Abstract
The aim of this study was to investigate fertiliser-derived N uptake of soybean from different depths of the soil under field conditions. In addition, soybean root growth in sandy and loess soil was evaluated to understand the impact of site and soybean variety characteristics on soybean N uptake under continental conditions in Central Europe. Root analysis to determine rooting depth and root length density (RLD) was carried out using the profile wall method at three growth stages and two soybean cultivars (Glycine max (L.) Merr. cvs. Merlin and Sultana) in three consecutive years at two locations in eastern Germany. Fertiliser-derived N uptake of soybean from the soil surface and the subsoil was determined at 0.3 and 0.6 m depths using 15N-labelled nitrate N. Root studies showed that soybean roots grew up to 1.4 m on sandy and loess soil sites. Root length densities of up to 2.4 cm cm−3 were documented in the topsoil. By means of 15N application, soybean was shown to take up 15% of the surface-applied nitrogen in the dry growing season and 67 % in high rainfall years, between 19 and 77 % of the nitrogen placed at 0.3 m soil depth, and between 2 and 64 % of the nitrogen placed at 0.6 m soil depth by flowering. The field trials showed that soybeans can absorb a high proportion of the nitrogen placed in the subsoil by flowering time. Due to a well-developed root system reaching deep into the soil, soybeans are able to cover their N demand from soil-borne sources and secure yield formation during dry periods by water uptake from the subsoil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soybeans have recently become one of the most important grain legumes in Central Europe, contributing to the protein supply for humans and animals. For example, the area planted with soybeans in Germany doubled to 47,000 ha between 2016 and 2022 (Statista 2022).
Under dry conditions in the growing season, conditions that are increasingly observed at many locations in Central Europe, even annual crops, such as soybeans, must have a deep-reaching root system to use plant-available water in the subsoil for yield formation. Especially during flowering, soybeans have an increased water demand. Therefore, several studies have concluded that drought stress during the reproductive phase of flowering and pod formation results in a lower grain yield in soybean (Demirtas et al. 2010; Frederick et al. 2001; Sionit and Kramer 1977). He et al. (2020) showed that low irrigation during flowering time leads to a better distribution of roots in the subsoil, resulting in greater flower and pod formation. In addition, root growth is subject to a temperature-dependent optimum curve. The ratio of shoot to root growth shifts in favour of the roots at low temperatures but also when there is a lack of water and nutrient availability in the soil (Kutschera et al. 2018; Turman et al. 1995). Here, even a few deep roots are sufficient to maintain the water supply during drought events (Reicosky et al. 1972). According to Allmaras et al. (1975), soybeans can reach a rooting depth of up to 1.2 m and an RLD of 0.2 cm cm−3 in the topsoil at the time of pod formation until pod filling. Turman et al. (1995) showed that an earlier sowing date in mid-April reduced soybean root growth compared to sowing in mid-June. In this regard, an average RLD of 0.73 cm cm−3 was measured for early seeding, and an average RLD of 1.65 cm cm−3 at a depth of 0.56 m was measured for later seeding dates. Hoogenboom et al. (1987) described that root growth up to 0.4 m has already taken place in the early vegetative stage. In this context, dry phases at the beginning are even beneficial for root growth because during drought stress, almost all photosynthetic products enter the root; thus, an appropriate root system is established. Therefore, plants are more tolerant to drought stress at sensitive times, such as flowering, pod formation, and pod filling. As a result, soybeans under drought stress reach a rooting depth of 2.0 m for pod filling, compared to 0.9 m for irrigated soybean. In contrast, in years without drought stress, a maximum rooting depth of 1.2 m is reached at the time of pod formation (Hoogenboom et al. 1987).
Little data are available on the root growth of currently cultivated, high-yielding soybean varieties, which are now increasingly grown in Central Europe. Kutschera et al. (2018) plotted the root profile of soybeans grown under Austrian conditions and documented a maximum soybean rooting depth of 1.4 m at pod filling, which is the only available data on soybean root growth in Central Europe.
Kautz et al. (2013) pointed out that studies on nutrient uptake from subsoil have been neglected thus far. There are indications, however, that the subsoil can contribute significantly to the N, P (phosphorus), and K (potassium) supply of plants. Especially in view of the increasing drought in summer, it is important to promote nutrient uptake from the subsoil. Han et al. (2022) investigated the P uptake potential of various perennial crops under field conditions. In this study, 33P-labelled soil was incorporated obliquely into ingrowth cores up to 4.2 m in the subsoil. P uptake was strongly plant species dependent and detectable across all depth levels. Rasmussen et al. (2020) studied nutrient uptake from subsoil using rhizoboxes in chicory. They demonstrated uptake of 15N from 3.5 m and trace elements from a 2.3 m soil depth. Furthermore, it was shown that nutrient uptake from the topsoil, which is limited by drought, could be compensated for by the subsoil. Chen et al. (2021) further complemented these results with their studies on chicory. In the plant samples studied, the 2H and 15N values were about 10 times higher at a depth of 1.1 m compared to 3.5 m. Time and nutrient availability in the soil are major influencing factors. This explains why the 15N values decreased in the topsoil and increased to a larger extent in deeper soil layers under dry conditions.
Measurements in sugar beets between 3 and 18 weeks after planting showed that N uptake from the topsoil (up to 0.3 m) was greatest throughout the growth phase, although nutrient uptake was detected at all depth levels (up to 1.2 m). In addition, lateral movement of labelled N fertiliser from the injection site of 0.1 m was documented (Zinati et al. 2001).
Swiss and French studies have investigated N2 fixation in soybean. There are no data available except for one study on surface enrichment with 15N-labelled fertilisers (Oberson et al. 2007). Soybeans did not contribute to an improvement in N supply in any of the systems investigated since N removal by the grains was higher than the symbiotic N2 fixation of the soybeans. In this context, Amarger et al. (1979) found that non-inoculated soybeans have a higher 15N content than inoculated soybeans because the value of 15N uptake decreases the more a plant fixes itself. Similarly, the results of Kohl et al. (1980) showed that the 15N value is higher in soybeans without nodules than in soybeans with nodules. They also reported that the percentage of fixed N from the atmosphere of total plant N (%Ndfa) ranged from 21.7 to 62.1% depending on the growth stage.
