Abstract
Purpose
Nitrogen fertilizer management is an important agricultural tool that must be optimized to promote sustainable practices since the nitrogen-fertilizer recovery by plants (NRP) is low, leading to nitrogen losses to the environment. In sugarcane, N-fertilization has been investigated over the years but little attention has been given to N-fertilizer application methods. Sugarcane crop production and environmental impact regarding N-fertilizer application methods (i.e., applied onto the sugarcane straw layer and incorporated into the soil) were investigated in the present study aiming to achieve an environmental-friendly cropping system.
Methods
Sugarcane yield and NRP, N2O emissions, relevant components of the soil microbiological community and N-fertilizer retention in soil layers were quantified. The experiment was carried out in field conditions where N-fertilizer application methods using 15N-labelled ammonium nitrate (15NH415NO3) were compared to a control treatment with no N-fertilization.
Results
Incorporation of N-fertilizer into the soil increased the sugarcane yield by 17% (two-year average) compared to N-fertilizer applied onto the sugarcane straw layer, which was similar to control treatment. There was an increase in NRP-fertilizer of 79% due to the application of N-fertilizer incorporated into the soil. Furthermore, soil incorporation of N-fertilizer decreased N2O emission by 22% with the fertilizer N emission factor reduced four-fold. The N2O emissions were mostly associated with ammonium-oxidizing bacteria (AOB).
Conclusions
Our results show that application of N-fertilizer incorporated into the soil is an environmental-friendly N-fertilization management which will improve agricultural sustainability and reduce environmental impacts.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The global pursuit of renewable energy to reduce the reliance on fossil fuels and mitigate greenhouse gas emissions (GHG), mainly nitrous oxide (N2O), has brought ethanol to prominence (Carvalho et al. 2019; Jaiswal et al. 2017; Long et al. 2015). While the USA is the world’s largest ethanol producer, the N2O emission factor for ethanol derived from maize is 4.6 higher than that from sugarcane (Otto et al. 2022).
Brazil is the world’s largest sugarcane producer, contributing 40% to global production. Over the past decade, concerns have arisen regarding Brazilian sugarcane production due to the country´s stagnant total volume (~ 630 million tons) and low yield (≤ 75 Mg ha−1 of stalk) (FAO 2018). Under favorable edaphoclimatic conditions and proper agronomic management, the potential sugarcane yield in Brazil reaches 310 Mg ha−1 of stalk (Dias and Sentelhas 2018; Waclawovsky et al. 2010). Factors limiting productivity encompass soil quality degradation (Bordonal et al. 2018a, b), extensive agricultural machinery traffic (Castioni et al. 2019), weather adversities throughout crop development (de Castro et al. 2022; Lisboa et al. 2018; Ruiz Corrêa et al. 2019), and the adoption of cost-effective agricultural practices rather than those beneficial to the crop, especially concerning N-fertilizer management (Otto et al. 2016; Quassi de Castro et al. 2018).
Effective N-fertilizer management plays an important role in ensuring productivity and sustainability of sugarcane (Cantarella and Rossetto 2014; de Castro et al. 2017; Thorburn et al. 2011). Understanding the dynamics of N-fertilizer in the soil–plant-atmosphere system is crucial in addressing the low nitrogen-fertilizer recovery (NRP) by sugarcane plants when N-fertilizer is applied to the soil (Boschiero et al. 2019; Franco et al. 2011; Otto et al. 2016; Thorburn et al. 2011). Inadequate N-fertilizer use contributes to soil and water contamination (Bowles et al. 2018a; Ghiberto et al. 2015; Khan et al. 2016; Skocaj et al. 2013), along with the emission of gases, for example, N2O and ammonia (NH3) (Degaspari et al. 2020; Gonzaga et al. 2018; Pan et al. 2016; Soares et al. 2015). To bypass NH3 emission from urea application, ammonium nitrate can be employed as N source in sugarcane fields (Boschiero et al. 2018). Nevertheless, there is a knowledge gap regarding nitrogen losses (e.g., leaching, runoff and N2O emission) and NRP when ammonium nitrate is applied in sugarcane fields using different N-fertilizer application methods.
The two most widespread methods for applying N-fertilizer in sugarcane fields are either onto the sugarcane straw layer or incorporated into the soil. To meet plant nutritional requirements under high yield conditions (> 100 Mg ha−1), new recommendations advocate for higher N-fertilizer rates (approx. 150 kg N ha−1 — Castro et al. 2019; Otto et al. 2019; Sanches et al. 2019). It is noteworthy that sugarcane is mechanically harvested without prior burning in Brazilian fields, leaving a layer of sugarcane straw residue on the soil surface (Carvalho et al. 2019). This aroused challenges in fertilization management since the straw layer hamper the incorporation of N-fertilizer into the soil. Then, application of N-fertilizer onto the sugarcane straw layer is the wide usage method, nevertheless it may lead to N-fertilizer immobilization during straw decomposition (Otto et al. 2016; Quassi de Castro et al. 2021) and significant emission of N2O (Borges et al. 2019; Carmo et al. 2013; da Silva Paredes et al. 2014; Signor and Cerri 2013; Soares et al. 2015).
The increased N2O emissions associated with sugarcane N-fertilization contradict recent Brazilian sectoral policies (Renovabio) and the Conference of the Parties (COP 23), both aiming to promote initiatives for the mitigation of GHG emissions while generating revenue for the sugar-energy sector by the commercialization of decarbonization credits (Gonçalves et al. 2021; Grangeia et al. 2022). Therefore, adjustments in crop agronomic management, particularly in N-fertilization, will enhance environmental and economic sustainability through reducing N-fertilizer losses from the soil–plant system by increased NRP (Bowles et al. 2018b; Otto et al. 2019; Thorburn et al. 2017). An important part of improving NRP involves identifying the N loss pathway (e.g., via gaseous emissions) and correlating those GHG emissions with the soil microbial communities that generate these emissions. There is over 60 types of bacteria and archaea associated to N2O emissions (Pitombo et al. 2016), with ammonia-oxidizing bacteria (AOB) and archaea (AOA) standing out (Lourenço et al. 2018; Soares et al. 2016).
Another pathway of N-fertilizer loss from the soil is via leaching, primarily of the nitrate anion. Nitrate leaching from N-fertilizer application in sugarcane fields in South-Central Brazil can reach up to 6% of the total N-fertilizer applied (Otto et al. 2016). In general, the magnitude of N-leaching losses from sugarcane ratoon fields is related not only to the frequency and intensity of rainfall but also to the season and N-fertilizer application methods (Khan et al. 2016; Otto et al. 2016).
Based on these statements, the present research aimed to test the hypothesis that the application of granular N-fertilizer incorporated into the soil reduces N losses and increases NRP by sugarcane plants compared to N-fertilizer applied onto the sugarcane straw layer. To examine this hypothesis, the research objectives were as follows: i) quantify the N-fertilizer recovery by sugarcane using different N-fertilizer application methods; ii) quantify N2O emissions throughout the crop cycle; iii) assess N-fertilizer losses via leaching and N-fertilizer retention in soil layers; and iv) quantify the microbial communities associated with N2O-N emissions.
2 Material and Methods
2.1 Field Description
The experiment was carried out in Sales Oliveira, São Paulo, Brazil (20º51’S 47º56’W), in a cultivated field that had grown sugarcane for eight years (experiment was established in the 7th ratoon). Mechanized harvesting without prior sugarcane straw burning was conducted in the three years preceding the experiment set up. The sugarcane variety was SP81-3280, harvested at the end of the crop season (from September to November). The mean annual precipitation and temperature of the field were 1553 mm and 23 ºC, respectively. Before initiating the experiment, soil samples were collected for chemical and physical characterization (Online Resource 2), according to the methodology described by Raij et al. (2001). The soil was classified as Rhodic Eutrudox (Soil Survey Staff 2014).
