Abstract
Soil salinity is abiotic stress of growing concern, whose effects can be potentially mitigated by the use of suitable fertilisers. Based on this, an experiment was conducted to determine the role of vegetable oil–coated urea on the performance of wheat (Triticum aestivum) under salinity. Neem oil–coated urea (NOCU), castor oil–coated urea (COCU), and normal urea (NU) were compared in wheat plants growing in pots at three soil salinity levels (0, 6, and 12 dS m-1). Plant morphology, growth, element contents (Na, Cl, K, and N), and several traits were assessed at the flag leaf stage; biological yield, grain yield, and its components were assessed at maturity. Salinity stunted growth (approximately -50% yield with high salinity vs. control); boosted Na and Cl concentrations while abating K and N concentrations in plant organs; impaired leaf water status; reduced photosynthetic pigments and increased antioxidant activities and osmo-regulating compounds. NOCU and, to a lesser degree, COCU mitigated salinity effects by upgrading antioxidant activities, reducing oxidative stress markers, increasing leaf water status, photosynthetic pigments, and osmo-regulating compounds. However, NOCU under high salinity could only achieve the levels of NU under intermediate salinity. Lastly, NOCU and COCU restricted plant entry of adverse ions (Na and Cl) while increasing K and N accumulation. Vegetable oil–coated urea, namely NOCU, significantly contributed to improving wheat behaviour and final yield under salinity. These outcomes are associated with the two fertilisers’ properties of slow nitrogen release.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soil salinity (SS) is a major threat to soil degradation, agro-ecosystem quality, and global food security. SS affects more than one billion hectares globally, particularly in arid and semi-arid regions where high evaporation promotes salt accumulation in the soil profile (FAO 2015; Bailey-Serres et al. 2019; Perri et al. 2022). The extent of SS is continuously soaring, and it is feared that half of the world’s arable land will be converted into salt-affected soils by the end of the century (Jamil et al. 2011; Zhu et al. 2022). Plants growing under salinity stress face various biochemical and physiological disturbances from seed germination to the reproductive stage (Sofy et al. 2002; Zhu et al. 2022). The increased concentration of toxic ions (Na+ and Cl−) alters the enzymatic activities, protein synthesis, and photosynthesis, reduces membrane stability, and impairs nutrient homeostasis (Kumar et al. 2019). Besides this, SS decreases the osmotic potential of soil solution, which results in limited water availability for plant transpiration and reduced photosynthesis (Shrivastava et al. 2015; Kumar et al. 2019).
Plant response to SS depends on salt concentration, plant species, and salt type (Joshi et al. 2022). Increased salt concentration in the growing medium reduces stomata opening, photosynthetic rates, chlorophyll contents, chloroplast functioning, and leaf area. All this determines lower photosynthetic efficiency (Kim et al., 2018), leading to a significant reduction in plant growth (El-Sabagh et al. 2020; Denaxa et al., 2022; Joshi et al. 2022; Van Zelm et al. 2020). One of the major effects of SS is the excessive production of reactive oxygen species (ROS) that damage proteins, lipids, and DNA (Kim et al. 2018). However, plants possess excellent defence systems comprising enzymatic and non-enzymatic compounds to cope with ROS increase (Joshi et al. 2022). Besides, plants accumulate various potential osmolytes and use ion exclusion mechanisms to mitigate SS deleterious impacts (Isayenkov and Maathuis 2019). In strawberries, the increased concentration of salts around plant roots was shown to cause metabolic disorders and leaf toxicity symptoms affecting photosynthetic efficiency and assimilate production, in turn reducing plant growth (Voutsinos-Frantzis et al. 2023). Increased salt concentration disturbs the soil physical structure and reduces nutrient and water availability, which is another main cause of the substantial reduction in plant growth observed under SS (Etesami et al. 2019). Therefore, containing the yield losses due to SS is a major concern in view of meeting the world’s rising food demand (Etesami et al. 2019).
Different strategies are envisaged to reduce the deleterious impacts of abiotic stresses. Fertilisation practices are also involved, as a nutrient application must be tailored to face the unfavourable growth conditions determined by stress agents. Nitrogen (N) application is considered important to improve plant growth and tolerance to SS (Nazir et al. 2023). In fact, N supply is an important premise for general crop production and becomes a key factor to sustain plant growth under stress (Zörb et al. 2018). Nitrogen use efficiency (NUE) is considered the most relevant parameter to be focused on under stress as well as no stress conditions, in view of sustainable crop production (Altaf et al. 2021). From a practical viewpoint, this reflects in the 4R principles for N fertilisation (right time, right amount, right source, and right place), which become more critical in view of supporting the growth of stressed plants (Ghafoor et al. 2021).