Despite their major importance, there are only a few studies on soybeans under field conditions that deal with root growth and nutrient uptake. To fill this gap, field trials were established at two locations over 3 years, and rooting depth, root length density, and N uptake from the soil surface and subsoil were investigated using 15N-labelled fertiliser.
In the field study presented here, the following hypotheses were tested: (i) The point enrichment method used provides valid data on the N uptake ability of soybeans in different soil layers, thus confirming the method used. This is to be expected because (ii) soybeans take up less N from deeper soil layers (0.6 m) than from topsoil (0.3 m). (iii) The studied genotypes differ in their N uptake capacity; therefore, deeper-rooted varieties can take up more N from the subsoil. Based on the difference in yield potential between the cultivars Merlin and Sultana, it was assumed that Sultana developed deeper roots and could thus tap the water and nutrient reserves in the subsoil better than Merlin.
2 Materials and Methods
2.1 Experimental Design and Site Description
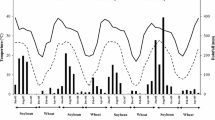
From 2017 to 2019, the field trials were conducted on agricultural land in Käbschütztal near Meißen (MEI) and on the experimental field of the Leibniz Centre for Agricultural Landscape Research (ZALF) e.V. (MÜ) in Germany. A description of the sites, as well as temperature and precipitation data for the experimental years, are presented in Supplementary Information (SI) Table S1 and Fig. 1. The field trials were performed in a split-plot design (main plots: cultivars; subplots: application depth of 15N-enriched nitrate) with four replicates and a single subplot size of 10.0 × 1.5 m in MEI and 8.0 × 3.0 m in MÜ. In this case, the experiment was conducted in a new area each year to ensure that the plots were free of 15N-labelled fertiliser.
The land at MEI was farmed conventionally long term, while at MÜ, it was farmed organically for more than 20 years. The preceding crop before soybean was winter wheat at the MEI site in all 3 years, oats at the MÜ site in 2018, and winter rye in 2019. For better weed control, MÜ was cultivated twice and ploughed once before seeding. In MEI, only two tillage operations were carried out with the cultivator before sowing. The soybean cultivars Merlin and Sultana were investigated. Inoculation was performed with two teaspoons of peat preparation (HiStick, BASF) per plot (equivalent to about 5.7 kg ha−1). The inoculant HiStick was mixed with the seeds directly before sowing and then applied together. The narrow-leafed lupin (Lupinus angustifolius L., cv. Boregine, non-inoculated seeds) was chosen as the reference crop for soybean in 2017, and niger seed (Guizotia abyssinica) was chosen in 2018 and 2019. Weed control was performed in MÜ with a harrow and hoe. Herbicides in the form of Clomazone (0.25 l ha−1 in 250 l of water) and Metribuzin (0.4 l ha−1 in 250 l of water) were used at MEI after sowing. The seeding densities were 80 germinating seeds m−2 (cvs. Merlin and Sultana) at MEI and 84 seeds m−2 for both varieties in MÜ. The row spacing was 0.33 m at MEI and 0.5 m at MÜ.
2.2 Determination of Soil Parameters
For a better characterisation of the locations, the sowing times and yields in the respective trial years are shown in Table 1. To classify the soil conditions, a basic soil nutrient analysis was carried out from the top 0.3 m (BBCH 11; V1) (Table 1). A basic chemical analysis was carried out on each soil sample in accordance with the guidelines for soil analysis of the Association of German Agricultural Analytic and Research Institutes e.V. (VDLUFA). The pH value (Association of German Agricultural Analytic and Research Institutes 1991d) and the phosphorus (P), potassium (K) (Association of German Agricultural Analytic and Research Institutes 1991b), magnesium (Mg) (Association of German Agricultural Analytic and Research Institutes 1991c), humus (DIN EN 15936:2012-11 2012), and total nitrogen (Nt) contents (Landwirtschaftliche Kommunikations- und Servicegesellschaft mbH LKS 2019; DIN ISO 11277:2002-08 2002) of the soil were determined accordingly.
To evaluate the inorganic soil N (Nmin) content, three soil samples per plot were collected from the sites using a Pürckhauer soil auger (diameter, 0.03 m) after sowing soybean. Mixed samples from three incremental samples were collected from depths of 0 to 0.3, 0.3 to 0.6, and 0.6 to 0.9 m. The samples were immediately cooled down with a cooler box to < 5 °C in the field for transport and were stored for further analysis at – 18 °C on the same day. To determine the Nmin content in the soil, 100 g of moist soil was mixed with 250 ml of 0.01 M CaCl2 solution for 60 min in a shaker (manufacturer GFL) with an overhead spinning agitation. Subsequently, the extract was filtered (folding filter, MN 615.25; diameter, 150 mm; filtration time, 22 s; thickness, 0.16 mm, retention, > 4 μm), and the supernatant was stored in polyethylene tubes (10 ml) at − 18 °C. The Nmin content of the extract was analysed using the VDLUFA method (Association of German Agricultural Analytic and Research Institutes 1991a). About 90 % was present as NO3− and the remainder as NH4+. The initial Nmin (NO3− + NH4+) values were at a low level, except at MEI in 2017.
The soil water content was determined volumetrically at MEI using a profile probe (PR2 profile probe, UP GmbH, accuracy ± 0.04 m3 m−3, 0 to 40 °C). For this purpose, after seeding in two plots per block, a fibreglass tube was inserted one metre deep into the soil and sealed with a lid. The profile probe was used to measure the volumetric soil water content at 0.1, 0.2, 0.3, 0.4, 0.6, and 1 m depths on five (2018) and six dates (2019) in the experimental field. In MÜ, the soil water content was determined gravimetrically. For this purpose, a soil sample was taken at different times in two plots per block with a drill stick (Pürckhauer soil auger; diameter, 0.03 m) to a depth of 1 m. The soil moisture content was determined every 0.1 m. The insertion of soil probes was not possible at the MÜ site.