2.2 Experimental Design and Treatments
The experiment was conducted in a randomized block design encompassing three treatments and three replicates over two consecutive years (1st and 2nd year, corresponding to the 7th and 8th sugarcane ratoon). The treatments consisted of i. control [without N-fertilization], and two N-fertilizer application methods, namely ii. Surface [N-fertilizer applied onto the sugarcane straw layer], and iii. Incorporated [N-fertilizer applied in a band at 0.08 m soil depth, positioned 0.15 m away from the sugarcane row, methodology described by Castro et al. (2017)]. Each plot consisted of seven sugarcane rows with inter-row spacing of 1.5 m and a length of 2 m, totaling an area of 21 m2. To evaluate the N-fertilizer losses by leaching and surface runoff, the plots were delimited by a galvanized steel gutter.
Nitrogen, phosphorus, and potassium (NPK) were applied 30 days after the harvest in both experimental years, except in the control treatment, in which N was not applied. The rates and sources adopted for NPK fertilization were 120 kg N ha−1 (applied as ammonium nitrate — 33% N), 35 kg P ha−1 (applied as triple superphosphate — 41% P2O5) and 120 kg K ha−1 (applied as potassium chloride — 60% K2O), respectively. In the 2nd year, microplots containing double-labeled 15N ammonium nitrate (15NH415NO3, with 5 atom% 15N) were installed at the center of each plot.
2.3 Sugarcane Stalk Population and Yield
Sugarcane stalk population (stalks ha−1) and yield were evaluated in both years. The stalks in each plot were counted and the aboveground biomass of sugarcane, comprising three plant tissues (dry leaves, tops and stalks), was quantified. The fresh mass of each plant tissue was determined to calculate the stalk yield per hectare.
2.4 Nitrogen-Fertilizer Losses via Leaching and Runoff
N-fertilizer losses through leaching and runoff were evaluated in the 2nd year following the methodology described by Scarpare et al. (2023). N-leaching was assessed using lysimeters installed at a depth of 0.90 m in the microplot. N-fertilizer at this depth was considered to have leached beyond the effective root zone since 80% of the sugarcane plant root system is located in the first 0.40 m of soil depth, with long roots extending to 0.60 m (Otto et al. 2009; Rossi Neto et al. 2018). To maintain lysimeters operational, weekly vacuuming was performed. Surface water runoff from the entire plot was collected in water tanks, where water sub-samples were obtained. Collected solutions were stored in a freezer to prevent N transformation until analysis.
Leachate and runoff water were analyzed using a mass spectrometer interfaced with an automatic nitrogen and carbon analyzer (IRMS-ANCA-GSL, Sercon Inc., Crewe, GBR) for determining total-N content and isotopic abundance, according to Ghiberto et al. (2009). As no difference in isotopic abundance between treatments was observed, ammonium and nitrate content in surface water runoff and leachate water were determined by flow injection analysis by conductimetry (Reis et al. 1997) and colorimetry (Giné et al. 1980), respectively.
2.5 N2O Emission
N2O emission was evaluated in the 2nd year. Gas sampling started one day before the N-fertilizer application and continued daily for 18 days. Afterward, sampling was conducted three times a week for three weeks, twice a week for the following three weeks, and once a week until the onset of sugarcane harvesting (46 sampling events). Flux of N2O were measured using static chambers (PVC cylinders with 0.20 m in height and 0.30 m in diameter) placed in row and inter-row. The chambers were sealed with caps and air samples were collected using BD nylon syringes (Becton Dickinson Industry Co. Ltd) after 0, 15, and 30 min of incubation, and transferred to evacuated glass vials. Simultaneously, soil moisture and temperature were measured with a MPS 2 Decagon® device installed at a 0.10 m depth.
Gas samples were analyzed using a Shimadzu® (GC-2014) gas chromatograph equipped with a packed column and an electron capture detector for N2O analyses. The N2O flux was calculated based on the linear change in concentration within the chamber during the incubation time (30 min). Atmospheric pressure, chamber height, and air temperature were measured simultaneously with gas sampling for determining air chamber volume and calculating N2O emissions. Daily N2O flux was calculated according to Eq. 1 (Parkin and Venterea 2010).
where: F is the N2O flux (μg N2O-N m−2 h−1), [ΔC/Δt] is the gas concentration exchange rate inside the chamber during the incubation time (μg mol−1 h−1), V is the chamber volume (L), Vm is the molar volume of the gas at the sampling temperature (L mol−1), m is the molecular mass of N2O (N = 14), and A is the soil area covered by the chamber (m2).
The N2O flux were converted and expressed in g N2O ha−1 day−1 using linear interpolation between the dates adjacent to the samples (Whittaker and Robinson 1967). The cumulative N2O emissions from the fertilized band were considered to represent 20% of the total area, with the area between rows accounting for 80%, as used in other studies (Borges et al. 2019; Carmo et al. 2013; Gonzaga et al. 2019). The N2O emission factors were calculated based on the N-fertilizer rate applied within the chamber according to Eq. 2.
where: EFN2O is the emission factor (N2O as percentage of nitrogen fertilizer applied); N2Otreatment and N2Ocontrol are the cumulative emissions of N2O from a specific treatment and a specific control; and Nfertilizer is the N-fertilizer rate applied to soil.
2.6 Nitrogen-Fertilizer Recovery by Aboveground Plant Tissues
The N-fertilizer recovery by the aboveground of sugarcane plant was determined by summing up the N-fertilizer recovery by each plant tissue (tops, stalks, and dry leaves) in the 2nd year. The plant tissues were sampled in both the central row and the two adjacent rows (Trivelin et al. 1994). After determining the fresh mass of each plant tissue, they were ground and sub-samples were taken for moisture and isotopic abundance determination. The sub-samples were oven-dried at 65 °C until reaching a constant mass followed by grinding in a Wiley mill with a 0.5 mm mesh sieve. The moisture content of the plant tissues was calculated, and their yield was corrected to dry matter, serving as the basis for calculating the NRP. The ground and dried samples were weighted (Mettler Toledo XPR6U, Columbus, EUA) in tin capsules and analyzed in a mass spectrometer coupled to an automatic nitrogen analyzer (IRMS-ANCA-GSL, Sercon Inc., Crewe, GBR). The results obtained were the isotopic abundance of the samples (atom% 15N) and the total-N content (Total-N, g kg−1). The results were used to calculate the nitrogen in plant derived from fertilizer (NPDFF), in percentage, the amount of nitrogen in plant derived from fertilizer (ANPDFF), kg ha−1, and the 15NRP in each plant tissue, following the equations provided (Trivelin et al. 1994).
where NPDFF is the N in each aboveground plant tissue derived from fertilizer (%); “a” and “b” are the isotopic abundance (atom % 15N excess) in the plant and in the fertilizer, respectively; ANPDFFCR and ANPDFFAR is the amount of N in the plant derived from the fertilizer at the center row and at the adjacent rows, respectively; Total-N is the plant N content (kg N ha−1); 15NRP is the N-fertilizer recovery by each aboveground plant tissue; and NR is the N-fertilizer rate applied (kg N ha−1).
2.7 Nitrogen-Fertilizer Recovery in the Soil and by the Belowground Plant Tissues
In the 2nd year of the experiment, the soil was sampled up to a depth of 0.9 m within the chamber, to assess the 15N-fertilizer recovery in the soil. Before soil sampling, the litter inside the chamber was collected, encompassing material from the superficial soil layer and residual straw (referred to as straw-soil). Soil samples were obtained using a Dutch auger at depths of 0.0–0.1, 0.1–0.2, 0.2–0.4, 0.4–0.6, and 0.6–0.9 m. Soil samplings were oven-dried at 50 °C for 72 h with air circulation and renovation. A sub-sample was ground in a ball mill, and analyzed for total-N (g kg−1) and isotopic abundance (atom% 15N) using a mass spectrometer (IRMS-ANCA-GSL, Sercon Inc., Crewe, GBR). The soil mass of each soil layer was determined by the ring volumetric method (Blake and Hartge, 1986). The average soil density at depths of 0.0–0.1, 0.1–0.2, 0.2–0.4, 0.4–0.6, and 0.6–0.9 m was 1.25, 1.18, 1.16, 1.16, and 1.15 g cm−3, respectively, in N-fertilizer applied incorporated into the soil treatment, and 1.28, 1.17, 1.15, 1.22, and 1.13 g cm−3, respectively, in N-fertilizer applied onto the sugarcane straw layer treatment. The N-fertilizer recovery in each soil depth (15NRS) was calculated according to Eqs. 3, 4, and 7.
