Abstract
Livestock grazing and dung deposition can increase soil nutrients, contributing to the dominance of exotic species. Recent research suggests that native herbivore grazing has positive effects on native vegetation and soil health. However, little is known about the effects of native herbivore dung on plant growth and its potential implications for the restoration of degraded grasslands. This study examined the effects of dung addition from a native herbivore, kangaroo (Macropus giganteus), and ruminant livestock, sheep (Ovis aries), on biomass production and nutrient uptake of the native perennial wallaby grass (Rytidosperma auriculatum) and the annual exotic wild oat (Avena barbata), two of the most abundant grasses from the temperate grasslands of southern Australia. We conducted a glasshouse experiment, adding each type of dung to each plant species grown without competition in pots containing soil with a nutrient composition similar to that of old fields. Kangaroo dung produced higher wallaby grass aboveground biomass than other treatments and less wild oat aboveground biomass than the control. Kangaroo dung affected nutrient uptake but not nutrient concentration. Sheep dung had no effect. We demonstrated that native herbivores and livestock dung can have different effects on the biomass of native and invasive grasses. The higher nutrient uptake in wallaby grass appeared to be a consequence of the higher biomass production, suggesting that the effects produced by kangaroo dung could be related to its chemical and biological characteristics rather than its nutrient composition. Incorporating native herbivores’ dung or facilitating their presence can improve restoration outcomes in degraded grasslands.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Temperate grasslands are valuable ecosystems that have been severely impacted and modified by humans (Hautier et al. 2015). They provide forage resources for livestock grazing and essential ecosystem services such as water supply and flow regulation, carbon sequestration, soil erosion control, and pollination (Bengtsson et al. 2019). Despite their importance, the continuous agricultural expansion has substantially reduced the extension of natural grasslands worldwide (Corbin and D’Antonio 2004; Hoekstra et al. 2005). Thus, the conservation and restoration of native grasslands are paramount.
Grazing is considered one of the main drivers of floristic and edaphic changes, and thus, all of its manyfold impacts need to be considered in the management of grasslands for the conservation of native vegetation (HilleRisLambers et al. 2010; Zemmrich et al. 2010; Eldridge et al. 2018). In managed grasslands, the positive effects of livestock grazing on soil fertility have been vastly studied (Lai and Kumar 2020; Rayne and Aula 2020). However, the presence of livestock in natural and semi-natural grasslands is often related to negative impacts on soils and the loss of biodiversity (Kimball and Schiffman 2003; Blumenthal et al. 2017). In contrast, native herbivores have been associated with positive effects on soil health and native vegetation (Hobbs 1996; van der Wal et al. 2004; Eldridge et al. 2017). These contrasting effects produced by livestock and native herbivores are likely to be influenced by their coevolution with vegetation and grasslands site-specific characteristics (Hobbs 1996; Forbes et al. 2019). A better understanding of the responses of grassland species to the various effects of grazing is essential for the conservation of native vegetation.
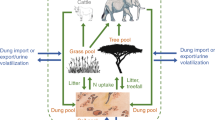
The activity of grazers encompasses several effects beyond defoliation, including dung deposition, which can influence the chemical and biological properties of soil, leading to changes in vegetation composition (Bardgett and Wardle 2003; Filazzola et al. 2020). The addition of dung represents a substantial input of nutrients that can modify nutrients and biological processes in soils, thus affecting vegetation (Peco et al. 2006; Nichols et al. 2016). Most grazers recycle a high portion of the nutrients they consumed through their dung and urine, which in the case of cattle could be as high as 80% for nitrogen (Wachendorf et al. 2008). Dung decomposition reduces the time and increases the rates at which nutrients, especially nitrogen (N), potassium (K), and phosphorus (P) become available to plants compared to plant residue decomposition (Bloor 2015; Milotić and Hoffmann 2016). The subsequent changes in soil chemical, physical, and biological properties can lead to indirect effects on nutrient cycling that can affect vegetation communities (Yoshitake et al. 2014). These effects will largely depend on the composition of grazers and the vegetation communities they feed from (Liu et al. 2018). The high nutrient content in livestock dung can alter the trade-offs in species resource-ratio requirements, favoring the dominance of species with elevated responses to nutrient increases like invasive annual graminoids (Davis et al. 2000; Harpole et al. 2016; Pearson et al. 2018). Conversely, native herbivores dung, from European ungulates and African large herbivores, facilitates the redistribution of nutrients and can have profound positive effects on native vegetation (Hobbs 1996; van der Wal et al. 2004; van der Waal et al. 2011). Yet, several herbivore groups and ecosystems remain to be studied.
