Abstract
Melatonin was found in plants in the late 1990s, but its function, signaling, and interaction with other phytohormones still unclear. Melatonin research in plants has increased substantially in recent years, including reports on the impact of this putative plant hormone under biotic and abiotic stress situations. Temperature extremes, salt, drought, hypoxia or anoxia, nutrient deficiency, herbicides, UV radiation stress, and heavy metal toxicity are all important obstacles to horticulture crop production worldwide. To deal with these environmental challenges, plants have evolved complex signaling networks. Phytohormones are essential for controlling plant growth, development, and stress responses. Melatonin, a pleiotropic chemical present in a variety of species, has recently emerged as a powerful regulator of plant abiotic stress tolerance. The purpose of this review is to investigate the interplay between melatonin and phytohormones in the control of abiotic stress responses in horticultural crops. We explore the interactions of melatonin with several phytohormones under various abiotic stresses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The growth, development, and yield (quality and quantity) of various crops are greatly influenced by abiotic stresses such as extreme temperatures (high or low temperature), drought, salinity, UV, nutrient deficiency, and heavy metal toxicity (Munns 2003; Saudy 2014; Annacondia et al. 2018; He et al. 2018; Saudy et al. 2021a, 2023a; El-Bially et al. 2022a; El-Metwally et al. 2022a; El-Yazied et al.2022; Nasser et al.2022; Shaaban et al. 2023a; Shahin et al. 2023). Similar adverse impacts on crop plants were reported under biotic stresses (Saudy 2015; Saudy and Mubarak 2015; Saudy et al. 2020a, 2021b; Abou El-Enin et al. 2023). Abiotic stress leads to an increase in free radicals within plant cells, where about 1–2% of consumed oxygen is transformed into reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide radical (O2•–), hydrogen peroxide (H2O2), hydroxyl radical (•OH), etc., as byproducts of aerobic metabolism, which make them highly vulnerable, dramatically disturbing plant metabolism and having negative impacts on crop productivity (Sachdev et al 2021; Abd El-Mageed et al. 2022; Hadid et al. 2023; Saudy et al. 2023b). It has been proved that these stressors affect normal plant growth and development, resulting in lower agricultural yields and lower quality (El-Metwally and Saudy 2021a; Saudy et al. 2021c, 2021d, 2022; Shabbir et al 2022; El-Bially et al. 2023). In response to these adverse environmental conditions, plants have developed sophisticated signaling networks to adapt and thrive; the regulatory network comprising enzymatic and non-enzymatic antioxidant systems tends to keep the magnitude of ROS within plant cells to a non-damaging level (Shahzad et al. 2019; Sharma et al. 2019b; Demirel et al. 2020; Sachdev et al 2021; El-Metwally et al. 2022c). Plant growth, development, and stress responses are intricately regulated by a range of phytohormones such as auxins (Zhu et al. 2013), gibberellic acid (GA) (Khan et al. 2015), abscisic acid (ABA) (Leubner-metzger 2012), salicylic acid (SA) (Horváth et al. 2007), jasmonic acid (JA) (Hashem and El-sherif 2019), ethylene (ET) (Sharma et al. 2019a), and cytokinins (CKs) (O’Brien and Benková 2013). Plant hormones assume vital roles in orchestrating the various aspects of plant life and their adaptive responses to stress (Saudy et al. 2020b; El-Metwally et al. 2022b; El-Sayed et al. 2022; Rizk et al. 2023; Swain et al. 2023). In recent times, melatonin, a multifaceted molecule primarily recognized for its role in animal physiology, has emerged as a potent and influential regulator of abiotic stress tolerance in the realm of plant biology (Altaf et al. 2023; Pan et al. 2023; Tiwari et al. 2022).
Melatonin (N-acetyl-5-methoxytryptamine) synthesis in plants can be stimulated by a range of factors, including light, temperature fluctuations, and UV radiation (Pan et al. 2023). Melatonin was discovered first in grapes and subsequently in olive oil. The highest melatonin concentrations were found in the berry exocarp (skin) of different Italian and French wine grape cultivars grown in northwestern Italy (0.9 and 0.8 ng g−1), while the lowest concentration was found in the Cabernet Franc cultivar (0.005 ng g−1) (Iriti et al 2006). In human, melatonin, a natural hormone secreted by the pineal gland, plays a role in the sleep–wake cycle and is generally safe for short-term use. It may help treat sleep disorders like delayed sleep phase and insomnia and may reduce response after repeated use (Iriti et al 2006). Owing to the intensive intake of melatonin-rich diets, cardiovascular health related to human nutrition improved (Meng et al. 2017). Since melatonin reduces the oxidation of molecules associated inflammation, it has anti-inflammatory potential, thus supplying food containing distinctive amount of melatonin is beneficial for reducing the harms of free radicals (Pandi-Perumal et al. 2006; Mauriz et al. 2013). Additionally, it has been documented that melatonin has antiestrogenic potential via reducing hormone-linked cancers (Sánchez-Barceló et al. 2005). Melatonin is currently recognized as an important compound in a variety of physiological processes in plants, such as seed germination (Liu et al. 2022b), root growth (Wang et al. 2022b), stomatal control (Jensen et al. 2023), and stress responses (Pan et al. 2023). Melatonin functions as a signaling molecule, regulating the growth and development of plants as well as their reactions to environmental challenges (Altaf et al. 2021a, 2021b; Jensen et al. 2023; Tiwari et al. 2022). In the horticulture sector, where the growing of crops under a variety of environmental circumstances is crucial for sustainable agriculture, the effects of melatonin on stress tolerance have particularly attracted attention.
2 Melatonin General Mechanism of Stress Mitigation
Recently, researches have focused on elucidating the complex interactions between phytohormones and melatonin in the control of abiotic stress responses in horticultural crops (Jensen et al. 2023; Wang, et al. 2022b). Melatonin lowered the levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Altaf et al. 2021a, 2021b; Peng et al. 2023). Melatonin had the potential to stabilize membrane systems (Tan et al. 2023) under exposure to harmful conditions like high temperatures, salinity, drought, chilling, and heavy metal toxicity (Altaf et al. 2023; Hasan et al. 2023; Kaya et al. 2023; Korkmaz et al. 2021; Tan et al. 2023). In these situations, melatonin can directly increase antioxidant enzyme activity and indirectly promote the gene expression of stress response and antioxidative systems under stress conditions. Melatonin induces the production of antioxidant enzymes, such as CAT, POD, and SOD, which help to detoxify excess hydrogen peroxide and maintain redox homeostasis. Melatonin also induces the expression of genes related to antioxidant enzymes (Sun et al. 2021). Melatonin can reduce the harms caused by salt stress via increasing the activity of antioxidant enzymes and removing hydrogen peroxide in plants (Pan et al. 2023; Zhang et al. 2021). Nevertheless, melatonin increases the production of respiratory burst oxidase homologs (RBOH), which generate superoxide radicals (O2•−), which in turn increase hydrogen peroxide (H2O2) levels (Arnao and Hernández-Ruiz 2021). Melatonin signaling activates pathways that respond to abiotic and biotic stresses, including ROS- and RNS-mediated pathways (Altaf et al. 2021a, 2021b; Peng et al. 2023). Moreover, melatonin regulates various antioxidant pathways, such as the AsA-GSH cycle in tomatoes (Xu et al. 2023). Melatonin can help plants to remove harmful levels of reactive oxygen species by regulating the hydrogen peroxide-mediated signaling pathway (Altaf et al. 2021b; Peng et al. 2023). Melatonin turns on and off genes that are involved in helping plants respond to stress. As well, melatonin turns on the genes for HSFA2, GhNHX1, GhSOS1, GhAKT1, and HSP90, which help tomato seedlings survive hot temperatures (Shen et al 2021; Jannatizadeh et al 2019). Melatonin controls the expression of many genes that are involved in making and breaking down the plant hormones IAA, GA, CK, ABA, ET, JA, SA, and BR. This suggests that melatonin may interact with these other hormones in complex ways (Arnao and Hernández-Ruiz 2021). Melatonin and IAA are similar in structure. Melatonin also turns on genes that are involved in auxin signaling and transport, which leads to the formation of new roots in tomato plants (Wen et al. 2016).
The interaction between phytohormones and melatonin has important ramifications for the creation of techniques to increase crop tolerance to abiotic challenges (Jensen et al. 2023). Understanding the principles underlying their interaction might help to develop creative and sustainable strategies to lessen the negative impacts of environmental difficulties on the growth of horticultural crops (Arnao and Hernández-Ruiz 2021; He et al. 2020; Sun et al. 2021; Wei et al. 2020).
This review investigates the regulation of abiotic stressors in horticultural crops by melatonin in conjunction with phytohormones. The possible effects of melatonin on mitigating the abiotic stresses on some horticulture crops are illustrated in Table 1. It draws attention to possible gains in crop stress resistance and output. Recent developments in the use of phytohormones and exogenous melatonin improve plant resistance to abiotic stressors, with particular emphasis on the practical implications for horticultural techniques. In addition, this brings together previous research on melatonin’s relationship to phytohormones, emphasizing how it controls abiotic stressors on horticultural crops. It seeks to stimulate research and create cutting-edge approaches for resilient and sustainable agricultural production in the face of global environmental issues. The probable functions of melatonin to alleviate the stresses in plants are summarized in Fig. 1 and will be explained later in this review.
3 Melatonin Biosynthesis and Degradation in Plants
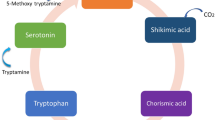
Briefly, in plants, melatonin is formed enzymatically from tryptophan (Back et al. 2016). As shown in Fig. 2, several enzymes catalyze tryptophan, tryptamine, serotonin, and 5-methoxytryptamine, and eventually, melatonin is formed. Contrariwise, melatonin could be decomposed via enzymatic and non-enzymatic fate. Plants could enzymatically stimulate the hydroxylation of melatonin, as proven by the synthesis of 2-, 4-, and 6-hydroxymelatonin (Hardeland 2015; Tan and Reiter 2020). Also, non-enzymatic degradation of melatonin occurs through hydroxylation and deoxygenation mechanisms (Tan et al. 2002). It has been documented that melatonin is non-enzymatically degraded by ROS and photolitically by UV light (Hardeland et al. 2009).
4 Melatonin Impact on Abiotic Stress
Melatonin is a plant-wide abiotic stress regulator (Pan et al. 2023; Tiwari et al. 2022). Plants are vulnerable to several environmental stresses throughout their lives. Plants have evolved several coping strategies to deal with a variety of stressful environments while preserving their survival and capacity to reproduce (Araújo et al. 2014; El-Metwally and Saudy 2021b; Zubair et al. 2022). Melatonin administered exogenously improves plant tolerance to abiotic stressors (Tiwari et al. 2022). Now, we will review how melatonin interacts with various abiotic stresses (high or low temperature, drought, salinity, UV radiation, and heavy metal toxicity) in horticultural crops.
4.1 Melatonin Impact on Extreme Temperatures
High temperatures have negative impacts on plants, such as speeding leaf senescence, creating physiological and metabolic imbalances, exacerbating plant oxidative damage, and decreasing photosynthetic efficiency (Hatfield and Prueger 2015; Hu et al. 2023). These consequences result in significantly reduced plant growth, hence poor plant yields. At high temperatures, exogenous melatonin on carnation (Dianthus caryophyllus) enhanced biomass, damage from oxidation, and osmotic accumulation, increased photosystem II efficiency, encouraged stomatal opening, and decreased chloroplast injury by upregulating the expression of heat tolerance genes (Hu et al. 2023). In tomato, melatonin significantly maintained photosynthetic systems under heat stress by balancing electron transfer, regulating phosphorylated and redox proteins, and adjusting antioxidant enzyme activity and ROS homeostasis (Sun et al. 2022).
Also, low-temperature stress is one of the most difficult issues with tropical plants development and growth. Low temperatures produce a variety of physiological, molecular, and metabolic changes in plants (Raza et al. 2023). One of the negative impacts of cold is that it causes cell membrane damage, resulting in reduced membrane fluidity and altered ion homeostasis in plants (Ding et al. 2017; Pan et al. 2023; Raza et al. 2023). Melatonin boosts photosynthetic carbon fixation in cold-stressed tomato plants, improves antioxidant capacity, increases expression of cold-responsive genes, increases metabolite accumulation in cold-stressed plants, and protects tomato plants from cold-induced harm (Ding et al. 2017). Postharvest exogenous melatonin treatment reduced chilling damage, weight loss rate, and was effective for retarding senescence in grape and cucumber during the storage by increasing the activity of peroxidase and catalase enzyme, thereby reducing O2•− and H2O2 rates in the tissue (Nasser et al. 2022; Liu et al. 2022a).