In this framework, the application of slow-release N fertilisers is seen as a way to support plant growth, NUE, and plant tolerance to abiotic stresses (Zörb et al. 2018; Altaf et al. 2021), while reducing environmental impact (Ghafoor et al. 2021). Among these fertilisers, coated urea was shown to reduce N losses and improve NUE and N recovery, resulting in improved yield and stress tolerance in plants (Li et al. 2018; Ain et al. 2020; Dimpka et al. 2020). Urea coating is associated with increases in nitrogen internal efficiency (NIE), apparent N recovery, and agronomic NUE, which are the basis for the better plant performance observed under stress conditions (Li et al. 2017; Affendi et al. 2018: Asghar et al. 2022). Among coating materials, vegetable oils are easily available, environmentally friendly, highly hydrophobic, and easily degraded by soil microorganisms. All these factors support their use in urea coating, significantly influencing fertiliser properties: in fact, vegetable oil coatings reduce N losses by slowing the rate of urea hydrolysis and the subsequent NH4+ nitrification to NO3-. This results in reduced N2O losses and improved N availability and NUE (Yuan et al. 2022). Moreover, vegetable oil coatings have good adhesion to the urea surface, which prolongs the release of urea and consequently improves crop yield, NUE, and stress tolerance (Dong et al. 2019; Bortoletto-Santos et al. 2016).
Despite the potential benefits of slow-release fertilisers in alleviating salinity, the literature hardly returns works conducted to determine the impact of these fertilisers on plant growth under SS. This is especially serious as it concerns wheat, one of the most important cereals representing a stable food source for 40% of the world population (Giraldo et al. 2019). Wheat is grown in 216 million hectares over 90 countries with an annual production of 778 million metric tons (FAOSTAT 2022; Langridge et al. 2022). The main statistical sources do not indicate which proportion of the wheat surface is affected by salinity. However, wheat is generally associated with rainfed cropping or limited irrigation use. This condition exacerbates salinity effects, as salts tend to accumulate in soil profile season after season. In this respect, wheat epitomises a scenario of rising difficulties faced by many crop plants in coping with salinity.
It is sensed that a strategical approach should be deployed to face the global threat posed by salinity in order to, at least, mitigate the stress effects on soil, cultivated plants, and the general environment. Adjusting nitrogen fertilisation to the specific needs under salinity could fit in this strategy, and slow-release nitrogen fertilisers could play a non-negligible role. However, this is confronted with the paucity of information on the role of these fertilisers, namely oi-coated urea, on plant growth, physiological activities, and element content under SS. In this light, this work was aimed at reducing the gap of knowledge hampering the efficient use of N fertilisers in wheat grown under SS. Therefore, a pot experiment was conducted to determine the impact of neem and castor oil–coated urea on wheat growth, physiological traits, antioxidant activities, element content, and NUE under SS.
2 Materials and Methods
2.1 Experimental Setting
A pot trial was conducted to evaluate the effect of urea with different coatings (neem and castor oil) on morphological, growth, compositional, and physiological attributes of wheat under SS in a greenhouse at the University of Agriculture, Faisalabad (Pakistan). During the study period, the average minimum and maximum daily temperatures were 14 °C and 31.4 °C, respectively; relative humidity ranged between 64.9% and 88.5%.
The soil for filling the pots was collected from the 0-10 cm depth of a field at the Agronomy experimental farm. Soil samples were taken and homogenised to create a representative sample to be analysed by standard procedures. The soil was a clay loam with pH 7.70, organic matter 8.4 g kg-1, EC 0.97 dS m-1, available phosphorus 9.6 mg kg-1, exchangeable potassium 181 mg kg-1, and total nitrogen 0.35 g kg-1. Plastic pots with a 5 L capacity were filled with soil, and in total 27 pots were used for the experiment.
Twelve seeds of wheat were sown in each pot on December 2, 2021. The cultivar Akbar-2019 (not acknowledged for being salt tolerant) was used for this experiment. The pots were visited regularly and watered on the basis of visual observation. The water was applied to pots by using a hand sprayer until all the plants were fully watered.
2.2 Experimental Treatments
The study involved different levels of SS: control, 6 dS m-1, and 12 dS m-1 cross combined with different fertilisers: normal urea (NU), neem (Azadirachta indica) oil–coated urea (NOCU), and castor (Ricinus communis) oil–coated urea (COCU). Table salt (NaCl) was used to determine SS levels according to treatments. Concentrations of NaCl needed as per treatment were calculated by the formula:
In the above equation, TSS indicates the total soluble salts that were calculated as TSS = (EC2 - EC1) × 10; ECI and EC2 were the original electrical conductivity (EC) and the required EC, respectively. Therefore, to achieve the target SS levels (6 and 12 dS m-1), NaCl salt was applied at the rate of 1.179 and 2.58 g/kg of soil. The saturation percentage in the above equation was calculated by the formula:
Therefore, to determine the soil saturation percentage, the saturated paste of soil was made by adding water and mixing it with a spatula. The mixture was allowed to reach equilibrium for 2 h then filtered with filter paper, and the extract was obtained. Thereafter, the soil was oven dried (65 °C), and the soil saturation percentage was determined with the above formula.
