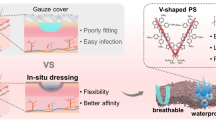
Abstract
Living cells and active factors are the two core elements of tissue repair, directly affecting the healing efficiency of damaged tissue. Nanofat (NF) can release living cells, such as adipose-derived stem cells (ADSCs), as well as active growth factors to promote angiogenesis, thus realizing cell-based wound healing. Herein, a novel living electrospun short fibrous sponge is constructed by modifying three-dimensional (3D) bionic short fibers with engineered NF. The uniform distribution of the polydopamine (PDA) modification endows the living sponges with stable mechanical properties, reversible water absorption and excellent adhesion even after repeated compression by an external force and long-term aqueous immersion. Meanwhile, the living electrospun short fibrous sponges with uniform NF modification contain living cells such as ADSCs and active growth factors such as vascular endothelial growth factor (VEGF), which can effectively promote the tube formation of human umbilical vein endothelial cells (HUVECs). In vivo, the living sponges can effectively and continuously act on wounds and act as a bionic living skin to prevent the loss of internal nutrients, creating a comfortable and favorable microenvironment for tissue regeneration and promoting the healing of diabetic wounds. Therefore, living electrospun short fibrous sponges via engineered NF are expected to achieve continuous wound healing with in situ living cells and active factors in injured tissues.







Similar content being viewed by others
References
Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen 1999;7:362.
Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: a cellular perspective. Physiol Rev 2019;99:665.
Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920.
Niu YM, Li Q, Ding Y, Dong L, Wang CM. Engineered delivery strategies for enhanced control of growth factor activities in wound healing. Adv Drug Deliv Rev 2019;146:190.
Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science 2014;346:941.
Bakhshandeh B, Zarrintaj P, Oftadeh MO, Keramati F, Fouladiha H, Sohrabi-Jahromi S, Ziraksaz Z. Tissue engineering; strategies, tissues, and biomaterials. Biotechnol Genet Eng Rev 2017;33:144.
Devalliere J, Dooley K, Hu Y, Kelangi SS, Uygun BE, Yarmush ML. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017;141:149.
Hui Chong LS, Zhang J, Bhat KS, Yong D, Song J. Bioinspired cell-in-shell systems in biomedical engineering and beyond: Comparative overview and prospects. Biomaterials 2021;266:120473.
Li N, Hua JL. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci 2017;74:2345.
Montemurro T, Viganò M, Ragni E, Barilani M, Parazzi V, Boldrin V, Lavazza C, Montelatici E, Banfi F, Lauri E, Giovanelli S, Baccarin M, Guerneri S, Giordano R, Lazzari L. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol 2016;95:228.
Ohkouchi S, Block GJ, Katsha AM, Kanehira M, Ebina M, Kikuchi T, Saijo Y, Nukiwa T, Prockop DJ. Mesenchymal stromal cells protect cancer cells from ROS-induced apoptosis and enhance the Warburg effect by secreting STC1. Mol Ther 2012;20:417.
Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010;116:829.
Sindrilaru A, Scharffetter-Kochanek K. Disclosure of the culprits: macrophages-versatile regulators of wound healing. Adv Wound Care (New Rochelle) 2013;2:357.
Kong D, Melo LG, Gnecchi M, Zhang L, Mostoslavsky G, Liew CC, Pratt RE, Dzau VJ. Cytokine-induced mobilization of circulating endothelial progenitor cells enhances repair of injured arteries. Circulation 2004;110:2039.
Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. J Clin Invest 2010;120:4207.
Nath C, Gulati SC. Role of cytokines in healing chronic skin wounds. Acta Haematol 1998;99:175.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835.
Katsuno Y, Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev Cell 2021;56:726.
Liu Y, Liu YE, Wu M, Zou RF, Mao S, Cong PF, Hou MX, Jin HX, Zhao Y, Bao YL. Adipose-derived mesenchymal stem cell-loaded β-chitin nanofiber hydrogel promote wound healing in rats. J Mater Sci Mater Med 2022;33:12.