4.2 Melatonin Impact on Drought
Drought is a global environmental challenge, negatively impacting plant growth and agricultural productivity. It reduces membrane hydration, damages proteins, and accumulates reactive oxygen species, damaging chloroplasts and mitochondria, causing cellular degeneration (El-Metwally et al. 2021; Doklega et al. 2023; Ramadan et al. 2023a, b; Shaaban et al. 2023b). Furthermore, under low water supply, the potential of crop plants to absorb water and nutrients dramatically reduced (Saudy and El-Metwally 2019, 2023; Makhlouf et al. 2022). Accordingly, drought could generate nutrient deficiency (Salem et al. 2022) which dramatically injures plant growth (Noureldin et al. 2013; Saudy et al. 2018, 2020c; Ali et al. 2023). Therefore, deficit water significantly decreased yield and quality (El-Bially et al. 2018; Abd–Elrahman et al. 2022; El-Bially et al. 2022b). Melatonin lessens drought stress in kiwifruit seedlings (Xia et al. 2020). Treatment of melatonin to wine grapes leads to reducing drought stress (Meng et al. 2014). Melatonin pretreatment improved seedling development, root properties, leaf photosynthesis, and antioxidant activity, enhancing tomato seedling adaptation to drought stress (Altaf et al. 2022b).
4.3 Melatonin Impact on Salinity
Salinity is a soil condition characterized by a high concentration of soluble salts such as NaCl, the soil’s most abundant and soluble salt (Munns and Tester 2008). Salinity stress has a negative influence on agricultural productivity all over the world, with a bigger impact in arid and semi-arid regions (Shahid et al. 2018). Salinity produces ionic imbalance both outside and inside the plant. First, soil salinity lowers soil water potential, resulting in a fall in water level in plants, and hence decreases in cell division, plant development, and productivity (Mubarak et al. 2021; Salem et al. 2021; Lasheen et al. 2023). The high concentration of Cl− and Na+ ions inside the cells, along with a low K+/Na+ ratio, impairs plant enzyme and membrane function, which induces cell dryness, stomata closing, and a reduction in CO2 levels inside the photosynthetic cell (Gupta and Huang 2014; Munns and Tester 2008). Melatonin improves salinity stress–induced damage to snap bean plants by increasing proline, sugar content, and enhancing antioxidant enzyme activity (Azizi et al. 2022). Melatonin pretreatment enhanced salinity tolerance of tomato seedlings (Altaf et al. 2020). Strawberry fruit production and quality were lowered by salinity stress. Foliar melatonin reduced fruit output and quality while increasing leaf antioxidant systems and abscisic acid levels induced by salt (Zahedi et al. 2020).
4.4 Melatonin Impact on UV
Ultraviolet radiation is a part of the nonionizing radiation area of the electromagnetic spectrum and accounts for approximately 9% of solar radiation. As reported by Aboul Fotouh et al. (2014), UV is classified into three categories, i.e. UV-C (200–280 nm), UV-B (280–315 nm), and UV-A (315–400 nm). High-intensity UV exposure causes stress, suppressing photosystem II, electron transport, photosynthesis, and affecting biomass accumulation, partitioning as well as cell development, abnormal growth, and yield loss in plants (Wei et al. 2019). In apples, melatonin treatment reduced plant growth and biomass output, hindered root system development, and eased the inhibitory effects of UV-B radiation on photosynthetic systems, which reduced chlorophyll levels, stomatal apertures, and leaf membrane damage (Wei et al. 2019).
4.5 Melatonin Impact on Heavy Metal Toxicity
Heavy metals like cadmium (Cd), chromium (Cr), nickel (Ni), and vanadium (V) in growth medium disrupt key physiological processes, negatively affecting plants’ life cycle from germination to production. Higher concentrations of heavy metal hinder nutrient absorption and transport, disrupting metabolic processes and affecting growth, development, and yield (Ghori et al. 2019). Melatonin boosted seedling development and leaf photosynthesis, inhibited heavy metal transfer from root to shoot, adjusted root architecture and mineral intake in pepper seedlings, and decreased oxidative damage by modulating antioxidant enzymes under heavy metal toxicity (Altaf et al. 2023). Cd-induced phytotoxicity in tomato was reduced by increasing biomass production, root system, and macro and micronutrient intake while lowering Cd uptake in tomato due to melatonin supply (Altaf et al. 2022a). Exogenous melatonin reduces Cd toxicity in Chinese cabbage (Wang et al. 2021b).
5 Crosstalk Between Melatonin and Phytohormones
Researchers have described the interactions of melatonin with other phytohormones. Because of their chemical similarities, auxin (IAA) was the primary focus of research on the interaction of melatonin and other plant hormones (Arnao and Hernández-Ruiz 2018; Yang et al. 2021). Numerous investigations, however, have discovered fascinating links between melatonin and almost all known plant hormones, including JA, SA, brassinosteroids, polyamines, strigolactones, plant peptide hormones, and nitric oxide, gibberellin, cytokinins, ethylene, and ABA (Arnao and Hernández-Ruiz 2018; Murch and Erland 2021; Yang et al. 2021, 2022a; Wang et al. 2022c; El-Beltagi et al. 2023). By surveying the relationship between melatonin and various plant hormones, which will be explained later in this article, we can illustrate their possible interactions in Fig. 3.
5.1 Melatonin and Auxin Crosstalk
IAA is the primary auxin in plants, controlling growth and development processes such as cell division and elongation, tissue differentiation, apical dominance, and responses to light, gravity, and pathogens (Fu et al. 2015). Melatonin stimulates growth in the same manner as auxin and indolyl-3-acetic acid (IAA) do (Hernández-Ruiz et al. 2004; Arnao and Hernández-Ruiz 2006).
Regarding the regulation of auxin biosynthesis and metabolism, both melatonin and auxin have the same precursor, tryptophan, and can be converted into IAA or IAA agonists. Melatonin, when applied exogenously, promotes root development in a manner similar to IAA (Wen et al. 2016). Melatonin therapy increases mustard and tomato seedling development and influences IAA and IBA biosynthesis. Melatonin treatment at 50 M has been demonstrated to increase the expression of auxin signal-transduction genes (IAA19 and IAA24) as well as auxin efflux genes (PIN1, PIN3, and PIN7), which promote the formation of adventitious roots in tomato (Chen et al. 2009; Wen et al. 2016). On the other hand, melatonin treatment at 600 M reduces auxin production levels and transport routes, as demonstrated by lower expression of YUC1, YUC2, YUC5, YUC6, TAA1, TAR2, PIN1, PIN3, and PIN7. Melatonin at concentrations ranging from 10 to 20 mM increases the expression of Os01g08320 (OsIAA1), Os02g56120 (OsIAA9), Os02g57250 (OsIAA10), Os06g07040 (OsIAA20), and Os011g11410 (OsIAA27) (Chen et al. 2009; Wen et al. 2016). These data imply that melatonin mimics IAA in plants at low doses (Chen et al. 2009; Wen et al. 2016).
To see if there was an interaction between melatonin and IAA, researchers looked at the expression of genes involved in IAA production and signal transduction. Melatonin increased the expression of MdYUCCA1, MdYUCCA10, MdARF7, and MdARF19. Melatonin also increased the expression of the IAA transport-related genes MdAUX1, MdPIN1, and MdPIN3 (Mao et al. 2020). Concerning the relation between IAA and melatonin in mitigating the stresses, exogenous IAA addition can reduce the toxic effect of Cd in Cinnamomum camphora leaves via increasing total chlorophyll and carotenoid content; decreasing proline, soluble sugar, and MDA content; and improving photosynthetic performance (Zhou et al. 2020). Exogenous IAA could lessen Cd adverse effects in tea via reduction its accumulation (Zhang et al. 2020). Mitigation of copper toxicity in germinating seeds of pea using IAA was observed (Ben Massoud et al. 2017). Using IAA boosted yield of broad beans in high salinity conditions (Latef et al. 2021). In this connection, melatonin treatment raised the accumulation of IAA and crosstalk happened between melatonin and IAA during adventitious root formation in apple (Mao et al. 2020). Melatonin could alleviate cold tolerance via IAA accumulation in watermelon (Li et al. 2017).
5.2 Melatonin and ABA Crosstalk
ABA is a vital phytohormone involved in several physiological processes, such as stomatal closure, reacting to stress, and seed dormancy (Liao et al. 2023; Ortiz-García et al. 2023; Seo and Koshiba 2002). In addition, ABA is known as the “universal stress hormone” because it simultaneously regulates plant growth and development and governs the production of a number of defensive metabolites that help plants fight off the negative effects of stressful environments (Singh et al. 2022). ABA played critical role in plants’ ability to mitigate the detrimental effects of abiotic stress through the regulation of gene expression, stomatal closure, production of protective metabolites, and other adaptive biochemical processes (Parwez et al. 2022). Plant responses to abiotic stressors are modulated by melatonin’s effects on ABA levels and signaling pathways. The crosstalk between melatonin and ABA plays a meaningful position in the regulation of abiotic stress responses in horticulture crops (Jensen et al. 2023). Here, we go through the workings of melatonin-ABA crosstalk in horticultural crops.
Exogenous melatonin treatment reinforces the expression of key ABA biosynthesis genes like 9-cis-epoxycarotenoid dioxygenase (Banerjee and Roychoudhury 2019; Fu et al. 2016; Singh et al. 2022). According to prior research, endogenous melatonin production and the expression of the EnCOR14a and EnCBFs genes were both dramatically elevated in response to cold stress (Fu et al. 2016). This shows that both ABA-dependent and ABA-independent mechanisms are involved in melatonin’s ability to promote cold tolerance (Fu et al. 2016). Melatonin can also control ABA metabolism by moderating the activity of enzymes that break down ABA, including ABA 8′-hydroxylase in watermelon (Li et al. 2020). The total ABA levels in plants are influenced by melatonin’s control of ABA production and metabolism.
Each of melatonin and ABA has the potential to control ROS scavenging and antioxidant defense mechanisms (Fu et al. 2016). ABA stimulates the genes that produce antioxidant enzymes, which improves the detoxification of ROS. There is evidence that the signal molecule of ABA can cause the expression of genes, start a signal transduction pathway, and prepare plants to respond to stress (Fujita et al. 2011; Nakashima et al. 2006; Xu et al. 2022). According to earlier research on pea, ABA enhanced the amount of ROS and the activity of antioxidant enzymes against oxidative stress, and calcium dioxide and ROS crosstalk is crucial to the ABA-induced antioxidant defense (Xiong et al. 2006). Like other powerful antioxidants, melatonin may directly scavenge ROS (Li et al. 2015). Melatonin can boost ABA-induced antioxidant enzyme activities, lower ROS levels, and lessen oxidative stress in horticultural crops that have been subjected to abiotic stressors, according to studies in apple (He et al. 2020; Li et al. 2020), cassava (Wei et al. 2020), pepper (Altaf et al. 2023; Kaya et al. 2023), tomato (Hasan et al. 2023; Peng et al. 2023), cucumber (Zhang et al. 2014), and strawberry (Hayat et al. 2022).
Under adverse circumstances, ABA prevents germination and encourages seed dormancy maintenance (Fujita et al. 2011). Melatonin could influence ABA-mediated seed germination and dormancy (Zhang et al. 2014). Melatonin controls seed germination by favorably upregulating ABA catabolism and GA biosynthesis. To the best of our knowledge, the cucumber research explains how melatonin reduces the inhibitory effect of salt stress on cucumber germination (Zhang et al. 2014). According to some reports, melatonin therapy can induce seed dormancy by lowering ABA concentrations and changing the expression of genes that respond to ABA (Zhang et al. 2022, 2014). Under salt stress, melatonin down-regulated ABA biosynthesis genes (e.g., CsNECD2) in cucumber (Zhang et al. 2014). Melatonin-treated seeds had high levels GA, low levels of ABA, and high levels of amylase and alpha-amylase activity during germination. The ABA 8-hydroxylase genes (LbCYP707A1 and LbCYP707A2), which mediate the changes in GA and ABA levels in seeds during germination, were upregulated by melatonin treatment, as were key genes involved in GA biosynthesis (GA20ox and GA3ox). Key genes involved in ABA biosynthesis (LbNCED1 and LbNCED3) were downregulated by melatonin treatment. Melatonin in seeds encourages food uptake and protein synthesis to speed up seed germination in sea-lavender (Li et al. 2019b). Under stress, melatonin and ABA’s interplay in seed dormancy control aids in the effective germination and establishment of horticultural crops such as cucumber (Zhang et al. 2022), sea-lavender (Li et al. 2019b), tomato (Cai et al. 2022), and almond (García-Sánchez et al. 2022).
Stomatal closure is a crucial adaptive response of plants to reduce water loss under abiotic stress conditions. Melatonin and ABA interact to control stomatal movements (Li et al. 2015; Yang et al. 2022b). Previous studies pointed out that melatonin may contribute in stomatal movement by regulating the ABA (Yang et al. 2022b). Another work presented that melatonin be capable of control stomatal movement in response to stress through interacting with the phytohormone ABA (Wang et al. 2023). Melatonin encouraged stoma closure by regulating the K+ channel (Wang et al. 2023). Under stressful circumstances, melatonin-ABA interaction leads to increased water usage effectiveness and decreased water loss (Li et al. 2020; Yang et al. 2022a).