The seeds of neem and castor were collected from trees growing in nearby areas. Seeds were crushed to extract the oil, which was used to coat granular urea. The coated urea was left to air dry under shade before applying it for experimental purposes (Rehman et al. 2021). In total, 625 mg urea (= 287.5 mg N) was applied to each pot, split into three doses: the first dose at sowing; the second and third dose during wheat growth. Soil incorporation was assured by irrigation.
2.3 Growth Traits
Plant samples were taken at the flag leaf stage, 83 days after sowing (DAS) (February 23, 2022), to determine growth traits. Three plants were randomly taken from each pot, and root and shoot lengths were measured and weighed to determine the fresh weight. After that, they were oven dried (70 °C) to determine the dry weight. Leaves from the three plants were also fresh and dry-weighed. Although root fresh and dry weight may be slightly underestimated because of pulling wheat plants from pots, this error influences all treatments in the same way, therefore the comparisons among treatments in terms of root dry weight and root-to-shoot ratio should not be affected.
Samples taken on the same date were used to assess physiological traits, osmo-regulating compounds, oxidative stress markers, antioxidant activities, and element concentrations, which are described in the following points.
2.4 Physiological Traits
The chlorophyll and carotenoid contents were determined with standard methods of Lichtenthaler (1987). A 0.5 g of fresh leaf sample was homogenised in 80% methanol solution by using a pestle and mortar. Then the extract was centrifuged and filtered, and absorbance was recorded at 663, 645, and 480 nm wavelengths with a spectrophotometer Hitachi U-2001 (Hitachi, Tokyo, Japan) to determine the respective chlorophyll a, chlorophyll b, and carotenoid contents. The same instrument was used in the other cases where specific wavelengths were read after extractions.
The standard procedure of Mostofa and Fujita (2013) was used for the determination of relative water contents (RWC). Fresh leaf samples were taken from the plant and weighed to determine fresh weight (FW), then leaves were dipped in distilled water for 24 h. Then leaves were taken out from the water, excess water was removed, and turgid weight (TW) was taken. Finally, leaves were oven dried (75 °C) to assess dry weight (DW), and RWC was determined with the following formula:
For electrolyte leakage (EL), 0.5 g of fresh leaf sample (chopped into small pieces) was dipped in distilled water for 30 min, and EC1 was measured by using an EC meter. Then, EC2 was recorded after heating the samples in a water bath at 90 °C for 50 min. Finally, EC% was determined with the following formula:
Membrane stability index (MSI) (Sairam 1994) was also calculated as the percent complement of EL, using the following formula:
2.5 Osmo-Regulating Compounds
For the determination of total soluble protein (TSP), whose role in salt stress mitigation has recently been underlined (Athar et al. 2022), 0.5 frozen plant sample was ground in 5 mL phosphate buffer, then centrifuged at 14,000 rpm for 15 min at 4 °C. Then the plant sample was treated with 2 mL Bradford reagent, left for 15-20 min and absorbance was recorded at 595 nm with the aforementioned spectrophotometer (Bradford 1976).
In the case of free amino acids (Free a.a.), a 0.5 g plant sample was ground with 5 mL phosphate buffer and centrifuged (15,000 rpm) for 15 min. Afterward, 1 mL crude extract was poured into a test tube and added with 1 mL pyridine and 1 mL ninhydrin. Then, these test tubes were placed in a water bath for 30 min at 90 °C, and after that, the volume of test tubes was brought to 25 mL, and absorbance was recorded at 570 nm (Hamilton and Van Slyke 1943).
The concentration of total soluble sugars (TSS) was measured by placing 1–2 drops of the supernatant on the prism of a refractometer. In the case of proline, again 0.5 g of plant sample was extracted by adding 10 mL sulpho-salicylic acid (3%) and centrifuged for 10 min at 10,000 rpm. Afterward, acid-ninhydrin was added to the supernatant and placed for 30 min in a water bath (90 °C). Then the absorbance was recorded at 520 nm to determine proline content (Bates et al. 1973).