After soil sampling, the sugarcane root system was sampled through trenches (1.5 × 2.0 x 1.0 m, width x length x depth). The samples were washed for soil removal and compartmentalized into two plant tissues (roots, and rhizome). The root system tissues were dried, ground, and analyzed in a mass spectrometer (IRMS-ANCA-GSL, Sercon Inc., Crewe, GBR) obtaining the total-N (g kg−1) and isotopic abundance (atom% 15N), following procedures described for the aboveground plant samples. The 15N-fertilizer recovery by the belowground plant tissues was calculated according to Eqs. 3, 4, and 7.
2.8 Microbiological Analyses: Genomic DNA Extraction and Quantification of amoA Genes
Soil samples for genomic DNA extraction was collected from the surface layer (0–0.1 m) where N-fertilizer was applied in the 2nd year of the experiment; samples were stores at -80 °C until the moment of use. The DNA extraction was performed using the PowerSoil PowerLyzer DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, USA) following the instructions of the manufacturer. All genes used were quantified by applying the quantitative PCR technique (qPCR) with the use of the ViiA7 Real-Time PCR System (Applied Biosystems, Foster City, USA).
Reactions were performed with 1 µL BSA, 5.5 µM of each primer with concentration of 10 nM, 10 µL of qPCR SYBR master mix 2x (Applied Biosystems, Foster City, CA, USA) and 10 to 20 ng of DNA. The primers used (Online Resource 3) were 16S rRNA of bacteria and archaea (Bakke et al. 2011; Yu et al. 2005), nitrogenase (nifH; Wallenstein and Vilgalys 2005), ammonium-oxidizing (amoA) for bacteria (AOB; Rotthauwe et al. 1997) and archaea (AOA; Leininger et al. 2006). The standard curve was undertaken with pre-amplification of primer sets, using known genes in serial dilution of 102 to 107 µL−1 genes. The amplifications were performed as described in Table S2; the specificity of the primer set was checked by observing a single melting peak (60 ºC to 95 ºC), thus validating the purity of the generated products and also for presenting a single band in the 1% agarose gel, corroborating the size of amplicons. Only r2 above 0.99 of the standard curve were accepted. Amplification efficiencies of quantitative PCR were of 97% ± 9.
2.9 Weather Conditions during the Experimental Period
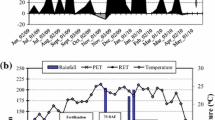
Climatological data was monitored by a meteorological station (Vantage Pro II, Decagon Devices, CA, USA). The water balance (Online Resource 1) was calculated according to the Penman–Monteith method (Howell and Evett 2004).
The precipitation was evenly distributed throughout the maximum plant growth season, from November to December, resulting in a slight water deficit in the soil (≤ 40 mm) during the autumn and winter (March to September). It is noteworthy that after N-fertilization a substantial volume of precipitation occurred (1090 mm accumulated precipitation), favoring the incorporation of N-fertilizer into the soil solution.
2.10 Statistical Analysis
The data set was subjected to analysis of variance (ANOVA) of the entirely randomized block design using the ‘ExpDes’ package (Ferreira et al. 2014), after performing the Shapiro–Wilk normality test. Means among treatments were compared by the Tukey test at p < 0.05 significance. The agronomic traits, such as stalk yield and population, and recovery of 15N-fertilizer in both aboveground and belowground plant tissues, as well as in the soil depths, and the environmental variables (GHG emissions, flux, and emission factor) were subjected to a non-metric multidimensional scaling (N-MDS) using Bray–Curtis dissimilarity matrix. All analysis were performed using Bray–Curtis dissimilarity matrix (R Core Team 2021).
3 Results
3.1 The Application of N-fertilizer Incorporated into the Soil Increased the Population and Yield of Sugarcane
The incorporation of N-fertilizer into the soil increased the number of stalks per hectare compared to the control treatment (Table 1). In the 1st year, the stalk population in incorporated treatment was greater than the surface application. In the 2nd year, the stalk population did not differ among the N-fertilizer application methods. The N-fertilizer application method influenced sugarcane stalk yield. Stalk yield in the incorporated treatment was 15 and 9 Mg stalk ha−1 greater than the control in the 1st and 2nd year, respectively (Table 1). In the 2nd year, sugarcane stalk yield in the incorporated treatment was also greater than surface treatment.
3.2 N-Fertilizer Recovery by the Plant (15NRP) Increased by Applying N-Fertilizer Incorporated into the Soil
The ANPDFF of aboveground plants in incorporated and surface treatment were 59.4 and 33.3 kg N ha−1, respectively (Table 2). The difference among N-fertilizer application methods is due to the ANPDFF in stalk and dry leaves. The average ANPDFF in the belowground did not differ among treatments, with an average of 4.8 kg N ha−1.
The 15NRP in the aboveground plant was 49.6% and 27.7% for incorporated and surface treatments, respectively (Table 2). N-fertilizer incorporated into the soil enhanced the N-fertilizer recovery in the stalk and the dry leaves (Table 2). The average of 15NRP in the belowground plant was 4%. The N-fertilizer recovery by the belowground tissues and in the top did not differ among the N-fertilizer application methods (Table 2). Sugarcane stalk was the main sink of N-fertilizer applied to the soil followed by top, dry leaves, rhizome and roots.
3.3 N-Fertilizer Application Method Altered the N-Fertilizer Recovery in Soil Layers (15NRS)
The highest 15N-fertilizer recovery in the soil (15NRS) was observed in the 0.2–0.4 m and 0.1–0.2 m soil layers for incorporated and surface treatment, respectively (Fig. 1). In these soil layers treatments differed in the 15NRS as well as at the deepest layer (0.6–0.9 m). The average of total NRS was 43.1 and 39.1% in the incorporated and surface treatments, respectively.
15N-fertilizer recovery in each soil layer (15NRS, %) within the N-fertilizer application method (i.e., Incorporated or Surface) in the 2nd year of the experiment. The data represent the average of three independent biological replicates ± standard error. Lowercase letters indicate differences among the N-fertilizer application methods within each soil layer according to Tukey post-hoc test (p < 0.05). “ns” means non-significant difference among N-fertilizer application methods
3.4 Influence of N-Fertilizer Application Method on the 15N-Fertilizer in the Plant-Soil-Atmosphere System
The 15N-fertilizer recovered in the plant-soil system is the sum of the total N-fertilizer recovery by the plant (NRP-total), in the soil (NRS-total), and in the litter (NRStraw-Soil). The total N-fertilizer recovery in the plant-soil system was 99.5 and 72.7% in the incorporated and surface treatments, respectively. The incorporated treatment increased by 76% the NRP-total compared to the surface treatment (Fig. 2). The NRS-total and the NRStraw-Soil did not differ among N-fertilizer application methods, an average of 41% and 2.4% respectively.