Melatonin and ABA act together in the adjustment of stress-responsive genes implicated in abiotic stress tolerance. Stress-related genes, such as those that code for the late embryogenesis abundant proteins in watermelon (Li et al. 2019a), osmo-protectants in tomato (Mushtaq et al. 2022), and stress-responsive transcription factors can be induced by both melatonin and ABA (Alam et al. 2018; Wang et al. 2021b). Melatonin has been found to increase ABA-induced gene expression, which improves ABA-induced stress tolerance in horticultural crops such as in grape (Xu et al. 2018) and tomato (Jahan et al. 2021).
5.3 Melatonin and SA Crosstalk
Since SA is involved in both plant immunity and defense mechanisms against pathogens and abiotic stressors (Nejat and Mantri 2017; Prakash et al. 2021). On the other hand, melatonin has been proven to be a powerful antioxidant and a regulator of plant stress responses (Pan et al. 2023; Tiwari et al. 2022). An essential component of plant defense mechanisms and stress tolerance in horticultural crops is the interaction between the melatonin and SA.
Melatonin can affect signaling and SA biosynthesis pathways (Esmaeili et al. 2023). Studies have revealed that melatonin can increase the expression of crucial genes involved in SA biosynthesis, such as isochorismate synthase and phenylalanine ammonia-lyase (Pérez-Llorca et al. 2019). Chorismate can be changed into isochorismate, and that compound can then be changed into SA when isochorismate synthase is activated by melatonin (Pérez-Llorca et al. 2019). In kiwi, due to the upregulation of the expression of SA-responsive phenylalanine ammonia-lyase (PAL), melatonin pretreatment enhanced endogenous SA production primarily via the PAL pathway, activating the kiwifruit defensive response to chilling stress (Guo et al. 2023). In another study on peach, melatonin pretreatment stimulated PAL activity, which in turn increased endogenous SA content, helping to counterbalance the ratio of unsaturated to saturated fatty acids in peach fruit and reducing membrane lipid peroxidation (Guo et al. 2023). Cherry tomato fruit may be more resistant to Botrytis cinerea infection via exogenous melatonin. Disease resistance to Botrytis cinerea caused by melatonin is reliant on SA buildup and the SA signaling system (Li et al. 2022). Melatonin can also modify SA signaling by influencing the expression of genes that are SA sensitive and interacting with SA receptors (Guo et al. 2023).
Melatonin and SA are capable to induce the expression of defense-related genes implicated in plant immunity(Guo et al. 2023; Li et al. 2022; Nejat and Mantri 2017). Melatonin has been demonstrated to increase the expression of genes producing pathogenesis-related (PR) proteins, which are important in plant defense against pathogens (Guo et al. 2022; Li et al. 2022). Melatonin treatment significantly inhibited gray mold development, induced ROS burst, increased SA accumulation accompanied by increased activity of its synthesis-related enzymes, upregulated expression of defense genes like SlWRKY70, SlTGA5, SlPR1, SlPR2, SlNPR1, and SlGLU, but decreased nitric oxide (NO) content during storage in cherry tomato. Furthermore, melatonin therapy boosted endogenous melatonin concentration in fruit during storage by upregulating the expressions of SlTDC, SlSNAT, and SlASMT (Li et al. 2022). The interaction between melatonin and SA results in a synergistic influence on the activation of defense-related genes, indicating increased resistance of horticulture plants against biotic and abiotic stresses.
Melatonin and SA are regarded as antioxidants that can affect the efficacy of plant antioxidant defense systems (Pieterse and Van Loon 1999; Yang et al. 2022b). Both compounds can scavenge ROS and reduce oxidative stress (Horváth et al. 2007; Pan et al. 2023). Melatonin has been demonstrated to boost the activity of antioxidant enzymes including superoxide dismutase (SOD) and catalase (CAT), as well as the accumulation of non-enzymatic antioxidants like glutathione and ascorbate. Melatonin caused a burst of ROS, elevated endogenous melatonin and SA, and boosted chitinase (CHI) and 1,3-glucanase (GLU) activity in tomatoes. Furthermore, the treatment altered the phenylpropanoid pathway by boosting the activities of PAL, 4-coumarate-coenzyme A ligase (4CL), and peroxidase (POD) in tomato, which was followed by greater levels of lignin, flavonoids, and total phenols (Li et al. 2019c). Melatonin-treated tomato seedlings show improved pigment content, antioxidant enzyme activity, and reduced malondialdehyde (MDA) levels (Debnath et al. 2020). To scavenge ROS and prevent membrane peroxidation, the antioxidant enzymes SOD, POD, CAT, APX, and GR are required. The activity of these enzymes increased when supplemented with single and combination melatonin and Trichoderma treatments in a pak choi research. Furthermore, the use of melatonin and Trichoderma inhibits the production of H2O2 in roots, which is consistent with an increase in CAT activity. The treatment of melatonin and Trichoderma controls pak choi clubroot disease by enhancing enzymatic activities (Gulzar et al. 2023). Melatonin increases the contents of ascorbic acid (AsA) and glutathione (GSH) in tomato (Xu et al. 2023). SA also stimulates the production of antioxidant enzymes and increases plant antioxidant capacity (Horváth et al. 2007; Pieterse and Van Loon 1999; Srivastava and Dwivedi 1998). SA application increased the content of SOD, CAT, APX, AsA, GSH, and DPPH scavenging capacity, but inhibited the accumulation of ROS and MDA in longans (Zhang et al. 2023). Thus, melatonin and SA interaction in regulating antioxidant defense systems influences the mitigation of oxidative injury in stress conditions.
Priming is the process by which plants are prepared to generate a quicker and stronger defensive response in the event of future stress exposure (Rakshit and Singh 2018). Melatonin and SA treatments are able to prime plants, resulting in greater defensive response and stress tolerance (Alam et al. 2022; Rajora et al. 2022). Seed priming with SA showed better performance, especially to severe soil moisture-deficit conditions. This is due to SA’s external supplementation maintaining high chlorophyll content (SPAD value), membrane stability index, osmotic potential, and reducing electrolyte leakage in cantaloupe (Alam et al. 2022). Melatonin priming (5, 10, 25 uM) was administered to leek seeds, and germination was examined at optimal (21 °C), chilling stress (7 °C), and high temperature (35 °C). Treatment with 5 M melatonin increased seedling resistance and germination in chilling stress (Jannatizadeh et al. 2019). The findings from the sweet corn study suggested that melatonin priming might increase sweet corn tolerance to oxidative stress by increasing antioxidant enzyme activity and decreasing herbicide harm (Fathi et al. 2023).
5.4 Melatonin and JA Crosstalk
Melatonin-jasmonic acid (JA) interaction is an important component of plant defense and stress responses in horticultural plants. JA is an important phytohormone that plays a role in a variety of physiological processes, including plant growth, development, and defense against biotic stressors (Wang et al. 2020a). The mechanisms and consequences of melatonin-JA crosstalk in the modulation of abiotic stressors in horticultural crops are discussed here.
A study on litchi fruit supports the effect of melatonin on jasmonic acid. Melatonin therapy has been demonstrated in studies to increase the expression of essential genes involved in JA production, like lipoxygenases (LOX) in litchi fruit (Wang et al. 2020b) and allene oxide synthase (AOS) in blueberries (Qu et al. 2022). Adjustment of JA biosynthesis and metabolism via melatonin contributes to the activation of defense responses against abiotic stresses. JA plays as a signaling molecule through binding to its receptor, coronatine-insensitive protein 1 (COI1), indicating to degradation of JASMONATE ZIM-domain (JAZ) repressor proteins and successive activation of JA-responsive genes (Abdelkareem et al. 2017; Ishiga et al. 2013; Wang et al. 2020a). Melatonin therapy has been demonstrated to increase the expression of JA-responsive genes, indicating a function in JA signaling control (Qu et al. 2022; Wang et al. 2020a). This connection between the melatonin and JA signaling pathways helps to activate stress-responsive genes implicated in abiotic stress tolerance.
Both melatonin and JA play important roles in plant defense against biotic stressors (Pan et al. 2023; Wang et al. 2020a). Melatonin has been found to improve plant tolerance to diseases and pests (Pan et al. 2023; Qu et al. 2022). In a similar manner, JA participates in the regulation of defense-related genes as well as the generation of secondary metabolites like phytoalexins and volatile organic compounds (VOCs), which aid in plant defense against herbivores and pathogens (Gols et al. 1999; Okada et al. 2015). The crosstalk of melatonin with JA has a synergistic impact on the activation of defensive mechanisms, resulting in increased plant tolerance to both biotic and abiotic stressors. Melatonin and JA could scavenge ROS and reduce oxidative stress. Melatonin treatment improves antioxidant enzyme activity and increases non-enzymatic antioxidant accumulation (Gulzar et al. 2023; Xu et al. 2023). As well, JA is involved in the regulation of antioxidant defense mechanisms (Okada et al. 2015). Tomato responses to Bemisia tabaci nymphs are mediated by JA signaling (Zhang et al. 2018). In guava, JA decreases chilling injury and stimulates fruit defense reaction as signaled by the behavior of total phenols and boost in sugar content, lipoxygenase (LOX) and phenylalanine-ammonia lyase (PAL) activities (González-Aguilar et al. 2004). In tomato, the pretreatment of tomato fruit with JA increases the synthesis of some stress proteins, like PR proteins, which leads to improved chilling tolerance and disease resistance, lessening the incidence of decay (Ding et al. 2002). Melatonin encourages disease tomato fruit resistant to Botrytis cinerea by activation the JA signaling pathway (Liu et al. 2019). Melatonin and JA boost antioxidant capacity, which reduces cold-induced oxidative damage in watermelon (Guo et al. 2021). Melatonin and JA’s interplay in altering antioxidant defense systems adds an extra layer of protection against oxidative damage under abiotic stress situations.
5.5 Melatonin and ET Crosstalk
Melatonin and ethylene (ET) had an important role in plant stress reactions and growth regulation in horticulture plants (Chen et al. 2021; Pan et al. 2023). ET is an essential phytohormone implicated in several physiological routes, with seed germination, fruit ripening, senescence, and responses to biotic and abiotic stresses (Chen et al. 2021; Sharma et al. 2019a). Ripening is a coordinated array of developmental and physiological activities that create changes in color, fragrance, and nutritional content in fleshy fruits such as bananas, apples, peaches, strawberries, melons, squash, and tomatoes (Chen et al. 2023; Gambhir et al. 2023; Gupta et al. 2022; Liu et al. 2023; Verde et al. 2023; Wei et al. 2022). Ripening and senescence are quite similar; however, ripening is a separate phase for fleshy fruit that precedes and may predispose the fruit to senescence, according to metabolic activity. Ethylene is a gaseous phytohormone that regulates plant development and fruit ripening. Extensive research on fruit ripening triggered by ethylene through its sensing, signaling, and gene regulation processes has investigated the role of ethylene in fruit ripening, which has been observed in fruit crops (Gupta et al. 2022).
Melatonin has the ability to affect ET biosynthesis and signaling pathways (Verde et al. 2023). ET is produced from methionine via enzymatic processes, with 1-aminocyclopropane-1-carboxylic acid (ACC) serving as an intermediary (Fatma et al. 2022; Gupta et al. 2022). Melatonin has been demonstrated to influence the activity of the major enzymes in ET production, ACC synthase (ACS) and ACC oxidase (ACO) (Wang et al. 2022a). In pak choi study, melatonin also significantly maintained chlorophyll concentration by limiting ethylene formation and action throughout shelf life, which is most likely due to decreased activities of 1-aminocyclopropane-1-carboxylate (ACC) synthase (ACS) and ACC oxidase (ACO), as well as expression levels of their associated genes (Wang et al. 2022a). Melatonin reduced postharvest pak choi leaf yellowing due to regulation of ethylene production and respiratory metabolism (Wang et al. 2022a). Furthermore, melatonin can also influence ET signaling by influencing the expression of ET-responsive genes and interacting with ET receptors in tomato (Yang et al. 2022a). Melatonin regulates ET production and transduction, which helps to modulate plant stress responses.
ET is well known for its function in plant development and growth, including cell elongation, leaf senescence, and fruit ripening (Gambhir et al. 2023). Melatonin has been linked to the control of these processes (Verde et al. 2023; Wang et al. 2022a; Yang et al. 2022b). Melatonin has been demonstrated in studies to impact ET-induced cell elongation and modulate the functioning of genes associated with leaf senescence and fruit ripening (Ahmad et al. 2023; Wang et al. 2022a). Melatonin and ET interact to regulate development and growth functions, which helps to fine-tune plant responses to environmental stimuli.
ET is known to influence leaf senescence, a closely regulated process that involves the intentional destruction of cellular components (Koyama 2014). Melatonin has been investigated to delay senescence in a variety of horticulture plant types (Zhao et al. 2021). In apples, melatonin consumption was connected to a considerable delay in drought-induced leaf senescence. This delayed the drought-related decreases in PSII photosynthesis and photochemistry efficiency. Melatonin’s regulatory role has a significant anti-senescence impact, in detached leaves (Wang et al. 2013). In peach, melatonin treatment delayed senescence and preserved peach fruit quality, decreased ROS generation and blocked membrane lipid peroxidation, promoted antioxidant enzyme activity, and reduced oxidative stress (Gao et al. 2016). In pears, mmelatonin inhibited, rather than increased, ethylene bursts during pear fruit senescence, as well as protected mature pear fruit from experiencing physiological problems and may also help to preserve the commercial worth of pear fruit (Zhai et al. 2018).