2.6 Antioxidant Activities
To determine catalase (CAT) activity, 0.5 g leaf sample and 5 mL potassium phosphate buffer were centrifuged and 10,000 rpm for 15 min, then absorbance was recorded at 240 nm (Aebi 1984).
To determine peroxidase (POD) activity, 0.5 g leaf sample was homogenised in 5 mL potassium phosphate buffer using a pestle and mortar. The solution was centrifuged at 10,000 rpm for 15 min, then the supernatant was collected, and absorbance was recorded at 470 nm (Guan et al. 2009).
Ascorbate peroxidase (APX) activity was determined by the method of Gutteridge and Halliwell (1990). A 0.5 g frozen leaf sample was homogenised in 5 mL potassium phosphate buffer (pH 7.8) using a pestle and mortar. The solution was centrifuged at 10,000 rpm for 15 min, the supernatant was collected, and absorbance was recorded at 290 nm to assess APX activity.
To determine Ascorbic acid (AsA), again 0.5 g leaf sample was homogenised using 5 mL trichloroacetic acid (TCA), and then centrifuged (8000 rpm) for 10 min. Then, 0.5 mL dithiocarbamate (DTC) reagent was added to 2 mL supernatant, incubated for 3 h, and cooled. Then, 2 mL sulfuric acid was added as dropwise and slightly shaken. The mixture was kept for 30 min at 30 °C, and the absorbance was recorded at 520 nm (Mukherjee and Choudhuri 1983).
2.7 Oxidative stress markers
In the case of malondialdehyde (MDA), a 0.5 g frozen plant sample was ground with 5 mL TCA and centrifuged for 15 min at 12,000 rpm. Afterward, a mixture containing 1 mL sample and 1 mL TCA was heated at 100 °C for 30 min and then rapidly cooled in an ice bath (4 °C). The concentration of MDA was determined by measuring absorbance at 532 nm (Rao et al. 2000).
The concentration of H2O2 in wheat samples was determined with the method of Velikova et al. (2000). A 0.5 g frozen plant sample was ground in 5 mL trichloroacetic acid (TCA) and centrifuged, then 1 mL extract was placed in test tubes supplemented with 1 M potassium iodide (166 mg) and 100 μL potassium phosphate buffer. Then, absorbance was read at 390 nm.
2.8 Element Concentrations
To determine element concentrations, samples of plant organs (roots, stem, and leaves) were taken, oven-dried (65 °C), and ground to make powder. Then, a 0.5 g powdered sample was taken and digested by adding HCl and HNO3 (1:2) at 180 °C. Afterward, the samples were diluted by adding distilled water, and concentrations of Na+ and K+ were determined with a flame photometer. Conversely, the concentration of Cl- in digested samples was measured using a chloride analyser. Lastly, the total N concentration was obtained by the Kjeldahl procedure. Element concentrations in the single organs are reported in the Supplementary Materials (Table S1 and S2). Element contents were obtained as the product of element concentrations × the dry weights of the respective plant organs.
2.9 Yield Traits
Three plants from each pot were taken at physiological maturity at 134 DAS (April 15, 2022). The following morphological and yield traits at harvest were determined: number of fertile (i.e., ear bearing) tillers, spike length, spikelets per spike, grains per spike, thousand-grain weight (TGW), biological (i.e., total above ground) and grain yield, and harvest index (HI), i.e., grain yield/biological yield. All weights were determined on a DW basis, and all data are reported on a per-plant basis.
2.10 Statistical and Principal Component Analysis
The experiment was conducted in a completely randomised factorial design with three replications. All the collected data were checked for normality of distribution (Kolmogorov-Smirnov test) and homogeneity of variances (Bartlett’s test). The data were analysed by two-way ANOVA for SS, urea coating (UC), and their interaction. Tukey’s HSD test at P ≤ 0.05 was used to indicate different levels in significant ANOVA sources. Besides the tables reporting means and ANOVA results, Table S3 in the Supplementary Materials reports the mean data and their standard errors.
To identify potential relationships among the measured traits, the data were explored through three principal component analyses, denominated PCA1, PCA2, and PC3. Trait acronyms used in the PCAs were further shortened with respect to tables, in order to avoid label overlapping. The new acronyms are reported in the respective PCA captions.