15N-fertilizer recovered in the soil–plant system in the 2nd year of the experiment within the N-fertilizer application method at sugarcane harvesting. NRS-total is the 15N-fertilizer recovery in the soil (sum of N-fertilizer recovered in each soil layer, from 0 to 0.9 m), NRStraw-Soil is the 15N-fertilizer recovered in the litter, and NRP-total is the 15N-fertilizer recovery by the plant (sum of N-fertilizer recovered in the aboveground and belowground tissues). The data represent the average of three independent biological replicates. Lowercase letters indicate differences among N-fertilizer application methods within each evaluated system (soil, straw-soil, plant, and non-recovered nitrogen) according to Tukey post-hoc test (p < 0.05)
3.5 Ammonium and Nitrate Lost in the Water Leaching and Surface Water Runoff
The isotopic abundance in the water samples from water tanks (runoff) and lysimeters (nitrogen leaching) did not differ among treatments (i.e., solutions presented the natural abundance of 15N, an average of 0.3663 atom% 15N). Therefore, the ammonium and nitrate content were quantified in the water samples. N-fertilization increased the ammonium and nitrate content in the surface water runoff regardless of the application method (Table 3). Ammonium content was two-fold higher in surface treatment compared to incorporated treatment, meanwhile, they did not differ in terms of nitrate content. In the leached water solution, N-fertilization increased the nitrate content regardless of the N-fertilizer application method compared to the control (Table 3).
3.6 N2O Emission Decreased when Applying N-Fertilizer Incorporated into the Soil
The N2O emission occurred more intensively after N-fertilization for up to three months (Fig. 3A). N-fertilization increased N2O emission compared to control treatment, regardless of the application method. Additionally, applying N-fertilizer incorporated into the soil reduced by 50% the N2O emissions compared to surface application. From the 3rd to 6th month after N-fertilization, a significant drop in N2O emissions (below 100 µg N2O m2 day−1) was observed (Fig. 3A). Following this period until the sugarcane harvest, the N2O emissions from control and incorporated treatments were nearly nil, while surface applications continued to emit N2O to the atmosphere (Fig. 3A).
Daily mean N2O emission (A), accumulated of N2O emission [aN2O] (B), N2O emission factor [EF] (C), and intensity of N2O emission [iN2O] (D) in the 2nd year of the experiment (from October 2015 to November 2016) after the application of ammonium nitrate incorporated into the soil (Incorporated), and over the sugarcane straw layer (Surface), and in the control treatment (without N-fertilization). The data represent the average of three independent biological replicates ± standard error. Lowercase letters indicate differences among the treatments according to Tukey post-hoc test (p < 0.05). “u.n.” means unavailable once the N2O emission factor is calculated in function of the control treatment
The lowest accumulated N2O emission (aN2O) was obtained in the control treatment (Fig. 3B), which was 2-folder and 1.7-folder lower than the surface and incorporated treatment, respectively. On the other hand, the incorporated treatment reduced the aN2O by 18% and the emission factor (EF%) by 75% (Fig. 3C) compared to surface treatment. No differences in N2O emission intensity (iN2O) were found among the control and incorporated treatments, meanwhile, the surface treatment doubled the iN2O (Fig. 3D).
3.7 Microbiological Analysis
The control treatment presented higher abundance of bacteria (Fig. 4A) and archaea (Fig. 4B) compared to the N-fertilization treatments. Even considering the higher abundance in the control treatment, no differences were observed among treatments.
Absolute quantification (copies per gram of dry soil) of microbial 16S rRNA genes from bacteria (A) and archaea (B) and genes associated with the biological nitrogen fixation [nifH, nitrogenase] (C), nitrifying bacteria [AOB, Ammonium-Oxidizing Bacteria] (D) and nitrifying archaea [AOA, Ammonium-Oxidizing Archaea] (E). The data represent the average of three independent biological replicates ± standard error. Small letters indicate difference between treatments according to the post-hoc Tukey test (p < 0.05)
The abundance of biological nitrogen fixation (nifH) and nitrifying bacteria (AOB) exhibited variations among treatments (respectively, Fig. 4C and 4D). The lowest abundance of nifH was obtained in the incorporated treatment differing from the control and surface treatments. Surface and control treatments exhibited a similar abundance of nifH (Fig. 4C). The incorporated treatment also presented the lowest abundance of AOB and did not differ from the control treatment (Fig. 4D). An increase in the abundance of AOB was observed in surface treatment compared to incorporated treatment (Fig. 4D). The abundance of nitrifying archaea (AOA) was not influenced by N-fertilization regardless the application method (Fig. 4E).
3.8 Multivariate Analysis
The PCA (principal component analysis) accounted for 67% of the data variation (Fig. 5). The parameters related to plant (15NRP-total and yield) are directed towards the incorporated treatment, while they displayed a negative correlation with the parameters of N2O emission (EF% and Daily N2O emission). The N2O emission parameters are directed towards the surface treatment and correlated positively to the abundance of AOB. It is noteworthy that the N-fertilizer recovered in the soil (15NRS-total) showed a negative correlation with the abundance of bacteria (Bacteria16S), archaea (Archaea16S), and AOA, being these directed toward the control treatment. Based on these statements, it indicates that applying N-fertilizer incorporated into the soil increases the N-fertilizer recovery by the plant leading to a high yield and low N2O emission.
Principal components analysis conducted on variables of interest. It includes the daily N2O emission (N2O emission), emission factor (EF%), total of 15N-fertilizer recovered in the soil (15NRS-total) and by the plant (15NRP-total), sugarcane stalk yield (Yield), the abundance of nitrifying bacteria (AOB), nitrifying Archaea (AOA), and nitrogen-fixing bacteria (nifH), and the total abundance of bacteria (Bacteria 16S) and archaea (Archaea 16S). Black arrows indicate the correlation between biological and environmental parameters by different N-fertilizer application methods. The significance of these correlations was evaluated via the Bray–Curtis distance permutation test
4 Discussion
To achieve an environmental-friendly sugarcane production system, advancements have been made in N-fertilization management in the main producing countries, for example, Brazil (Otto et al. 2016), India (Ghosh et al. 2018), Australia (Bell 2014; Kroon et al. 2016), and Mexico (Bautista et al. 2019). However, the comparison of the application of granular N-fertilizer incorporated into the soil or onto the sugarcane straw layer exploring effects on soil, plant, and losses remains unexplored.
The understanding of the N-fertilizer management is crucial in Brazil since the absence of N-fertilization reduces the sugarcane yield (Sanches and Otto 2022). Furthermore, the tropical conditions, characterized by high temperatures, radiation, and precipitation during the spring and summer, potentially lead to high N-losses (Bowles et al. 2018a; Otto et al. 2016). These risks are associated with the length of the sugarcane growing season, 12 to 18 months in Brazil (de Castro et al. 2022), and the application of N-fertilization in several sugarcane stages (Franco et al. 2011).
To improve fertilization efficiency, a guide for best fertilizer management practices was developed by the International Plant Nutrition Institute (IPNI). This highlights the importance of adjusting the fertilization method to achieve the goals of sustainable agriculture. Adjusting the N-fertilizer application method has been promoting great outcomes, such as a yield increase (de Castro et al. 2017), a reduction in N2O emissions (Borges et al. 2019), and an enhancement in the N-fertilizer use efficiency (Boschiero et al. 2019; de Castro et al. 2022). However, further progress in refining N-fertilizer application methods is essential due to a lack of comprehensive studies providing information on both agronomic and environmental benefits. So, our work comes to fill this gap, highlighting that by applying N-fertilizer incorporated into the soil, great crop yield and lower nitrogen losses will be obtained.
Sugarcane commonly presents low NRP in soil-based N-fertilization (< 40%) with potential high nitrogen losses (Bowles et al. 2018a; Franco et al. 2015; Thorburn et al. 2017; Trivelin et al. 2013). Recent studies highlighted the benefits of the application of N-fertilizer incorporated into the soil (Borges et al. 2019; de Castro et al. 2017, 2022), supporting findings outlined in our study (average yield increase of 17% compared to surface application). However, none of these studies quantified N-fertilizer recovery in the soil–plant system, leaving uncertainties regarding potential increases in this parameter due to the incorporation of N-fertilizer into the soil. Our investigation addressed this gap, conclusively demonstrating that the previously observed benefits are indeed linked to an increase in NRP (an enhancement of 23.5% in incorporated application compared to surface application).