Melatonin and ET play key responsibilities in plant stress responses and tolerance (Ahmad et al. 2023; Chen et al. 2021). ET influences the regulation of stress-responsive genes as well as the antioxidant defense mechanisms (Chen et al. 2021). The antagonistic interactions between several components of the ABA and JA-ET signaling pathways alter defense- and stress-responsive gene expression in response to biotic and abiotic stimuli (Anderson et al. 2004). ET governs physiological, developmental, and reaction to stress by activating ET response Factors (ERFs), which are members of a vast multigene family of transcription factors. ERF genes are important regulators of abiotic stress responses and may be suitable for tomato stress resistance (Klay et al. 2018). Melatonin and ET interact, resulting in a synergistic impact on stress tolerance (Wang et al. 2022a). Ethylene could contribute to effective melatonin alleviation of Cd toxicity in radish roots (Xu et al. 2023).
5.6 Melatonin and CKs Crosstalk
CKs are important phytohormones that govern cell division, shoot growth, root formation, and other physiological activities (O’Brien and Benková 2013; Hassan et al. 2018; Saidi and Hajibarat 2021; Tawfik et al. 2022). Melatonin has been demonstrated in studies to alter CKs (Abd El-Naby et al. 2019; Arnao and Hernández-Ruiz 2021; Zhang et al. 2017). Using melatonin enhances plant tolerance to abiotic stressors by increasing CK-induced gene expression and antioxidant enzyme activity (Abd El-Naby et al. 2019; Mao et al. 2020; Zhang et al. 2017).
Melatonin has been shown to alter plant hormonal levels, physiological activities, and gene synthesis. Melatonin could affect CK biosynthesis and signaling pathways. Through a sequence of enzymatic processes, CKs are produced from isopentenyladenine (iP) and adenosine phosphate (Márquez-López et al. 2019). Melatonin has been demonstrated to impact CK levels in plants via modulating the activity of enzymes involved in CK production, such as isopentenyl transferase (IPT) (Arnao and Hernández-Ruiz 2021). Exogenous melatonin supply raised CK levels under heat stress by upregulating LpIPT2 and LpOG1, essential CK-biosynthesis-related genes, but melatonin had little effect on CK levels in non-stress settings. Melatonin also affected the CK signaling route by modulating A-ARRs and B-ARRs, transcription factors implicated in CK signaling pathways (Zhang et al. 2017). Melatonin’s control of CK biosynthesis and signaling leads to the modulation of plant growth and reactions to stress.
CKs play a vital action in promoting cell division, shoot growth, and overall plant development (Márquez-López et al. 2019; Saidi and Hajibarat 2021). CKs improve shoot growth and yield in salinized tomato (Ghanem et al. 2011). Synthetic cytokinins prolong the period of division of parenchyma cells in growing pears (Shargal et al. 2015). Melatonin has been shown to control plant growth and development, such as shoot elongation, root development, and flower induction in apricot (Abd El-Naby et al. 2019). Melatonin has been demonstrated in studies to alter CK-induced shoot elongation as well as the expression of genes involved in root development and branching (Abd El-Naby et al. 2019; Arnao and Hernández-Ruiz 2021; Zhang et al. 2017). Melatonin encourages adventitious root formation in apple (Mao et al. 2020). Melatonin appears to increase adventitious root growth in cucumber seedlings by influencing the expression of genes involved in hormone production, signaling, and cell wall construction (Wang et al. 2022c).
The interplay of melatonin and CKs in senescence control might entail the modulation of senescence-related gene expression as well as the regulation of ROS levels. In cucumber, leaf senescence increased the accumulation of endogenous melatonin by causing differential expression of CST5H, CsASMT, and CsTDC. The application of melatonin alleviated leaf senescence caused by darkness or age by downregulating the mRNA abundances of SAG12 and cell death-related gene PDCD, improving chlorophyll content due to normal chloroplast ultrastructure and reduce mRNA abundances of chlorophyll degradation genes, upregulating stages of proteins related to the Calvin-Benson cycle and PSI and PSII reaction centers, and in the end razing photosynthetic efficiency and yield of cucumber (Liu et al. 2022a).
Together, melatonin and CKs are implicated in the regulation of stress responses in horticulture crops (O’Brien and Benková 2013; Pan et al. 2023; Shargal et al. 2015). CKs have been shown to enhance plant tolerance to abiotic stresses, such as drought and salinity (O’Brien and Benková 2013). Cytokinin converse salinity stress tolerance in Vicia faba (Abdel- Latef et al. 2021). In eggplant, CK may be able to mitigate the negative effects of salt stress on plant development by boosting photosynthetic efficiency and strengthening antioxidant enzyme systems in leaves (Wu et al. 2012). Regarding melatonin and CKs interaction, melatonin has been found to promote plant tolerance to abiotic stressors by increasing CK-induced stress-responsive gene expression and antioxidant enzyme activity (Abd El-Naby et al. 2019; Mao et al. 2020; Wang et al. 2022c; Zhang et al. 2017).
6 Conclusions and Future Prospects
Understanding the complex interplay between melatonin and phytohormones gives important insights into the processes underpinning abiotic stress tolerance in horticultural crops. Melatonin-phytohormone interactions govern a variety of physiological and biochemical processes involved in stress adaption, including as ROS scavenging, antioxidant defense, transcriptional regulation, stomatal regulation, and ion homeostasis. Using creative agronomic methodologies and genetic engineering technologies to harness these interactions may offer interesting possibilities for improving abiotic stress tolerance in horticultural crops, assuring sustainable crop production in tough conditions.
One of the most promising prospects for using melatonin in conjunction with plant hormones is to develop new strategies to reduce the effects of climate change. Climate change is expected to lead to increased frequency and severity of abiotic stresses, such as drought, salinity, and extreme temperatures. Melatonin and plant hormones can be used to develop new crop varieties that are more tolerant to these stresses (Nawaz et al. 2016). For example, researchers have shown that melatonin can be used to improve thermotolerance drought, and salt tolerance in some crops. Plant hormones can also be used to improve stress tolerance in crops. Herein, ABA can be used to increase stomatal closure, which can help to reduce water loss during drought. Auxin can be used to promote root growth, which can help plants to access water and nutrients during drought. Cytokinin can be used to delay senescence, which can help plants to survive longer periods of stress. By combining melatonin and plant hormones, researchers can develop new crop varieties that are highly tolerant to a variety of abiotic stresses. This will help to ensure food security in the face of climate change.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abd El-Mageed TA, Mekdad AAA, Rady MOA, Abdelbaky AS, Saudy HS, Shaaban A (2022) Physio-biochemical and agronomic changes of two sugar beet cultivars grown in saline soil as influenced by potassium fertilizer. J Soil Sci Plant Nutr 22:3636–3654. https://doi.org/10.1007/s42729-022-00916-7
Abd El-Naby SKM, Ahmed MAA, El-Naggar YIM (2019) Effect of melatonin, GA3 and NAA on vegetative growth, yield and quality of ‘Canino’ apricot fruits. Acta Sci Pol Hortorum Cultus 18:167–174. https://doi.org/10.24326/ASPHC.2019.3.16
Abdel- Latef AAH, Akter A, Tahjib-Ul-arif M (2021) Foliar application of auxin or cytokinin can confer salinity stress tolerance in Vicia faba L. Agron 11:1–17. https://doi.org/10.3390/agronomy11040790
Abdelkareem A, Thagun C, Nakayasu M, Mizutani M, Hashimoto T, Shoji T (2017) Jasmonate-induced biosynthesis of steroidal glycoalkaloids depends on COI1 proteins in tomato. Biochem Biophysic Res Commun 489:206–210. https://doi.org/10.1016/J.BBRC.2017.05.132
Abd-Elrahman ShH, Saudy HS, Abd El–Fattah DA, Hashem FA (2022) Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J Soil Sci Plant Nutr 22:2144–2155. https://doi.org/10.1007/s42729-022-00799-8
Abou El-Enin MM, Sheha AM, El-Serafy Rasha S, Ali OAM, Saudy HS, Shaaban A (2023) Foliage-sprayed nano-chitosan-loaded nitrogen boosts yield potentials, competitive ability, and profitability of intercropped maize-soybean. Int J Plant Prod 17:517–542. https://doi.org/10.1007/s42106-023-00253-4
Aboul Fotouh MM, Moawad F, Tag El-Din M, Sharaf Eldeen H (2014) Influence of seed treatment with UV-C on saline stress tolerance in green beans (Phaseolus vulgaris L.). J Biol Chem Environ Sci 9:391–414 (https://www.researchgate.net/publication/263279203)
Ahmad I, Zhu G, Zhou G, Liu J, Younas MU, Zhu Y (2023) Melatonin role in plant growth and physiology under abiotic stress. Int J Mol Sci 24:1–14. https://doi.org/10.3390/IJMS24108759
Alam MN, Zhang L, Yang L, Islam MR, Liu Y, Luo H, Yang P, Wang Q, Chan Z (2018) Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genom 19:1–14. https://doi.org/10.1186/s12864-018-4588-y
Alam A, Ullah H, Thuenprom N, Tisarum R, Cha-um S, Datta A (2022) Seed priming with salicylic acid enhances growth, physiological traits, fruit yield, and quality parameters of cantaloupe under water-deficit stress. South Afr J Bot 150:1–12. https://doi.org/10.1016/J.SAJB.2022.06.056
Ali IAA, Hassan SE, Abdelhafez AA, Hewidy M, Nasser MA, Saudy HS, Hassan KM, Abou-Hadid AF (2023) Modifying the growing media and bio stimulants supply for healthy gerbera (Gerbera jamesonii) flowers. Gesun Pflanz. https://doi.org/10.1007/s10343-023-00943-z
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Qadir A, Anwar M, Shakoor A, Hayat F (2020) Exogenous melatonin enhances salt stress tolerance in tomato seedlings. Biol Plantar 64:604–615. https://doi.org/10.32615/BP.2020.090
Altaf MA, Shahid R, Ren MX, Altaf MM, Jahan MS, Khan LU (2021a) Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in Tomato seedling. J Soil Sci Plant Nutr 21:1842–1855. https://doi.org/10.1007/S42729-021-00484-2
Altaf MA, Shahid R, Ren MX, Altaf MM, Khan LU, Shahid S, Jahan MS (2021b) Melatonin alleviates salt damage in tomato seedling: A root architecture system, photosynthetic capacity, ion homeostasis, and antioxidant enzymes analysis. Sci Hort 285:1–10. https://doi.org/10.1016/J.SCIENTA.2021.110145
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Khan LU, Lal MK, Tiwari RK, Shakoor A (2022a) Melatonin mitigates cadmium toxicity by promoting root architecture and mineral homeostasis of tomato genotypes. J Soil Sci Plant Nutr 22:1112–1128. https://doi.org/10.1007/s42729-021-00720-9
Altaf MA, Shahid R, Ren MX, Naz S, Altaf MM, Khan LU, Tiwari RK, Lal MK, Shahid MA, Kumar R, Nawaz MA, Jahan MS, Jan BL, Ahmad P (2022b) Melatonin improves drought stress tolerance of tomato by modulation plant growth, root architecture, photosynthesis, and antioxidant defense system. Antiox 11:1–16. https://doi.org/10.3390/antiox11020309
Altaf MA, Hao Y, Shu H, Mumtaz MA, Cheng S, Alyemeni MN, Ahmad P, Wang Z (2023) Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J Hazard Mat 454:1–12. https://doi.org/10.1016/J.JHAZMAT.2023.131468
Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16:3460–3479. https://doi.org/10.1105/TPC.104.025833