In PCA1, the traits describing plant morphology, growth, and yield (i.e., root dry weight, stem dry weight, leaf dry weight, root to shoot ratio, leaf number, tiller number, spike length, spikelets per spike, grains per spike, thousand-grain weight, biological yield, grain yield, and harvest index) were plotted against the traits describing plant water status, photosynthetic pigments, and osmo-regulating compounds (i.e., total chlorophyll, carotenoids, relative water content, electrolyte leakage, membrane stability index, proline, total soluble sugars, total soluble proteins, free amino acids). In PCA2, the above-cited traits describing plant morphology, growth, and yield were plotted against the traits describing the plant oxidative markers and antioxidative enzymes (i.e., malondialdehyde, hydrogen peroxide, ascorbate peroxidase, catalase, peroxidase, ascorbic acid). Finally, in PCA3, the above-cited traits describing plant morphology, growth, and yield were plotted against the traits describing the content of ions and their translocation across plant organs (i.e., total sodium content, total chloride content, total potassium content, total nitrogen content, Na translocation index to roots, stems, and leaves, Cl translocation index to roots, stems, and leaves, K translocation index to roots stem and leaves, N translocation index to roots, stem and leaves).
The three urea treatments (NU, normal urea; NOCU, neem oil coated urea; COCU, castor oil coated urea), and the three soil salinity levels (0, 6, and 12 dS m-1) were used as supplementary categorical variables, i.e., variables that are not included in PCA computation. In each PCA, the P-values of the Pearson correlation coefficients between the variables and each PC are reported in the Supplementary Materials (Table S4, S5, and S6). The eigenanalysis is also displayed in the Supplementary Materials (Figure S1). The analyses were performed with the R software (RStudio Team 2020), using the packages Car (Fox and Weisberg 2018) and Emmeans (Lenth 2018) for the analysis of variance and post hoc tests, and the package FactoMineR (Le 2008) for principal component analysis.
3 (Results
3.1 Morphological and Growth Traits
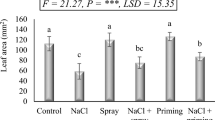
Soil salinity determined relevant reductions in all morphological and growth traits at the flag leaf stage (DAS 83) (Table 1). The leaf number was more than halved when passing from no salinity to high salinity (12 dS m-1). The same occurred to shoot DW, whereas root DW underwent only a ~25% decrease. As a result, the root-to-shoot ratio was more than doubled under high salinity vs. no salinity.
Urea coated with the two oils (NOCU and COCU) could mitigate the above-described adverse effects of salinity (Table 1). NOCU performed consistently better than COCU in alleviating salinity growth impairment. However, NOCU under high salinity could not restore the levels each trait possessed under non-saline conditions.
The significant interactions for all traits except root DW indicate, in general, a loss of mitigating activity for COCU when passing from the intermediate (6 dS m-1) to the high salinity level (12 dS m-1) (Table 1).
3.2 Leaf Water Status and Photosynthetic Pigments
The three traits indicating plant ability to maintain leaf hydration (RWC) and cell solute retention (EL and MSI) were severely affected by salinity (Table 2): in fact, under high salinity vs. no salinity, RWC and MSI were more than halved, while EL was more than doubled. The three photosynthetic pigments (chlorophyll a and b, and carotenoids) underwent sharp losses, too, under high salinity.
Urea coating could alleviate the strong damage to cell integrity and the photosynthetic machinery (Table 2). Again, NOCU performed consistently better than COCU, which in turn always passed the NU level in all traits.
The significant interactions for all traits except EL, indicate a slight decrease for COCU vs. NOCU activity at high salinity (Table 2). However, at high salinity, even NOCU could hardly maintain adequate levels of leaf hydration and electrolyte retention, as RWC fell to 29.7% while EL rose to 72.4%.
3.3 Osmo-Regulating Compounds
Salinity depressed TSP and Free a.a., while concurrently boosting TSS and proline (Table 3). Urea coating enhanced all four compounds according to the ranking: NOCU > COCU > NU. However, in TSP and Free a.a., NOCU could not attain the levels found under no salinity, whereas in TSS and Proline, NOCU increased their levels with respect to no salinity (Table 3).
The significant interactions in all compounds except TSS, outline stronger relative differences among urea coatings at high salinity in the case of TSP and, to a lesser extent, Free a.a. and Proline (Table 3).
3.4 Antioxidant Enzymes and Oxidative Stress Markers
Salinity enhanced the four antioxidant enzymes (CAT, POD, APX, and ascorbic acid) and the two oxidative stress markers (MDA and H2O2), with increases ranging from ~15% (Ascorbic acid) to ~1.3 fold (POD), when passing from no salinity to high salinity (Table 4). Urea coating also enhanced the antioxidant enzymes, according to the ranking: NOCU > COCU > NU (Table 4). Conversely, urea coating reduced oxidative stress markers, showing the opposite ranking: NOCU < COCU < NU. In antioxidant enzymes, NOCU attained almost the same levels determined by high salinity, whereas in oxidative stress markers, NOCU maintained levels quite close to no salinity. It is, therefore, sensed that NOCU exerted an eliciting activity of stress response mechanisms (the antioxidant enzymes) while restraining the oxidative stress signalling associated with salinity.