The addition of dung to soils may also affect substantially seed germination and plant growth (Milotić and Hoffmann 2017). Responses to dung addition can vary among species and depend on the biology of the animal producing the dung (Milotić and Hoffmann 2016). Dung chemical compounds can be beneficial by promoting the production of secondary metabolites that improve antioxidant activity and the health of plants (Baghdadi et al. 2018). Dung can also contain concentrations of phytotoxic compounds such as phenols and fatty acids that can affect germination and seedling growth (Milotic and Hoffmann 2016). Moreover, dung nutrients become available to plants at different rates depending on the diet of herbivores and their digestive physiology (Sitters et al. 2014; Milotić and Hoffmann 2016; Dhiman et al. 2021). In turn, plants have different nutrient requirements and/or absorption efficiencies depending on their physiology (Padgett and Allen 1999; Chapman et al. 2014). Dung also contains microorganisms that could potentially favor the establishment and growth of plants or become pathogens (Bardgett and van der Putten 2014; Qi et al. 2019). Thus, herbivores with different digestive physiologies could produce dung with species-specific effects on plant growth and development (Jørgensen and Jensen 1997; Dai 2000). For instance, cattle (ruminant) and horse (non-ruminant) dung have been reported to produce different effects on seed germination (Milotic and Hoffmann 2016) and plant growth (Milotić and Hoffmann 2017) for graminoid species with different life strategies. However, there is still much to be explored concerning the effects of dung on grasses (Bloor 2015), especially in scenarios where native herbivores are non-ruminant (Dai 2000).
Assessing the effects of dung deposition in semi-natural grasslands, where livestock and native grazers coexist, could strengthen our insight into the effects of grazing and improve its management for native vegetation conservation. Livestock grazing is often employed to manage plant communities with the intention of maintaining species richness and reducing the presence of invasive species with high levels of productivity (Bailey et al. 2019). Moreover, some management practices consider the exclusion of native herbivores to avoid losing young plants in restoration projects (Freeman and Pobke 2021). However, there is growing evidence concerning the negative effects of livestock grazing on native vegetation (Kimball and Schiffman 2003; Beever et al. 2006; Blumenthal et al. 2017; Filazzola et al. 2020). Furthermore, recent research has noticed that native herbivores produce positive effects on native plant richness, soil health, and productivity (Eldridge et al. 2021; Flojgaard et al. 2018; Travers et al. 2018; Snape et al. 2021; Hawkins and Zeglin 2022). Contrasting the effects of native herbivores and livestock dung on native vegetation could provide new insight into the relationship between native herbivores and native vegetation, which can benefit the establishment and growth of native vegetation in grasslands. Management and restoration projects could benefit from such information, by limiting or promoting the presence of native herbivores or by using their dung as fertilizer. This study focuses on the temperate grasslands of southern Australia, an ecosystem characterized by dominant perennial tussock grasses (Austrostipa, Rytidosperma, and Themeda) that have been severely affected by invasive species, mainly annual grasses (Lenz and Facelli 2005). We asked what is the effect of native herbivores and livestock dung on the growth of grasses and how it differs depending on dung type. We conducted a glasshouse experiment that simulated the soils rich in nutrients found in restoration projects at old fields (former farmlands). We assessed the effects of the addition of dung from gray kangaroo (Macropus giganteus) and sheep (Ovis aries) on the growth of the perennial native wallaby grass (Rytidosperma auriculatum) and the annual invasive wild oat (Avena barbata). Sheep dung is a rich source of nutrients that can reduce acidification and enhance soil nutrition (Rolando et al. 2018; Teixeira et al. 2019). Little is known about kangaroo dung composition, properties, or effects on soils.
2 Materials and Methods
2.1 Resources
To conduct the glasshouse experiment, the Plant Research Centre of the University of Adelaide provided sterilized loamy soil rich in nutrients that mimicked the composition and texture of old-field soils from the Mediterranean-type climate region of South Australia, “rich soil” hereafter (Table 1). A local plant nursery (Seeding Natives) donated wallaby grass seeds for the experiment, while wild oat seeds were obtained from Para Woodlands Nature Reserve (34.6420° S, 138.8147° E), former farmland currently managed for native grassy woodlands restoration. At this site, pulse grazing by sheep is used to attempt weed control, and wild kangaroos graze freely. We collected fresh kangaroo and sheep dung samples at Para Woodlands in spring, just before starting the experiment. A random subsample of 250 g for each type of dung was homogenized and sent for chemical analyses at CSBP laboratories (Bibra Lake, WA, www.csbp-fertilisers.com.au). Sheep dung presented higher concentrations of N, K, and P. while kangaroo dung presented higher iron (Fe) and zinc (Zn) concentrations (Table 2).
2.2 Species
Two of the most abundant native and invasive species in the temperate grasslands in southern Australia are wallaby grass and wild oat respectively. The former is a slow-growing perennial grass adapted to droughts and soils with low nutrient availability that has a low response to P and N fertilizers compared to wild oats (Waddell et al. 2016; Mitchell et al. 2019; McIntyre et al. 2022; Ba and Facelli 2022). This species is used as a grazing pasture (McIntyre et al. 2022) and is commonly used in restoration projects (Jellinek et al. 2020). The latter is an annual invasive grass from central Asia and the Mediterranean Basin, associated with higher fertility soils in southern Australia, such as old fields (Lenz and Facelli 2005). Previous glasshouse studies reported a faster response of wild oat to higher nutrients in old-field soil than wallaby grass, which is consistent with the rapid response to resources that annual species have (Smith et al. 2018).