The bulk density of the soil was determined at three soil layers, 0 – 0.3, 0.3 – 0.8, and 0.8 – 1.2 m depth. For this purpose, a pit for root determination was used. Eight cylinders (100 cm3) were collected from each soil layer. The samples were dried for 24 h at 105 °C in a drying oven (Heraeus Instruments, UT 6760). Subsequently, the weight of the soil sample was recorded without cylinders, and the bulk density (dB) was calculated.
2.3 15N Enrichment and Analysis
The application of 15N-enriched nitrate was performed at three levels: at the soil surface (1.35 × 2.0 m), at a soil depth of 0.3 m, and at a depth of 0.6 m. The surface application was carried out with a battery-powered backpack sprayer immediately after seeding before the soybeans had germinated. For this purpose, the enriched 15N fertiliser was dissolved in water and applied to the plots (600 ml per plot). Deep enrichment was applied to the side of the plot when the soybeans had up to two leaves. For this purpose, a wooden angle (45°) was used to hammer a Pürckhauer soil auger (diameter, 0.03 m) into the ground for 0.43 or 0.85 m. Thus, the soil was removed to the point of enrichment. Subsequently, a plastic tube (diameter, 0.025 m) was inserted into the pre-drilled hole. An aqueous solution (50 ml) containing the labelled nitrogen was then added to the tube with a syringe and rinsed with 30 ml of water to prevent the fertiliser residue from remaining in the tube. Finally, the tube was covered with adhesive tape and left in the soil until the final harvest. From the end of the tube, 0.3 or 0.6 m was measured in the direction of lay, and the place was marked for later harvesting (similar to the principle of Han et al. (2022)).
The advantage of this approach is that soybeans should not root along the shaft, making it easier to reach the 15N-enriched fertiliser depot. In addition, the tube was covered to prevent the water from reaching the enrichment point.
Potassium nitrate and ammonium sulphate labelled with 15N from Merck with a purity of > 99% were used for the experiment. The following placement of the 15N-enriched fertiliser was conducted (Table 2):
At the MEI site, enrichment with 15N-labelled fertiliser was carried out in all three trial years. For MÜ, only data for two depths were available for 2017.
Harvesting of the fertilised plots was performed in two steps. First, the samples with enrichment at 0.3 or 0.6 m depth were harvested at the end of flowering or the beginning of pod formation, respectively, as it was assumed that the time of maximum N demand had already been exceeded at this time. For harvesting at MEI, a metal ring with a 0.5 m2 area was placed in the centre of the already marked point. Within the ring, the entire stand of plants was cut off by hand just above the soil surface. Due to the wider row space at the MÜ site, a ring was not used, but 0.5 linear metres were harvested in the marked row. As a control, in addition to the enriched samples, a non-enriched sample was also collected from each plot.
Harvesting of the surface-enriched plots was done at the beginning of pod maturity (BBCH 80/85 (Meier 2018); R7 (Fehr et al. 1971)). Since the plants had already lost their first leaves by this time, two collection baskets (1.0 × 0.2 m) were placed in the enriched plots before the first leaf fall. The leaves, as well as the other plant samples, were regularly collected and processed. For this purpose, the middle three rows (0.81 m2) of each plot were harvested by hand. The processing of the harvest took place immediately afterward. The harvested plants were weighed, shredded, and dried at 55 °C until a constant dry weight was reached. Microanalysis (EuroEA3000-Hekatech) was used to determine the contents of total N and carbon (C) in shoots (chromatographic separation of the oxidation gases). The principle of analysis in this machine is based on dynamic flash combustion, followed by gas chromatography separation of the resultant gaseous species (Karasek and Ray 1988). For this purpose, the samples were ground to a particle size of ≤ 0.2 mm, and the material was filled into 3 × 6 mm tin capsules (IVA Analysentechnik, Meerbusch) and weighed with a fine balance (Mettler Toledo XA 105 Dual Range) with an accuracy of ± 0.01 mg. The amount weighed was based on the C to N ratio of the plant material, which ranged from 2.8 to 3.0 mg for soybeans.
Stable nitrogen isotope ratio analysis of 15N and 14N was performed at the UC Davis Stable Isotope Facility Laboratory, USA (PDZ Europa ANCA-GSL elemental analyser coupled with PDZ Europa 20 - 20 isotope mass spectrometer). The delta (δ) 15N values of the applied fertiliser were calculated to be 29.222 ‰ and 266.540 ‰ (Peoples et al. 1989), where atmospheric N2 is usually the ultimate standard (0.3663 at.% 15N; (Mariotti 1983)).
The proportion of nitrogen derived from fertiliser (% Ndff) in the soybean shoots is calculated using Eq. (1) (Jensen 1994):
- Se:
-
15N enrichment level enriched soybean, δ15N (‰)
- Sne:
-
15N enrichment level non-enriched soybean, δ15N (‰)
- Afert:
-
15N enrichment level of the applied fertiliser δ15N (‰)
The 15N recovery of the applied fertiliser rate (RR) is calculated following Eq. (2) (Jensen 1994):
- % Ndff:
-
Nitrogen derived from fertiliser in the shoot (%)
- Ntshoot:
-
Soybean shoot Nt content (g/harvest area)
- ANfert:
-
applied amount of fertiliser (g)
2.4 Root Analysis
Root examinations were performed using the profile wall method (Böhm 1979). For this purpose, a soil pit was made in the experimental field when the soybean plants had two to three leaves. The pit ran the full width of the trial in MEI to allow two replicates per cultivar. In MÜ, only one plot per cultivar was examined. An overview of the individual trial dates is presented in SI Table S2.
