Abstract
The objective of this study is to evaluate the role of dopamine hydrochloride (DH) in alleviating the detrimental effects of salt stress on Glycine max (L.) plant. Soybean seeds were treated with 150 mM NaCl and DH (100 µM or 200 µM) after they had been grown in plastic pots then the growth parameters, physiological and molecular analyses were assessed. Data showed that salinity stress decreased the germination percentage by 63.6%, the tolerance index (TI) and the seedling vigor index (SVI) were highly decreased. Salinity stress led to a markedly decline in the photosynthetic efficiency and the content of chlorophyll a and chlorophyll b by 43.5%, 77.4% and 44.6%, respectively. Salinity stress increased MDA and activity of CAT, SOD, POD, APX, GST and GR by 150%, 39.8%, 75%, 160%, 77.7%, 50% and 57%, respectively. However, DH (100 µM or 200 µM) significantly alleviated the toxic effects of salinity stress, marinated ions absorption, and enhanced the molecular level. Wherein out of 30 ISSR amplified fragments were formed. There were 10 unique bands (587 bp, 453 bp, 393 bp, 435 bp, 157 bp, 679 bp, 473 bp, 675 bp, 758 bp and 531 bp) were appeared in response to DH (100 µM and 200 µM) compared with untreated plants. Our analysis suggests a constructive effect of DH (100 µM and 200 µM) in alleviating the toxic effects of salinity stress on Glycine max (L.) plant not only at the level of antioxidative defense but also by regulating the molecular response highlighting the potential use of DH to improve the sustainability of horticultural production under climate change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
One of the most challenging objectives of our time is to increase food production by 70% in 2050 and feed an additional 2.3 billion people worldwide (Zaid and Wani 2019). Soybean (Glycine max L.) is the most commercial legume crop which produces 29% of oil and 70% of the protein consumed worldwide (Teixeira et al. 2020). Soybean oil is essential for both human diets and animal feed because of its high unsaturated fatty acid content and is renowned as a potent source of protein due to its variety of amino acids (Zhao et al. 2022). Soybean seeds are also regarded as a source of hormones, phospholipids, and antioxidant compounds (Lin et al. 2022). Significant increases in agricultural productivity are urgently needed nowadays as a result of population growth, climate change, and global warming. Nonetheless, a range of environmental limitations, such as biotic and abiotic stresses, function as challenges to agricultural productivity (Ashour et al. 2023; Dawood et al. 2022).
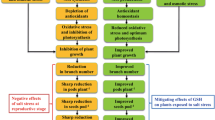
Abiotic stressors have negative impacts on the growth, development, and production of soybean plants (Godoy et al. 2021). The detrimental impacts of ecological stress on plant production have increased due to recent climate change and significant human activity worldwide (Fariduddin et al. 2019; Zhang et al. 2020). Plant growth, development, and crop yield are significantly harmed by abiotic threats, especially salt stress, heavy metal stress, drought stress, and nutritional deficiency (Dawood et al. 2022; Duan et al. 2023; Noor et al. 2022). Salinity stress causes osmotic stress, nutritional deficiencies, ion toxicity, and harmful damage to DNA of plants at the molecular level (Snehi et al. 2023). Furthermore, salinity stress reduces the number of root nodules, the effectiveness of nitrogen fixation, growth, and ultimately production of crops especially soybean plants, which are categorized as a highly salt-sensitive crop (Zaid and Wani 2019; Fariduddin et al. 2019). Plants can use inappropriate molecular and biochemical processes, such as ion uptake control, osmolyte synthesis, modification of the photosynthetic pathway, membrane structure modification, and increase in anti-oxidative enzymes, gene expression regulation, and growth promotion in order to adapt to salinity stress (Gao et al. 2021).
Recently, several approaches have been thoroughly investigated to assist plants in overcoming such these abiotic limitations (Duan et al. 2023; Saddhe et al. 2021). Exogenous supplementation of hormones, bio-stimulants, and growth regulators were still apparent as an appropriate strategy for alleviating the harmful effects induced by abiotic stress (Ahammed et al. 2020a). The ability of plants to tolerate these stresses may generally be modified by chemical application in a variety of plant species (Sako et al. 2021; Gul et al. 2022). Catecholamines group such as dopamine plays an important role as neuromodulators or neurotransmitter in humans, animals, and plants like the potato (Solanum tuberosum), mucuna (Mucuna pruriens), plantain (Plantago major), banana (Musa acuminata), and avocado (Persea americana) (Akula and Mukherjee 2020; Liu et al. 2020a). Nearly 50 years ago, catecholamines were found in different 44 plants tissues, proving that higher plants have a high rate of catecholamine production (Cui et al. 2020). In plants, dopamine is a precursor of melanin and produced from the tyrosine using tyramine or L-3, 4-dihydroxyphenylalanine (L-DOPA) (Marchiosi et al. 2020). Dopamine is a water-soluble compound with chemical formula of C8H11NO2, has a prevailing antioxidant capacity comparing with glutathione and other flavonoids like quercetin and catechin (Bala 2020), plays an important role in alleviating the toxic effect of abiotic stress, regulates a variety of metabolic activities inside plant cells, such as active oxygen scavenging processes and sugars metabolism (Gao et al. 2021), regulation of ions permeability and photophosphorylation of chloroplasts (Mohammadi et al. 2021). It can also interact with plant hormones and affect the growth and development of plants (Wang et al. 2021). Both biotic and abiotic stresses have considerable impacts on the production of dopamine in plants (Roshchina 2022). Li et al. (2015) indicated that 200 mM, NaCl reduced plant growth, the photosynthetic rate and the maximum quantum yield of PSII in the dark (Fv/Fm) of salt-stressed Malus hupehensis Rehd. However, these inhibitory effects of salt stress were alleviated by pretreatment of plants with 100 or 200 µM dopamine. In addition, the application of dopamine stimulated the uptake of K, N, P, S, Cu, and Mn but inhibited the uptake of Na and Cl furthermore; dopamine also reduced the formation of H2O2 due to the activation of the antioxidant system enzymes and improvement of the ascorbate-glutathione cycle (Li et al. 2015). Liang et al. (2017) reported that the exogenous dopamine (100 μM) markedly alleviated the nutrient deficiency, maintained the photosynthetic capacity and development of the root system, promoted the uptake of N, P, K, Ca, Mg, Fe, Mn, Cu, Zn, and B in Malus hupehensis plants under abiotic stress. Moreover, Liu et al. (2020b) observed that the exogenous application of different dopamine concentrations between 5 µM and 100 µM promoted the production of ethylene in Beta vulgaris (L.).
The overall objective of this research was to study the role of dopamine hydrochloride DH (100 µM and 200 µM) in alleviating the toxic effect induced by salinity stress in the Glycine max (L.) Merr. plant during the early growth stage through studying its effect on the photosynthetic and antioxidative systems of salt-stressed soybean seedlings. Moreover, to know the important role of DH in regulating the absorption of mineral ions and its role to maintain the distortion that might be occurred to DNA fragments by salinity stress, trying to find a suitable potential way to improve the tolerance of soybean plant for defense against related damage caused by salinity stress.
2 Materials and Methods
2.1 Growing Conditions of the Plant Material
Soybean seeds (cv. Giza 111) were provided by the agronomy department of the Agriculture Center, Research Station in Sakha, Kafr El-Sheikh, Egypt. Dopamine hydrochloride (DH) (M.wt; 189.64, Chemical formula; C8H11NO2.HCl) was provided from Sigma Aldrich Co. in Egypt. The different concentrations of NaCl (0, 50, 100, 150, 200, 250, and 300 mM) and DH (0, 50, 100, 200, or 400 µM) were prepared. A preliminary study was conducted to identify the lethal and sub-lethal of NaCl and the most efficient concentrations of DH.
During the growing season, seeds of Glycine max (L.) with uniform size and shape were divided into six groups of three replicates (n=3, 18 pots) in a randomized complete block design as follows: 1; Control, 2; Salt stress (150 mM NaCl), 3; DH (100 µM), 4; DH (200 µM), 5; 150 mM NaCl + DH (100 µM) and 6; 150 mM NaCl + DH (200 µM). Five seeds were sown in each plastic pot (25 cm diameter and 30 cm depth) filled with 5 kg clay-sandy soil (2:1w/w) and watered every 2 days with water for the first week (before seedling emergence). After that, at 7th and 14th days after sowing, seedlings were watered with the different solutions till 80 % field capacity as follows: 1st group (control) was watered with water, 2nd group was watered with 150 mM NaCl, 3rd group was watered with DH (100 µM), 4th group was watered with DH (200 µM), 5th group was watered with 150 mM NaCl and DH (100 µM) and finally 6th group was watered with 150 mM NaCl and DH (200 µM). Under the conditions of 13 h light and 11 h dark, at 35°C ±2 and 21°C ± 2, respectively with 62 % relative humidity, the sown seeds were left to grow in the greenhouse for 21 days. All experiments were carried out in the greenhouse of the Botany Department, Faculty of Science, Tanta University.