2.3 Experimental Design
We conducted a fully factorial experiment in a glasshouse from 13/08 to 21/10 in 2020. We prepared 60 pots (r: 4.5 cm, h 12.5 cm) filled with rich soil and watered them before sowing. We allocated 30 pots to each species and each pot received 14 seeds of the corresponding species. We applied three dung treatments to the pots: kangaroo dung, sheep dung, and dung-free control treatment, each replicated 10 times. Previous to application, dung samples from each species were crushed and homogenized. Dung application consisted of adding 10 g kangaroo or sheep dung distributed over the surface of the pots. Pots were located in an unheated greenhouse without artificial light at the Benham building, Adelaide, Australia (34.918°N, 138.6047°E).
2.4 Harvesting and Measurements
We assessed seedling emergence and germination rates for each species separately: for wild oats, 3 days after the first seedling emerged, and for wallaby grass, 3 and 9 days after the first emergence. After 1 month, plants in each pot were thinned to three individuals to reduce intraspecific competition. Plants were harvested after 10 weeks when wild oats started to flower. Below and aboveground plant parts were dried separately to a constant weight at 65 °C. To assess the effect of dung addition on plant growth, we measured the aboveground biomass, belowground biomass, and total biomass for each pot. We also calculated the biomass ratio by dividing aboveground by belowground biomass. The total biomass was calculated by adding aboveground and belowground biomass. The aboveground nutrient concentration and nutrient uptake (aboveground biomass × nutrient concentration) of N, K, and P were analyzed for each species separately to explore any potential effects produced by dung addition. The nutrient analysis was conducted by the Australian Precision Ag Laboratory (APAL). We focused our results on the aboveground biomass since rich soils promote the allocation of resources to this part of the plant (Stevens et al. 2010; Nogueira et al. 2018).
2.5 Data Analysis
We conducted statistics tests for each species separately due to differences in life-history traits. Seedling emergence and germination rate were compared between treatments using one-way ANOVA followed by Tukey’s HSD test for each species. The same tests were used to compare biomass (aboveground, root, ratio, and total) production, nutrient concentration, and nutrient uptake (N, K, and P) between treatments for wallaby grass. In the case of wild oats, aboveground biomass, nutrient concentration, and nutrient uptake were compared between treatments employing the ANOVA type III test due to the unbalanced number of samples caused by the loss of part of the samples. All statistics were conducted using R v. 4.1.2 (R Core Team, 2021).
3 Results
3.1 Seedling Emergence
Seedling emergence started after 3 days for wild oat and 9 days for wallaby grass, with no significant differences between dung treatments (Supplementary material). The germination rate of wild oats was higher than 85% for all treatments. The germination rate of Wallaby grass was lower than 50% 3 days after emergence and presented a small increase after 9 days. Only pots with sheep dung presented a germination rate of wallaby grass higher than 50% (Table 3).
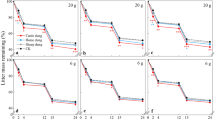
3.2 Biomass Production
The addition of kangaroo dung to wallaby grass resulted in significantly higher aboveground, belowground, and total biomass than the control treatment and the addition of sheep dung (P < 0.001, Fig. 1a, Table 4). In the case of wild oats, the addition of kangaroo dung resulted in significantly lower aboveground biomass than the control treatment (P < 0.05, Fig. 1b, Table 4). The addition of sheep dung had no significant effects on any of the plant species (Table 4). Further detail of biomass production for both species can be found in the Supplementary material.
3.3 Nutrient Uptake
The aboveground nutrient concentrations and uptake varied across species and treatments (Supplementary material). In the case of wallaby grass, there were no significant differences between the concentration of nutrients among treatments. The uptake of N, K, and phosphorous by wallaby grass was the highest in pots with kangaroo dung. The nutrient concentration and uptake by wild oat pots did not differ among treatments (Table 5).