Five steps were performed as part of the profile wall method: (1) digging the trench; (2) preparing the profile wall; (3) exposing the roots; (4) mapping the roots; and (5) counting procedure. At each examination date, the profile wall was removed for a further 0.2 to 0.25 m to record a new root image. An acrylic glass sheet (1 m × 1 m) was used to map the roots. A transparent film with a grid (0.05 m × 0.05 m) was placed on it. Marking was done with one point per 0.005 m root length. The root length per 0.05 m soil depth is recorded in 20 parallel squares (0.05 × 0.05 m), and the mean root length density and the standard deviation of the mean are calculated, which are shown in Supplementary Material Tables S5–S7. Plots were weed free to ensure that only soybean roots were documented.
2.5 Statistical Analysis
The statistical analysis system (SAS) programme version 9.3 of SAS Institute Inc. 2013 was used for the statistical analysis. The normal distribution of the data was tested according to Shapiro–Wilk (univariate normal procedure) (Munzert 2015). A one-factor analysis of variance (ANOVA) was performed for the yield comparisons of the two cultivars and for the evaluation of the areal enrichment. Cultivars and fertilisation depth were assessed using a two-factor analysis of variance. For the multiple mean value comparison of the balanced data, Tukey’s test was used. Significant differences between cultivars were indicated, with error probabilities (α) of < 0.05, < 0.01, and < 0.001 and are indicated by different letters in the tables.
3 Results
3.1 Root Examination
The root length density and rooting depth of soybean cultivars on both loess and sandy soils reached a maximum rooting depth between 0.6 and 0.85 m at the time of flowering (Figs. 2 and 3). At the time of pod formation or the beginning of maturity, a maximum rooting depth of 1.35 m was documented. The highest root length density was recorded between 0.15 and 0.2 m soil depth and ranged from 0.5 to 2.4 cm cm−3. Root length density gradually decreased to a soil depth of 0.3 to 0.4 m, and only a few roots were detected in deep soil layers.
Root length density of soybean cultivars (●Merlin; ○Sultana) at the Meißen (MEI) site in A 2017; B 2018; and C 2019 and at three growth stages of soybeans. One point corresponds to the average of an area 5 × 100 cm; there were two root profiles per variety on each day of investigation (N = 2); Tables S5–S7 in the Supplementary material provide additional information on standard deviation
Root length density of soybean cultivars (●Merlin; ○Sultana) at the Müncheberg (MÜ) site in A 2018 and B 2019 and at three growth stages of soybeans. One point corresponds to the average of an area 5 × 100 cm; there was one root profile per variety on each day of investigation (N = 1); Table S6–S7 in the Supplementary material provides additional information on standard deviation
There were hardly any visible differences between the cultivars Merlin and Sultana. Sultana appeared to have a higher root length density in the topsoil layer, up to 0.4 m, while Merlin was mostly rooted slightly deeper.
Differences between the years were observed at the MEI site. In 2017, a maximum rooting depth of 1.0 m was documented on the last survey date. In addition, the root length density was more pronounced in the upper soil layers, up to about 0.3 m, than in 2018 and 2019. In 2018, the root growth in the upper soil layer was the lowest, and a maximum rooting depth of 1.35 m was measured, which could be related to low water availability. In 2019, stronger growth in the upper soil layer, up to 1.0 cm cm−3, and a maximum rooting depth of 1.35 m were observed. At the MÜ site, growth similar to MEI was observed. The maximum rooting depth in 2018 was only 0.9 m, and in 2019, there were stronger fluctuations in the lower soil layer between 0.6 and 0.8 m for the Merlin cultivar on the second and third investigation dates. The measurement of soil water content confirmed a strong decrease in soil moisture during vegetation (Supplementary Material Fig. S1). Due to site differences, the soil water content in spring was only half as high at MÜ as at MEI in 2018 and 2019. The decrease in soil moisture was also visible in the lower soil layer of 0.6–1 m during flowering to pod formation. This indicates that both soybean varieties drew water from the lower soil layer at flowering. However, despite the large site differences due to soil properties, soybean root characteristics for 2018 and 2019 and varieties were very similar.
3.2 Soybean N Uptake
For the non-fertilised soybeans and the reference crop, a decrease in N yield in the soybean shoots was evident over the course of 2017–2019 (SI Table S3). Therefore, a significantly higher N yield was found at MEI than at MÜ for individual years. Comparing the varieties or the reference crops, the Merlin variety achieved the highest N yield in four out of five cases. The reference crop, as a non-legume, yielded the lowest N yield in all years and locations. Compared to the 15N-fertilised samples, the N content of the unfertilised samples was in a similar range, sometimes slightly above or below.
In 2017, the variants with surface 15N fertilisation also showed the highest N yield (Table 3). The N yield decreased in 2018 and increased again in 2019. A higher N yield was documented at MEI than at MÜ. When comparing the cultivars, there were no significant differences at MEI. However, at MÜ, the shoot N yield of the Merlin cultivar was significantly higher than that of Sultana. The reference crop showed the lowest N yield in all years and locations.
Except at MÜ in 2018, there were no significant differences in shoot N content between varieties or fertilisation depths. Table 4 shows that the shoot N content decreased from year to year. As in previous evaluations, N yields were also higher in MEI than in MÜ. In four out of five cases, the shoot N content was higher in Merlin than in Sultana, although the difference was only significant at MÜ in 2018 (Merlin, 67.5 kg ha−1; Sultana, 50.9 kg ha−1) (Table 4). A more differentiated examination for the 15N enrichment showed that Merlin had a higher N shoot content (70.3 kg ha−1) than Sultana (36.8 kg ha−1) in microplots of 15N application at a 0.30 m depth. In contrast, in microplots of N application at 0.6 m depth, the N shoot content of both varieties was similar (Sultana, 64.9 kg ha−1; Merlin, 64.8 kg ha−1; Table 5).