2.2 Growth Measurements
The 21-day old Glycine max (L.) seedlings were harvested, washed with water to get rid of soil particles, and separated into roots, shoots and leaves. The fresh weight (FW) of all samples was determined then the samples were dried at 50 °C in the oven to a constant weight for at least 72 h for determination of dry weight (DW). The growth criteria {germination percentage, shoot length, root length, leaf area, shoot fresh weight (SFW), root fresh weight (RFW), shoot dry weight (SDW), and root dry weight (RDW)} of soybean seedlings were determined.
The tolerance index (TI) of soybean seedlings (length of shoots and roots, leaf area, dry and fresh weights of shoots and roots) was expressed as a comparative tolerance to salt stress and calculated according to the formula of Turner and Marshall (1972), using the following equation; TI= (Mean of increase or decrease of the measured parameter)/control. The seedling vigor index was calculated according to Abdul-Baki and Anderson (1973). The formula SVI = seedling length (sum of shoot and root length) x germination percentage or SVII = seedling dry weight (sum dry weight of shoot and root) x germination percentage.
2.3 Evaluation of Photosynthetic Efficiency (Fv/Fm)
The photosynthetic activity was measured before collecting the seedlings and assayed as fluorescence kinetic (Fv /Fm) of dark-adapted leaves at the seedling stage by using OS-30 p chlorophyll fluorometer (Hudson, NH03051 USA). It makes use of a dual-mode pulse-modulated sensing technique that enables a range of tests to be carried out in wildly different environmental settings. Each treatment was measured three times. The initial fluorescence (Fo) is identified once the photosystem (PSII) reaction centers open (dark-adapted), whereas the maximal fluorescence (Fm) is recorded during exposure to saturating flash while PS II reaction centers are closed (light-adapted). Fluorescence fluctuation is determined as (Fv = Fm - Fo).
2.4 Determination of Chlorophyll Content
The photosynthetic pigments (Chlorophyll a, Chlorophyll b, and Carotenoid) of fresh leaves of soybean seedlings were determined according to Metzner et al. (1965) using a known fresh weight of plant samples that were homogenized in 80% cold aqueous acetone for 5 min then was centrifuged for 15 min at 1000 rpm and then, the extract was completed to an appropriate volume using cold acetone. The color intensities of these extracts were measured against a blank of pure 80% aqueous acetone at three wavelengths of 663, 644, and 480 nm, using a spectrophotometer and were expressed in mg g-1 FW.
2.5 Evaluation of Antioxidant Enzymes’ Activity
The extraction of antioxidant enzymes was carried out according to Beauchamp and Fridovich (1971) using 0.5 g of fresh plant material was frozen and homogenized in 8 ml of cold phosphate buffer (50 mM, pH 7) then was centrifuged at 4000 rpm for 20 min. The supernatant was used as a raw extract for enzymatic assay.
Catalase (CAT, EC: 1.11.1.6) activity was assayed according to Havir and McHale (1987) by using 0.1 M sodium phosphate buffer (2 mM, pH 7), H2O2 and 0.1 ml of enzyme extract. The decrease in H2O2 was followed as a decline in the absorbance at 240 nm and the activity was calculated using the extinction coefficient (36 x 103 mM-1 m-1) and expressed as µM g-1 FW min-1.
Superoxide dismutase (SOD, EC: 1.15.1.1) activity was determined according to van Rossum et al. (1997) by using 75 µM p-nitroblue tetrazolium (NBT), 100 µM EDTA, 2 µM riboflavin, and 13 mM L-methionine then the NBT reduction product (formazan) was measured at 560 nm and the activity was calculated using the molar extinction coefficient (21.1 mM-1 cm-1) and expressed as µM g-1 FW min-1.
Ascorbate peroxidase (APX, EC 1.11.1.11) activity was measured according to Foyer and Halliwell (1976) by using K-phosphate buffer (5 mM, pH 7), ascorbic acid (0.5 mM), H2O2 (0.25 mM), and EDTA (0.2 mM). The reaction was started by adding 0.1 ml of the extract to the mixture. The decline of the reaction was measured at 290 nm using the extinction coefficient (2.8 mM-1 m-1) and expressed as expressed as µM g-1 FW min-1.
The activity of guaiacol peroxidase (POD, EC: 1.11.1.7) was assayed according to Kato and Shimizu (1987). The reaction was initiated by mixing 0.1 ml of the extract with 7.2 mM guaiacol, 100 mM K-phosphate buffer (pH 5.8), 11.8 mM H2O2, and the activity was calculated at 470 nm using extinction coefficient (26.6 mM–1 cm–1) and expressed as expressed as µM g-1 FW min-1.
The glutathione reductase (GR, EC: 1.6.4.2) activity was determined according to Dalton et al. (1986) using a 0.2 M Na-PO4−2 buffer (pH 7.0), 2 mM EDTA, 20 mM GSSG, 2 mM NADPH and 100 μl enzyme extract. Oxidized glutathione (GSSG)-dependent oxidation of NADPH was monitored by recording the decrease in absorbance at 340 nm for 2 min with a molar extinction coefficient of 6.22 mM–1 cm–1 and expressed as µMol min-1 mg-1 protein.
The activity of glutathione-s-transferase (GST, EC: 2.5.1.18) was determined according to Habig et al. (1974) at 340 nm by measuring the rate of l-chloro-2,4-dinitrobenzene conjugation with reduced glutathione as a function of time. The assay mixture contained 0.1 ml 30 mM GSH, 0.1 ml (approximately 3-4 mg protein.ml-1) plant extract, 0.1 ml 30 mM CDNB and 2.7 ml 100 mM pH 6.5 phosphate buffer. The enzyme activity was expressed as µMol min-1 mg-1 protein.
2.6 Determination of Malondialdehyde Content
Malondialdehyde (MDA) was determined according to Heath and Packer (1968). Using 0.5 g of fresh plant sample was homogenized by trichloroacetic acid (5% w/v). The homogenate was centrifuged at 4000 rpm for 10 min then incubated for 20 min at 100 °C in a boiling water bath then 2 ml of the supernatant and two ml of thiobarbituric acid (0.67% w/v) were added to the homogenate. The absorbance was measured at both 532 and 600 nm. The MDA content was calculated using the extinction coefficient (155 mM-1 cm-1) and expressed as µM g-1 FW.
2.7 Determination of Contents of Osmo-Regulatory Compounds
The content of glycine betaine was estimated according to Grieve and Grattan (1983) and Valadez et al. (2016). The dried plant sample was diluted using an equal volume of H2SO4 (1M) then divided into 0.5 ml and placed in centrifuge tubes, then 0.2 ml of cold potassium iodide reagent was added and then cooled for 1 h over the ice. To obtain the precipitated per iodide crystals, the reactants were gently mixed, kept at 4ºC overnight, and then centrifuged for 15 min at 4ºC at 10,000 rpm. After 2 h, the crystals were dissolved in 1,2-dichloroethane, and the absorbance at 365 nm was recorded and expressed in mg g-1 DW.
The Proline content was estimated according to Bates et al. (1973). Using 0.5 g of dried plant sample was homogenized in 3% aqueous sulfosalicylic acid then the homogenate was centrifuged for 15 min at 10.000 rpm. After adding 2 ml of the supernatant, 2 ml of acid ninhydrin, and 2 ml of glacial acetic acid, the mixture was boiled at 100 °C for 1 h. Following the reaction's termination in an ice bath, 4 ml of toluene was used to extract the reaction mixture, and the absorbance was measured at 520 nm and expressed in mg g-1 DW.
The content of total amino acid t was estimated according to Lee and Takahashi (1966). A total of 0.5 g of dried plant sample was homogenized using 80% ethanol. The extract was then centrifuged for 15 min at 800 rpm, neutralized with 0.1 N NaOH using methyl red as an indicator, and ninhydrin reagent was added. The content was heated for 20 min in a boiling water bath and then diluted with distilled water. The substance was detected at 570 nm and quantified in mg g-1 DW.
Total soluble protein content was determined as described by Bradford (1976). Exactly 0.1 ml borate buffer extract was well mixed with 3 ml Commasie Brilliant Blue (CBB) reagent. After 2 min, the absorbance was measured at 595 nm and expressed in mg g-1 DW. The phenol sulfuric acid method was estimated to determine the content of total soluble carbohydrates according to Dubois et al. (1956). A 0.1 ml borate buffer was added to 1ml of 5% phenol and 5 ml concentrated H2O2 then placed in a boiling water bath at 30ºC for 20 min. The absorption was read at 490 nm and expressed in mg g-1 DW.