The benefits observed stem from the placement of the N-fertilizer close to the sugarcane root system. This can influence the activity of microorganisms surrounding the fertilizer granule and potentially increase the availability of N-fertilizer for plants (Craig et al. 2021; Mariano et al. 2016). Soil microorganisms also benefit from the nitrogen from the fertilizer, crucial for balancing the C:N ratio to maintain their functionalities (Hodge et al. 2000; Kuzyakov and Xu 2013). For instance, they aid in the mineralization of sugarcane plant residues deposited onto the soil, characterized by a high C:N ratio (Fortes et al. 2013). Placing the N-fertilizer at a depth of 0.08 m bypasses the soil layer with the highest microbial activity, where N-fertilizer immobilization tends to be greater (Fracetto et al. 2017).
Currently, the primary concern on nitrogen loss relies on the escalation of greenhouse gas (GHG) emissions, prominently emphasizing N2O emissions (Masson-Delmotte et al. 2018; Peters et al. 2013). In the sugarcane crop system, maintaining a straw layer on the soil surface alongside N-fertilization has the potential to elevate losses through GHG emissions (Carmo et al. 2013; Degaspari et al. 2020; Gonzaga et al. 2018; Sanches and Otto 2022). Conversely, the application of N-fertilizer incorporated into the soil has minimized N2O emissions compared to applying N-fertilizer onto the sugarcane straw layer (Borges et al. 2019). The majority of N2O emissions during the sugarcane crop cycle occur within a short period following fertilization (Fracetto et al. 2017; Gonzaga et al. 2018), which was also observed in our study, that is, great emissions between the 7th and 25th days after N-fertilization. Moreover, applying the N-fertilizer into the soil reduced the N2O emission factor throughout the crop cycle, contributing substantially to mitigate emissions linked to N-fertilization (Borges et al. 2019; Bowles et al. 2018b, a).
The N2O emissions from agricultural soils rely on microorganism communities, mainly bacterial communities, which can increase the emission due to N-fertilization (Fracetto et al. 2017; Lourenço et al. 2018; Prosser and Martiny 2020; Soares et al. 2016; Vasconcelos et al. 2022). The incorporation of N-fertilizer into the soil can create localized regions with elevated nitrogen concentrations, leading to shifts in soil pH, influencing microbial activity and selecting microbial populations (Scarlett et al. 2021; Wang et al. 2019; Zhong et al. 2023). The interaction between N-fertilization, ammonia-oxidizing bacteria (AOB), and increased N2O emissions has been observed (Fracetto et al. 2017; Vasconcelos et al. 2022). This interaction might be influenced by potential changes in soil pH and the NH4+/NH3− ratio resulting from N-fertilizer application, which could favor the AOB community (Prosser and Martiny 2020). Moreover, soil characteristics such as pH, texture, organic matter content, and moisture levels are widely recognized to impact N2O emissions (Oliveira et al. 2022; Otto et al. 2022). These factors play a significant role in regulating soil biological activity, which in turn affects the transformation of nitrogen in the soil, ultimately influencing the emission of nitrogen gases (Fracetto et al. 2017; Soares et al. 2016).
It is also noteworthy that the application of N-fertilizer incorporated into the soil emerges as a viable strategy to mitigate N loss by water runoff and water leaching. Rainfall intensity influences nutrient losses through water runoff (Khan et al. 2016; Skocaj et al. 2013). In our study, despite applying N-fertilization during the rainy season, isotopic abundance in surface water runoff did not exhibit significant differences between treatments. This could be attributed to the characteristics of the experimental field's soil, classified as Rhodic Eutrudox, featuring substantial A and B horizons and efficient water infiltration throughout the profile (Barbosa et al. 2018; Castioni et al. 2019; Prado et al. 2013). Additionally, the volume and distribution of rainfall during the experimental period (1,548 mm) played a crucial role; the proportion of water drained via surface runoff throughout the study accounted for less than 1% of the total rainfall.
N-leaching is also influenced by rainfall intensity as well as soil texture, and N-fertilization management (Fontes and Alleoni 2006; Ghiberto et al. 2009, 2015; Scarpare et al. 2023). In soils with limited cation and anion retention, N-fertilization during high moisture periods can amplify N movement through the soil profile, increasing N-leaching (Bowles et al. 2018a). Ghiberto et al. (2015) showed a loss of 22 kg N ha−1 by nitrate leaching. On the other hand, a recent study indicated that nitrogen leaching and runoff in sugarcane fields are lower where straw is kept onto the soil surface, respectively, 1.9 and 10 kg N ha−1 (Scarpare et al. 2023). Similarly, experiments across Brazilian sites under N-fertilization reported low levels of N-fertilizer enriched in 15N atoms in leached water (Cantarella and Montezano 2010). Our study aligns with these findings, indicating an average N-leaching loss (15NRS at 0.6–0.9 m depth) of only 8%, regardless of the N-fertilizer application method.
5 Conclusion
The choice of the N-fertilizer application method holds significant relevance to achieving the goals of sustainable and environmental-friendly agriculture, promoting agronomic and environmental benefits. Agronomically, applying N-fertilizer incorporated into the soil (at 0.08 m depth) on both sides of the sugarcane row enhanced sugarcane stalk yield and the N-fertilizer recovery by the plant compared to the traditional surface method (application of N-fertilizer over the sugarcane straw layer). Environmentally, the application of N-fertilizer incorporated into the soil reduced N2O emissions and minimized the N-fertilizer recovery in the soil that might otherwise be lost through N-leaching or runoff. So, the incorporation of N-fertilizer into the soil emerges as a practical and field-adaptable alternative. Opting for this method not only promotes an environmentally friendly N-fertilization system but also contributes to mitigating global warming by reducing greenhouse gas emissions.
Data Availability
The data presented in this study are available on request from the corresponding author.
Code Availability
Not applicable.