Hasturk O, Kaplan DL. Cell armor for protection against environmental stress: advances, challenges and applications in micro- and nanoencapsulation of mammalian cells. Acta Biomater 2019;95:3.
Nguyen PQ, Courchesne ND, Duraj-Thatte A, Praveschotinunt P, Joshi NS. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv Mater 2018;30:1704847.
Liu XY, Inda ME, Lai Y, Lu TK, Zhao XH. Engineered living hydrogels. Adv Mater 2022;34:2201326.
Hernández-Arriaga AM, Campano C, Rivero-Buceta V, Prieto MA. When microbial biotechnology meets material engineering. Microb Biotechnol 2022;15:149.
Bhusari S, Sankaran S, Del Campo A. Regulating bacterial behavior within hydrogels of tunable viscoelasticity. Adv Sci (Weinh) 2022;9:2106026.
Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 2012;125:3025.
Li YL, Xiao Y, Liu CS. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem Rev 2017;117:4376.
Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials 2017;129:188.
Zhao ZQ, Sun YX, Qiao QC, Zhang L, Xie XJ, Weir MD, Schneider A, Xu HHK, Zhang N, Zhang K, Bai YX. Human periodontal ligament stem cell and umbilical vein endothelial cell co-culture to prevascularize scaffolds for angiogenic and osteogenic tissue engineering. Int J Mol Sci 2021;22:12363.
Yang Y, Xia T, Zhi W, Wei L, Weng J, Zhang C, Li XH. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011;32:4243.
Mohamadyar-Toupkanlou F, Vasheghani-Farahani E, Bakhshandeh B, Soleimani M, Ardeshirylajimi A. In vitro and in vivo investigations on fibronectin coated and hydroxyapatite incorporated scaffolds. Cell Mol Biol (Noisy-le-grand) 2015;61:1.
Martens W, Bronckaers A, Politis C, Jacobs R, Lambrichts I. Dental stem cells and their promising role in neural regeneration: an update. Clin Oral Investig 1969;2013:17.
Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater 2012;8:1401.
Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res 2002;60:613.
Yao QQ, Cosme JG, Xu T, Miszuk JM, Picciani PH, Fong H, Sun HL. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017;115:115.
Wang J, Cheng Y, Wang HY, Wang YH, Zhang KH, Fan CY, Wang HJ, Mo XM. Biomimetic and hierarchical nerve conduits from multifunctional nanofibers for guided peripheral nerve regeneration. Acta Biomater 2020;117:180.
Li Y, Wang J, Qian DJ, Chen L, Mo XM, Wang L, Wang Y, Cui WG. Electrospun fibrous sponge via short fiber for mimicking 3D ECM. J Nanobiotechnol 2021;19:131.
Wang J, Lin JW, Chen L, Deng LF, Cui WG. Endogenous electric-field-coupled electrospun short fiber via collecting wound exudation. Adv Mater 2022;34:2108325.
Qian ST, Wang J, Liu ZM, Mao JY, Zhao BF, Mao XY, Zhang LC, Cheng LY, Zhang YG, Sun XM, Cui WG. Secretory fluid-aggregated janus electrospun short fiber scaffold for wound healing. Small 2022;18:e2200799.
Miguel SP, Figueira DR, Simões D, Ribeiro MP, Coutinho P, Ferreira P, Correia IJ. Electrospun polymeric nanofibres as wound dressings: a review. Colloids Surf B Biointerfaces 2018;169:60.
Asiri A, Saidin S, Sani MH, Al-Ashwal RH. Epidermal and fibroblast growth factors incorporated polyvinyl alcohol electrospun nanofibers as biological dressing scaffold. Sci Rep 2021;11:5634.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211.
Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes 2006;55:1537.
Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obes Relat Metab Disord 1998;22:1145.
Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg 2013;132:1017.