2.8 Determination of Minerals Elements
The amount of sodium, potassium and calcium was determined according to Allen et al. (1974) method. A 0.5 g of dried plant sample, 4 ml of nitric acid, and 2 ml of perchloric acid (30 %) were heated over time until the odors disappeared and the mixture changed into a clear solution without charring. The solution was diluted to a volume of 50 ml. and analyzed with an atomic absorption flame emission spectrophotometer to determine the amount of minerals present. The amount of nitrogen (N) was determined using 0.2 ml of the digested sample and a few drops of phenolphthalein as an indicator. The mixture was titrated against sodium hydroxide (0.63 N) until the color turned pink then 1 ml of sodium hydroxide-sodium hypochlorite and 1 ml of phenol-sodium nitroprusside were added then the mixture was incubated at 37 °C for 15 min. The absorption was read at 630 nm by spectrophotometer.
2.9 Intersimple Sequence Repeats (ISSR) Analysis
2.9.1 DNA Extraction, Optimization of PCR Conditions, Primers Sequences, and Electrophoresis of ISSR Amplification Products
The modified method of CTAB with minor adjustments made to the PCR amplification conditions was used according to Williams et al (1990). Five ISSR primers (UBC8897, UBC811, UBC819, UBC822 and UBC874) were chosen to develop the amplification products from isolated genomic DNA samples (Operan Nippon EGT CO., LTD. USA) in a gradient thermal cycler (The Biometera Thermal Cycler, manufactured in Germany). The reaction mixture (25 µl) was prepared as follows: 12.5 µl of green master mix (NIPPON Genetics Europe Master Mix Taq Ready Mix with Dye), 7.5 µl of distilled water, 3.0 µl of DNA, and 2 µl of each primer (10 p moles/l), then the amplification conditions were adjusted as represented in Table S1.
A standardized PCR program was adjusted at 1 min at 94°C for 45 cycles, 2 min at 72°C, then 1 min at 57°C. The reaction was then maintained for 10 min at 72°C. The reaction products were separated in 1X Tris-borate-ethylenediaminetetraacetic acid (TBE) buffer and added directly on 1.5% agarose gels, stained with ethidium bromide, and photographed with UVP photoDoc-It Imaging System under a UV trans-illuminator. When at least one polymorphic band was found, profiles were deemed to be distinct.
2.9.2 Statistical Analysis
The measured parameters in all treatments were represented by the mean of replicates with computed standard deviation (±SD). The mean values were compared according to Duncan’s multiple-range test (p ≤ 0.05). Person correlations between attributes were also calculated using the SPSS 15 program.
3 Results
3.1 Preliminary Experiment
Data represented in Fig. S1 show that the germination percentage of soybean seeds was significant decreased at 150 mM NaCl by 65% compared with control after that the germination percentage was inhibited after this concentration. On the other hand, among the different concentrations of DH, only both concentrations (DH 100 µM and DH 200 μM) were the most effective concentrations improved the germination percentage of soybean seeds compared with other DH concentrations.
3.2 Effect of Dopamine Hydrochloride (DH) on Plant Growth, Tolerance Index, and Seedling Vigor Indices of the Salt-stressed Soybean Plant
Data represented in Fig. 1 and Table 1, show that all growth parameters and the tolerance index (TI) of Glycine max (L.) were significantly decreased in response to salt stress (150 mM NaCl). The germination percentage, shoot length, root length, leaf area, shoot fresh weight (SFW), root fresh weight (RFW), shoot dry weight (SDW) and root dry weight (RDW) of salt-stressed Glycine max (L.) were significant decreased by 63.6%, 36.8%, 35.9%, 39.4%, 45.5%, 47.5%, 61.5% and 46.1%, respectively, compared to the control. On the other hand, data showed that DH (100 µM and 200 µM) markedly increased the germination percentage, shoot length, root length, leaf area, SFW, RFW, SDW and RDW of salt-stressed Glycine max (L.) by 120%, 150%, 52.2%, 48.3%, 50.2%, 44.3%, 44.3%, 47%, 35.9%, 43.6%, 59.3%, 56.2%, 100%, 120%, 157% and 57.1%, respectively compared with stressed seedlings reflecting the effective role of DH in reducing the hazardous effects of salinity stress on the morphological characteristic of Glycine max (L.) seedlings (Fig. 1 and Table 1).
Effect of salinity stress (150 mM NaCl) and different concentrations of dopamine hydrochloride (DH 100 µM and DH 200 µM) on a the germination percentage, b shoot length, c root length, d leaf area, e shoot fresh weight, f root fresh weight, g shoot dry weight and h root dry weight of 21-day old salt stressed Glycine max (L.) Merr. seedlings. Values are means of 3 replicates with bars showing ± SD. Different small letters on each column indicate significant differences at p ≤ 0.05 as analyzed according to Duncan’s multiple-range test
Besides, the germination of seeds is an important point in seedling establishment and subsequent plant health and vigor. Results in Table 2 show that the length and dry weight vigor indices of salt-stressed Glycine max (L.) seedlings were significantly decreased in response to salinity stress (150 mM NaCl) by 76.9% and 84.8%, respectively compared with the control. While both concentrations of DH (100 µM and 200 µM) increased the length and dry weight vigor indices retarding the effects of salinity stress on Glycine max (L.) Merr. as seedlings showed a significant increase in length vigor index by 230% and 270%, respectively compared with salt-stressed seedlings, as well as the pretreatment of soybean seedlings with DH (100 µM and 200 µM) high significant increased the dry weight vigor index by the same percentage of 400% compared with salt-stressed seedlings (Table 2).
3.3 Role of Dopamine on Photosynthetic Efficiency, Chlorophyll and Carotenoids Contents of Salt-stressed Soybean Plant
Data represented in Fig. 2 show that salinity stress (150 mM NaCl) significantly decreased the maximum potential efficiency of PSII (Fv/Fm) by 43.5% compared with the control, although this decrease was counteracted by the pretreatment of salt-stressed seedlings with both concentrations of DH (100 µM and 200 µM) by 35.5% and 30.6%, respectively compared with untreated salt-stressed seedlings (Fig. 2a). Our results represented in Fig. 3 b, c show that salinity stress (150 mM NaCl) markedly decreased the content of chlorophyll a (Chl a) and chlorophyll b (Chl b) by 77.4% and 44.6%, respectively compared with control. While, treatment of salt-stressed seedlings with both concentrations of DH (100 µM and 200 µM) ameliorated the negative effects induced by salinity stress on the content of the photosynthetic pigments in salt-stressed seedlings whereas, DH 100 µM and DH 200 µM high significant increased the content of Chl a by 102% and 162%, respectively and increased the content of Chl b by 41.7% and 61.3%, respectively compared with untreated salt-stressed seedlings (Fig. 3 b, c). In contrast with data represented in Fig. 3d showed that salinity stress led to a significant increase in the content of carotenoids (Carot.) by 137% compared with the control, while the pretreatment of salt-stressed soybean seedlings with DH (100 µM and 200 µM) significantly decreased the content of Carot. in salt-stressed seedlings by 41.5% and 30.4%, respectively compared with untreated salt-stressed seedlings.
Effect of salinity stress (150 mM NaCl) and different concentrations of dopamine hydrochloride (DH 100 µM and DH 200 µM) on a photosynthetic activity (Fv/Fm), b chlorophyll a (Chl a), c chlorophyll b (Chl b) and d carotenoids (Carot.) of 21-day old salt-stressed Glycine max (L.) Merr. seedlings. Values are means of 3 replicates with bars showing ± SD. Different small letters on each column indicate significant differences at p ≤ 0.05 as analyzed according to Duncan’s multiple-range test
Effect of salinity stress (150 mM NaCl) and different concentrations of dopamine hydrochloride (DH 100 µM and DH 200 µM) on the activity of antioxidant enzymes a catalase enzyme (CAT), b superoxide dismutase (SOD), c peroxidase (POD), d ascorbate peroxidase (APX), e glutathione reductase (GR) and f glutathione s transferase (GST) of 21-day old salt stressed Glycine max (L.) Merr. seedlings. Values are means of 3 replicates with bars showing ± SD. Different small letters on each column indicate significant differences at p ≤ 0.05 as analyzed according to Duncan’s multiple-range test
3.4 Improvement of Antioxidant System in Dopamine Hydrochloride Treated Soybean Plant
Results represented in Fig. 4 show that salinity stress (150 mM NaCl) significantly increased the activity of antioxidant enzymes (CAT, SOD, POD, APX, GST and GR) in salt-stressed soybean seedlings by 39.8%, 75%, 160%, 77.7%, 50% and 57%, respectively compared with the control. On the other hand, both concentrations of DH (100 µM and 200 µM) caused high significant decrease in the activity of CAT by 26.8% and 23%, compared with untreated salt-stressed soybean seedlings (Fig. 4a). Additionally, the pretreatment of salt-stressed seedlings with DH (200 µM) led to noticeable decrease in the activity of SOD, POD and APX by 50%, 58.8% and 62.5%, respectively compared with untreated salt-stressed soybean seedlings (Fig. 4b, c, d). Besides, data showed a similar trend of DH 100 µM and DH 200 µM to reduce the activity of GST and GR enzymes in salt-stressed soybean seedlings by the same percentages of 32.3% and 38.2%, respectively compared to untreated salt-stressed seedlings (Fig. 4e, f).