The significant interactions (CAT, MDA, and H2O2) depict lower relative differences among urea coatings at high vs. low salinity in CAT and, to a lesser extent, MDA and H2O2 (Table 4).
3.5 Element Concentrations, Contents, and Partitioning to Plant Organs
Soil salinity determined by NaCl supply resulted in strong increases in the plant concentrations of the two ions Na+ and Cl- (Table 5). A relevant decrease in K concentration was observed at high salinity, as the likely consequence of excess Na in the growth medium, resulting in competition for the uptake of either cation. Nitrogen, too, underwent a relevant decrease in concentration under high salinity, as the likely consequence of impaired root apparatus. Since high salinity drastically curbed total DW (−50%), the increases in Na and Cl contents were mitigated with respect to their increases in concentrations, whereas the decreases in K and N contents were enhanced with respect to their decreases in concentrations (Table 5).
Urea coating could modestly mitigate the above large variations in element concentrations and contents (Table 5). The usual ranking (NOCU > COCU > NU) was observed in the containment of both Na+ and Cl- increase, and K+ and N decrease.
The significant interactions in all cases except Cl content, outline a picture of non-univocal behaviour in element concentrations and contents at high vs. no salinity levels.
The allocation of the four elements to the three vegetative organs is expressed by the translocation index (TI) (Fig. 1). To better evidence the main changes, data at the intermediate salinity level (6 dS m-1) were not included in TI assessment. The four elements showed a similar behaviour under the influence of salinity and urea coatings: high salinity (12 dS m-1) vs. no salinity enhanced element partitioning to roots up to ~50% (Na, Cl, and K) and 40–50% (N), at the expenses of the stem. The effect of urea coating could not be clearly detected under no salinity, whereas under high salinity NOCU determined a small but significant decrease in the partitioning of all four elements to roots, in exchange for a slight increase in the partitioning to leaves. It is perceived in the four elements, that the increase in the partitioning to roots under high salinity was mainly driven by the increase in the root-to-shoot ratio (Table 1), leading the roots to become the main portion of plant biomass under salinity.
Translocation index (TI) of (A) sodium, (B) chloride, (C) potassium, (D) nitrogen to plant organs. SS 0 and SS 12 mean no salinity and high salinity (12 dS m-1), respectively; NU, NOCU, and COCU mean normal urea, neem oil–coated urea, and castor oil–coated urea, respectively. Vertical bars, ± SE (n=3). Different letters indicate significant differences for the same organ (Tukey test at P ≤ 0.05)
3.6 Morphological and Yield Traits at Maturity
Wheat morphological traits at harvest (DAS 134) were substantially curbed by salinity (Table 6). Under high salinity vs. no salinity, the number of fertile tillers, spikelets per spike, and the thousand-grain weight were reduced by approximately two-thirds, while spike length and the number of grains per spike were more than halved (Table 6). As a result, the biological and grain yield were equally reduced by more than 50%, while the harvest index varied modestly.
Urea coating could mitigate salinity adverse effects on these traits, although no urea coating could restore the trait levels obtained under no salinity (Table 6). In all the morphological and yield traits, the usual ranking was shown in the effectiveness of the three urea types: NOCU > COCU > NU.
The significant interactions for all traits indicate generally wider relative differences among urea types at high salinity. This allowed especially NOCU to be more effective in upholding biological and grain yield under high salinity, although in both traits the levels obtained by NU under no salinity were not attained (Table 6).
3.7 Principal Component Analysis
Three PCAs were performed to visualise the relationships among the main traits and extrapolate the salient information from the results obtained. In all three PCAs, most of the variance was explained by the first component.
In PCA1, the traits describing plant biometry, growth, and yield were put in relation with those describing plant water status, the content of photosynthetic pigments, and osmo-regulating compounds (Fig. 2). The first PC, explaining 87.6% of the data variance, clearly represents the effect of salinity, as the three salinity treatments were aligned along this PC, with the control (0 dS m-1) at the extreme right side, and the high salinity level (12 dS m-1) at the extreme left side. Indeed, almost all the traits related to growth, water status, and membrane stability were oriented in the same direction as the control treatment and opposite to the 12 dS m-1 treatment, in contrast to proline, total soluble sugars, electrolyte leakage, and root to shoot ratio, which increased with soil salinity. The barycentres of the three urea coatings were aligned along a diagonal, with NU on the negative side of the first PC, NOCU on the positive side, and COCU in the middle, at the intersection of the axes. This placement may suggest that NOCU was the most effective treatment in counteracting the noxious effects of salinity, allowing the plant to maintain growth, hydration, and photosynthetic pigments at values similar to those of the control.