4 Discussion
Wallaby grass and wild oats biomass production responded in unexpected ways to the addition of dung. We expected that the addition of dung, particularly the nutrient-richer sheep dung, would trigger an increase in growth in wild oat, which is documented as responding to increased nutrient availability (Ba and Facelli 2022). We also expected a minor response, if any from, wallaby grass which is less responsive to nutrient additions (Waddell et al. 2016; Mitchell et al. 2019; McIntyre et al. 2022). Instead, our results revealed that only kangaroo dung positively affected the biomass production of wallaby grass. Kangaroo dung also produced a negative effect on wild oats, despite the capacity of this species to respond rapidly to increased nutrient availability. Furthermore, neither grasses were affected by sheep dung, which had a higher nutrient concentration than kangaroo dung. Differences in nutrient uptake might be principally related to aboveground biomass production since there were no differences in nutrient concentrations between treatments. Likewise, other studies employing organic fertilizers have not found an increase in nutrient concentration on the aboveground biomass (Banik et al. 2021; Tshikalange et al. 2022). The absence of changes in nutrient concentrations suggests that the addition of dung could have increased the available nutrients in the soil, thus increasing biomass production (Han et al. 2016; Tshikalange et al. 2022). Differences in proportions of mineral to organic nutrients related to sheep and dung digestive systems could have affected nutrient availability (Sitters and Olde Venterink 2021). However, the nutrient content in soils employed in this study was already high and might have already supplied the nutrient requirements for both species. Hence, the biomass production in our experiment may not be directly related to dung nutrient content, but to dung characteristics that affect plant growth.
Dung chemical and biological characteristics, factors outside the scope of our study, could provide possible explanations for the kangaroo dung effects reported here. Dung micronutrients can increase the production of secondary metabolites that benefit plant health (Bimova and Pokluda 2009; Ibrahim et al. 2013), while dung phytotoxic compounds can affect the initial growth of seedlings (Milotić and Hoffmann 2017). In addition, plant-soil-microbial interactions can play a significant role for plants, as microbes can either favor the establishment of plants or become pathogens for them (Bardgett and van der Putten 2014). The impact of grazing on soil-microbial communities varies between herbivores. Sheep have been shown to cause a negative effect on plant pathogens which affect plant-soil-microbial interactions (Eldridge and Delgado-Baquerizo 2018). These effects could be related to changes in soil pH and nutrient availability caused by trampling and the addition of dung (Ling et al. 2017; Eldridge et al. 2020) but also to the microbial content in dung. Recent research has shown that dung from native herbivores could serve as a dispersal and homogenization vector for soil-microbial communities in grasslands (Hawkins and Zeglin 2022). Furthermore, a few studies have shown that cow dung contains bacteria that promote plant growth due to their capacity to solubilize nutrients (Qi et al. 2019; Bhatt and Maheshwari 2020; Naghizadeh et al. 2022). However, dung can also contain pathogenic fungi and bacteria that negatively affect plant growth (El Barnossi et al. 2020; Semenov et al. 2021). Regarding the plants employed in this study, or closely related ones, glasshouse experiments have shown that alterations in soil-microbial communities affect graminoids biomass production. Ba et al. (2018) reported that soil inoculated with Rytidosperma caespitosum roots as a source of microbial inoculum had a positive effect on the total biomass of R. caespitosum and a negative one on wild oat total biomass. Similarly, Smith et al. (2018) reported a negative effect on wild oat biomass production in soils inoculated with soil from a remnant native perennial grassland.
Differences in diet and digestive physiology between kangaroos and sheep can provide insight into the chemical and biological composition of dung. Kangaroos and sheep share several grass species in their diets (Squires 1982) and have similar bacterial flora and anaerobic fungi in the forestomach (Smith 2009). However, macropods’ dietary preferences (Taylor 1983; Wilson 1991) and differences in the fermentation processes of rumen and macropods’ forestomach (Smith 2009) could result in different chemicals and biological characteristics in dung. Further research concerning microbial communities in dung and soils could provide new insight into mechanisms acting behind fertilization. Possible steps to follow could consider contrasting our results to the addition of sterilized dung, as well as conducting microbial phylogenetic analysis on soils before and after the addition of dung. It must be noted that our results were obtained in conditions of high fertility that simulated the restoration site. Complementing our results with an experiment in soils with lower nutrient contents, adding or not adding the two types of dung could provide a broader insight into the role of dung addition on the growth of these species.
Our results may contribute to improving the management and restoration of semi-natural grasslands. Research in ecosystems like tundra and savannahs has shown that the effects of wildlife dung addition on soil belowground components can significantly impact vegetation (van der Wal et al. 2004; van der Waal et al. 2011; Veldhuis et al. 2018). Favoring the presence of native herbivores may contribute to improving the biomass production of native plants. However, if their presence presents a problem for revegetation, like kangaroos in southern Australia, the allocation of wildlife dung as a fertilizer could be an option to be considered.
5 Conclusions
Our results provide evidence that native herbivores and ruminant livestock dung addition can have different effects on native and exotic grasses. We demonstrated that kangaroo dung could be beneficial for the biomass production of wallaby grass and detrimental for wild oats. In contrast, the addition of sheep dung does not seem to affect the biomass production of wallaby grass or wild oat in soils with high nutrient levels. However, we could not find the expected evidence linking the effects of dung nutrient content to plant nutrient uptake and growth. Hence, we suggest that other dung properties, such as bio-chemical compounds and the effects of dung microbes on plant-soil microbe interactions, could be responsible for the effects documented. It could then be possible to maintain a controlled presence of kangaroos in restoration sites or add kangaroo dung in their absence to promote native grass establishment and biomass production.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the following link to the electronic supplementary material.