Effect of salinity stress (150 mM NaCl) and different concentrations of dopamine hydrochloride (DH 100 µM and DH 200 µM) on the minerals content {nitrogen (N+3), sodium (Na+), potassium (K+) and calcium (Ca+2)} of 21-day old salt stressed Glycine max (L.) Merr. seedlings. Values are means of 3 replicates with bars showing ± SD. Different small letters on each column indicate significant differences at p ≤ 0.05 as analyzed according to Duncan’s multiple-range test
3.5 Implication of Dopamine Hydrochloride on the Contents of Malondialdehyde (MDA) and Compatible Osmolytes in Soybean Plant
As illustrated in Table 3, salt stress (150 mM NaCl) significantly increased the content of MDA in salt-stressed soybean seedlings by 150% compared to control. While the pretreatment of salt-stressed soybean seedlings with DH (100 µM and 200 µM), the content of MDA was significantly decreased by an approximate percentage of 43% compared with untreated salt-stressed seedlings. On the other hand, the contents of glycine betaine (GB), total amino acids (TAA), total soluble proteins (TSP) and proline (Pro.) were significant increased in response to salinity stress (150 mM NaCl) by 160%, 48.5%, 75% and 25.5%, respectively compared to control but this increase was relieved in response to DH treatment whereas, the pretreatment of salt-stressed seedlings with DH (100 µM and 200 µM) significant decreased the content of GB, TAA, TSP and Pro. by approximate percentages of 29%, 30%, 52.5% and 12%, respectively compared with untreated salt-stressed soybean seedlings (Table 3).
3.6 Effect of Dopamine Hydrochloride on the Content of Minerals in Soybean Plant
Data represented in Fig. 4 show the content of sodium (Na+) ions concentrated in leaves of soybean seedlings was significantly increased in response to salinity stress (150 mM NaCl) by 219% compared to control. In contrast, 150 mM NaCl decreased the contents of potassium (K+), nitrogen (N+3), and calcium (Ca+2) ions in salt-stressed soybean seedlings by65.7%, 28.5 and 12.3%, respectively, compared to control. On the other hand, the pretreatment of salt-stressed soybean seedlings with both concentrations of DH (100 µM and 200 µM) led to noticeable decrease in the content of Na+ by 74% and 69.3%, respectively compared with untreated salt-stressed seedlings. Besides, both concentrations of DH (100 µM and 200 µM) significant increased the content of K+, N+3, and Ca+2 ions in all salt-stressed seedlings by the approximate same percentage of 180%, 33% and 14%, respectively compared with untreated salt-stressed seedlings (Fig. 4).
3.7 Implication of Dopamine Hydrochloride on Intersimple Sequence Repeats (ISSR-PCR) Polymorphism
Data represented in Table S3 and Fig. 5 show 30 amplified DNA fragments (19 polymorphic bands, 10 unique bands, and one monomorphic band) were produced by the selected five primers (Table S1). Moreover, the percentage of polymorphic bands was represented in Table S4. Results in Table S3 and Fig. 5 indicate 2 unique bands (587 bp; UBC807 and 453 bp; UBC811) have appeared in response to DH (100 µM) treatment while 4 unique bands (473 bp; UBC811, 675 bp; UBC822, 758 bp and 531 bp; UBC874) were created in response to DH (200 µM) treatment compared with control. Moreover, 4 unique bands produced only in salt-stressed plants (150 mM NaCl) under DH (100 µM and 200 µM) treatments with molecular sizes (393 bp; UBC822, 435 bp; UBC811, 157 bp; UBC807 and 679 bp; UBC811, respectively) compared with control. Additionally 6 polymorphic bands were appeared in response to both concentration of DH (100 µM and 200 µM) and 150 mM NaCl compared with control.
Effect of salinity stress (150 mM NaCl) and different concentrations of dopamine hydrochloride (DH 100 µM and DH 200 µM) on the electrophoretic pattern of the inter simple sequence repeats (ISSR-PCR) analysis produced by five primers a UBC807, b UBC811, c UBC819, d UBC822 and e UBC874 of 21-day-old salt stressed Glycine max (L.) Merr. seedlings. M=Marker, 1=Control, 2=150 mM NaCl, 3=DH 100 µM, 4=DH 200 µM, 5=150 mM NaCl + DH 100 µM, 6=150 mM NaCl + DH 200 µM
One monomorphic band was found in all samples by primer UBC819 with a band size of 315 bp (Table S3 and Fig. 5c). The other four primers (UBC807, UBC811, UBC822 and UB874) were not amplified any monomorphic bands. The largest band size of 861 bp was polymorphic and appeared by primer UBC807 in samples of control, DH 100 µM, DH 200 µM, and 150 mM NaCl + DH 200 µM (Fig. 5a). While the smallest band size of 117 bp appeared by primer UBC874 was a polymorphic band and appeared in samples of control, DH 100 µM and DH 200 µM (Fig. 5e). Three additional bands (632bp and 469 bp; UBC822 and 165 bp; UBC874) were formed in response to 150 mM NaCl and they were disappeared under both concentrations of DH (100 µM and 200 µM) in salt-stressed samples (Fig. 5e). Four DNA bands specific in control sample appeared in the treatment of 150 mM NaCl + DH 200 µM with molecular sizes of 861 bp, 790 bp and 726 bp with primer UBC 807, 190 bp with primer UBC819 (Fig. 5a, c).
3.8 Person correlation
Pearson correlation analysis was created to identify the correlation between all morphological and biochemical parameters obtained in treated and untreated salt-stressed Glycine max (L.) Merr. seedlings with DH (100 µM and DH 200 µM). Results represented in Table S2 show an obvious negative correlation between salinity stress (150 mM NaCl) and SFW, SDW, RFW, RDW, Leaf area (LA), shoot length (SL), root length (RL), Chl a, Chl b, photosynthetic activity (PhA) at p ≤ 0.001 (r2 = 0.600** to 1.000***), p ≤ 0.005 (r2 = 0.500* to 0.600**). While, data showed positive correlation between the salinity stress and MDA, Carot., and the activity of CAT, SOD, POD, APX, GR, and GST, the content of GB, TAA, TSP and Pro. at p ≤ 0.001 (r2 = 0.600** to 1.000***), p ≤ 0.005 (r2 = 0.500* to 0.600**) (Table S2).
4 Discussion
Dopamine hydrochloride (DH) greatly reduced the effects of salt stress on soybean plants and enhanced their growth. The current study demonstrated that changes at the physiological, biochemical, and molecular levels under salt stress which resulted in significant reductions in the germination percentage, all measured growth parameters and the tolerance and vigor indices of soybean plants as a result to overabundance of sodium (Na+) and chloride (Cl−) ions in plant tissues can have a number of unfavorable hyperionic and hyperosmotic effects (Fariduddin et al. 2019), which can disrupt membrane structure and impair essential physiological and biochemical functions like photosynthesis, respiration, protein synthesis, uptake of nutrients, transpiration rate, metabolism of sugar and lipids, and water potential reduction (Fariduddin et al. 2019; Dawood et al. 2022). Ultimately, these factors can alter the growth and development of crop plants. On the other hand, the supplementation of soybean plants with dopamine DH (100 µM and 200 µM) significantly decreased the inhibitory effects of salinity stress on the growth of the seedlings and increased the tolerance index as well as the seedlings vigor indices by increasing the length and dry weight of the shoots and roots of the soybean seedlings. Several studies suggested that Plant growth regulators (PGRs) are naturally derived signaling molecules, known to play significant and intricate roles to regulate growth, physiology, development, morphology, and responses to abiotic stresses (Fariduddin et al. 2019). Dopamine may be an interaction factor between catecholamines and plant hormones causing stimulated the gibberellic acid (GA3) (Bamel and Prabhavathi 2020). Dopamine plays an important role as a cofactor in the oxidation of indole acetic acid (IAA) to raise the amount of auxin in the shoot and root of plants causing the development of plant growth (Liu et al. 2020a).