References
Bakke I, De Schryver P, Boon N, Vadstein O (2011) PCR-based community structure studies of Bacteria associated with eukaryotic organisms: A simple PCR strategy to avoid co-amplification of eukaryotic DNA. J Microbiol Methods 84:349–351. https://doi.org/10.1016/j.mimet.2010.12.015
Barbosa LC, de Souza ZM, Franco HCJ, Otto R, Rossi Neto J, Garside AL, Carvalho JLN (2018) Soil texture affects root penetration in Oxisols under sugarcane in Brazil. Geoderma Reg 13:15–25. https://doi.org/10.1016/j.geodrs.2018.03.002
Bautista EAP, López IG, Salgado-Garcia S, Rivera SI, Sánchez SC (2019) Fertilization Alternatives for Sugarcane Crop in Pujiltic Sugarcane Mill, Chiapas, Mexico. Sugar Tech 21:756–764. https://doi.org/10.1007/s12355-018-0692-1
Bell MJ (2014) A review of nitrogen use efficiency in sugarcane. Sugar Research Australia
Borges CD, Carvalho JLN, Kölln OT, Sanches GM, Silva MJ, Castro SGQ, Castro SAQ, Sousa LL, Oliveira JVC, Cantarella H, Vargas VP, Tsai SM, Franco HCJ (2019) Can alternative N-fertilization methods influence GHG emissions and biomass production in sugarcane fields? Biomass Bioenerg 120:21–27. https://doi.org/10.1016/j.biombioe.2018.10.017
Boschiero BN, Mariano E, Trivelin PCO (2018) “Preferential” ammonium uptake by sugarcane does not increase the 15N recovery of fertilizer sources. Plant Soil 429:253–269. https://doi.org/10.1007/s11104-018-3672-z
Blake GR, Hartge KH (1986) Bulk Density. In: Klute A (ed) Methods of Soil Analysis. Part 1: Physical and Mineralogical Methods. ASA, SSSA, Madison, pp 363–375
Boschiero BN, Mariano E, Azevedo RA, Ocheuze Trivelin PC (2019) Influence of nitrate - ammonium ratio on the growth, nutrition, and metabolism of sugarcane. Plant Physiol Biochem 139:246–255. https://doi.org/10.1016/j.plaphy.2019.03.024
Bowles TM, Atallah SS, Campbell EE, Gaudin ACM, Wieder WR, Grandy AS (2018a) Addressing agricultural nitrogen losses in a changing climate. Nat Sustain 1:399–408. https://doi.org/10.1038/s41893-018-0106-0
Bowles TM, Jackson LE, Cavagnaro TR (2018b) Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob Chang Biol 24:e171–e182. https://doi.org/10.1111/gcb.13884
Cantarella H, Montezano ZF (2010) Nitrogênio e enxofre (in Portuguese). In: Prochnow LI, Casarin V, Stipp SR (eds) Boas práticas para uso eficiente de fertilizantes: nutrientes. IPNI Brasil, Piracicaba, SP, pp 5–70
Cantarella H, Rossetto R (2014) FERTILIZERS FOR SUGARCANE. In: Cortez LAB (ed) Sugarcane bioethanol — R&D for Productivity and Sustainability. Editora Edgard Blücher, São Paulo, pp 405–422
Carvalho JLN, Cerri CEP, Karlen DL (2019) Sustainable Sugarcane Straw Special Issue: Considerations for Brazilian Bioenergy Production. Energy Res 12:746–748. https://doi.org/10.1007/s12155-019-10063-0
Castioni GAF, Cherubin MR, de Bordonal RO, Barbosa LC, Menandro LMS, Carvalho JLN (2019) Straw removal affects soil physical quality and sugarcane yield in brazil. BioEnergy Res 12:789–800. https://doi.org/10.1007/s12155-019-10000-1
Craig H, Antwis RE, Cordero I, Ashworth D, Robinson CH, Osborne TZ, Bardgett RD, Rowntree JK, Simpson LT (2021) Nitrogen addition alters composition, diversity, and functioning of microbial communities in mangrove soils: An incubation experiment. Soil Biol Biochem 153:108076. https://doi.org/10.1016/j.soilbio.2020.108076
da Silva Paredes D, da Lessa ACR, de Sant’Anna SAC, Boddey RM, Urquiaga S, Alves BJR (2014) Nitrous oxide emission and ammonia volatilization induced by vinasse and N fertilizer application in a sugarcane crop at Rio de Janeiro, Brazil. Nutr Cycl Agroecosystems 98:41–55. https://doi.org/10.1007/s10705-013-9594-5
de Bordonal RO, Carvalho JLN, Lal R, de Figueiredo EB, de Oliveira BG, La Scala N (2018a) Sustainability of sugarcane production in Brazil A review. Agron Sustain Dev 38:13. https://doi.org/10.1007/s13593-018-0490-x
de Bordonal RO, Menandro LMS, Barbosa LC, Lal R, Milori DMBP, Kolln OT, Franco HCJ, Carvalho JLN (2018b) Sugarcane yield and soil carbon response to straw removal in south-central Brazil. Geoderma 328:79–90. https://doi.org/10.1016/j.geoderma.2018.05.003
de Castro SGQ, Decaro ST, Franco HCJ, Graziano Magalhães PS, Garside A, Mutton MA (2017) Best Practices of Nitrogen Fertilization Management for Sugarcane Under Green Cane Trash Blanket in Brazil. Sugar Tech 19:51–56. https://doi.org/10.1007/s12355-016-0443-0
de Castro SGQ, Rossi Neto J, Kölln OT, Borges BMMN, Franco HCJ (2019) Decision-making on the optimum timing for nitrogen fertilization on sugarcane ratoon. Sci Agric 76:237–242. https://doi.org/10.1590/1678-992x-2017-0365
de Castro SGQ, Magalhães PSG, de Castro SAQ, Kölln OT, Franco HCJ (2022) Optimizing Nitrogen Fertilizer Rates at Distinct In-season Application Moments in Sugarcane. Int J Plant Prod 16:137–152. https://doi.org/10.1007/s42106-021-00175-z
Degaspari IAM, Soares JR, Montezano ZF, Del Grosso SJ, Vitti AC, Rossetto R, Cantarella H (2020) Nitrogen sources and application rates affect emissions of N2O and NH3 in sugarcane. Nutr Cycl Agroecosystems 116:329–344. https://doi.org/10.1007/s10705-019-10045-w
Dias HB, Sentelhas PC (2018) Sugarcane yield gap analysis in Brazil – A multi-model approach for determining magnitudes and causes. Sci Total Environ 637–638:1127–1136. https://doi.org/10.1016/j.scitotenv.2018.05.017
do Carmo JB, Filoso S, Zotelli LC, de Sousa NER, Pitombo LM, Duarte-Neto PJ, Vargas VP, Andrade CA, Gava GJC, Rossetto R, Cantarella H, Neto AE, Martinelli LA (2013) Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 5:267–280. https://doi.org/10.1111/j.1757-1707.2012.01199.x
FAO (2018) Statistics Division. In: Food Agric. Organ. United Nations. http://www.fao.org/faostat/en/#data/QC. Accessed 10 Feb 2022
Ferreira EB, Cavalcanti PP, Nogueira DA (2014) ExpDes: An R Package for ANOVA and Experimental Designs. Appl Math 05:2952–2958. https://doi.org/10.4236/am.2014.519280
Fontes MPF, Alleoni LRF (2006) Electrochemical attributes and availability of nutrients, toxic elements, and heavy metals in tropical soils. Sci Agric 63:589–608. https://doi.org/10.1590/S0103-90162006000600014
Fortes C, Vitti AC, Otto R, Ferreira DA, Franco HCJ, Trivelin PCO (2013) Contribution of nitrogen from sugarcane harvest residues and urea for crop nutrition. Sci Agric 70:313–320. https://doi.org/10.1590/S0103-90162013000500005
Fracetto FJC, Fracetto GGM, Bertini SCB, Cerri CC, Feigl BJ, Siqueira Neto M (2017) Effect of agricultural management on N2O emissions in the Brazilian sugarcane yield. Soil Biol Biochem 109:205–213. https://doi.org/10.1016/j.soilbio.2017.02.004
Franco HCJ, Otto R, Faroni CE, Vitti AC, Almeida de Oliveira EC, Trivelin PCO (2011) Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. F Crop Res 121:29–41. https://doi.org/10.1016/j.fcr.2010.11.011
Franco HCJ, Otto R, Vitti AC, Faroni CE, de Oliveira ECA, Fortes C, Ferreira DA, Kölln OT, Garside AL, Trivelin PCO (2015) Residual recovery and yield performance of nitrogen fertilizer applied at sugarcane planting. Sci Agric 72:528–534. https://doi.org/10.1590/0103-9016-2015-0170
Ghiberto PJ, Libardi PL, Brito AS, Trivelin PCO (2009) Leaching of nutrients from a sugarcane crop growing on an Ultisol in Brazil. Agric Water Manag 96:1443–1448. https://doi.org/10.1016/j.agwat.2009.04.020