PCA1 biplot. Red squares indicate the barycentres of the three urea treatments (NU, normal urea; NOCU, neem oil–coated urea; COCU, castor oil–coated urea), the green triangle represents three soil salinity levels ( 0, 6, and 12 dS m-1), while the violet circles indicate the barycentres of the quantitative variables (root dry weight (DW(r)), stem dry weight (DW(s)), leaf dry weight (DW(l)), root to shoot ratio (R:S), leaf number (LN), tiller number (TN), spike length (SL), spikelets per spike (SLPS), grains per spike (GPS), MSI (Membrane stability index), thousand-grain weight (TGW), biological yield (BY), grain yield (GY), harvest index (HI), total chlorophyll (Chl_T), carotenoids (Car), relative water content (RWC), electrolyte leakage (EL), proline (PRO), total soluble sugars (TSS), total soluble proteins (TSP), free amino acids (Free aa))
In PCA2, the traits describing plant biometry, growth, and yield were put in relation with the traits describing plant oxidative status and content of antioxidant compounds (Fig. 3). Once again, the first PC, explaining 79.5% of the data variance, resembles the effect of salinity, with the control treatment (0 dS m-1) located at the extreme right side of this PC, the high salinity level (12 dS m-1) at the extreme left side of the same PC, and the intermediate salinity level in between. Indeed, except for the root-to-shoot ratio, all the growth-related traits were opposite to the 12 dS m-1 salinity level, as they decreased with increasing salinity. Conversely, the barycentres of the enzymes and oxidative stress markers were oriented toward the barycentre of the highest salinity levels, as their content increased with salinity. Also in this case, the barycentre of the NOCU treatment was located on the upper right quadrant, on the positive side of the first PC, the COCU at the intersection of the biplot axis, and the NU in the lower-left quadrant, on the negative side of the first PC. This placement suggests that NOCU was the treatment that most stimulated the enzymatic activity, boosting the plant response to salinity and helping to counteract the oxidative stress, thereby allowing maintaining performance levels more similar to those of the control.
PCA2 biplot. Red squares indicate the barycentres of the three urea treatments (NU, normal urea; NOCU, neem oil–coated urea; COCU, castor oil–coated urea), the green triangle represents three soil salinity levels ( 0, 6, and 12 dS m-1), while the violet circles indicate the barycentres of the quantitative variables (root dry weight (DW(r)), stem dry weight (DW(s)), leaf dry weight (DW(l)), root to shoot ratio (R:S), leaf number (LN), tiller number (TN), spike length (SL), spikelets per spike (SLPS), grains per spike (GPS), thousand-grain weight (TGW), biological yield (BY), grain yield (GY), harvest index (HI), malondialdehyde (MDA), hydrogen peroxide (H2O2), ascorbate peroxidase (APX), catalase (CAT), peroxidase (POD), ascorbic acid (AsA))
In PCA3, the traits describing plant biometry, growth, and yield were put in relation with the traits describing the total content of ions and their translocation across the plant organs (Fig. 4). As in the previous two PCAs, the first PC, accounting for 78.9% of the data variance, reproduces the effects of salinity, with the high salinity level placed on the right side of the first PC and the control on the left side. Indeed, the barycentres of the primary growth traits and total content of K and N were positioned on the positive side of PC1, as they reached the highest values under control conditions. In contrast, the barycentres of the total content of Na and Cl were positioned on the negative side of the first PC, as they increased proportionally with salinity. The barycentre of the NOCU treatment was oriented in the same direction as the leaf translocation indices, indicating that a major translocation of ions to the photosynthetic organs occurred under this treatment. The NU barycentre, instead, was aligned to the barycentres of the root translocation indices, as this treatment promoted ion accumulation at the root level in exchange for lower accumulation at the stem levels. Lastly, the COCU treatment resulted to be placed in the same direction as the stem translocation indices, indicating that a major (although not significantly different) accumulation of ions at the stem level occurred in correspondence with this treatment.