Variations in photosynthetic efficiency (Fv/Fm) indicate that salinity stress led to a dramatic reduction in the content of photosynthetic pigments. Results indicated that salinity stress significant decreased the content of chlorophyll a and chlorophyll b as well as they caused high significant increase in the content of carotenoids. The amount of damage to the photosynthetic machinery during the stress response is shown by the ratio of variable fluorescence to maximum fluorescence, which represents the maximum photochemical efficiency of PSII (Khatri and Rathore 2022). These findings could be explained by how salt stress causes plants to close or constrict their stomata in order to stop losing water (Hameed et al. 2021). But shutting stomata also limits CO2's ability to enter leaf cells, which prevents photosynthesis (Dadasoglu et al. 2022). Our current study's findings also demonstrated that salt stress reduced PSII's maximum potential efficiency. However, the dopamine therapy counteracted this drop in Fv/Fm levels. Dopamine can decrease chlorophyll b concentration, raising the Chl a/b ratio and preventing the accumulation of excess electrons as an adaptive mechanism in the photosynthetic electron transport chain (Liu et al. 2020a). Studies have shown that exogenous dopamine increases the degree to which stomata open, causing the plants under salt stress to grow longer and wider. Less than 100 μM was the largest stomatal opening observed in Malus subjects treated with dopamine (Jiao et al. 2019). Additional study has revealed that dopamine can affect ABA concentration and sugar metabolism and it has shown that ABA and sugar can control stomatal behavior in a range of environmental circumstances (Liu et al. 2020a). Li et al. (2013) suggest that this may be the process by which dopamine regulates stomatal activity in plants that are stressed by salt. It has been demonstrated that giving dopamine to cucumber seedlings under nitrate stress reduces the negative effects on plants by improving nitrogen and carbon metabolism and the expression of genes linked to these processes (Liu et al. 2020a). For instance, dopamine acts as the chemical equivalent of a naturally occurring arbitrator, or oxygen-reducing factor, allowing for the use of reduced oxygen levels in energy transduction during photosynthesis, hence controlling this elimination of photosynthetic activities (Abdulmajeed et al. 2022). Due to stomatal-related constraints that change photosynthetic metabolism, decreased diffusion through the stomata and mesophyll tissues, or both, dopamine hydrochloride can directly or indirectly affect the photosynthetic apparatus and lessen the toxic effect of salt-induced stress by lowering the availability of CO2 (Ji et al. 2022). Moreover, dopamine treatment accelerates the formation of chlorophyll by increasing the concentration of N in leaf tissue (Farouk et al. 2023).
According to our findings, soybean seedlings under high salinity stress had higher levels of MDA and antioxidant enzymes (CAT, SOD, POD, APX, GST, and GR). The observed outcomes could be explained by the disturbance of cellular redox equilibrium, which in turn affects the increased generation of oxidative impairment biomarkers (ROS) such as superoxide, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. These ROS can cause havoc with cellular metabolism, directly damage membrane lipids, and impair ion absorption (Huo et al. 2022; Senousy et al. 2023; Zaid and Wani 2019). On the other hand, the start of oxidative damage in cells is a sign of both salt stress and cell death. H2O2 has received special attention among ROS since it has been discussed as a significant oxidant accumulation with oxidative rupture. Membrane lipid peroxidation is a critical limitation that is widely used to assess the extent of oxidative rupture. It is mostly caused by increasing lipoxygenase activity (Zaid and Wani 2019; Desoky et al. 2020; Dumanović et al. 2021). Conversely, we discovered that pretreating soybean seedlings with dopamine hydrochloride (DH) at 100 µM and 200 µM decreased the amount of MDA and the antioxidant activity levels in comparison to untreated stressed seedlings. These results support the hypothesis that, in plants under salt stress, dopamine plays a crucial protective role in oxidative stress defence and cellular damage mitigation (Ahammed et al. 2020b). There are two reasons for this result. Dopamine either helps the organism adjust how it responds to stressful situations or it serves as a potent water-soluble antioxidant that may control the decrease in oxygen generation during photosynthetic activities (Ahammed and Li 2023). In addition to their removal from the cell walls, chemicals and their derivatives may cause this process due to their antioxidative properties. For instance, the oxidation of dopamine produces melanin, which is a highly effective ROS scavenger. Our results corroborate those of Gao et al. (2020a), who reported that exogenous dopamine protected apple plants from oxidative stress caused by an excessive build-up of ROS due to salt stress. Additionally, Raza et al. (2022) found that dopamine treatment increases antioxidant enzyme activity and the efficiency of the ascorbate (AsA)-glutathione (GSH) cycle, which in turn greatly influences plants' ability to withstand salt. Dopamine treatment also plays a major role in ROS exclusion during salt stress. Several studies have demonstrated that exogenous dopamine administration can significantly increase the activities of salt-stressed plant dehydroascorbate reductase (DHAR) and monodehydroascorbate reductase (MDHAR), also regulates the process of oxygen reduction during photosynthetic respiration moreover, acts as an oxygen reduction factor, enabling oxygen reduction to take part in energy conversion during photosynthesis, and a natural media for chemical analogs (Gao et al. 2020a). These properties may account for its protective effect on plants under salt stress (Bala 2020). Melanin, a consequence of dopamine oxidation and a strong active oxygen scavenger, is assumed to be the primary source of dopamine's strong antioxidant activity (Gao et al. 2020b; Ahmad et al. 2021).
The present investigation revealed that, in contrast to control and untreated seedlings, the soybean seedlings under salt stress exhibited elevated levels of osmolyte accumulations, such as total soluble proteins, proline, glycine betaine, and total free amino acids. In contrast, when soybean plants were treated with varying concentrations of dopamine hydrochloride DH (100 µM or DH 200 µM), the contents of these compatible osmolytes were significantly higher than in untreated plants. This improved osmotic adjustment and significantly protected cells from salt toxicity. Plants produce osmolytes in response to salt stress, which is an essential mechanism for shielding cellular components from damage and stopping the deterioration of cellular membranes in addition to getting rid of ROS (Rouphael et al. 2017, Semida et al. 2020, Desoky et al. 2021). These findings align with the findings of Ni et al. (2018) and Pal et al. (2018) that showed high accumulation of osmolytes in wheat plants under salt stress as a result of protection the plant tissues and adjust their osmotic balance, allowing the plants to reserve the growth. Proline is an essential osmolyte, a significant source of energy and nitrogen, and a complex molecule that is involved in osmotic adjustment, enzyme protection, and ROS scavenging (Pal et al. 2018; Angon et al. 2022). It is becoming evident that total soluble proteins play a critical role in counteracting the negative effects of stress damage on plants by making them resistant to dehydration and excessive osmotic regulation (Rady et al. 2021). The study's findings also investigated the greater rise in osmolyte levels induced by dopamine hydrochloride (DH) treatments at 100 µM or 200 µM. These treatments may shield soybean seedling metabolisms from excessive ROS caused by salinity stress, as demonstrated by the reduction of MDA and the activation of antioxidant enzymes.
According to our findings, the salt stress caused a much higher accumulation of Na+ ions and a significantly smaller accumulation of K+, N+3, and Ca+2 ions in the leaves of soybean seedlings. But when same plants were pretreated with different amounts of dopamine hydrochloride DH (100 µM or DH 200 µM), their levels of K+, N+3, and Ca+2 in the soybean leaves remained much greater than those of the untreated plants. Plant development can be promoted in both normal and salt-stressed settings through the mechanisms of ion absorption.
Dopamine's advantageous effects on ion uptake and its inhibitory effects on the uptake of Na+ and Cl- may be linked to our salt-stressed soybean seedlings' ability to withstand high salinities. A high K+/Na+ ratio is maintained in the cytosol via the extrusion of Na+ and/or their intracellular compartmentalization, especially in vacuoles (Blumwald et al. 2000). The expression of Na+/H+ antiporter genes in the roots and leaves of salt-stressed apple plants has also been shown to be enhanced by exogenous dopamine administration, maintaining a higher K+/Na+ ratio in the plants and lessening the damage caused by salt stress (Li et al. 2015). Furthermore, Liu et al. (2020b) showed that plants receiving insufficient nutrients may tolerate a significantly higher quantity of deadly nutrients than untreated plants when dopamine (100 µM) is applied. Moreover, exogenous dopamine injection has a number of advantageous effects, such as antisenescence and antioxidative qualities that promote nutrient absorption (Liang et al. 2017). Li et al. (2015) suggest that the ability of dopamine to modify the root structure is what determines the efficiency of element absorption in the absence of nourishment. For example, apple trees were able to alter the width and length of their roots to enhance the uptake and utilization of potassium when there was an inadequate amount of the mineral. In addition to improving nutrient uptake in roots and the transfer of nutrients from roots to stems and leaves, exogenous dopamine therapy can have an impact on root structure. Therefore, dopamine can enhance plants' ability to respond to nutritional stress by regulating the intake of nutrients by plants as well as their mobility and distribution within plants (Li et al. 2015).