References
Ba L, Facelli JM (2022) Invasive success of exotic wild oat depends on nutrient availability and competition in temperate grasslands of southern Australia. Plant Soil 472:465–478. https://doi.org/10.1007/s11104-021-05262-8
Ba L, Facelli E, Facelli JM (2018) Plant-mycorrhizal fungi feedbacks: potential accomplices of Avena barbata’s high invasiveness. Plant Ecol 219:1045–1052. https://doi.org/10.1007/s11258-018-0857-8
Baghdadi A, Halim RA, Ghasemzadeh A, Ramlan MF, Sakimin SZ (2018) Impact of organic and inorganic fertilizers on the yield and quality of silage corn intercropped with soybean. PeerJ 6:e5280. https://doi.org/10.7717/peerj.5280
Bailey DW, Mosley JC, Estell RE et al (2019) Synthesis paper: targeted livestock grazing: prescription for healthy rangelands. Rangeland Ecol Manage 72:865–877. https://doi.org/10.1016/j.rama.2019.06.003
Banik C, Koziel JA, Bonds D, Singh AK, Licht MA (2021) Comparing biochar-swine manure mixture to conventional manure impact on soil nutrient availability and plant uptake-a greenhouse study. Land-Basel 10. https://doi.org/10.3390/land10040372
Bardgett RD, van der Putten WH (2014) Belowground biodiversity and ecosystem functioning. Nature 515(7528):505–511. https://doi.org/10.1038/nature13855
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268. https://doi.org/10.1890/02-0274
Beever EA, Huso M, Pyke DA (2006) Multiscale responses of soil stability and invasive plants to removal of non-native grazers from an arid conservation reserve. Divers Distrib 12:258–268. https://doi.org/10.1111/j.1366-9516.2006.00253.x
Bengtsson J, Bullock JM, Egoh B et al (2019) Grasslands-more important for ecosystem services than you might think. Ecosphere 10:e02582. https://doi.org/10.1002/ecs2.2582
Bhatt K, Maheshwari DK (2020) Zinc solubilizing bacteria (Bacillus megaterium) with multifarious plant growth promoting activities alleviates growth in Capsicum annuum L. 3 Biotech 10:1–10. https://doi.org/10.1007/s13205-019-2033-9
Bimova P, Pokluda R (2009) Impact of organic fertilizers on total antioxidant capacity in head cabbage. Horticult Sci 36:21–25. https://doi.org/10.17221/9/2008-Hortsci
Bloor JMG (2015) Additive effects of dung amendment and plant species identity on soil processes and soil inorganic nitrogen in grass monocultures. Plant Soil 396:189–200. https://doi.org/10.1007/s11104-015-2591-5
Blumenthal DM, LeCain DR, Augustine DJ (2017) Composted manure application promotes long-term invasion of semi-arid rangeland by Bromus tectorum. Ecosphere 8. https://doi.org/10.1002/ecs2.1960
Chapman DF, Lee JM, Waghorn GC (2014) Interaction between plant physiology and pasture feeding value: a review. Crop Pasture Sci 65:721–734. https://doi.org/10.1071/Cp13379
Corbin JD, D’Antonio CM (2004) Competition between native perennial and exotic annual grasses: implications for an historical invasion. Ecology 85:1273–1283. https://doi.org/10.1890/02-0744
Dai XB (2000) Impact of cattle dung deposition on the distribution pattern of plant species in an alvar limestone grassland. J Veg Sci 11:715–724. https://doi.org/10.2307/3236578
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88(3):528–534. https://doi.org/10.1046/j.1365-2745.2000.00473.x
Dhiman S, Kumar S, Baliyan N, Dheeman S, Maheshwari DK (2021) Cattle dung manure microbiota as a substitute for mineral nutrients and growth management practices in plants. In: Endophytes: Mineral Nutrient Management, Volume 3. Springer pp 77–103. https://doi.org/10.1007/978-3-030-65447-4_4
El Barnossi A, Saghrouchni H, Moussaid F, Chahmi N, Housseini AI (2020) Microbiological study of effects of solid organic waste (chicken droppings and sheep manure) decomposed in the soil used for Pisum sativum cultivation. Int J Environ Stud 77:830–842. https://doi.org/10.1080/00207233.2019.1704116
Eldridge DJ, Delgado-Baquerizo M (2018) Functional groups of soil fungi decline under grazing. Plant Soil 426:51–60. https://doi.org/10.1007/s11104-018-3617-6
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I (2017) Do grazing intensity and herbivore type affect soil health? Insights from a semi-arid productivity gradient. J Appl Ecol 54:976–985. https://doi.org/10.1111/1365-2664.12834