In the regression analysis of average root length density (cm/cm3) in the root profile and total shoot N uptake (kg/ha), data for all sites, varieties, and experimental years revealed a weakly significant relationship (R2 = 0.41; p = 0.002; N = 20).
3.3 N Acquisition from Different Soil Depths
Soybean and the reference crop from the plots not enriched in 15N had 15N enrichment levels between 2.2 and 4.4 δ15N‰ and between 4.4 and 6.8 δ15N‰, respectively (SI Table S4). Compared to non-fertilised soybean, the measured 15N values were significantly higher in the 15N-fertilised soybean samples (Fig. 4). In 2017, the results were low at all depths of investigation, and in 2019, the highest delta 15N values were obtained. The surface 15N fertilisation results were higher than after 15N application at 0.3 and 0.6 m in three of the five cases. Nitrogen was obviously better taken up by plants at MÜ than at MEI, as the measured delta N values were higher across all depth levels studied. In 2018 and 2019, the non-N2-fixing reference plant also showed the highest 15N enrichment of all plants. In 2018, at MÜ, the value of the reference plant deviated. Due to the severe drought in the summer of 2018, the niger seed hardly grew at MÜ. Therefore, in 2019, regular irrigation was conducted to provide the plants with sufficient water. Delta 15N values at MÜ showed that niger seed roots reached a depth of 0.6 m in 2018 to absorb fertiliser adequately. When comparing soybean varieties, the 15N content of Sultana at MÜ was higher than that of Merlin across all years and enrichment levels. At MEI, Merlin had higher delta 15N values only for surface enrichment.
Delta 15N values (‰) in above ground plant biomass after 15N fertilisation to surface, at 0.3 m and 0.6 m subsoil in A 2017, B 2018, and C 2019 at two experimental sites. Ref., reference plant; cultivar: S, Sultana; M, Merlin; trial sites: MEI, Meißen; MÜ, Müncheberg; bars indicate the standard error (SE); four replicates per fertilisation depth and variety (N = 4)
Delta N (‰) in soybean shoots varied highly between years, sites, and cultivars: delta N values in soybean shoots increased annually, reaching the highest values in 2019 (Tables 6 and 7). Within the individual years of investigation, no significant differences were found between the varieties Merlin and Sultana. However, as a result, delta N values in the shoots of Sultana were higher than those for Merlin in four out of six cases. The reference crop had higher delta N values than soybean, except for MÜ in 2018 (Table 6).
In comparison, significantly higher delta N values in soybean shoots occurred after 15N application at 0.3 m depth compared to 0.6 m depth at both study sites. The only non-significant result occurred at MEI in 2019, but again, values were higher after 15N application at 0.3 m depth than at 0.6 m depth. On average, among the cultivars, the measured delta N values at MÜ were higher than at MEI in all cases (Table 7).
The percentage of N derived from fertiliser (% Ndff) in soybean shoots was lowest after surface 15N application in 2017 (MEI 17 reference = 0.354 %) and increased annually (MÜ 19 reference = 3.328 %) (Table 8). In five out of six cases, the % Ndff in soybean shoots was higher at MÜ than at MEI. There was no significant difference between the cultivars within the individual years. At MEI, the Merlin variety showed a higher % Ndff, whereas at MÜ, this trend was observed for Sultana.
Except for at MÜ in 2018, no significant interactions between cultivar*depth were found for % Ndff in soybean shoots. No significant differences were identified between the soybean cultivars. At MÜ, the cultivar Sultana tended to take up more of the 15N fertiliser, but the difference was not statistically significant (Table 9).
After 15N application at a depth of 0.3 m, plants took up significantly more fertiliser N compared to 15N application at a depth of 0.6 m in all years and locations. Only at MÜ in 2018 was no significant difference observed with the depth of 15N application (Table 10). After 15N application at 0.3 m, the average value of % Ndff was 1.218 for Merlin and 0.824 for Sultana; at 0.6 m, Merlin averaged 0.383, and Sultana averaged 1.864.
No differences were found between the varieties when calculating the 15N recovery rate. Soybean at MEI in 2017 showed the highest 15N uptake efficiency of about 67% after surface application. In dry years (2018 and 2019), the recovery rate decreased sharply at both sites (7.5 – 26.8 %) after surface application. For subsoil application, the recovery rate of 15N fertiliser at 0.3 m depth was, on average, more than twice as high (about 45 %) as at 0.6 m depth (21 %). Thus, the data show plausible results and confirm the methodology used.
4 Discussion
Roots serve plants primarily for water and nutrient uptake, with rooting depth and root length density in space and time representing the central characteristics of their appropriation. Thus far, only a few studies on soybean root growth in the field are available (Allmaras et al. 1975; Kutschera et al. 2018; Ordóñez et al. 2018; Turman et al. 1995). This is especially true for central European conditions. The determined maximum rooting depth of almost 1.4 m corresponds to the data of Kutschera et al. (2018). However, the rooting depth depends on various influencing factors, such as sowing time, water availability, and soil conditions. Ordóñez et al. (2018) also measured a maximum soybean rooting depth of 1.2 to 1.57 m at various locations in the USA and demonstrated that rooting depth is significantly related to water table depth. Due to the deep root growth of soybeans, they were also able to use water reserves in the subsoil, especially in low precipitation years 2018 and 2019. The determined decrease in soil water reserves in the subsoil showed this very clearly (see figures in the Supplementary Material Fig. S1). Therefore, soybeans have a significantly higher capacity to absorb water from the subsoil than, for example, pea or faba bean, which generally do not root deeper than 100 cm (Li et al. 2005; Liu et al. 2011). However, at flowering, soybeans always only reached a maximum rooting depth of 60 cm (Figs. 2 and 3); thus, at this stage, they still confer an insufficient ability to absorb water from the subsoil. In 2018 and 2019, this could be observed at both experimental sites. Dry phases at the flowering stage of soybean are often associated with sensitive yield losses. Serraj and Sinclairs (1998) showed that soybeans are sensitive to soil drought and that nodulation and N2 fixation are also reduced by drought.