According to our findings, the five ISSR primers yielded 30 ISSR-derived fragments, of which 19 were polymorphic bands, 10 were unique bands, and 1 was monomorphic band. According to Zorb et al. (2009), the ISSR patterns in this study showed the emergence of novel bands in salt-stressed soybean, which may be the consequence of the ion toxicity caused by salinity stress. Ions pass through the nucleus and initiate signaling events that modify DNA remodeling and change gene expression. Aly et al. (2019) suggested that structural DNA rearrangements caused by different kinds of DNA damage could be linked to the effects of salt stress on ISSR fingerprinting. Furthermore, the removal of some bands brought on by salt stress may be due to the generation of harmful ROS, which damages genes by interacting with macromolecules like DNA and taking electrons from them (Pandey and Gupta 2018; Sharma et al. 2019). These structural alterations include chromosomal rearrangements, base deletions, mutations, pyrimidine dimers, base modifications, cross-links, modified or double-strand breaks, oxidized bases, bulky adducts, and strand breaks (Gill and Tuteja 2010). When the Taq-polymerase enzyme comes into contact with damaged DNA, a number of things could happen, such as blockage and likely enzyme-DNA separation, which will cause the bands to vanish (Gruber et al. 2000). Alternatively, the formation of novel bands may be associated with mutations rather than DNA damage as a result of changes in oligonucleotide priming-sites affecting DNA mutations if new annealing events occur at the same locus in a sufficient number of cells (Enan 2006). However, when external dopamine hydrochloride DH (100 µM and 200 µM) was given to soybean plants under salt stress, some of the bands vanished. This could have been due to a variety of molecular and biochemical alterations as well as an adaptation for altering gene expression, which is an indicator of salinity tolerance (Arif et al. 2020). The presence or lack of DNA fragments in ISSR fingerprinting patterns may indicate DNA damage from point mutations brought on by ROS production from salt stress (Sharma et al. 2012). An increase in ROS may make genomic DNA damage worse, which would lead to ISSR polymorphism. DNA bases are altered as a result of ROS attacking leading to irreversible single- and double-strand breaks in the molecule (Banerjee and Roy 2021). One of the most frequent forms of DNA damage under ROS stress is double-strand breaks, which can lead to genomic instability and DNA fragmentation (Cannan and Pederson 2016). As the concentration of NaCl increased in the current study, the fingerprinting variation of ISSR markers showed an increase in polymorphism. As a result, this fingerprinting variation could be used to measure the qualitative genotoxic activity of salinity and other environmental stress factors like heat, pollution, drought, and so on, as well as to identify target genes for particular genotoxic agents. Our results showed that treatment with dopamine hydrochloride mitigated the negative effects of salinity stress, leading to the regeneration of most lost bands as well as the removal of certain salt-induced bands. This improvement may be linked to either the mitigation of the effects of salt stress or the decrease of ROS and consequently their harmful effects. Concurrent with these findings, Gao et al. (2020b) showed that dopamine treatment reduces the accumulation of DNA damage in apple plants. This may be explained by the fact that dopamine application raises tolerance mechanisms within salt-stressed plants by altering critical processes like DNA elimination and chromosomal structural changes, which were linked to the suppression of ROS formation under salinity stress. Therefore, exogenous dopamine hydrochloride (DH) at 100 µM and 200 µM improved the growth, photosynthetic rate, and membrane stability in soybean plants. Hence, dopamine presents a fresh opportunity for horticulture uses, safeguarding its significance in resolving the problem of nutrient depletion in salted environments.
5 Conclusions
This study demonstrates that dopamine plays a crucial role in enhancing the tolerance of soybean plants to salinity stress. Exogenously applied low concentrations of dopamine hydrochloride alleviated the detrimental effects of salt stress on soybean plants. Dopamine maintained photosynthetic efficiency and pigment levels, inhibited the accumulation of reactive oxygen species, and protected the plant cells from lipid peroxidation under salinity conditions. Furthermore, dopamine promoted the accumulation of compatible osmolytes, antioxidant enzymes, and facilitated the absorption of essential ions such as potassium, nitrogen, and calcium during salt stress. At the molecular level, the application of dopamine resulted in the appearance of newly synthesized DNA bands and the disappearance of bands formed under salinity stress alone. Additionally, four DNA bands specific to the control sample appeared in the dopamine treatment, indicating dopamine's role in modulating gene expression and cellular processes in response to salinity stress. These findings suggest that dopamine plays a vital role in regulating multiple physiological processes that confer tolerance to abiotic stresses, such as salinity, in soybean plants. The ability of dopamine to increase resistance to abiotic stresses opens up new avenues for its application in horticultural production, particularly in the context of climate change. Further research is needed to elucidate the mechanistic links between dopamine and the various physiological and molecular processes involved in stress tolerance.
Data Availability
The authors declare that the data presented in this manuscript is their own work. All the data collected in this experiment were given in the manuscript and supplementary data. The data would be made available on request to the corresponding author.
Abbreviations
- DH:
-
Dopamine Hydrochloride
- L-DOPA:
-
L-3, 4-dihydroxyphenylalanine
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
- H2O2 :
-
Hydrogen peroxide
- POX:
-
peroxides
- ROS:
-
Reactive Oxygen Species
- HO- :
-
Hydroxyl radical
- O2 • :
-
Superoxide anion
References
Abdul-Baki AA, Anderson JD (1973) Relationship between decarboxylation of glutamic acid and vigor in soybean seed. Crop Sci 13(2):227–232. https://doi.org/10.2135/cropsci1973.0011183X001300020023x
Abdulmajeed AM, Alharbi BM, Alharby HF, Abualresh AM, Badawy GA, Semida WM, Rady MM (2022) Simultaneous action of silymarin and dopamine enhances defense mechanisms related to antioxidants, polyamine metabolic enzymes, and tolerance to cadmium stress in Phaseolus vulgaris. Plants 11(22):3069. https://doi.org/10.3390/plants11223069
Ahammed GJ, Li X (2023) Dopamine-induced abiotic stress tolerance in horticultural plants. Sci Hortic 307:111506. https://doi.org/10.1016/j.scienta.2022.111506
Ahammed GJ, Li X, Liu A, Chen S (2020a) Brassinosteroids in plant tolerance to abiotic stress. J Plant Growth Regul 39:1451–1464. https://doi.org/10.3390/ijms232314577
Ahammed GJ, Wang Y, Mao Q, Wu M, Yan Y, Ren J, Wang X, Liu A, Chen S (2020b) Dopamine alleviates bisphenol A-induced phytotoxicity by enhancing antioxidant and detoxification potential in cucumber. Environ Pollut 259:113957. https://doi.org/10.1016/j.envpol.2020.113957
Ahmad A, Khan WU, Shah AA, Yasin NA, Ali A, Rizwan M, Ali S (2021) Dopamine alleviates hydrocarbon stress in Brassica oleracea through modulation of physiobiochemical attributes and antioxidant defense systems. Chemosphere 270:128633. https://doi.org/10.1016/j.chemosphere.2020.128633
Akula R, Mukherjee S (2020) New insights on neurotransmitters signaling mechanisms in plants. Plant Signal Behav 15:1737450. https://doi.org/10.1080/15592324.2020.1737450
Allen GC, Curtis MT, Hooper AJ, Tucker PM (1974) X-ray photoelectron spectroscopy of iron oxygen systems. J Chem Soc Dalton Transactions 14:1525–1530. https://doi.org/10.1039/DT9740001525