Ghiberto PJ, Libardi PL, Trivelin PCO (2015) Nutrient leaching in an Ultisol cultivated with sugarcane. Agric Water Manag 148:141–149. https://doi.org/10.1016/j.agwat.2014.09.027
Ghosh A, Bhattacharyya R, Meena MC, Dwivedi BS, Singh G, Agnihotri R, Sharma C (2018) Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res 177:134–144. https://doi.org/10.1016/J.STILL.2017.12.006
Giné MF, Bergamin FH, Zagatto EAG, Reis BF (1980) Simultaneous determination of nitrate and nitrite by flow injection analysis. Anal Chim Acta 114:191–197. https://doi.org/10.1016/S0003-2670(01)84290-2
Gonçalves F, Perna R, Lopes E, Maciel R, Tovar L, Lopes M (2021) Strategies to improve the environmental efficiency and the profitability of sugarcane mills. Biomass Bioenerg 148:106052. https://doi.org/10.1016/j.biombioe.2021.106052
Gonzaga LC, Carvalho JLN, de Oliveira BG, Soares JR, Cantarella H (2018) Crop residue removal and nitrification inhibitor application as strategies to mitigate N2O emissions in sugarcane fields. Biomass Bioenerg 119:206–216. https://doi.org/10.1016/j.biombioe.2018.09.015
Gonzaga LC, do Zotelli LC, de Castro SGQ, de Oliveira BG, de Bordonal RO, Cantarella H, Carvalho JLN (2019) Implications of Sugarcane Straw Removal for Soil Greenhouse Gas Emissions in São Paulo State, Brazil. BioEnergy Res 12:843–857. https://doi.org/10.1007/s12155-019-10006-9
Grangeia C, Santos L, Lazaro LLB (2022) The Brazilian biofuel policy (RenovaBio) and its uncertainties: An assessment of technical, socioeconomic and institutional aspects. Energy Convers Manag X 13:100156. https://doi.org/10.1016/j.ecmx.2021.100156
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308. https://doi.org/10.1016/S1360-1385(00)01656-3
Howell TA, Evett SR (2004) The Penman-Monteith method. Section 3 in evapotranspiration: determination of consumptive use in water rights proceedings. Continuing legal education in colorado, Inc. Denver, CO
Jaiswal D, De Souza AP, Larsen S, LeBauer DS, Miguez FE, Sparovek G, Bollero G, Buckeridge MS, Long SP (2017) Brazilian sugarcane ethanol as an expandable green alternative to crude oil use. Nat Clim Chang 7:788–792. https://doi.org/10.1038/nclimate3410
Khan KS, Mack R, Castillo X, Kaiser M, Joergensen RG (2016) Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 271:115–123. https://doi.org/10.1016/j.geoderma.2016.02.019
Kroon FJ, Thorburn P, Schaffelke B, Whitten S (2016) Towards protecting the Great Barrier Reef from land-based pollution. Glob Chang Biol 22:1985–2002. https://doi.org/10.1111/gcb.13262
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. https://doi.org/10.1038/nature04983
Lisboa IP, Cherubin MR, Lima RP, Cerri CC, Satiro LS, Wienhold BJ, Schmer MR, Jin VL, Cerri CEP (2018) Sugarcane straw removal effects on plant growth and stalk yield. Ind Crops Prod 111:794–806. https://doi.org/10.1016/j.indcrop.2017.11.049
Long SP, Karp A, Buckeridge MS, Davis SC, Jaiswal D, Moore PH, Moose SP, Murphy DJ, Onwona-Agyeman S, Vonshak A (2015) Feedstocks for biofuels and bioenergy. In: Souza GM, Victoria RL, Joly CA, Verdade LM (eds) Bioenergy & Sustainability: bridging the gaps. Scientific committee on problems of the environment (SCOPE), São Paulo, pp 302–347. https://bioenfapesp.org/scopebioenergy/images/chapters/bioenergy_sustainability_scope.pdf
Lourenço KS, Cassman NA, Pijl AS, van Veen JA, Cantarella H, Kuramae EE (2018) Nitrosospira sp. govern nitrous oxide emissions in a tropical soil amended with residues of bioenergy crop. Front Microbiol 9:339319. https://doi.org/10.3389/fmicb.2018.00674
Mariano E, Jones DL, Hill PW, Trivelin PCO (2016) Mineral nitrogen forms alter 14C-glucose mineralisation and nitrogen transformations in litter and soil from two sugarcane fields. Appl Soil Ecol 107:154–161. https://doi.org/10.1016/j.apsoil.2016.05.019
Masson-Delmotte V, Zhai P, Pörtner H-O, Roberts D, Skea J, Shukla PR, Pirani A, Moufouma-Okia W, Péan C, Pidcock R, Connors S, Matthews JBR, Chen Y, Zhou X, Gomis MI, Lonnoy E, Maycock T, Tignor M, Waterfield T (2018) Global Warming 1.5°C. An IPCC Special Report on the impacts of global warming 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. IPCC, Geneva. 616 p. https://www.ipcc.ch/site/assets/uploads/sites/2/2019/06/SR15_Full_Report_High_Res.pdf
Oliveira DMS, Pimentel LG, Barreto MSC, Weiler DA, Bayer C (2022) Greenhouse gas emissions and C costs of N release associated with cover crop decomposition are plant specific and depend on soil moisture: A microcosm study. J Environ Qual 51:193–204. https://doi.org/10.1002/jeq2.20330
Otto R, Trivelin PCO, Franco HCJ, Faroni CE, Vitti AC (2009) Root system distribution of sugar cane as related to nitrogen fertilization, evaluated by two methods: monolith and probes. Rev Bras Ciência Do Solo 33:601–611. https://doi.org/10.1590/S0100-06832009000300013
Otto R, Castro SAQ, Mariano E, Castro SGQ, Franco HCJ, Trivelin PCO (2016) Nitrogen Use Efficiency for Sugarcane-Biofuel Production: What Is Next? BioEnergy Res 9:1272–1289. https://doi.org/10.1007/s12155-016-9763-x
Otto R, Mariano E, Mulvaney RL, Khan SA, Boschiero BN, Tenelli S, Trivelin PCO (2019) Effect of previous soil management on sugarcane response to nitrogen fertilization. Sci Agric 76:72–81. https://doi.org/10.1590/1678-992x-2017-0147
Otto R, Ferraz-Almeida R, Sanches GM, Lisboa IP, Cherubin MR (2022) Nitrogen fertilizer consumption and nitrous oxide emissions associated with ethanol production – A national-scale comparison between Brazilian sugarcane and corn in the United States. J Clean Prod 350:131482. https://doi.org/10.1016/j.jclepro.2022.131482
Pan B, Lam SK, Mosier A, Luo Y, Chen D (2016) Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric Ecosyst Environ 232:283–289. https://doi.org/10.1016/j.agee.2016.08.019
Parkin TB, Venterea RT (2010) Chapter 3. Chamber-Based Trace Gas Flux Measurements. In: Follett RF (ed) Sampling Protocols. USDA, Agricultural Research Service, pp 1–39
Peters GP, Andrew RM, Boden T, Canadell JG, Ciais P, Le Quéré C, Marland G, Raupach MR, Wilson C (2013) The challenge to keep global warming below 2 °C. Nat Clim Chang 3:4–6
Pitombo LM, do Carmo JB, de Hollander M, Rossetto R, López MV, Cantarella H, Kuramae EE (2016) Exploring soil microbial 16S rRNA sequence data to increase carbon yield and nitrogen efficiency of a bioenergy crop. GCB Bioenergy 8:867–879. https://doi.org/10.1111/gcbb.12284
Prado RDM, Caione G, Campos CNS (2013) Filter Cake and Vinasse as Fertilizers Contributing to Conservation Agriculture. Appl Environ Soil Sci 2013:1–8. https://doi.org/10.1155/2013/581984
Prosser JI, Martiny JBH (2020) Conceptual challenges in microbial community ecology. Philos Trans R Soc B Biol Sci 375:20190241. https://doi.org/10.1098/rstb.2019.0241
Quassi de Castro SG, Graziano Magalhães PS, Coutinho Junqueira Franco H, Mutton MÂ (2018) Harvesting Systems, Soil Cultivation, and Nitrogen Rate Associated with Sugarcane Yield. BioEnergy Res 11:583–591. https://doi.org/10.1007/s12155-018-9917-0