Annacondia ML, Magerøy MH, Martinez G (2018) Stress response regulation by epigenetic mechanisms: changing of the guards. Physiol Plant 162:239–250. https://doi.org/10.1111/PPL.12662
Araújo SS, Beebe S, Crespi M, Delbreil B, González EM, Gruber V, Lejeune-Henaut I, Link W, Monteros MJ, Prats E, Rao I, Vadez V, Patto MCV (2014) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci 34:237–280. https://doi.org/10.1080/07352689.2014.898450
Arnao MB, Hernández-Ruiz J (2006) The physiological function of melatonin in plants. Plant Sign Behav 1:89–95. https://doi.org/10.4161/psb.1.3.2640
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Ann Bot 121:195–207. https://doi.org/10.1093/AOB/MCX114
Arnao MB, Hernández-Ruiz J (2021) Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol 23:7–19. https://doi.org/10.1111/PLB.13202
Azizi F, Amiri H, Ismaili A (2022) Melatonin improves salinity stress tolerance of Phaseolus vulgaris L. cv. Pak by changing antioxidant enzymes and photosynthetic parameters. Acta Physiol Plantar 44:1–12. https://doi.org/10.1007/s11738-022-03373-y
Back K, Tan DX, Reiter RJ (2016) Melatonin biosynthesis in plants: multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J Pin Res 61:426–437. https://doi.org/10.1111/jpi.12364
Banerjee A, Roychoudhury A (2019) Melatonin application reduces fluoride uptake and toxicity in rice seedlings by altering abscisic acid, gibberellin, auxin and antioxidant homeostasis. Plant Physiol Biochem 145:164–173. https://doi.org/10.1016/J.PLAPHY.2019.10.033
Ben Massoud M, Karmous I, El Ferjani E, Chaoui A (2017) Alleviation of copper toxicity in germinating pea seeds by IAA, GA3, Ca and citric acid. J Plant Interact 13:21–29. https://doi.org/10.1080/17429145.2017.1410733
Cai S, Liu J, Wang G, Wu L, Song J (2022) Analysis of regulatory mechanism of melatonin on tomato seed germination under Cd2+ stress. Chinese Bull Bot 58:1–23. https://doi.org/10.11983/CBB22202
Chen Q, Qi W, Reiter RJ, Wei W, Wang B (2009) Exogenously applied melatonin stimulates root growth and raises endogenous indoleacetic acid in roots of etiolated seedlings of Brassica juncea. J Plant Physiol 166:324–328. https://doi.org/10.1016/J.JPLPH.2008.06.002
Chen H, Bullock DA, Alonso JM, Stepanova AN (2021) To fight or to grow: the balancing role of ethylene in plant abiotic stress responses. Plants 11:1–25. https://doi.org/10.3390/PLANTS11010033
Chen R, Wu Y, Wei X, Huang Z, Mao L (2023) Ethylene promotes ABA biosynthesis by repressing the expression of miR161 in postharvest strawberry fruit. Postharv Biol Technol 199:1–8. https://doi.org/10.1016/J.POSTHARVBIO.2023.112302
Debnath B, Li M, Liu S, Pan T, Ma C, Qiu D (2020) Melatonin-mediate acid rain stress tolerance mechanism through alteration of transcriptional factors and secondary metabolites gene expression in tomato. Ecotox Environ Saf 200:1–9. https://doi.org/10.1016/J.ECOENV.2020.110720
Demirel U, Morris WL, Ducreux LJM, Yavuz C, Asim A, Tindas I, Campbell R, Morris JA, Verrall SR, Hedley PE, Gokce ZNO, Caliskan S, Aksoy E, Caliskan ME, Taylor MA, Hancock RD (2020) Physiological, biochemical, and transcriptional responses to single and combined abiotic stress in stress-tolerant and stress-sensitive Potato genotypes. Front Plant Sci 11:1–21. https://doi.org/10.3389/fpls.2020.00169
Ding CK, Wang CY, Gross KC, Smith DL (2002) Jasmonate and salicylate induce the expression of pathogenesis-related-protein genes and increase resistance to chilling injury in tomato fruit. Planta 214:895–901. https://doi.org/10.1007/s00425-001-0698-9
Ding F, Liu B, Zhang S (2017) Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci Hort 219:264–271. https://doi.org/10.1016/J.SCIENTA.2017.03.029
Doklega SMA, Saudy HS, El-Sherpiny MA, Abou El-Yazied A, Abd El-Gawad HG, Ibrahim MFM, Abd El-Hady MAM, Omar MMA, Metwally AA (2023) Rhizospheric addition of hydrogel polymer and zeolite plus glutathione mitigate the hazard effects of water deficiency on common bean plants through enhancing the defensive antioxidants. Gesun Pflanz. https://doi.org/10.1007/s10343-023-00947-9
El-Beltagi HS, El-Sayed SM, Abdelhamid AN, Hassan KM, Elshalakany WA, Nossier MI, Alabdallah NM, Al-Harbi NA, Al-Qahtani SM, Darwish DBE, Abbas ZK, Ibrahim HA (2023) Potentiating biosynthesis of alkaloids and polyphenolic substances in Catharanthus roseus plant using κ-Carrageenan. Mol 28:3642. https://doi.org/10.3390/molecules28083642
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2018) Efficacy of ascorbic acid as a cofactor for alleviating water deficit impacts and enhancing sunflower yield and irrigation water–use efficiency. Agric Wat Manage 208:132–139. https://doi.org/10.1016/j.agwat.2018.06.016
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2022) Sunflower response to application of L–ascorbate under thermal stress associated with different sowing dates. Gesun Pflanz 74:87–96. https://doi.org/10.1007/s10343-021-00590-2
El-Bially MA, Saudy HS, Hashem FA, El–Gabry YA, Shahin MG, (2022b) Salicylic acid as a tolerance inducer of drought stress on sunflower grown in sandy soil. Gesun Pflanz 74:603–613. https://doi.org/10.1007/s10343-022-00635-0
El-Bially MA, El-Metwally IM, Saudy HS, Aisa KH, Abd El-Samad GA (2023) Mycorrhiza-inoculated biochar as an eco-friendly tool improves the broomrape control efficacy in two faba bean cultivars. Rhizosphere 26:100706. https://doi.org/10.1016/j.rhisph.2023.100706
El-Metwally IM, Saudy HS (2021a) Interactional impacts of drought and weed stresses on nutritional status of seeds and water use efficiency of peanut plants grown in arid conditions. Gesun Pflanz 73:407–416. https://doi.org/10.1007/s10343-021-00557-3
El-Metwally IM, Saudy HS (2021b) Interactive application of zinc and herbicides affects broad–leaved weeds, nutrient uptake, and yield in rice. J Soil Sci Plant Nutr 21:238–248. https://doi.org/10.1007/s42729-020-00356-1
El-Metwally IM, Saudy HS, Abdelhamid MT (2021) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Italian J Agromet 2:81–90. https://doi.org/10.36253/ijam-872
El-Metwally IM, Geries L, Saudy HS (2022a) Interactive effect of soil mulching and irrigation regime on yield, irrigation water use efficiency and weeds of trickle–irrigated onion. Archiv Agron Soil Sci 68:1103–1116. https://doi.org/10.1080/03650340.2020.1869723
El-Metwally IM, Sadak MSh, Saudy HS (2022b) Stimulation effects of glutamic and 5-Aminolevulinic acids on photosynthetic pigments, physio-biochemical constituents, antioxidant activity, and yield of peanut. Gesun Pflanz 74:915–924. https://doi.org/10.1007/s10343-022-00663-w
El-Metwally IM, Saudy HS, Elewa TA (2022c) Natural plant by-products and mulching materials to suppress weeds and improve sugar beet (Beta vulgaris L.) yield and quality. J Soil Sci Plant Nutr 22:5217–5230. https://doi.org/10.1007/s42729-022-00997-4
El-Sayed SM, Hassan KM, Abdelhamid AN, Yousef EE, Abdellatif YMR, Abu-Hussien SH, Nasser MA, Elshalakany WA, Darwish DB, Abdulmajeed AM, Alabdallah NM, Al-Qahtani SM, Al-Harbi NA, Dessoky ES, Ashour H, Ibrahim MS (2022) Exogenous paclobutrazol reinforces the antioxidant and antimicrobial properties of lavender (Lavandula officinalis) oil through modulating its composition of oxygenated terpenes. Plants 11:1607. https://doi.org/10.3390/plants11121607
El-Yazied AA, Ibrahim MFM, Ibrahim MAR, Nasef IN, Al-Qahtani SM, Al-Harbi NA, Alzuaibr FM, Alaklabi A, Dessoky ES, Alabdallah NM (2022) Melatonin mitigates drought induced oxidative stress in potato plants through modulation of osmolytes, sugar metabolism, ABA homeostasis and antioxidant enzymes. Plants 11:1–16. https://doi.org/10.3390/plants11091151
Esmaeili S, Sharifi M, Ghanati F, Soltani BM, Samari E, Sagharyan M (2023) Exogenous melatonin induces phenolic compounds production in Linum album cells by altering nitric oxide and salicylic acid. Sci Rep 13:1–12. https://doi.org/10.1038/s41598-023-30954-9
Fathi N, Kazemeini SA, Alinia M, Mastinu A (2023) The effect of seed priming with melatonin on improving the tolerance of Zea mays L. var Saccharata to paraquat-induced oxidative stress through photosynthetic systems and enzymatic antioxidant activities. Physiol Mol Plant Pathol 124:1–9. https://doi.org/10.1016/J.PMPP.2023.101967
Fatma M, Asgher M, Iqbal N, Rasheed F, Sehar Z, Sofo A, Khan NA (2022) Ethylene signaling under stressful environments: Analyzing collaborative knowledge. Plants 11:1–29. https://doi.org/10.3390/PLANTS11172211
Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY (2015) Indole-3-acetic acid: a widespread physiological code in interactions of fungi with other organisms. Plant Sign Behav 10:1–9. https://doi.org/10.1080/15592324.2015.1048052
Fu J, Wu Y, Miao Y, Xu Y, Zhao E, Wang J, Sun H, Liu Q, Xue Y, Xu Y, Hu T (2016) Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci Rep 7:1–11. https://doi.org/10.1038/srep39865
Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124:509–525. https://doi.org/10.1007/S10265-011-0412-3
Gambhir P, Raghuvanshi U, Parida AP, Kujur S, Sharma S, Sopory SK, Kumar R, Sharma AK (2023) Elevated methylglyoxal levels inhibit tomato fruit ripening by preventing ethylene biosynthesis. Plant Physiol 192:2161–2184. https://doi.org/10.1093/PLPHYS/KIAD142
Gao H, Zhang ZK, Chai HK, Cheng N, Yang Y, Wang DN, Yang T, Cao W (2016) Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharv Biol Technol 118:03–110. https://doi.org/10.1016/J.POSTHARVBIO.2016.03.006
García-Sánchez S, Cano A, Hernández-Ruiz J, Arnao MB (2022) Effects of temperature and light on the germination-promoting activity by melatonin in almond seeds without stratification. Agron 12:1–7. https://doi.org/10.3390/AGRONOMY12092070
Ghanem ME, Albacete A, Smigocki AC, Frébort I, Pospíilová H, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, Pérez-Alfocea F (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140. https://doi.org/10.1093/JXB/ERQ266
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16:1807–1828. https://doi.org/10.1007/S13762-019-02215-8
Gols R, Posthumus MA, Dicke M (1999) Jasmonic acid induces the production of gerbera volatiles that attract the biological control agent Phytoseiulus persimilis. Entom Exp App 93:77–86. https://doi.org/10.1046/J.1570-7458.1999.00564.X
González-Aguilar GA, Tiznado-Hernández ME, Zavaleta-Gatica R, Martínez-Téllez MA (2004) Methyl jasmonate treatments reduce chilling injury and activate the defense response of guava fruits. Biochem Biophysic Res Comm 313:694–701. https://doi.org/10.1016/J.BBRC.2003.11.165
Gulzar S, Manzoor MA, Liaquat F, Shah IH, Rehman A, Hameed MK, Arif S, Zhou X, Zhang Y (2023) Effects of melatonin and Trichoderma harzianum on pak choi yield, chlorophyll contents and antioxidant defense system under clubroot disease. South Afr J Bot 58:292–300. https://doi.org/10.1016/J.SAJB.2023.05.021
Guo Y, Yan J, Su Z, Chang J, Yang J, Wei C, Zhang Y, Ma J, Zhang X, Li H (2021) Abscisic acid mediates grafting-induced cold tolerance of Watermelon via interaction with melatonin and methyl jasmonate. Front Plant Sci 12:1–13. https://doi.org/10.3389/fpls.2021.785317
Guo J, Bai Y, Wei Y, Dong Y, Zeng H, Reiter RJ, Shi H (2022) Fine-tuning of pathogenesis-related protein 1 (PR1) activity by the melatonin biosynthetic enzyme ASMT2 in defense response to cassava bacterial blight. J Pin Res 72:e12784. https://doi.org/10.1111/JPI.12784