Aly AA, Eliwa NE, Maraei RW (2019) Physiological and molecular studies on ISSR in two wheat cultivars after exposing to gamma radiation. Sci Asia 45(5):436–445. https://doi.org/10.2306/scienceasia1513-1874.2019.45.436
Angon PB, Tahjib AM, Samin SI, Habiba U, Hossain MA, Brestic M (2022) How do plants respond to combined drought and salinity stress? A systematic review. Plants 11:2884. https://doi.org/10.3390/plants11212884
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
Ashour M, Al-Souti AS, Hassan SM, Ammar GAG, Goda AMAS, El-Shenody R, Abomohra AE, El-Haroun E, Elshobary ME (2023) Commercial seaweed liquid extract as strawberry biostimulants and bioethanol production. Life 13:85. https://doi.org/10.3390/life13010085
Bala K (2020) Beyond a neurotransmitter Physiological role of dopamine in plants. In: Baluska F, Mukherjee S, Ramakrishna A (eds) Neurotransmitters in plant signaling and communication. Signaling and communication in plants. Springer, Cham, pp 169–187. https://doi.org/10.1007/978-3-030-54478-2_9
Bamel K, Prabhavathi (2020) Dopamine in plant development and redox signaling. In: Baluska F, Mukherjee S, Ramakrishna A (eds) Neurotransmitters in plant signaling and communication. Signaling and communication in plants. Springer: Cham, p 123-139. https://doi.org/10.1007/978-3-030-54478-2_7
Banerjee S, Roy S (2021) An insight into understanding the coupling between homologous recombination mediated DNA repair and chromatin remodeling mechanisms in plant genome: an update. Cell Cycle 20:1760–1784. https://doi.org/10.1080/15384101.2021.1966584
Bates LS, Waldron RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–208. https://doi.org/10.1007/BF00018060
Beauchamp C, Fridovich I (1971) Superoxide dismutase improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287. https://doi.org/10.1016/0003-2697(71)90370-8
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochimica Biophysica Acta (BBA) Biomembranes 1465(1-2):140–151. https://doi.org/10.1016/s0005-2736(00)00135-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Cannan WJ, Pederson DS (2016) Mechanisms and consequences of double-strand DNA break formation in chromatin. J Cell Physiol 231:3–14. https://doi.org/10.1002/jcp.25048
Cui J, Lamade E, Tcherkez G (2020) Potassium deficiency reconfigures sugar export and induces catecholamine accumulation in oil palm leaves. Plant Sci 300:110628. https://doi.org/10.1016/j.plantsci.2020.110628
Dadasoglu E, Turan M, Ekinci M, Argin S, Yildirim E (2022) Alleviation mechanism of melatonin in chickpea Cicer arietinum L under the salt stress conditions. Hortic 8(11):1066. https://doi.org/10.3390/horticulturae8111066
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83(11):3811–3815. https://doi.org/10.1073/pnas.83.11.3811
Dawood MFA, Zaid A, Latef AAHA (2022) Salicylic acid spraying-induced resilience strategies against the damaging impacts of drought and/or salinity stress in two varieties of Vicia faba L. seedlings. J Plant Growth Regul 41:1919–1942. https://doi.org/10.1007/s00344-021-10381-8
Desoky EM, El-Maghraby LMM, Awad AE, Abdo AI, Rady MM, Semida WM (2020) Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata L). Sci Hortic 272:109576. https://doi.org/10.1016/j.scienta.2020.109576
Desoky EM, Elrys AS, Mansour E, Eid RSM, Selem E, Rady MM, Ali EF, Mersal GAM, Semida WM (2021) Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci Hortic 288:110340. https://doi.org/10.1016/j.scienta.2021.110340
Duan H, Liu W, Zhou L, Han B, Huo S, El-Sheekh M, Dong H, Li X, Xu T, Elshobary M (2023) Improving saline-alkali soil and promoting wheat growth by co-applying potassium-solubilizing bacteria and cyanobacteria produced from brewery wastewater. Front Environ Sci 11:1–12. https://doi.org/10.3389/fenvs.2023.1170734
Dubois M, Gilles K, Hamilton J, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chimica Acta 28:350–356. https://doi.org/10.1021/ac60111a017
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front Plant Sci 11:552969. https://doi.org/10.3389/fpls.2020.552969
Enan MR (2006) Application of random amplified polymorphic DNA (RAPD) to detect the genotoxic effect of heavy metals. Biotechnol Applied Biochem 43(3):47–154. https://doi.org/10.1042/BA20050172
Fariduddin Q, Zaid A, Mohammad F (2019) Plant growth regulators and salt stress: Mechanism of tolerance trade-off. In: Akhtar M (ed) Salt Stress, microbes, and plant interactions: Causes and solution. Springer, Singapore, pp 1:91-111 https://doi.org/10.1007/978-981-13-8801-9_4
Farouk S, El-Hady MAA, El-Sherpiny MA, Hassan MM, Alame KH, Al-Robai SA, El-Bauome HA (2023) Effect of dopamine on growth, some biochemical attributes, and the yield of Crisphead Lettuce under nitrogen deficiency. Hortic 9(8):945. https://doi.org/10.3390/horticulturae9080945
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 133:21–25
Gao T, Liu X, Shan L, Wu Q, Liu Y, Zhang Z, Li C (2020a) Dopamine and arbuscular mycorrhizal fungi act synergistically to promote apple growth under salt stress. Environ Exp Bot 178:104159. https://doi.org/10.1016/j.envexpbot.2020.104159
Gao T, Zhang Z, Liu X, Wu Q, Chen Q, Liu Q, Li C (2020b) Physiological and transcriptome analyses of the effects of exogenous dopamine on drought tolerance in apple. Plant Physiol Biochem 148:260–272. https://doi.org/10.1016/j.plaphy.2020.01.022
Gao T, Liu Y, Liu X, Zhao K, Shan L, Wu Q, Liu Y, Zhang Z, Ma F, Li C (2021) Exogenous dopamine and overexpression of the dopamine synthase gene MdTYDC alleviated apple replant disease. Tree Physiol 41:1524–1541. https://doi.org/10.1093/treephys/tpaa154
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Godoy F, Olivos-Hernández K, Stange C, Handford M (2021) Abiotic stress in crop species improving tolerance by applying plant metabolites. Plants10(2):186 https://doi.org/10.3390/plants10020186
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307. https://doi.org/10.1007/BF02374789
Gruber M, Wellinger RE, Sogo JM (2000) Architecture of the replication fork stalled at the 3′ end of yeast ribosomal genes. Mol cell Biol 20(15):5777–5787. https://doi.org/10.1128/MCB.20.15.5777-5787.2000
Gul Z, Tang ZH, Arif M, Ye Z (2022) An insight into abiotic stress and influx tolerance mechanisms in plants to cope in saline environments. Biol 11(4):597. https://doi.org/10.3390/biology11040597
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–9. https://doi.org/10.1016/S0021-9258(19)42083-8
Hameed A, Ahmed MZ, Hussain T, Aziz I, Ahmad N, Gul B, Nielsen BL (2021) Effects of salinity stress on chloroplast structure and function. Cells 10(8):2023. https://doi.org/10.3390/cells10082023
Havir EA, McHale NA (1987) Biochemical and developmental characterization of multiple forms of catalase in tobacco leaves. Physiol Plant 84(2):450–455. https://doi.org/10.1104/pp.84.2.450
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–98. https://doi.org/10.1016/0003-9861(68)90654-1
Huo S, Wang H, Chen J, Hu X, Zan X, Zhang C, Qian J, Zhu F, Ma H, Elshobary M (2022) A preliminary study on polysaccharide extraction, purification, and antioxidant properties of sugar-rich filamentous microalgae Tribonema minus. J Appl Phycol 34:2755–2767. https://doi.org/10.1007/s10811-021-02630-w
Ji Z, Liu Z, Han Y, Sun Y (2022) Exogenous dopamine promotes photosynthesis and carbohydrate metabolism of downy mildew-infected cucumber. Sci Hortic 295:110842. https://doi.org/10.1016/j.scienta.2021.110842
Jiao XY, Li YX, Zhang XZ, Liu CL, Liang W, Li C, Ma FW, Li CY (2019) Exogenous dopamine application promotes alkali tolerance of apple seedlings. Plants 8:580. https://doi.org/10.3390/plants8120580
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot 65:729–735. https://doi.org/10.1139/b87-097
Khatri K, Rathore MS (2022) Salt and osmotic stress induced changes in physio chemical responses, PSII photochemistry and chlorophyll a fluorescence in peanut. Plant Stress 3:100063. https://doi.org/10.1016/j.stress.2022.100063
Lee YP, Takahashi T (1966) An improved colorimeteric determination of amino acids with the use of ninhydrine. Anal Biochem 14:71–77. https://doi.org/10.1016/0003-2697(66)90057-1