Quassi de Castro SA, Otto R, Bohórquez Sánchez CE, Tenelli S, Sermarini RA, Trivelin PCO (2021) Sugarcane straw preservation results in limited immobilization and improves crop N-fertilizer recovery. Biomass Bioenerg 144:105889. https://doi.org/10.1016/j.biombioe.2020.105889
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org
Raij B van, Andrade JC, Cantarella H, Tavares S, Quaggio JA (2001) Análise química para avaliação da fertilidade de solos tropicais (in Portuguese). Instituto Agronômico, Campinas
Reis BF, Vieira JA, Krug FJ, Giné MF (1997) Development of a flow injection system with two analytical paths for ammonium determination in soil extracts by conductometry. J Braz Chem Soc 8:523–528. https://doi.org/10.1590/S0103-50531997000500015
Rossi Neto J, de Souza ZM, Kölln OT, Carvalho JLN, Ferreira DA, Castioni GAF, Barbosa LC, de Castro SGQ, Braunbeck OA, Garside AL, Franco HCJ (2018) The Arrangement and Spacing of Sugarcane Planting Influence Root Distribution and Crop Yield. BioEnergy Res 11:291–304. https://doi.org/10.1007/s12155-018-9896-1
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712. https://doi.org/10.1128/aem.63.12.4704-4712.1997
Ruiz Corrêa ST, Barbosa LC, Menandro LMS, Scarpare FV, Reichardt K, de Moraes LO, Hernandes TAD, Franco HCJ, Carvalho JLN (2019) Straw Removal Effects on Soil Water Dynamics, Soil Temperature, and Sugarcane Yield in South-Central Brazil. Bioenergy Res 12:749–763. https://doi.org/10.1007/s12155-019-09981-w
Sanches GM, Otto R (2022) A novel approach for determining nitrogen requirement based on a new agronomic principle—sugarcane as a crop model. Plant Soil 472:29–43. https://doi.org/10.1007/s11104-021-05263-7
Sanches GM, de Paula MTN, Magalhães PSG, Duft DG, Vitti AC, Kolln OT, Borges BMMN, Franco HCJ (2019) Precision production environments for sugarcane fields. Sci Agric 76:10–17. https://doi.org/10.1590/1678-992x-2017-0128
Scarlett K, Denman S, Clark DR, Forster J, Vanguelova E, Brown N, Whitby C (2021) Relationships between nitrogen cycling microbial community abundance and composition reveal the indirect effect of soil pH on oak decline. ISME J 15:623–635. https://doi.org/10.1038/s41396-020-00801-0
Scarpare FV, do Zotelli LC, Barizon R, de Castro SGQ, Bezerra AHF (2023) Leaching Runoff Fraction for Nitrate and Herbicides on Sugarcane Fields: Implications for Grey Water Footprint. Sustainability 15:6990. https://doi.org/10.3390/su15086990
Signor D, Cerri CEP (2013) Nitrous oxide emissions in agricultural soils: a review. Pesqui Agropecuária Trop 43:322–338. https://doi.org/10.1590/S1983-40632013000300014
Skocaj DM, Everingham YL, Schroeder BL (2013) Nitrogen Management Guidelines for Sugarcane Production in Australia: Can These Be Modified for Wet Tropical Conditions Using Seasonal Climate Forecasting? Springer Sci Rev 1:51–71. https://doi.org/10.1007/s40362-013-0004-9
Soares JR, Cantarella H, Vargas VP, Carmo JB, Martins AA, Sousa RM, Andrade CA (2015) Enhanced-Efficiency Fertilizers in Nitrous Oxide Emissions from Urea Applied to Sugarcane. J Environ Qual 44:423–430. https://doi.org/10.2134/jeq2014.02.0096
Soares JR, Cassman NA, Kielak AM, Pijl A, Carmo JB, Lourenço KS, Laanbroek HJ, Cantarella H, Kuramae EE (2016) Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci Rep 6:30349. https://doi.org/10.1038/srep30349
Soil Survey Staff (2014) Keys to soil taxonomy, 12th edn. USDA-NRCS, Washington, DC
Thorburn PJ, Biggs JS, Attard SJ, Kemei J (2011) Environmental impacts of irrigated sugarcane production: Nitrogen lost through runoff and leaching. Agric Ecosyst Environ 144:1–12. https://doi.org/10.1016/j.agee.2011.08.003
Thorburn PJ, Biggs JS, Palmer J, Meier EA, Verburg K, Skocaj DM (2017) Prioritizing Crop Management to Increase Nitrogen Use Efficiency in Australian Sugarcane Crops. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.01504
Trivelin PCO, Lara Cabezas WAR, Victoria RL, Reichardt K (1994) Evaluation of a 15N plot design for estimating plant recovery of fertilizer nitrogen applied to sugar cane. Sci Agric 51:226–234. https://doi.org/10.1590/S0103-90161994000200005
Trivelin PCO, Franco HCJ, Otto R, Ferreira DA, Vitti AC, Fortes C, Faroni CE, Oliveira ECA, Cantarella H (2013) Impact of sugarcane trash on fertilizer requirements for São Paulo, Brazil. Sci Agric 70:345–352. https://doi.org/10.1590/S0103-90162013000500009
Vasconcelos ALS, Cherubin MR, Cerri CEP, Feigl BJ, Borja Reis AF, Siqueira-Neto M (2022) Sugarcane residue and N-fertilization effects on soil GHG emissions in south-central. Brazil Biomass and Bioenergy 158:106342. https://doi.org/10.1016/j.biombioe.2022.106342
Waclawovsky AJ, Sato PM, Lembke CG, Moore PH, Souza GM (2010) Sugarcane for bioenergy production: An assessment of yield and regulation of sucrose content. Plant Biotechnol J 8:263–276
Wallenstein MD, Vilgalys RJ (2005) Quantitative analyses of nitrogen cycling genes in soils. Pedobiologia (jena) 49:665–672. https://doi.org/10.1016/j.pedobi.2005.05.005
Wang C, Zhou X, Guo D, Zhao J, Yan L, Feng G, Gao Q, Yu H, Zhao L (2019) Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann Microbiol 69:1461–1473. https://doi.org/10.1007/s13213-019-01529-9
Whittaker EJ, Robinson G (1967) The Calculus of Observations: an introduction to numerical analysis, 4th edn. Dover Publications, New York
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679. https://doi.org/10.1002/bit.20347
Zhong Y, Yan W, Canisares LP, Wang S, Brodie EL (2023) Alterations in soil pH emerge as a key driver of the impact of global change on soil microbial nitrogen cycling: Evidence from a global meta-analysis. Glob Ecol Biogeogr 32:145–165. https://doi.org/10.1111/geb.13616
Acknowledgements
The authors thank the São Paulo Research Foundation (FAPESP) for the financial support provided for this project (grant #2017/13619-0) and for granted PhD scholarship to SAQC (grant #2017/25489-3). We are also very thankful to Center of Isotopes of the São Paulo State University (CIE/UNESP) for the support granted to our research in isotopic measurement and analysis. Our special thanks to the Brazilian Biorenewables National Laboratory (LNBR/CNPEM) for the structural and operational support for this project.
Funding
Open access funding provided by Copenhagen University. This research was funded by São Paulo Research Foundation (FAPESP), grant #2017/13619–0 to SGQC. SAQC received Ph.D. scholarship from FAPESP, grant #2017/25489–3.
Author information
Authors and Affiliations
Contributions
Writing-original draft: SGQC, SAQC, JLNC and RAC. Data curation: SGQC, VEC, and CDB. Writing, revision and methodology: VEC, SAQC, JLNC, and CDB. Formal analysis: SGQC, OTK, and RAC. Project administration: SGQC and HCJF. Resources: SGQC, VEC and HCJF. All authors contributed to the article and approved the final manuscript version.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflicts of Interest
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quassi de Castro, S.G., Costa, V.E., Quassi de Castro, S.A. et al. Fertilizer Application Method Provides an Environmental-Friendly Nitrogen Management Option for Sugarcane. J Soil Sci Plant Nutr 24, 3195–3208 (2024). https://doi.org/10.1007/s42729-024-01744-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-024-01744-7