Guo W, Zhang C, Yang R, Zhao S, Han X, Wang Z, Li S, Gao H (2023) Endogenous salicylic acid mediates melatonin-induced chilling-and oxidative-stress tolerance in harvested kiwifruit. Postharv Biol Technol 201:1–11. https://doi.org/10.1016/J.POSTHARVBIO.2023.112341
Gupta S, Gupta K, Kheni J, Panigrahi J (2022) Current understanding of ethylene and fruit ripening. In: Singh S, Husain T, Singh VP, Tripathi DK, Prasad SM, Dubey NK (eds) Ethylene in Plant Biology. John Wiley & Sons Ltd, pp 109–124. https://doi.org/10.1002/9781119744719.CH6
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int J Genom 1–19. https://doi.org/10.1155/2014/701596
Hadid ML, Ramadan KhMA, El-Beltagi HS, Ramadan AA, El-Metwally IM, Shalaby TA, Bendary ESA, Saudy HS (2023) Modulating the antioxidant defense systems and nutrients content by proline for higher yielding of wheat under water deficit. Not Bot Horti Agrobo 51:13291. https://doi.org/10.15835/nbha51313291
Hardeland R (2015) Melatonin in plants and other phototrophs: advances and gaps concerning the diversity of functions. J Exp Bot 66:627–646. https://doi.org/10.1093/jxb/eru386
Hardeland R, Tan DX, Reiter RJ (2009) Kynuramines, metabolites of melatonin and other indoles: the resurrection of an almost forgotten class of biogenic amines. J Pin Res 47:109–126. https://doi.org/10.1111/j.1600-079x.2009.00701.x
Hasan MK, Xing QF, Zhou CY, Wang KX, Xu T, Yang P, Qi ZY, Shao SJ, Ahammed GJ, Zhou J (2023) Melatonin mediates elevated carbon dioxide-induced photosynthesis and thermotolerance in tomato. J Pin Res 74:1–13. https://doi.org/10.1111/JPI.12858
Hassan KM, Hosni AM, Hewidy M, Abdel Razik AB (2018) Micropropagation and evaluation of genetic stability of foxglove tree (Paulownia tomentosa). Arab Univ J Agric Sci (2D):2287–2296. https://doi.org/10.21608/ajs.2018.35343
Hashem HA, El-sherif NA (2019) Exogenous jasmonic acid induces lead stress tolerance in kidney bean (Phaseolus vulgaris l.) by changing amino acid profile and stimulating antioxidant defense system. Jordan J Biol Sci 12:345–353
Hatfield JL, Prueger JH (2015) Temperature extremes: effect on plant growth and development. Weather Clim Extrem 10:4–10. https://doi.org/10.1016/J.WACE.2015.08.001
Hayat F, Sun Z, Ni Z, Iqbal S, Xu W, Gao Z, Qiao Y, Tufail MA, Jahan MS, Khan U, Wan C, Gu X (2022) Exogenous melatonin improves cold tolerance of strawberry (Fragaria × ananassa Duch.) through modulation of DREB/CBF-COR pathway and antioxidant defense system. Hort 8:1–18. https://doi.org/10.3390/horticulturae8030194
He M, He CQ, Ding NZ (2018) Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front Plant Sci 9:1–18. https://doi.org/10.3389/fpls.2018.01771
He J, Zhuang X, Zhou J, Sun L, Wan H, Li H, Lyu D (2020) Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol 40:746–761. https://doi.org/10.1093/TREEPHYS/TPAA024
Hernández-Ruiz J, Cano A, Arnao MB (2004) Melatonin: A growth-stimulating compound present in lupin tissues. Planta 220:140–144. https://doi.org/10.1007/s00425-004-1317-3
Horváth E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300. https://doi.org/10.1007/s00344-007-9017-4
Hu D, Zhang X, Xue P, Nie Y, Liu J, Li Y, Wang C, Wan X (2023) Exogenous melatonin ameliorates heat damages by regulating growth, photosynthetic efficiency and leaf ultrastructure of carnation. Plant Physiol Biochem 198:1–13. https://doi.org/10.1016/J.PLAPHY.2023.107698
Iriti M, Rossoni M, Faoro F (2006) Melatonin content in grape: myth or panacea. J Sci Food Agric 86:1432–1438. https://doi.org/10.1002/jsfa.2537
Ishiga Y, Ishiga T, Uppalapati SR, Mysore KS (2013) Jasmonate ZIM-domain (JAZ) protein regulates host and nonhost pathogen-induced cell death in tomato and Nicotiana benthamiana. PLoS ONE 8:1–7. https://doi.org/10.1371/JOURNAL.PONE.0075728
Jahan MS, Shu S, Wang Y, Hasan MM, El-Yazied AA, Alabdallah NM, Hajjar D, Altaf MA, Sun J, Guo S (2021) Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in Tomato through the modulation of ABA- and GA-mediated pathways. Front Plant Sci 12:1–14. https://doi.org/10.3389/fpls.2021.650955
Jannatizadeh A, Aghdam MS, Luo Z, Razavi F (2019) Impact of exogenous melatonin application on chilling injury in tomato fruits during cold storage. Food and Bioproc Technol 12:741–750. https://doi.org/10.1007/s11947-019-2247-1
Jensen NB, Ottosen CO, Zhou R (2023) Exogenous melatonin alters stomatal regulation in tomato seedlings subjected to combined heat and drought stress through mechanisms distinct from ABA signaling. Plants 12:1–18. https://doi.org/10.3390/plants12051156
Kaya C, Shabala S, Kaya C, Shabala S (2023) Melatonin improves drought stress tolerance of pepper (Capsicum annuum) plants via upregulating nitrogen metabolism. Func Plant Biol 50:1–14. https://doi.org/10.1071/FP23060
Khan AL, Waqas M, Lee IJ (2015) Resilience of Penicillium resedanum LK6 and exogenous gibberellin in improving Capsicum annuum growth under abiotic stresses. J Plant Res 128:259–268. https://doi.org/10.1007/s10265-014-0688-1
Klay I, Gouia S, Liu M, Mila I, Khoudi H, Bernadac A, Bouzayen M, Pirrello J (2018) Ethylene response factors (ERF) are differentially regulated by different abiotic stress types in tomato plants. Plant Sci 274:137–145. https://doi.org/10.1016/J.PLANTSCI.2018.05.023
Korkmaz A, Değer Ö, Szafrańska K, Köklü Ş, Karaca A, Yakupoğlu G, Kocaçinar F (2021) Melatonin effects in enhancing chilling stress tolerance of pepper. Sci Hort 289:1–9. https://doi.org/10.1016/J.SCIENTA.2021.110434
Koyama T (2014) The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front Plant Sci 5:1–8. https://doi.org/10.3389/fpls.2014.00650
Lasheen FF, Hewidy M, Abdelhamid AN, Thabet RS, Abass MMM, Fahmy Asmaa A, Saudy HS, Hassan KM (2023) Exogenous application of humic acid mitigates salinity stress on pittosporum (Pittosporum tobira) plant by adjusting the osmolytes and nutrient homeostasis. Gesun Pflanz. https://doi.org/10.1007/s10343-023-00939-9
Latef AAHA, Tahjib-Ul-Arif M, Rhaman MS (2021) Exogenous auxin-mediated salt stress alleviation in faba bean (Vicia faba L.). Agron 11:1–16. https://doi.org/10.3390/AGRONOMY11030547
Leubner-metzger ALG (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31:253–270. https://doi.org/10.1007/s00299-011-1180-1
Li C, Tan DX, Liang D, Chang C, Jia D, Ma F (2015) Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J Exp Bot 66:669–680. https://doi.org/10.1093/JXB/ERU476
Li H, Chang J, Zheng J, Dong Y, Liu Q, Yang X, Wei C, Zhang Y, Ma J, Zhang X (2017) Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci Rep 7:1–15. https://doi.org/10.1038/srep40858
Li H, Mo YL, Cui Q, Yang XZ, Guo YL, Wei CH, Yang J, Zhang Y, Ma JX, Zhang X (2019a) Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci 278:32–43. https://doi.org/10.1016/J.PLANTSCI.2018.10.016
Li J, Zhao C, Zhang M, Yuan F, Chen M (2019b) Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signal Behav 14:1–10. https://doi.org/10.1080/2F15592324.2019.1659705
Li S, Xu Y, Bi Y, Zhang B, Shen S, Jiang T, Zheng X (2019c) Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharv Biol Technol 157:1–9. https://doi.org/10.1016/J.POSTHARVBIO.2019.110962
Li H, Guo Y, Cui Q, Zhang Z, Yan X, Ahammed GJ, Yang X, Yang J, Wei C, Zhang X (2020) Alkanes (C29 and C31)-Mediated intracuticular wax accumulation contributes to melatonin- and ABA-induced drought tolerance in Watermelon. J Plant Growth Regul 39:1441–1450. https://doi.org/10.1007/s00344-020-10099-z
Li S, Huan C, Liu Y, Zheng X, Bi Y (2022) Melatonin induces improved protection against Botrytis cinerea in cherry tomato fruit by activating salicylic acid signaling pathway. Sci Hort 304:1–12. https://doi.org/10.1016/J.SCIENTA.2022.111299
Liao Y, Tang Y, Wang S, Su H, Chen J, Zhang D, Wu J, Zhou D, Yan M, Liu L (2023) Abscisic acid modulates differential physiological and biochemical responses to cadmium stress in Brassica napus. Environ Poll Bioavail 35:1–13. https://doi.org/10.1080/26395940.2023.2168216
Liu C, Chen L, Zhao R, Li R, Zhang S, Yu W, Sheng J, Shen L (2019) Melatonin induces disease resistance to botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J Agric Food Chem 67:6116–6124. https://doi.org/10.1021/acs.jafc.9b00058
Liu Q, Xin D, Xi L, Gu T, Jia Z, Zhang B, Kou L (2022a) Novel applications of exogenous melatonin on cold stress mitigation in postharvest cucumbers. J Agric Food Res 10:1–8. https://doi.org/10.1016/J.JAFR.2022.100459
Liu Y, Li Z, Zhong C, Zhang Y, Wang-Pruski G, Zhang Z, Wu J (2022b) Alleviating effect of melatonin on Melon seed germination under autotoxicity and saline-alkali combined stress. J Plant Growth Regul 42:2474–2485. https://doi.org/10.1007/s00344-022-10720-3
Liu Z, Shao M, Junejo SA, Zhang B, Jiang H, Fu X, Huang Q (2023) Controlled ethylene-releasing from V-type starch coated with sodium alginate for banana ripening via changing the humidity of environment. Postharv Biol Technol 202:1–10. https://doi.org/10.1016/J.POSTHARVBIO.2023.112377
Makhlouf BSI, Khalil SRA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr 22:1676–1691. https://doi.org/10.1007/s42729-022-00762-7
Mao J, Niu C, Li K, Chen S, Tahir MM, Han M, Zhang D (2020) Melatonin promotes adventitious root formation in apple by promoting the function of MdWOX11. BMC Plant Biol 20:1–11. https://doi.org/10.1186/S12870-020-02747-Z/FIGURES/8
Márquez-López RE, Quintana-Escobar AO, Loyola-Vargas VM (2019) Cytokinins, the Cinderella of plant growth regulators. Phytochem Rev 18:1387–1408. https://doi.org/10.1007/S11101-019-09656-6
Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J (2013) A review of the molecular aspects of melatonin’s anti-inflammatory actions: recent insights and new perspectives. J Pin Res 54:1–14. https://doi.org/10.1111/j.1600-079x.2012.01014.x
Meng JF, Xu TF, Wang ZZ, Fang YL, Xi ZM, Zhang ZW (2014) The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: antioxidant metabolites, leaf anatomy, and chloroplast morphology. J Pin Res 57:200–212. https://doi.org/10.1111/JPI.12159
Meng X, Li Y, Li S, Zhou Y, Gan R-Y, Xu D-P, Li H-B (2017) Dietary sources and bioactivities of melatonin. Nutr 9:367. https://doi.org/10.3390/nu9040367
Mubarak M, Salem EMM, Kenawey MKM, Saudy HS (2021) Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J Soil Sci Plant Nutr 21:2020–2031. https://doi.org/10.1007/s42729-021-00498-w
Munns R (2003) Handbook of plant and crop stress. Field Crops Res 38:60–61. https://doi.org/10.1016/0378-4290(94)90035-3
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Murch SJ, Erland LAE (2021) A systematic review of melatonin in plants: an example of evolution of literature. Front Plant Sci 12:1–24. https://doi.org/10.3389/fpls.2021.683047
Mushtaq N, Iqbal S, Hayat F, Raziq A, Ayaz A, Zaman W (2022) Melatonin in micro-tom tomato: improved drought tolerance via the regulation of the photosynthetic apparatus, membrane stability, osmoprotectants, and root system. Life 12:1–23. https://doi.org/10.3390/life12111922
Nakashima K, Fujita Y, Katsura K, Maruyama K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Transcriptional regulation of ABI3- and ABA-responsive genes including RD29B and RD29A in seeds, germinating embryos, and seedlings of Arabidopsis. Plant Mol Biol 60:51–68. https://doi.org/10.1007/s11103-005-2418-5
Nasser MA, El-Mogy MM, Samaan MSF, Hassan KM, El-Sayed SM, Alsubeie MS, Darwish DB, Mahmoud SF, Al-Harbi NA, Al-Qahtani SM (2022) Postharvest exogenous melatonin treatment of table grape berry enhances quality and maintains bioactive compounds during refrigerated storage. Hort 8:1–10. https://doi.org/10.3390/horticulturae8100860