Li C, Wei Z, Liang D, Zhou S, Li Y, Liu C, Ma F (2013) Enhanced salt resistance in apple plants overexpressing a Malus vacuolar Na+/H+ antiporter gene is associated with differences in stomatal behavior and photosynthesis. Plant Physiol Biochem 70:164–173. https://doi.org/10.1016/j.plaphy.2013.05.005
Li C, Sun X, Chang C, Jia D, Wei Z, Li C, Ma F (2015) Dopamine alleviates salt-induced stress in Malus hupehensis. Physiol Plant 153(4):584–602. https://doi.org/10.1111/ppl.12264
Liang B, Li C, Ma C, Wei Z, Wang Q, Huang D, Chen Q, Li C, Ma F (2017) Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol Biochem 119:346–359. https://doi.org/10.1016/j.plaphy.2017.09.012
Lin YX, Xu HJ, Yin GK, Zhou YC, Lu XX, Xin X (2022) Dynamic changes in membrane lipid metabolism and antioxidant defense during soybean (Glycine max L Merr) seed aging. Front Plant Sci 13:908949 https://doi.org/10.3389/fpls.2022.908949
Liu Q, Tengteng G, Wenxuan L, Yusong L, Yongjuan Z, Yuerong L, Wenjing L, Ke D, Fengwang M, Chao L (2020a) Functions of dopamine in plants: A review. Plant Signal Behav 15(12):1827782. https://doi.org/10.1080/15592324.2020.1827782
Liu XM, Gao TT, Zhang ZJ, Tan KX, Jin YB, Zhao YJ, Ma FW, Li C (2020b) The mitigation effects of exogenous dopamine on low nitrogen stress in Malus hupehensis. J Integr Agric 19:2709–2724. https://doi.org/10.1016/S2095-3119(20)63344-5
Marchiosi R, Soares AR, Abrahao J, dos Santos WD, Ferrarese-Filho O (2020) L-DOPA and dopamine in plant metabolism. In: Baluska F, Mukherjee S, Ramakrishna A (eds) Neurotransmitters in plant signaling and communication. Signaling and communication in plants. Springer, Cham, pp 141-167. https://doi.org/10.1007/978-3-030-54478-2_8
Metzner H, Rau H Senger H (1965) Investigations on the synchronizability of individual pigment deficiency mutants of Chlorella. Planta 65:186. https://doi.org/10.1007/BF00384998
Mohammadi AM, Moradi H, Ghasemi K, Biparva P (2021) Elicitation of dopamine biosynthesis in common purslane as affected by methyl jasmonate and silicon. J Plant Nutr 44:3083–3098. https://doi.org/10.1080/01904167.2021.1936027
Ni J, Wang Q, Shah FA, Liu W, Wang D, Huang S, Fu S, Wu L (2018) Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molec 23:799. https://doi.org/10.3390/molecules23040799
Noor I, Sohail H, Sun J, Nawaz MA, Li G, Hasanuzzaman M, Liu J (2022) Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 303:135196. https://doi.org/10.1016/j.chemosphere.2022.135196
Pal M, Tajti J, Szalai G, Peeva V, Vegh B, Janda T (2018) Interaction of polyamines, abscisic acid and proline under osmotic stress in the leaves of wheat plants. Sci Rep 8:12812–12839. https://doi.org/10.1038/s41598-018-31297-6
Pandey C, Gupta M (2018) Selenium amelioration of arsenic toxicity in rice shows genotypic variation: a transcriptomic and biochemical analysis. J Plant Physiol 231:168–181. https://doi.org/10.1016/j.jplph.2018.09.013
Rady MM, Boriek SHK, Abd El-Mageed TA, Seif El-Yazal MA, Ali EF, Hassan FAS, Abdelkhalik A (2021) Exogenous gibberellic acid or dilute bee honey boosts drought stress tolerance in Vicia faba by rebalancing osmoprotectants, antioxidants, nutrients, and phytohormones. Plants 10:748. https://doi.org/10.3390/plants10040748
Raza A, Salehi H, Rahman MA, Zahid Z, Madadkar Haghjou M, Najafi-Kakavand S, Charagh S, Osman HS, Albaqami M, Zhuang Y, Siddique KH (2022) Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front Plant Sci 13:1–36. https://doi.org/10.3389/fpls.2022.961872
Roshchina VV (2022) Biogenic amines in plant cell at normal and stress: Probes for dopamine and histamine. In Aftab T, Naeem M (eds) Emerging plant growth and regulation in Agriculture Roles in stress tolerance. Academic press, pp 357-376 https://doi.org/10.1016/B978-0-323-91005-7.00009-6
Rouphael Y, Cardarelli M, Bonini P, Colla G (2017) Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front Plant Sci 8:131. https://doi.org/10.3389/fpls.2017.00131
Saddhe AA, Manuka R, Penna S (2021) Plant sugars: Homeostasis and transport under abiotic stress in plants. Physiol Plant 171:739–755. https://doi.org/10.1111/ppl.13283
Sako K, Nguyen HM, Seki M (2021) Advances in chemical priming to enhance abiotic stress tolerance in plants. Plant Cell Physiol 61:1995–2003. https://doi.org/10.1093/pcp/pcaa119
Semida WM, Abdelkhalik A, Rady MOA, Marey RA, El-Mageed TAA (2020) Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci Hortic 272:109580. https://doi.org/10.1016/j.scienta.2020.109580
Senousy HH, Khairy HM, El-Sayed HS, Sallam ER, El-Sheikh MA, Elshobary ME (2023) Interactive adverse effects of low-density polyethylene microplastics on marine microalga Chaetoceros calcitrans. Chemosphere 311:137182. https://doi.org/10.1016/j.chemosphere.2022.137182
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Sharma A, Gupta P, Prabhakar PK (2019) Endogenous repair system of oxidative damage of DNA. Current Chem Biol 13(2):110–119. https://doi.org/10.2174/2212796813666190221152908
Snehi S, Kumar S, Rathi SR, Prakash NR (2023) Augmenting salinity tolerance in rice through genetic enhancement in the post-genomic era. In: Sharma D, Singh S, Sharma SK, Singh R. (eds) Smart plant breeding for field crops in post-genomics era . Springer, Singapore, pp 137-164. https://doi.org/10.1007/978-981-19-8218-7_4
Teixeira WF, Soares LH, Fagon EB, Costa S, Mello KR, Doirado ND (2020) Amino acids as stress reducers in soybean plant growth under different water deficit conditions. J Plant Growth Regul 7581-7595. https://doi.org/10.1007/s00344-019-10032-z
Turner RG, Marshall C (1972) The accumulation of zinc by subcellular fractions of roots of Agrostis tenuis Sibth in relation to zinc tolerance. New Phytol 71(4):671–676. https://doi.org/10.1111/j.1469-8137.1972.tb01277.x
Valadez BMG, Aguado SGA, Tiessen FA, Robledo PA, Munoz OA, Rascon CQ, Santacruz VA (2016) A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal Biochem 498:47–52. https://doi.org/10.1016/j.ab.2015.12.015
van Rossum MW, Alberda M, van der Plas LH (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130(2):207–216. https://doi.org/10.1016/S0168-9452(97)00215
Wang Y, Zhang Z, Wang X, Yuan X, Wu Q, Chen S, Zou Y, Ma F, Li C (2021) Exogenous dopamine improves apple fruit quality via increasing flavonoids and soluble sugar contents. Sci Hortic 280:109903. https://doi.org/10.1016/j.scienta.2021.109903
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531–6535. https://doi.org/10.1093/nar/18.22.6531
Zaid A, Wani SH (2019) Reactive oxygen species generation, scavenging and signaling in plant defense responses. In: Jogaiah S, Abdelrahman M (eds) Bioactive molecules in plant defense. Springer, Cham, pp 111–132. https://doi.org/10.1007/978-3-030-27165-7_7
Zhang H, Zhao Y, Zhu JK (2020) Thriving under stress: how plants balance growth and the stress response. Develop Cell 55(5):529–543. https://doi.org/10.1016/j.devcel.2020.10.012
Zhao Q, Xu Y, Liu Y (2022) Soybean oil bodies: A review on composition, properties, food applications, and future research aspects. Food Hydrocolloids 124:107296. https://doi.org/10.1016/j.foodhyd.2021.107296
Zorb C, Herbst R, Forreite C, Schubert S (2009) Short-term effects of salt exposure on the maize chloroplast protein pattern. Proteomics 9:4209–4220. https://doi.org/10.1002/pmic.200800791
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funds present
Author information
Authors and Affiliations
Contributions
Walaa A. Abo-Shanab: Conceptualization, visualization, methodology, data validation, software, collecting data, writing (creating the first draft), writing-reviewing, and editing.
Rana H. Diab: Conceptualization, visualization, methodology, data validation, investigation, writing- reviewing and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abo-Shanab, W.A., Diab, R.H. Dopamine Hydrochloride Alleviates the Salt-induced Stress in Glycine max (L.) Merr. plant. J Soil Sci Plant Nutr (2024). https://doi.org/10.1007/s42729-024-01768-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42729-024-01768-z