Eldridge DJ, Delgado-Baquerizo M, Travers SK, Val J, Oliver I, Dorrough JW, Soliveres S (2018) Livestock activity increases exotic plant richness, but wildlife increases native richness, with stronger effects under low productivity. J Appl Ecol 55:766–776. https://doi.org/10.1111/1365-2664.12995
Eldridge DJ, Travers SK, Val J, Wang JT, Liu HW, Singh BK, Delgado-Baquerizo M (2020) Grazing regulates the spatial heterogeneity of soil microbial communities within ecological networks. Ecosystems 23:932–942. https://doi.org/10.1007/s10021-019-00448-9
Eldridge DJ, Ding JY, Travers SK (2021) Low-intensity kangaroo grazing has largely benign effects on soil health. Ecol Manage Restor 22:58–63. https://doi.org/10.1111/emr.12439
Filazzola A, Brown C, Dettlaff MA et al (2020) The effects of livestock grazing on biodiversity are multi-trophic: a meta-analysis. Ecol Lett 23:1298–1309. https://doi.org/10.1111/ele.13527
Flojgaard C, Bruun HH, Hansen MDD, Heilmann-Clausen J, Svenning JC (2018) Are ungulates in forests concerns or key species for conservation and biodiversity? Reply to Boulanger etal. (DOI: 10.1111/gcb.13899). Global Change Biol 24:869–871. https://doi.org/10.1111/gcb.14029
Forbes ES, Cushman JH, Burkepile DE, Young TP, Klope M, Young HS (2019) Synthesizing the effects of large, wild herbivore exclusion on ecosystem function. Funct Ecol 33:1597–1610. https://doi.org/10.1111/1365-2435.13376
Freeman A, Pobke K (2021) Macropod management is critical for recovery of Sheoak Grassy Woodlands on Eyre Peninsula, South Australia. Ecol Manage Restor 22:44–49. https://doi.org/10.1111/emr.12478
Han SH, An JY, Hwang J, Kim SB, Park BB (2016) The effects of organic manure and chemical fertilizer on the growth and nutrient concentrations of yellow poplar (Liriodendron tulipifera Lin.) in a nursery system. For Sci Technol 12:137–143. https://doi.org/10.1080/21580103.2015.1135827
Harpole WS, Sullivan LL, Lind EM et al (2016) Addition of multiple limiting resources reduces grassland diversity. Nature 537:93–96. https://doi.org/10.1038/nature19324
Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB (2015) Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348:336–340. (https://www.science.org/doi/10.1126/science.aaa1788)
Hawkins JH, Zeglin LH (2022) Microbial dispersal, including bison dung vectored dispersal, increases soil microbial diversity in a grassland ecosystem. Front Microbiol 13. https://doi.org/10.3389/fmicb.2022.825193
HilleRisLambers J, Yelenik SG, Colman BP, Levine JM (2010) California annual grass invaders: the drivers or passengers of change? J Ecol 98:1147–1156. https://doi.org/10.1111/j.1365-2745.2010.01706.x
Hobbs NT (1996) Modification of ecosystems by ungulates. J Wildl Manage 60:695–713. https://doi.org/10.2307/3802368
Hoekstra JM, Boucher TM, Ricketts TH, Roberts C (2005) Confronting a biome crisis: global disparities of habitat loss and protection. Ecol Lett 8:23–29. https://doi.org/10.1111/j.1461-0248.2004.00686.x
Ibrahim MH, Jaafar HZ, Karimi E, Ghasemzadeh A (2013) Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 18:10973–10988. https://doi.org/10.3390/molecules180910973
Jellinek S, Harrison PA, Tuck J, Te T (2020) Replanting agricultural landscapes: how well do plants survive after habitat restoration? Restor Ecol 28(6):1454–1463. https://doi.org/10.1111/rec.13242
Jørgensen FV, Jensen ES (1997) Short-term effects of a dung pat on N2 fixation and total N uptake in a perennial ryegrass/white clover mixture. Plant Soil 196:133–141. https://doi.org/10.1023/A:1004234029920
Kimball S, Schiffman PM (2003) Differing effects of cattle grazing on native and alien plants. Conserv Biol 17:1681–1693. https://doi.org/10.1111/j.1523-1739.2003.00205.x
Lai L, Kumar S (2020) A global meta-analysis of livestock grazing impacts on soil properties. PLoS One 15:e0236638. https://doi.org/10.1371/journal.pone.0236638
Lenz TI, Facelli JM (2005) The role of seed limitation and resource availability in the recruitment of native perennial grasses and exotics in a South Australian grassland. Austral Ecol 30:684–694. https://doi.org/10.1111/j.1442-9993.2005.01508.x
Ling N, Chen D, Guo H, Wei J, Bai Y, Shen Q, Hu S (2017) Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 292:25–33. https://doi.org/10.1016/j.geoderma.2017.01.013
Liu C, Wang L, Song XX et al (2018) Towards a mechanistic understanding of the effect that different species of large grazers have on grassland soil N availability. J Ecol 106:357–366. https://doi.org/10.1111/1365-2745.12809