Nawaz MA, Huang Y, Bie Z, Ahmed W, Reiter RJ, Niu M, Hameed S (2016) Melatonin: current status and future perspectives in plant science. Front Plant Sci 6:1–13. https://doi.org/10.3389/fpls.2015.01230
Nejat N, Mantri N (2017) Plant immune system: crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defense. Curr Iss Mol Biol 23:1–16. https://doi.org/10.21775/CIMB.023.001
Noureldin NA, Saudy HS, Ashmawy F, Saed HM (2013) Grain yield response index of bread wheat cultivars as influenced by nitrogen levels. Ann Agric Sci, Ain Shams Univ 58:147–152. https://doi.org/10.1016/j.aoas.2013.07.012
O’Brien JA, Benková E (2013) Cytokinin cross-talking during biotic and abiotic stress responses. Front Plant Sci 4:1–11. https://doi.org/10.3389/fpls.2013.00451
Okada K, Abe H, Arimura GI (2015) Jasmonates induce both defense responses and communication in monocotyledonous and dicotyledonous plants. Plant Cell Physiol 56:16–27. https://doi.org/10.1093/PCP/PCU158
Ortiz-García P, Ortega-Villaizán AG, Onejeme FC, Müller M, Pollmann S (2023) Do opposites attract? Auxin-abscisic acid crosstalk: new perspectives. Int J Mol Sci 24:1–19. https://doi.org/10.3390/IJMS24043090
Pan Y, Xu X, Li L, Sun Q, Wang Q, Huang H, Tong Z, Zhang J (2023) Melatonin-mediated development and abiotic stress tolerance in plants. Front Plant Sci 14:1–13. https://doi.org/10.3389/fpls.2023.1100827
Pandi-Perumal SR, Srinivasan V, Maestroni GJ, Cardinali DP, Poeggeler B, Hardeland R (2006) Melatonin: nature’s most versatile biological signal? FEBS J 273:2813–2838. https://doi.org/10.1111/j.1742-4658.2006.05322.x
Parwez R, Aftab T, Gill SS, Naeem M (2022) Abscisic acid signaling and crosstalk with phytohormones in regulation of environmental stress responses. Environ Exp Bot 199:1–20. https://doi.org/10.1016/J.ENVEXPBOT.2022.104885
Peng X, Wang N, Sun S, Geng L, Guo N, Liu A, Chen S, Ahammed GJ (2023) Reactive oxygen species signaling is involved in melatonin-induced reduction of chlorothalonil residue in tomato leaves. J Hazard Mat 443:1–12. https://doi.org/10.1016/J.JHAZMAT.2022.130212
Pérez-Llorca M, Muñoz P, Müller M, Munné-Bosch S (2019) Biosynthesis, metabolism and function of auxin, salicylic acid and melatonin in climacteric and non-climacteric fruits. Front Plant Sci 10:1–10. https://doi.org/10.3389/fpls.2019.00136
Pieterse CMJ, Van Loon LC (1999) Salicylic acid-independent plant defence pathways. Tren Plant Sci 4:52–58. https://doi.org/10.1016/S1360-1385(98)01364-8
Prakash V, Singh VP, Tripathi DK, Sharma S, Corpas FJ, Durgesh C, Tripathi K, Sharma S, Corpas FJ (2021) Nitric oxide (NO) and salicylic acid (SA): a framework for their relationship in plant development under abiotic stress. Plant Biol 23:39–49. https://doi.org/10.1111/PLB.13246
Qu G, Wu W, Ba L, Ma C, Ji N, Cao S (2022) Melatonin enhances the postharvest disease resistance of blueberries fruit by modulating the jasmonic acid signaling pathway and phenylpropanoid metabolites. Front Chem 10:1–10. https://doi.org/10.3389/fchem.2022.957581
Rajora N, Vats S, Raturi G, Thakral V, Kaur S, Rachappanavar V, Kumar M, Kesarwani AK, Sonah H, Sharma TR, Deshmukh R (2022) Seed priming with melatonin: a promising approach to combat abiotic stress in plants. Plant Stress 4:1–10. https://doi.org/10.1016/J.STRESS.2022.100071
Rakshit A, Singh HB (2018) Advances in seed priming. In Springer Nature Singapore 1–307 https://doi.org/10.1007/978-981-13-0032-5
Ramadan KMA, El-Beltag HS, Abd El Mageed TA, Mazrou KE, Mohamed GF, El-Saadony MT, El-Saadony FMA, Roby MHH, Saudy HS, Abou-Sreea AIB (2023a) Significance of selenium in ameliorating the effects of irrigation deficit via improving photosynthesis efficiency, cell integrity, osmo-protectants, and oil profile of anise crop. Not Bot Horti Agrobo 51:13437. https://doi.org/10.15835/nbha51413437
Ramadan KMA, El-Beltagi HS, Abd El-Mageed TAA, Saudy HS, Al-Otaibi HH, Mahmoud MAA (2023b) The changes in various physio-biochemical parameters and yield traits of faba bean due to humic acid plus 6-benzylaminopurine application under deficit irrigation. Agron 13:1227. https://doi.org/10.3390/agronomy13051227
Raza A, Charagh S, Najafi-Kakavand S, Abbas S, Shoaib Y, Anwar S, Sharifi S, Lu G, Siddique KHM (2023) Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 8:1–24. https://doi.org/10.1016/J.STRESS.2023.100152
Rizk TY, Kholousy ASO, Saudy HS, Sultan ShS, Abd Alwahed SHA (2023) Breaking dormancy and enhancing germination of Avena sterilis L. and Amaranthus retroflexus L. weeds by gibberellic acid and potassium nitrate to keep soil and crops healthy. Gesun Pflanz 75:757–763. https://doi.org/10.1007/s10343-022-00780-6
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: generation, signaling, and defense mechanisms. Antiox 10:1–37. https://doi.org/10.3390/antiox10020277
Saidi A, Hajibarat Z (2021) Phytohormones: plant switchers in developmental and growth stages in potato. J Gen Engin Biotechnol 19:1–17. https://doi.org/10.1186/S43141-021-00192-5
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2021) Soil mulching and deficit irrigation effect on sustainability of nutrients availability and uptake, and productivity of maize grown in calcareous soils. Comm Soil Sci Plant Anal 52:1745–1761. https://doi.org/10.1080/00103624.2021.1892733
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2022) Influence of silicon forms on nutrient accumulation and grain yield of wheat under water deficit conditions. Gesun Pflanz 74:539–548. https://doi.org/10.1007/s10343-022-00629-y
Sánchez-Barceló EJ, Cos S, Mediavilla D, Martínez-Campa C, González A, Alonso-González C (2005) Melatonin-estrogen interactions in breast cancer. J Pin Res 38:217–222. https://doi.org/10.1111/j.1600-079x.2004.00207.x
Saudy HS (2014) Chlorophyll meter as a tool for forecasting wheat nitrogen requirements after application of herbicides. Archiv Agron Soil Sci 60:1077–1090. https://doi.org/10.1080/03650340.2013.866226
Saudy HS (2015) Maize–cowpea intercropping as an ecological approach for nitrogen-use rationalization and weed suppression. Archiv Agron Soil Sci 61:1–14. https://doi.org/10.1080/03650340.2014.920499
Saudy HS, El-Metwally IM (2019) Nutrient utilization indices of NPK and drought management in groundnut under sandy soil conditions. Comm Soil Sci Plant Anal 50:1821–1828. https://doi.org/10.1080/00103624.2019.1635147
Saudy HS, El-Metwally IM (2023) Effect of irrigation, nitrogen sources and metribuzin on performance of maize and its weeds. Comm Soil Sci Plant Anal 54:22–31. https://doi.org/10.1080/00103624.2022.2109659
Saudy HS, Mubarak M (2015) Mitigating the detrimental impacts of nitrogen deficit and fenoxaprop-p-ethyl herbicide on wheat using silicon. Comm Soil Sci Plant Anal 46:913–923. https://doi.org/10.1080/00103624.2015.1011753
Saudy HS, Abd El–Momen WR, El–khouly NS (2018) Diversified nitrogen rates influence nitrogen agronomic efficiency and seed yield response index of sesame (Sesamum indicum, L.) cultivars. Comm Soil Sci Plant Anal 49:2387–2395. https://doi.org/10.1080/00103624.2018.1510949
Saudy HS, El-Metwally IM, Abd El-Samad GA (2020a) Physio–biochemical and nutrient constituents of peanut plants under bentazone herbicide for broad–leaved weed control and water regimes in dry land areas. J Arid Land 12:630–639. https://doi.org/10.1007/s40333-020-0020-y
Saudy HS, Hamed MF, Abd El–Momen WR, Hussein H (2020) Nitrogen use rationalization and boosting wheat productivity by applying packages of humic, amino acids and microorganisms. Comm Soil Sci Plant Anal 51:1036–1047. https://doi.org/10.1080/00103624.2020.1744631
Saudy HS, Noureldin NA, Mubarak M, Fares W, Elsayed M (2020c) Cultivar selection as a tool for managing soil phosphorus and faba bean yield sustainability. Archiv Agron Soil Sci 66:414–425. https://doi.org/10.1080/03650340.2019.1619078
Saudy HS, El-Bially MA, El-Metwally IM, Shahin MG (2021a) Physio–biochemical and agronomic response of ascorbic acid–treated sunflower (Helianthus annuus) grown at different sowing dates and under various irrigation regimes. Gesun Pflanz 73:169–179. https://doi.org/10.1007/s10343-020-00535-1
Saudy HS, El–Bially MA, Ramadan KhA, Abo El–Nasr EKh, Abd El–Samad GA (2021b) Potentiality of soil mulch and sorghum extract to reduce the biotic stress of weeds with enhancing yield and nutrient uptake of maize crop. Gesun Pflanz 73:555–564. https://doi.org/10.1007/s10343-021-00577-z
Saudy HS, El-Metwally IM, Shahin MG (2021c) Co–application effect of herbicides and micronutrients on weeds and nutrient uptake in flooded irrigated rice: does it have a synergistic or an antagonistic effect? Crop Prot 149:105755. https://doi.org/10.1016/j.cropro.2021.105755
Saudy HS, Hamed MF, El–Metwally IM, Ramadan KhA, Aisa KH (2021) Assessing the effect of biochar or compost application as a spot placement on broomrape control in two cultivars of faba bean. J Soil ci Plant Nutr 21:1856–1866. https://doi.org/10.1007/s42729-021-00485-1
Saudy HS, El-Metwally IM, Sobieh ST, Abd-Alwahed SHA (2022) Mycorrhiza, charcoal, and rocket salad powder as eco-friendly methods for controlling broomrape weed in inter-planted faba bean with flax. J Soil Sci Plant Nutr 22:5195–5206. https://doi.org/10.1007/s42729-022-00995-6
Saudy HS, El–Bially MA, Hashem FA, Shahin MG, El–Gabry YA (2023a) The changes in yield response factor, water use efficiency, and physiology of sunflower owing to ascorbic and citric acids application under mild deficit irrigation. Gesun Pflanz 75:899–909. https://doi.org/10.1007/s10343-022-00736-w
Saudy HS, Salem EMM, Abd El–Momen WR (2023b) Effect of potassium silicate and irrigation on grain nutrient uptake and water use efficiency of wheat under calcareous soils. Gesun Pflanz 75:647–654. https://doi.org/10.1007/s10343-022-00729-9
Seo M, Koshiba T (2002) Complex regulation of ABA biosynthesis in plants. Tren Plant Sci 7:41–48. https://doi.org/10.1016/S1360-1385(01)02187-2
Shaaban A, Abd El-Mageed TA, Abd El-Momen WR, Saudy HS, Al-Elwany OAAI (2023a) The integrated application of phosphorous and zinc affects the physiological status, yield and quality of canola grown in phosphorus-suffered deficiency saline soil. Gesun Pflanz 75:1813–1821. https://doi.org/10.1007/s10343-023-00843-2
Shaaban A, Mahfouz H, Megawer EA, Saudy HS (2023b) Physiological changes and nutritional value of forage clitoria grown in arid agro-ecosystem as influenced by plant density and water deficit. J Soil Sci Plant Nutr 23:3735–3750. https://doi.org/10.1007/s42729-023-01294-4
Shabbir R, Singhal RK, Mishra UN, Chauhan J, Javed T, Hussain S, Kumar S, Anuragi H, Lal D, Chen P (2022) Combined abiotic stresses: challenges and potential for crop improvement. Agron 12:1–21. https://doi.org/10.3390/AGRONOMY12112795
Shahid SA, Zaman M, Heng L (2018) Soil salinity: Historical perspectives and a world overview of the problem. guideline for salinity assessment, Mitigation and Adaptation Using Nuclear and Related Techniques, pp 43–53. https://doi.org/10.1007/978-3-319-96190-3_2
Shahin MG, Saudy HS, El-Bially ME, Abd El-Momen WR, El-Gabry YA, Abd El-Samad GA, Sayed AN (2023) Physiological and agronomic responses and nutrient uptake of soybean genotypes cultivated under various sowing dates. J Soil Sci Plant Nutr. 23:5145-5158https://doi.org/10.1007/s42729-023-01389-y
Shargal A, Golobovich S, Yablovich Z, Shlizerman LA, Stern RA, Grafi G, Lev-Yadun S, Flaishman MA (2015) Synthetic cytokinins extend the phase of division of parenchyma cells in developing pear (Pyrus communis L.) fruits. J Hort Sci Biotechnol 81:915–920. https://doi.org/10.1080/14620316.2006.11512159
