effect of high-humidity hot air impingement blanching (HHAIB) pretreatment on drying characteristic and quality attributes of red pepper (Capsicum annuum L.). Food Chemistry, 220, 145–152.
Wang, J., Yang, X. H., Mujumdar, A. S., Wang, D., Zhao, J. H., Fang, X. M., & Xiao, H. W. (2017b). Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure, and drying kinetics of red bell pepper (Capsicum annuum L.). LWT-Food Science and Technology, 77, 337–347. https://doi.org/10.1016/j.lwt.2016.11.070
Wang, Q., Li, S., Han, X., Ni, Y., Zhao, D., & Hao, J. (2019). Quality evaluation and drying kinetics of shitake mushrooms dried by hot air, infrared and intermittent microwave–assisted drying methods. LWT-Food Science and Technology, 107, 236–242. https://doi.org/10.1016/j.lwt.2019.03.020
Won, M. Y., Lee, S. J., & Min, S. C. (2017). Mandarin preservation by microwave-powered cold plasma treatment. Innovative Food Science and Emerging Technologies, 39, 25–32. https://doi.org/10.1016/j.ifset.2016.10.021
Workneh, T., & Oke, M. (2012). The influence of the combined microwave power and hot-air ventilation on the drying kinetics and colour quality of tomato slices. African Journal of Biotechnology, 11(87), 15353–15364. https://doi.org/10.5897/AJB11.318
Wu, X. F., Zhang, M., Ye, Y. F., & Yu, D. X. (2020). Influence of ultrasonic pretreatments on drying kinetics and quality attributes of sweet potato slices in infrared freeze drying (IRFD). LWT-Food Science Technology, 131, 109801. https://doi.org/10.1016/j.lwt.2020.109801
Xiao, H. W., Law, C. L., Sun, D. W., & Gao, Z. J. (2014). Colour change kinetics of American ginseng (Panax quinquefolium) slices during air impingement drying. Drying Technology, 32(4), 418–427. https://doi.org/10.1080/07373937.2013.834928
Xiao, H. W., Pan, Z., Deng, L. Z., El-Mashad, H. M., Yang, X. H., Mujumdar, A. S., ... & Zhang, Q. (2017). Recent developments and trends in thermal blanching–A comprehensive review. Information Processing in Agriculture, 4(2), 101–127. https://doi.org/10.1016/j.inpa.2017.02.001
Yadav, B., Spinelli, A. C., Govindan, B. N., Tsui, Y. Y., McMullen, L. M., & Roopesh, M. S. (2019). Cold plasma treatment of ready-to-eat ham: Influence of process conditions and storage on inactivation of Listeria innocua. Food Research International, 123, 276–285. https://doi.org/10.1016/j.foodres.2019.04.065
Yanclo, L. A., Sigge, G., Belay, Z. A., October, F., & Caleb, O. J. (2022). Microstructural, biochemical, and drying characteristics of dehydrated ‘Sunectwentyone’ nectarines as affected by sodium metabisulphite. Food Science and Biotechnology, 31(3), 311–322. https://doi.org/10.1007/s10068-022-01039-6
Yanclo, L. J. A. O., Sigge, G. O., Belay, Z. A., & Caleb, O. J. (2023). Impact of electrolyzed water as pre-treatments on drying properties and total colour difference of fresh cut ‘Tommy Atkins’ mangoes. Heliyon, 9(8), e18555. https://doi.org/10.1016/j.heliyon.2023.e18555
Yarabbi, H., Soltani, K., Sangatash, M. M., Yavarmanesh, M., & Zenoozian, M. S. (2023). Reduction of microbial population of fresh vegetables (carrot, white radish) and dried fruits (dried fig, dried peach) using atmospheric cold plasma and its effect on physicochemical properties. Journal of Agriculture and Food Research, 14, 100789. https://doi.org/10.1016/j.jafr.2023.100789
Yepez, X., Illera, A. E., Baykara, H., & Keener, K. (2022). Recent advances and potential applications of atmospheric pressure cold plasma technology for sustainable food processing. Foods, 11(13), 1833. https://doi.org/10.3390/foods11131833
Yi, J. Y., Lyu, J., Bi, J. F., Zhou, L. Y., & Zhou, M. (2017). Hot air drying and freeze-drying pre-treatments coupled to explosion puffing drying in terms of quality attributes of mango, pitaya, and papaya fruit chips. Journal of Food Processing and Preservation, 41(6), e13300. https://doi.org/10.1111/jfpp.13300
Zanoelo, E. F., Cardozo-Filho, L., & Cardozo-Junior, E. L. (2006). Superheated steam drying of mate leaves and effect of drying conditions on the phenol content. Journal of Food Process Engineering, 29(3), 253–268. https://doi.org/10.1111/j.1745-4530.2006.00064.x
Zhang, X. L., Zhong, C. S., Mujumdar, A. S., Yang, X. H., Deng, L. Z., Wang, J., & Xiao, H. W. (2019). Cold plasma pretreatment enhances drying kinetics and quality attributes of chili pepper (Capsicum annuum L.). Journal of Food Engineering, 241, 51–57. https://doi.org/10.1016/j.jfoodeng.2018.08.002
Zhang, Y., Gao, Z., Hu, M., Pan, Y., Xu, X., & Zhang, Z. (2022). Delay of ripening and senescence in mango fruit by 6-benzylaminopurine is associated with inhibition of ethylene biosynthesis and membrane lipid catabolism. Postharvest Biology and Technology, 185, 111797. https://doi.org/10.1016/j.postharvbio.2021.111797
Zou, K., Teng, J., Huang, L., Dai, X., & Wei, B. (2013). Effect of osmotic pretreatment on quality of mango chips by explosion puffing drying. LWT-Food Science and Technology, 51(1), 253–259. https://doi.org/10.1016/j.lwt.2012.11.005
Acknowledgements
We are grateful for the research platform provided by the Agricultural Research Council (ARC) Infruitec-Nietvoorbij, Stellenbosch, South Africa.
Funding
Open access funding provided by Stellenbosch University. This work is based upon research supported by the National Research Foundation (NRF) of South Africa (Grant Nos. 137990 and 146360) awarded to Dr OJ Caleb. The NRF Research grant (Grant No. 138128) awarded to Dr ZA Belay is also gratefully acknowledged. The PhD Fellowship award to Ms. Loriane A Yanclo (OWSD, Rev. No. 3240309373), by the Organization for Women in Science for the Developing World (OWSD) and Swedish International Development Cooperation Agency (SIDA), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanclo, L.A., Sigge, G., Belay, Z.A. et al. Effects of Cold Plasma Pretreatment and Cultivar on the Drying Characteristics, Biochemical, and Bioactive Compounds of 'Tropica' and 'Keitt'Mangoes. J. Biosyst. Eng. 49, 135–155 (2024). https://doi.org/10.1007/s42853-024-00222-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42853-024-00222-3