Tonnard P, Verpaele A, Carvas M. Fat grafting for facial rejuvenation with nanofat grafts. Clin Plast Surg 2020;47:53.
Chaput B, Laloze J, Grolleau JL, Espagnolle N, Bertheuil N, Varin A. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg 2017;139:1024e.
Chen WM, Chen S, Morsi Y, El-Hamshary H, El-Newhy M, Fan CY, Mo XM. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl Mater Interfaces 2016;8:24415.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007;318:426.
Li DW, He FL, He J, Deng XD, Liu YL, Liu YY, Ye YJ, Yin DC. From 2D to 3D: the morphology, proliferation and differentiation of MC3T3-E1 on silk fibroin/chitosan matrices. Carbohydr Polym 2017;178:69.
Yeo M, Lee H, Kim G. Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, β-tricalcium phosphate, and collagen nanofibers: fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromol 2011;12:502.
Li X, Yin HM, Su K, Zheng GS, Mao CY, Liu W, Wang P, Zhang Z, Xu JZ, Li ZM, Liao GQ. Polydopamine-assisted anchor of chitosan onto porous composite scaffolds for accelerating bone regeneration. ACS Biomater Sci Eng 2019;5:2998.
Shi LX, Liu X, Wang WS, Jiang L, Wang ST. A self-pumping dressing for draining excessive biofluid around wounds. Adv Mater 2019;31:1804187.
Yang ZB, Jin SY, He Y, Zhang XY, Han XF, Li FC. Comparison of microfat, nanofat, and extracellular matrix/stromal vascular fraction gel for skin rejuvenation: basic research and clinical applications. Aesthet Surg J 2021;41:1557.
Kurokawa I, Mizutani H, Kusumoto K, Nishijima S, Tsujita-Kyutoku M, Shikata N, Tsubura A. Cytokeratin, filaggrin, and p63 expression in reepithelialization during human cutaneous wound healing. Wound Repair Regen 2006;14:38.
Rebling J, Ben-Yehuda Greenwald M, Wietecha M, Werner S, Razansky D. Long-term imaging of wound angiogenesis with large scale optoacoustic microscopy. Adv Sci (Weinh) 2021;8:2004226.
Hinz B. The role of myofibroblasts in wound healing. Curr Res Transl Med 2016;64:171.
Rakocevic J, Orlic D, Mitrovic-Ajtic O, Tomasevic M, Dobric M, Zlatic N, Milasinovic D, Stankovic G, Ostojić M, Labudovic-Borovic M. Endothelial cell markers from clinician’s perspective. Exp Mol Pathol 2017;102:303.
Rodríguez-Cabello JC, González de Torre I, Ibañez-Fonseca A, Alonso M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv Drug Deliv Rev 2018;129:118.
Ganeshkumar M, Ponrasu T, Krithika R, Iyappan K, Gayathri VS, Suguna L. Topical application of Acalypha indica accelerates rat cutaneous wound healing by up-regulating the expression of Type I and III collagen. J Ethnopharmacol 2012;142:14.
Acknowledgements
X. F and J. W contributed equally to this work. This research was funded by the National Key Research and Development Program of China (2020YFA0908200), National Natural Science Foundation of China General Program (32000937 and 51873107), Shanghai Municipal Health Commission (20204Y0354), Youth Innovation Technology Project of Higher School in Shandong Province (20190919), China Postdoctoral Science Foundation (2022T150426) and Program of Shanghai Academic Research Leader (22XD1422600).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors state that there are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 596 KB)
Supplementary file2 (MP4 989 KB)
Supplementary file3 (MP4 652 KB)
Supplementary file4 (MP4 575 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, X., Wang, J., Qian, D. et al. Living Electrospun Short Fibrous Sponge via Engineered Nanofat for Wound Healing. Adv. Fiber Mater. 5, 979–993 (2023). https://doi.org/10.1007/s42765-022-00229-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-022-00229-5