At MEI, the maximum rooting depth in 2018 and 2019 was about 0.15 m deeper than in 2017 (Fig. 2) when there was corresponding rainfall in the summer. This confirms the findings of Hoogenboom et al. (1987), who found that drought stress during the early stages of plant growth promotes deep root growth.
Similarly, the measured RLD shows that soybeans tend to grow deeper roots rather than wider roots when water is scarce during flowering. Therefore, topsoil RLD values in 2018 (0.5 to 0.6 cm cm−3) and 2019 (0.25 to 0.5 cm cm−3) were lower than those in the 2017 experimental year (0.8 to 1.1 cm cm−3). In addition, the documented RLD confirms the magnitude of Turman et al. (1995) and Allmaras et al. (1975). The applied profile wall method represents a suitable possibility, as confirmed by Bublitz et al. (2021) in their study on intercrops. In this context, the absolute values in heavily rooted areas are below the measurement results of methods used to study root development in soil cores and monoliths and are thus probably underestimated (Bublitz et al. 2021).
RLD in the topsoil decisively contributes to the P uptake of plants. Grain legumes have a high P requirement. However, Kautz et al. (2013) pointed out that subsoil can take up over two-thirds of the N, P, and K supply during severe drought. Thus, deep roots can also ensure nutrient supply during pod formation and the pod-filling phase of soybean. This can be justified by the better water availability in the subsoil, especially during dry periods, as shown here (Supplementary Material Fig. S1). Even if the RLD is better developed in the topsoil, the roots cannot take up the nutrients from the soil if soil moisture is lacking (Farmaha et al. 2011). Han et al. (2022) also demonstrated in their field experiments that roots are more effective in taking up P in the subsoil than in the topsoil, but this is highly dependent on the plant species.
It should be emphasised that there were no differences in root growth between the tested soybean cultivars or between sites and their different soil conditions. Due to the lower soil bulk density, it was assumed that the roots would root deeper in the sandy soil at MÜ than at MEI.
In addition, other factors, such as soil water and nutrient supply, play a significant role. In principle, the results can be used to demonstrate that soybeans can absorb inorganic N and water from deep soil layers during flowering until pod filling. Furthermore, it is likely that soybeans are exposed to less water stress during the pod-filling phase than during the flowering phase due to their deeper root growth at a later time.
The two soybean cultivars also showed no striking differences in shoot N accumulation, analogous to the results of the root tests. Only in one case was there a significant difference between the two investigated cultivars, which occurred at MÜ in 2018, with both a surface 15N application and a subsoil spot enrichment with 15N. The apparent greatest influence on N yield was water availability in each year of the experiment. In 2017, the shoot N yield was highest (219 to 249 kg ha−1), but it declined distinctly in 2018 (102 to 167 kg ha−1) and increased again in 2019 (184 to 211 kg ha−1). Figure 1 shows the course of precipitation in the individual years. From this, it can be inferred that the precipitation in July 2019 at MÜ, with over 100 l m−2, likely had a positive effect on soybean plant growth and, thus, N yield. Based on this series of experiments via a corresponding regression analysis, the intensive root growth of soybeans in the field was apparently a prerequisite for high N accumulation in the shoots. Intensive root growth ensures soybean not only a water supply but also inorganic nitrogen uptake from the subsoil, as shown by the results of 15N application in the subsoil. This is because mineral nitrogen uptake contributes to soybean yield stability under drought conditions, as shown by Purcell and King (2008).
Purcell and King (2008) showed that N fertilisation increases soybean drought tolerance, as measured by the biomass rate, N accumulation, and the reduction in flower and pod dieback.
From a sustainability point of view, plants that accumulate N via symbiotic N2 fixation should not be fertilised with N. However, due to the increasing dry periods, Purcell and King (2008) and Salvagiotti et al. (2008) agree that to ensure a stable soybean yield and thus an appropriate protein supply in Central Europe under dry conditions.
Following the calculations of Shearer and Kohl (1986), plants fertilised with 15N absorbed an average of 118 kg N ha−1 from the soil. In comparison, the non-fertilised control samples took up between 55 and 271 kg N ha−1 (mean 99 kg ha−1).
Subsoil spot enrichment with 15N-labelled nitrate, tested here for the first time in soybeans in field trials, has been shown to be well-suited to directly quantify the appropriate amount of inorganic nitrogen under field conditions. 15N application resulted in correspondingly distinct 15N signals in soybean shoots during flowering (Fig. 4), which was also attenuated with application depth and was stronger under dry conditions than under wet conditions. This was highlighted by the progression of soybean root depth growth (Figs. 2 and 3). Rasmussen et al. (2020) and Chen et al. (2021) showed the depth-dependent capacity for N uptake from the subsoil in chicory, with a spot 15N application in the subsoil, analogous to the results shown here on soybean. However, to improve the precision of the method chosen here, in further projects, several, e.g. 3 to 5, point applications should be made per square metre. It would also be advisable to use 15N-labelled ammonium enrichment instead of nitrate since ammonium is less mobile in the soil than nitrate.
5 Conclusions
Field trials with two soybean varieties carried out over two locations and 3 years have shown that
-
(i)
A valid estimation of the uptake of mineral nitrogen from the subsoil by soybeans under field conditions can be made by means of a point enrichment with 15N-labelled nitrate carried out at a depth of 0.3 and 0.6 m; the soybeans took up an average of 45 % (0.3 m) and 21 % (0.6 m) of the applied nitrogen until flowering.
-
(ii)
Independent of variety and location, but depending on the water supply at the location, the soybeans reached a maximum rooting depth of approximately 0.6 m until flowering and 1.4 m until the end of pod filling.
-
(iii)
the genotypes studied did not differ significantly in their rooting patterns and N uptake capacity.