McIntyre S, Muller WJ, Lewis J (2022) Habitat distributions of 12 co-occurring wallaby grasses (Rytidosperma spp., Poaceae) and their response to a transition from pastoral to conservation land use. Aust J Bot 70:131–145. https://doi.org/10.1071/Bt21100
Milotic T, Hoffmann M (2016) Reduced germination success of temperate grassland seeds sown in dung: consequences for post-dispersal seed fate. Plant Biol (stuttg) 18:1038–1047. https://doi.org/10.1111/plb.12506
Milotić T, Hoffmann M (2016) Cost or benefit for growth and flowering of seedlings and juvenile grassland plants in a dung environment. Plant Ecol 217:1025–1042. https://doi.org/10.1007/s11258-016-0629-2
Milotić T, Hoffmann M (2017) The impact of dung on inter-and intraspecific competition of temperate grassland seeds. J Veg Sci 28:774–786. https://doi.org/10.1111/jvs.12535
Mitchell ML, McCaskill MR, Armstrong RD (2019) Phosphorus fertiliser management for pastures based on native grasses in south-eastern Australia. Crop Pasture Sci 70:1044–1052. https://doi.org/10.1071/Cp19217
Naghizadeh M, Klaver L, Schoenherz AA, Rani S, Dalgaard TS, Engberg RM (2022) Impact of dietary sodium butyrate and salinomycin on performance and intestinal microbiota in a broiler gut leakage model. Animals-Basel 12:111. https://doi.org/10.3390/ani12010111
Nichols KL, Del Grosso SJ, Derner JD, Follett RF, Archibeque SL, Stewart CE, Paustian KH (2016) Nitrous oxide and methane fluxes from cattle excrement on C3 pasture and C4-dominated shortgrass steppe. Agr Ecosyst Environ 225:104–115. https://doi.org/10.1016/j.agee.2016.03.026
Nogueira C, Nunes A, Bugalho MN, Branquinho C, McCulley RL, Caldeira MC (2018) Nutrient addition and drought interact to change the structure and decrease the functional diversity of a Mediterranean grassland. Front Ecol Evol 6:155. https://doi.org/10.3389/fevo.2018.00155
Padgett PE, Allen EB (1999) Differential responses to nitrogen fertilization in native shrubs and exotic annuals common to Mediterranean coastal sage scrub of California. Plant Ecol 144:93–101. https://doi.org/10.1023/A:1009895720067
Pearson DE, Ortega YK, Villarreal D, Lekberg Y, Cock MC, Eren Ö, Hierro JL (2018) The fluctuating resource hypothesis explains invasibility, but not exotic advantage following disturbance. Ecology 99:1296–1305. https://doi.org/10.1002/ecy.2235
Peco B, Sanchez AM, Azcarate FM (2006) Abandonment in grazing systems: consequences for vegetation and soil. Agr Ecosyst Environ 113:284–294. https://doi.org/10.1016/j.agee.2005.09.017
Qi G, Pan Z, Yamamoto Y et al (2019) The survival of pathogenic bacteria and plant growth promoting bacteria during mesophilic anaerobic digestion in full-scale biogas plants. Anim Sci J 90:297–303. https://doi.org/10.1111/asj.13137
Rayne N, Aula L (2020) Livestock manure and the impacts on soil health: a review. Soil Systems 4:64. https://doi.org/10.3390/soilsystems4040064
Rolando JL, Dubeux JCB, Ramirez DA et al (2018) Land use effects on soil fertility and nutrient cycling in the Peruvian high-Andean Puna grasslands. Soil Sci Soc Am J 82:463–474. https://doi.org/10.2136/sssaj2017.09.0309
Semenov MV, Krasnov GS, Semenov VM, Ksenofontova N, Zinyakova NB, van Bruggen AHC (2021) Does fresh farmyard manure introduce surviving microbes into soil or activate soil-borne microbiota? J Environ Manage 294:113018. https://doi.org/10.1016/j.jenvman.2021.113018
Sitters J, Olde Venterink H (2021) Stoichiometric impact of herbivore dung versus urine on soils and plants. Plant Soil 462:59–65. https://doi.org/10.1007/s11104-021-04960-7
Sitters J, Maechler MJ, Edwards PJ, Suter W, Venterink HO (2014) Interactions between C:N: P stoichiometry and soil macrofauna control dung decomposition of savanna herbivores. Funct Ecol 28:776–786. https://doi.org/10.1111/1365-2435.12213
Smith ME, Facelli JM, Cavagnaro TR (2018) Interactions between soil properties, soil microbes and plants in remnant-grassland and old-field areas: a reciprocal transplant approach. Plant Soil 433:127–145. https://doi.org/10.1007/s11104-018-3823-2
Smith JA (2009) Macropod nutrition. Vet Clin North Am Exot Anim Pract 12:197–208, xiii. https://doi.org/10.1016/j.cvex.2009.01.010