Sharma A, Shahzad B, Kumar V, Kohli SK (2019b) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecul 9:284–283. https://doi.org/10.3390/biom9070285
Sharma A, Kumar V, Kumar GPS, Kumar R, Kohli SK, Yadav P, Kapoor D, Bali AS, Shahzad B, Khanna K, Kumar S, Thukral AK, Bhardwaj R (2019a) Abiotic stress management in plants: role of ethylene. Molecular Plant Abiotic Stress: Biol Biotechnol 185–208. https://doi.org/10.1002/9781119463665.CH10
Shen J, Chen D, Zhang X, Song L, Dong J, Xu Q, Hu M, Cheng Y, Shen F, Wang W (2021) Mitigation of salt stress response in upland cotton (Gossypium hirsutum) by exogenous melatonin. J Plant Res 134:857–871. https://doi.org/10.1007/s10265-021-01284-6
Singh A, Banerjee A, Roychoudhury A (2022) Fluoride tolerance in rice is negatively regulated by the ‘stress-phytohormone’ abscisic acid (ABA), but promoted by ABA-antagonist growth regulators, melatonin, and gibberellic acid. Protoplasma 259:1331–1350. https://doi.org/10.1007/s00709-022-01740-7
Srivastava MK, Dwivedi UN (1998) Salicylic acid modulates glutathione metabolism in pea seedlings. J Plant Physiol 153:409–414. https://doi.org/10.1016/S0176-1617(98)80168-5
Sun C, Liu L, Wang L, Li B, Jin C, Lin X (2021) Melatonin: a master regulator of plant development and stress responses. J Integ Plant Biol 63:126–145. https://doi.org/10.1111/JIPB.12993
Sun C, Meng S, Qi M, Li T, Yin Z (2022) Melatonin alleviates oxidative damage of photosynthesis under heat stress in Tomato. SSRN 17:1–21. https://doi.org/10.2139/SSRN.4030610
Swain R, Sahoo S, Behera M, Rout GR (2023) Instigating prevalent abiotic stress resilience in crop by exogenous application of phytohormones and nutrient. Front Plant Sci 14:1–22. https://doi.org/10.3389/fpls.2023.1104874
Tan D, Reiter RJ (2020) An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J Exp Bot 71:4677–4689. https://doi.org/10.1093/jxb/eraa235
Tan D, Reiter RJ, Manchester LC, Yan M, El-Sawi M, Sainz RM, Mayo JC, Kohen R, Allegra M, Hardeland R (2002) Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2:181–197. https://doi.org/10.2174/1568026023394443
Tan K, Jing G, Liu X, Liu C, Liu X, Gao T, Deng T, Wei Z, Ma F, Li C (2023) Heterologous overexpression of HIOMT alleviates alkaline stress in apple plants by increasing melatonin concentration. Sci Hort 309:1–12. https://doi.org/10.1016/J.SCIENTA.2022.111598
Tawfik E, Ahmed MF, Albalawi DA, Aljuaid BS, Darwish DB, Mahmoud SF, Hassan KM, Ibrahim MF, Abdel Razik AB (2022) Molecular identification of Zantedeschia culture with determination of its morphometric and metabolic activities for Mediterranean acclimatization. Plants. 11:2311. https://doi.org/10.3390/plants11172311
Tiwari RK, Lal MK, Kumar R, Mangal V, Altaf MA, Sharma S, Singh B, Kumar M (2022) Insight into melatonin-mediated response and signaling in the regulation of plant defense under biotic stress. Plant Mol Biol 109:385–399. https://doi.org/10.1007/s11103-021-01202-3
Verde A, Míguez JM, Gallardo M (2023) Melatonin stimulates postharvest ripening of apples by up-regulating gene expression of ethylene synthesis enzymes. Postharv Biol Technol 195:1–17. https://doi.org/10.1016/J.POSTHARVBIO.2022.112133
Wang P, Sun X, Li C, Wei Z, Liang D, Ma F (2013) Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J Pin Re 54:292–302. https://doi.org/10.1111/JPI.12017
Wang J, Song L, Gong X, Xu J, Li M (2020a) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21:1–17. https://doi.org/10.3390/IJMS21041446
Wang T, Hu M, Yuan D, Yun Z, Gao Z, Su Z, Zhang Z (2020b) Melatonin alleviates pericarp browning in litchi fruit by regulating membrane lipid and energy metabolisms. Postharv Biol Technol 160:1–9. https://doi.org/10.1016/J.POSTHARVBIO.2019.111066
Wang D, Chen Q, Chen W, Guo Q, Xia Y, Wang S, Jing D, Liang G (2021a) Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ Exp Bot 181:1–12. https://doi.org/10.1016/J.ENVEXPBOT.2020.104291
Wang T, Song J, Liu Z, Liu Z, Cui J (2021b) Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ Sci Poll Res 28:15394–15405. https://doi.org/10.1007/s11356-020-11689-w
Wang N, Fang H, Yang Q, Liu Z, Feng H, Ji S (2022a) Exogenous melatonin alleviated leaf yellowing via inhibiting respiration and ethylene biosynthesis during shelf life in pakchoi. Plants 11:1–15. https://doi.org/10.3390/plants11162102
Wang Y, Li J, Yang L, Chan Z (2022b) Melatonin antagonizes cytokinin responses to stimulate root growth in Arabidopsis. J Plant Growth Regul 42:1833–1845. https://doi.org/10.1007/s00344-022-10663-9
Wang Y, Zhao H, Hu X, Zhang Y, Zhang Z, Zhang L, Li L, Hou L, Li M (2022c) Transcriptome and hormone analyses reveal that melatonin promotes adventitious rooting in shaded cucumber hypocotyls. Front Plant Sci 13:1–14. https://doi.org/10.3389/fpls.2022.1059482
Wang Z, Mu Y, Zhang L, Liu Z, Liu D, Jin Z, Pei Y (2023) Hydrogen sulfide mediated the melatonin induced stoma closure by regulating the K+ channel in Arabidopsis thaliana. Environ Exp Bot 205:1–11. https://doi.org/10.1016/J.ENVEXPBOT.2022.105125
Wei Z, Li C, Gao T, Zhang Z, Liang B, Lv Z, Zou Y, Ma F (2019) Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol Biochem 139:630–641. https://doi.org/10.1016/J.PLAPHY.2019.04.026
Wei Y, Bai Y, Cheng X, Zhu B, Reiter RJ, Shi H (2020) The dual roles of melatonin biosynthesis enzymes in the coordination of melatonin biosynthesis and autophagy in cassava. J Pin Res 69:1–13. https://doi.org/10.1111/JPI.12652
Wei J, Zhang Z, Zhang P, Wu B (2022) Regulation of ethylene biosynthesis and signal transduction by nitric oxide leading to resistance against Alternaria alternata in Hami melon. J Science Food Agric 102:3535–3542. https://doi.org/10.1002/JSFA.11697
Wen D, Gong B, Sun S, Liu S, Wang X, Wei M, Yang F, Li Y, Shi Q (2016) Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front Plant Sci 7:1–11. https://doi.org/10.3389/fpls.2016.00718
Wu X, Zhu Z, Li X, Zha D (2012) Effects of cytokinin on photosynthetic gas exchange, chlorophyll fluorescence parameters and antioxidative system in seedlings of eggplant (Solanum melongena L.) under salinity stress. Acta Physiol Plantar 34:2105–2114. https://doi.org/10.1007/s11738-012-1010-2
Xia H, Ni Z, Hu R, Lin L, Deng H, Wang J, Tang Y, Sun G, Wang X, Li H, Liao M, Lv X, Liang D (2020) Melatonin alleviates drought stress by a non-enzymatic and enzymatic antioxidative system in kiwifruit seedlings. Int J Mol Sci 21:1–17. https://doi.org/10.3390/IJMS21030852
Xiong YC, Xing GM, Gong CM, Li FM, Wang SM, Li ZX, Wang YF (2006) Dual role of abscisic acid on antioxidative defense in grass pea seedling (Lathyrus sativus L.). Pak J Bot 38:999–1014
Xu L, Yue Q, Xiang G, Bian F, Yao Y (2018) Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hort Res 5:1–11. https://doi.org/10.1038/s41438-018-0045-y
Xu Z, Wang J, Zhen W, Sun T, Hu X (2022) Abscisic acid alleviates harmful effect of saline–alkaline stress on tomato seedlings. Plant Physiol Biochem 175:58–67. https://doi.org/10.1016/J.PLAPHY.2022.01.018
Xu J, Wei Z, Lu X, Liu Y, Yu W, Li C (2023) Involvement of nitric oxide and melatonin enhances cadmium resistance of tomato seedlings through regulation of the Ascorbate–glutathione cycle and Ros metabolism. Int J Mol Sci 24:1–16. https://doi.org/10.3390/IJMS24119526
Yang L, You J, Li J, Wang Y, Chan Z (2021) Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J Exp Bot 72:5599–5611. https://doi.org/10.1093/JXB/ERAB196
Yang L, Bu S, Zhao S, Wang N, Xiao J, He F, Gao X (2022a) Transcriptome and physiological analysis of increase in drought stress tolerance by melatonin in tomato. PLoS ONE 17:1–23. https://doi.org/10.1371/JOURNAL.PONE.0267594
Yang S, Zhao Y, Qin X, Ding C, Chen Y, Tang Z, Huang Y, Reiter RJ, Yuan S, Yuan M (2022b) New insights into the role of melatonin in photosynthesis. J Exp Bot 73:5918–5927. https://doi.org/10.1093/JXB/ERAC230
Zahedi SM, Hosseini MS, Abadía J, Marjani M (2020) Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria × ananassa Duch.). Plant Physiol Biochem 149:313–323. https://doi.org/10.1016/J.PLAPHY.2020.02.021
Zhai R, Liu J, Liu F, Zhao Y, Liu L, Fang C, Wang H, Li X, Wang Z, Ma F, Xu L (2018) Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharv Biol Technol 139:38–46. https://doi.org/10.1016/J.POSTHARVBIO.2018.01.017
Zhang HJ, Zhang N, Yang RC, Wang L, Sun QQ, Li DB, Cao YY, Weeda S, Zhao B, Ren S, Guo YD (2014) Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J Pin Res 57:269–279. https://doi.org/10.1111/JPI.12167
Zhang J, Shi Y, Zhang X, Du H, Xu B, Huang B (2017) Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ Exp Bot 138:36–45. https://doi.org/10.1016/J.ENVEXPBOT.2017.02.012
Zhang PJ, He YC, Zhao C, Ye ZH, Yu XP (2018) Jasmonic acid-dependent defenses play a key role in defending tomato against Bemisia tabaci nymphs, but not adults. Front Plant Sci 9:1–10. https://doi.org/10.3389/fpls.2018.01065
Zhang C, He Q, Wang M, Gao X, Chen J, Shen C (2020) Exogenous indole acetic acid alleviates Cd toxicity in tea (Camellia sinensis). Ecotox Environ Saf 190:1–12. https://doi.org/10.1016/J.ECOENV.2019.110090
Zhang Y, Fan Y, Rui C, Zhang H, Xu N, Dai M, Chen X, Lu X, Wang D, Wang J, Wang J, Wang Q, Wang S, Chen C, Guo L, Zhao L, Ye W (2021) Melatonin improves cotton salt tolerance by regulating ROS scavenging system and Ca2+ signal transduction. Front Plant Sci 12:1–16. https://doi.org/10.3389/fpls.2021.693690
Zhang H, Qiu Y, Ji Y, Wu X, Xu X, Wu P (2022) Melatonin promotes seed germination via regulation of ABA signaling under low temperature stress in cucumber. J Plant Growth Regul 42:2232–2245. https://doi.org/10.1007/s00344-022-10698-y
Zhang H, Shan T, Chen Y, Lin M, Chen Y, Lin L, Chen Y, Wang H, Fan Z, Lin H, Lin Y (2023) Salicylic acid treatment delayed the browning development in the pericarp of fresh longan by regulating the metabolisms of ROS and membrane lipid. Sci Hort 318:112073. https://doi.org/10.1016/J.SCIENTA.2023.112073
Zhao YQ, Zhang ZW, Chen YE, Ding CB, Yuan S, Reiter RJ, Yuan M (2021) Melatonin: a potential agent in delaying leaf senescence. Crit Rev Plant Sci 40:1–22. https://doi.org/10.1080/07352689.2020.1865637
Zhou J, Cheng K, Huang G, Chen G, Zhou S, Huang Y, Zhang J, Duan H, Fan H (2020) Effects of exogenous 3-indoleacetic acid and cadmium stress on the physiological and biochemical characteristics of Cinnamomum camphora. Ecotox Environ Saf 191:1–7. https://doi.org/10.1016/J.ECOENV.2019.109998
Zhu XF, Wang ZW, Dong F, Lei GJ, Shi YZ, Li GX, Zheng SJ (2013) Exogenous auxin alleviates cadmium toxicity in Arabidopsis thaliana by stimulating synthesis of hemicellulose 1 and increasing the cadmium fixation capacity of root cell walls. J Hazard Mat 263:398–403. https://doi.org/10.1016/J.JHAZMAT.2013.09.018
Zubair MS, Munis MFH, Alsudays IM, Alamer KH, Haroon U, Kamal A, Ali M, Ahmed J, Ahmad Z, Attia H (2022) First report of fruit rot of cherry and its control using Fe2O3 nanoparticles synthesized in Calotropis procera. Mol 14:1–11. https://doi.org/10.3390/molecules27144461
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, M.A.A., Nasser, M.A., Abdelhamid, A.N. et al. Melatonin as a Key Factor for Regulating and Relieving Abiotic Stresses in Harmony with Phytohormones in Horticultural Plants — a Review. J Soil Sci Plant Nutr 24, 54–73 (2024). https://doi.org/10.1007/s42729-023-01586-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01586-9