Due to a well-developed root system reaching deep into the soil, soybeans are able to additionally cover their Nitrogen requirement from soil-borne sources and to secure yield formation in dry periods by water uptake from the subsoil.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Allmaras RR, Nelson WW, Voorhees WB (1975) Soybean and corn rooting in southwestern Minnesota: II. Root distributions and related water inflow. Soil Sci Soc Am J 39:771–777. https://doi.org/10.2136/sssaj1975.03615995003900040046x
Amarger N, Mariotti A, Mariotti F, Durr JC, Bourguignon C, Lagacherie B (1979) Estimate of symbiotically fixed nitrogen in field grown soybeans using variations in15N Natural abundance. Plant Soil 52:269–280. https://doi.org/10.1007/BF02184565
Association of German Agricultural Analytic and Research Institutes (German) (1991a) Methode A 6.1.4.1: Bestimmung von mineralischem Stickstoff (Nitrat und Ammonium) in Bodenprofilen (Nmin-Labormethode). 3. Teillieferung 2002 zu Band I. In: Association of German Agricultural Analytic and Research Institutes (eds) Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch). VDLUFA-Verlag, Darmstadt
Association of German Agricultural Analytic and Research Institutes (German) (1991b) Methode A 6.2.1.1: Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug: 6. Teillieferung 2012. In: Association of German Agricultural Analytic and Research Institutes (eds) Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch). VDLUFA-Verlag, Darmstadt
Association of German Agricultural Analytic and Research Institutes (German) (1991c) Methode A 6.2.4.1: Bestimmung des pflanzenverfügbaren Magnesiums im Calciumchlorid-Auszug. In: Association of German Agricultural Analytic and Research Institutes (eds) Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch). VDLUFA-Verlag, Darmstadt
Association of German Agricultural Analytic and Research Institutes (German) (1991d) Methode A 5.1.1: Bestimmung des pH-Wertes. 7. Teillieferung 2016. In: Association of German Agricultural Analytic and Research Institutes (eds) Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch). VDLUFA-Verlag, Darmstadt
Böhm W (1979) Methods of studying root systems. Springer-Verlag, Berlin
Bublitz TA, Kemper R, Müller P, Kautz T, Döring TF, Athmann M (2021) Relating profile wall root-length density estimates to monolith root-length density measurements of cover crops. Agronomy 12:48. https://doi.org/10.3390/agronomy12010048
Chen G, Dresbøll DB, Thorup-Kristensen K (2021) Dual labelling by 2H and 15N revealed differences in uptake potential by deep roots of chicory. Rhizosphere 19:100368. https://doi.org/10.1016/j.rhisph.2021.100368
Demirtas C, Yazgan S, Candogan BN, Sincik M, Büyükcangaz H, Göksoy AT (2010) Quality and yield response of soybean (Glycine max L. Merrill) to drought stress in subhumid environment. Afr J Biotech 9(41):6873–6881
DIN EN 15936:2012-11 (German) (2012) Schlamm, Behandelter Bioabfall, Boden und Abfall—Bestimmung des Gesamten Organischen Kohlenstoffs (TOC) Mittels Trockener Verbrennung. Deutsche Fassung EN 15936:2012. Beuth Verlag GmbH, Berlin
DIN ISO 11277:2002-08 (German) (2002) Bodenbeschaffenheit—Bestimmung der Partikelgrößenverteilung in Mineralböden—Verfahren mittels Siebung und Sedimentation (ISO 11277:1998 + ISO 11277:1998 Corrigendum 1:2002). Beuth Verlag, Berlin
Farmaha BS, Fernández FG, Nafziger ED (2011) Distribution of soybean roots, soil water, phosphorus and potassium concentrations with broadcast and subsurface-band fertilization. Soil Sci Soc Am J 76:1079–1089. https://doi.org/10.2136/sssaj2011.0202
Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merrill 1. Crop Sci 11:929–931. https://doi.org/10.2135/cropsci1971.0011183X001100060051x
Frederick JR, Camp CR, Bauer PJ (2001) Drought-Stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Sci 41:759–763. https://doi.org/10.2135/cropsci2001.413759x
Han E, Dresbøll DB, Thorup-Kristensen K (2022) Tracing deep P uptake potential in arable subsoil using radioactive 33P isotope. Plant Soil 472:91–104. https://doi.org/10.1007/s11104-021-05178-3
He J, Jin Y, Turner NC, Li F-M (2020) Irrigation during flowering improves subsoil water uptake and grain yield in rainfed soybean. Agronomy 10:120. https://doi.org/10.3390/agronomy10010120
Hoogenboom G, Huck MG, Peterson CM (1987) Root growth rate of soybean as affected by drought stress 1. Agron J 79:607–614. https://doi.org/10.2134/agronj1987.00021962007900040004x
Jensen ES (1994) Availability of nitrogen in 15N-labelled mature pea residues to subsequent crops in the field. Soil Biol Biochem 26:465–472. https://doi.org/10.1016/0038-0717(94)90178-3
Karasek FW, Ray EC (1988) Basic gas chromatography-mass spectrometry: principles and technique. Elsevier Science, Amsterdam
Kautz T, Amelung W, Ewert F, Gaiser T, Horn R, Jahn R, Javaux M, Kemna A, Kuzyakov Y, Munch J-C, Pätzold S, Peth S, Scherer HW, Schloter M, Schneider H, Vanderborght J, Vetterlein D, Walter A, Wiesenberg GL, Köpke U (2013) Nutrient acquisition from arable subsoils in temperate climates: A review. Soil Biol Biochem 57:1003–1022. https://doi.org/10.1016/j.soilbio.2012.09.014
Kohl DH, Shearer G, Harper JE (1980) Estimates of N2 fixation based on differences in the natural abundance of N in nodulating and nonnodulating isolines of soybeans. Plant Physiol 66:61–65. https://doi.org/10.1104/pp.66.1.61