Snape MA, Fletcher D, Caley P (2021) Species composition, herbage mass and grass productivity influence pasture responses to kangaroo grazing in a temperate environment. Ecol Manage Restor 22:16–23. https://doi.org/10.1111/emr.12477
Squires VR (1982) Competitive Interactions in the dietary preference of kangaroos and sheep, cattle and goats in inland Australia. J Arid Environ 5:337–345. https://doi.org/10.1016/S0140-1963(18)31615-X
Stevens CJ, Dupre C, Dorland E et al (2010) Nitrogen deposition threatens species richness of grasslands across Europe. Environ Pollut 158:2940–2945. https://doi.org/10.1016/j.envpol.2010.06.006
Taylor RJ (1983) The diet of the eastern grey-kangaroo and wallaroo in areas of improved and native pasture in the New-England Tablelands. Australian Wildl Res 10:203–211. https://doi.org/10.1071/WR9830203
Teixeira DS, Rezende AA, Lannes LS (2019) Response of vegetation to sheep dung addition in a degraded Cerrado area. Rev Bras Eng Agric Ambient 23:47–52. https://doi.org/10.1590/1807-1929/agriambi.v23n1p47-52
Travers SK, Eldridge DJ, Dorrough J, Val J, Oliver I (2018) Introduced and native herbivores have different effects on plant composition in low productivity ecosystems. Appl Veg Sci 21:45–54. https://doi.org/10.1111/avsc.12334
Tshikalange B, Ololade O, Jonas C, Bello ZAA (2022) Effectiveness of cattle dung biogas digestate on spinach growth and nutrient uptake. Heliyon 8. https://doi.org/10.1016/j.heliyon.2022.e09195
van der Waal C, Kool A, Meijer SS et al (2011) Large herbivores may alter vegetation structure of semi-arid savannas through soil nutrient mediation. Oecologia 165:1095–1107. https://doi.org/10.1007/s00442-010-1899-3
van der Wal R, Bardgett RD, Harrison KA, Stien A (2004) Vertebrate herbivores and ecosystem control: cascading effects of faeces on tundra ecosystems. Ecography 27:242–252. https://doi.org/10.1111/j.0906-7590.2004.03688.x
Veldhuis MP, Gommers MI, Olff H, Berg MP (2018) Spatial redistribution of nutrients by large herbivores and dung beetles in a savanna ecosystem. J Ecol 106:422–433. https://doi.org/10.1111/1365-2745.12874
Wachendorf C, Lampe C, Taube F, Dittert K (2008) Nitrous oxide emissions and dynamics of soil nitrogen under N-15-labeled cow urine and dung patches on a sandy grassland soil. J Plant Nutr Soil Sci 171:171–180. https://doi.org/10.1002/jpln.200625217
Waddell H, Simpson R, Henderson B, Ryan M, Lambers H, Garden D, Richardson A (2016) Differential growth response of Rytidosperma species (wallaby grass) to phosphorus application and its implications for grassland management. Grass Forage Sci 71:245–258. https://doi.org/10.1111/gfs.12170
Wilson A (1991) Forage utilization by sheep and kangaroos in a semi-arid woodland. Rangel J 13:81–90. https://doi.org/10.1071/RJ9910081
Yoshitake S, Soutome H, Koizumi H (2014) Deposition and decomposition of cattle dung and its impact on soil properties and plant growth in a cool-temperate pasture. Ecol Res 29:673–684. https://doi.org/10.1007/s11284-014-1153-2
Zemmrich A, Manthey M, Zerbe S, Oyunchimeg D (2010) Driving environmental factors and the role of grazing in grassland communities: a comparative study along an altitudinal gradient in Western Mongolia. J Arid Environ 74:1271–1280. https://doi.org/10.1016/j.jaridenv.2010.05.014
Acknowledgements
We would like to thank the Nature Foundation for allowing us to access Para Woodlands Nature Reserve to collect kangaroo and sheep dung. Also, thanks to Andrew Randell from Seeding Natives Incorporated for providing us with wallaby grass seeds. We used the facilities of the University of Adelaide for this experiment.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
DR Guevara-Torres originally conceived the idea. DR Guevara-Torres and JM Facelli contributed to the study design. Material preparation, data collection, and analysis were performed by DR Guevara-Torres. The first draft of the manuscript was written by DR Guevara-Torres, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guevara-Torres, D.R., Facelli, J.M. Choose Local: Dung Addition from Native Herbivores Can Produce Substantial Positive Effects on the Growth of Native Grasses Compared to Livestock Dung. J Soil Sci Plant Nutr 23, 4647–4655 (2023). https://doi.org/10.1007/s42729-023-01380-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01380-7