Kutschera L, Lichtenegger E, Sobotik M (German) (2018) Wurzelatlas der Kulturpflanzen gemäßigter Gebiete mit Arten des Feldgemüsebaues, 2nd edn. DLG-Verlag, Frankfurt
Landwirtschaftliche Kommunikations- und Servicegesellschaft mbH LKS (German) (2019) LMUAA 027 Bestimmung des Gesamtstickstoffs in Böden durch Verbrennung und Gasanalyse. In: DAkkS (ed), Anlage zur Akkreditierungsurkunde D-PL-14632–01–00 nach DIN EN ISO/IEC 17025:2018. Amberg, Germany
Li L, Sun J, Zhang F, Guo T, Bao X, Smith FA, Smith ES (2005) Root distribution and interactions between intercropped species. Oecologia 147:280–290. https://doi.org/10.1007/s00442-005-0256-4
Liu L, Gan Y, Bueckert R, Van Rees K (2011) Rooting systems of oilseed and pulse crops. II: Vertical distribution patterns across the soil profile. Field Crop Res 122:248–255. https://doi.org/10.1016/j.fcr.2011.04.003
Mariotti A (1983) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–687. https://doi.org/10.1038/303685a0
Meier U (German) (2018) Entwicklungsstadien Mono- und Dikotyler Pflanzen: BBCH Monografie. Blackwell, Berlin
Munzert M (German) (2015) Landwirtschaftliche und Gartenbauliche Versuche mit SAS. Springer, Berlin/Heidelberg
Oberson A, Nanzer S, Bosshard C, Dubois D, Mäder P, Frossard E (2007) Symbiotic N2 fixation by soybean in organic and conventional cropping systems estimated by 15N dilution and 15N natural abundance. Plant Soil 290:69–83. https://doi.org/10.1007/s11104-006-9122-3
Ordóñez RA, Castellano MJ, Hatfield JL, Helmers MJ, Licht MA, Liebman M, Dietzel R, Martinez-Feria R, Iqbal J, Puntel LA, Córdova S, Togliatti K, Wright EE, Archontoulis SV (2018) Maize and soybean root front velocity and maximum depth in Iowa, USA. Field Crop Res 215:122–131. https://doi.org/10.1016/j.fcr.2017.09.003
Peoples MB, Faizah AW, Rerkasem B, Herridge DF (1989) Methods for evaluating nitrogen fixation by nodulated legumes in the field. ACIAR (Australian Centre for International Research), Canberra, pp. 1–69
Purcell LC, King CA (2008) Drought and nitrogen source effects on nitrogen nutrition, seed growth, and yield in soybean. J Plant Nutr 19:969–993. https://doi.org/10.1080/01904169609365173
Rasmussen CR, Thorup-Kristensen K, Dresbøll DB (2020) The effect of drought and intercropping on chicory nutrient uptake from below 2 m studied in a multiple tracer setup. Plant Soil 446:543–561. https://doi.org/10.1007/s11104-019-04348-8
Reicosky DC, Millington RJ, Klute A, Peters DB (1972) Patterns of water uptake and root distribution of soybeans (Glycine max) in the presence of a water table 1. Agron J 64:292–297. https://doi.org/10.2134/agronj1972.00021962006400030011x
Salvagiotti F, Cassman KG, Specht JE, Walters DT, Weiss A, Dobermann A (2008) Nitrogen uptake, fixation and response to fertilizer N in soybeans: a review. Field Crop Res 108:1–13. https://doi.org/10.1016/j.fcr.2008.03.001
Serraj R, Sinclair TR (1998) Soybean cultivar variability for nodule formation and growth under drought. Plant Soil 202:159–166. https://doi.org/10.1023/A:1004300819535
Shearer G, Kohl DH (1986) N2-fixation in field settings: Estimations based on natural 15N abundance. Aust J Plant Physiol 13:699–756. https://doi.org/10.1071/PP9860699
Sionit N, Kramer PJ (1977) Effect of water stress during different stages of growth of soybean. Agron J 69:274–278. https://doi.org/10.2134/agronj1977.00021962006900020018x
Statista (ed.) (German) (2022) Anbaufläche von Sojabohnen in Deutschland in den Jahren 2016 bis 2022. Available via https://de.statista.com/statistik/daten/studie/1172777/umfrage/anbauflaeche-sojabohnen-deutschland/. Accessed 10/10/2023
Turman PC, Wiebold WJ, Wrather JA, Tracy PW (1995) Cultivar and planting date effects on soybean root growth. Plant Soil 176:235–241. https://doi.org/10.1007/BF00011787
Zinati GM, Christenson DR, Harris D (2001) Spital and temporal distribution of 15N tracer and temporal pattern of N uptake from various depths by sugarbeet. Commun Soil Sci Plant Anal 32:1445–1456. https://doi.org/10.1081/CSS-100104204
Acknowledgements
This research could only be carried out with the help of many supporters. Therefore, my special thanks go to the Mucke family from Nimtitz and Gunhild Rosner (Zalf) for the provided experimental plots and the organisation of the experimental facility. Furthermore, I would like to thank Felix Tzschoch and Dr. Guido Lux for their support during the root experiments and Gerhard Porte for the construction of the wooden angle. Thank you to Dr. Annett Weigel and Dr. Annika Eisenschmidt for putting the finishing touches on the text and, of course, Marcel Porte, who always had my back to complete this work.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was funded by the European Social Fund and Sächsische Aufbaubank under grant number 100327910.
Author information
Authors and Affiliations
Contributions
Anne Porte and Knut Schmidtke contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Anne Porte. The first draft of the manuscript was written by Anne Porte, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Porte, A., Bellingrath-Kimura, S.D. & Schmidtke, K. Root Development and Subsoil 15N-labelled N Uptake in Soybean (Glycine max (L.) Merr.). J Soil Sci Plant Nutr 23, 6257–6272 (2023). https://doi.org/10.1007/s42729-023-01482-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01482-2