PCA3 biplot. Red squares indicate the barycentres of the three urea treatments (NU, normal urea; NOCU, neem oil–coated urea; COCU, castor oil–coated urea), the green triangle represents three soil salinity levels ( 0, 6, and 12 dS m-1), while the violet circles indicate the barycentres of the quantitative variables (root dry weight (DW(r)), stem dry weight (DW(s)), leaf dry weight (DW(l)), root to shoot ratio (R:S), leaf number (LN), tiller number (TN), spike length (SL), spikelets per spike (SLPS), grains per spike (GPS), thousand grain weight (TGW), biological yield (BY), grain yield (GY), harvest index (HI), total sodium content (Na_T), total chloride content (CL_T), total potassium content (K_T), total nitrogen content (N_T), Na translocation index to root (TI_Na(r)), Na translocation index to stem (TI_Na(s)), Na translocation index to leaf (TI_Na(l)), Cl translocation index to root (TI_Cl(r)), Cl translocation index to stem (TI_Cl(s)), Cl translocation index to leaf (TI_Cl(l)), K translocation index to root (TI_K(r)), K translocation index to stem (TI_K(s)), K translocation index to leaf (TI_K(l)), N translocation index to root (TI_N(r)), N translocation index to stem (TI_N(s)), N translocation index to leaf (TI_N(l)))
4 Discussion
Soil salinity represents a severe abiotic stress restricting crop growth and productivity to a growing extent in many world areas (Stocker et al. 2013). The present study, besides a non-saline control, involved two salinity levels, of which the intermediate (6 dS m-1) is considered the threshold for initial losses in wheat yield at the field level, whereas the highest (12 dS m-1) is assumed to determine an approximate 50% yield loss (Ayers et al. 1985). Compared to this, in our pot experiment at both the flag leaf stage and physiological maturity (DAS 83 and 134, respectively), stronger restraints in growth and final yield were incurred by the two salinity levels under the scenario of no mitigation represented by the use of NU. Therefore, the experimental conditions were shown to be quite challenging for any external input to support wheat growth and the related physiological processes.
Stunted growth under salinity originated from impaired physiological functions under many viewpoints: increased oxidative stress markers, disturbed nutrient homeostasis, and reduced photosynthetic pigments, plus reduced cell division and growth, as reported in other sources (Hassan et al. 2014; Al-Yasi et al. 2020).
The application of coated fertilisers has already been proven beneficial for nutrient uptake and use efficiency (Noor et al. 2017; Yaseen et al. 2017), and the availability of nutrients, namely nitrogen and potassium, is considered a pivotal point in supporting the wheat plant exposed to salinity (Ahanger et al. 2017; Giambalvo et al. 2022). In the case of urea, coating with vegetable oils is a technique established for decades (Singh and Singh 1989). However, it has not achieved general consensus among methods for obtaining controlled-release fertilisers, despite relatively simple industrial technology (Suri 1995; Aggarwal and De 2013). Vegetable oils are also proposed as components of polymers for fertiliser coating that perhaps have higher added value, yet do not always perform better from an agronomic viewpoint (Martinez et al. 2021). Both castor oil and neem oil are widely studied in urea coating, but especially the latter oil coating has proved able to maintain N availability in the soil for a longer time and reduce N losses, including volatilisation of the highly noxious N2O (Gupta et al. 2016). This is consistent with the stronger effect exerted by NOCU vs. COCU in almost all the plant traits examined in our experiment.
To our best knowledge, NOCU and COCU have hardly been investigated in response to salinity. However, some of their properties evidenced in previous studies help us to interpret their behaviour in response to salinity. The first point is element uptake and allocation to plant organs, both in the case of potentially adverse elements (Na and Cl), as well as major nutrients (K and N). NOCU and COCU favoured N uptake with respect to NU, which is consistent with a root system less severely affected by salinity, and with a nutrient availability maintained for a longer time thanks to slow N release (Gupta et al. 2016). The two circumstances combined determined higher N content in the whole plant under saline as well as non-saline conditions, and higher N allocation to the root system under salinity. The same pattern can be observed for potassium, although this nutrient was not supplied with the investigated fertilisers. It may, therefore, be evinced that nitrogen exerts a drag on potassium, favouring K uptake against the competition determined at the soil-root interface by increased Na availability. Conversely, the increased concentration of the two adverse elements (Na and Cl) supplied with saline treatments was responsible for disturbing ion homeostasis and decreasing nutrient concentrations in plant organs, a circumstance which is already echoed in the literature (Chen et al. 2012; Tarighaleslami et al. 2012). The salinity-driven increase in Na accumulation inhibits K uptake with a consequential impact on stomata guard cells (Munns and Tester 2008). Moreover, due to the lower concentration of external protons, salinity reduces the ability of Na/K antiporters to exclude excessive Na, resulting in increased Na accumulation in plant tissues at the expense of K accumulation (Munns and Tester 2008). Similarly, excess Na and Cl reduce N accumulation in plant tissues because of Na+ competition with the cationic form (NH4+) and Cl- competition with the anionic form (NO3-) of available nitrogen (Carpici et al. 2010; Baghbani et al. 2012). In our experiment, all the nutritional drawbacks engendered by salinity were mitigated by the two oil-coated fertilisers (NOCU and COCU), which succeeded in upkeeping nutrient (K and N) uptake while restraining adverse element (Na and Cl) access to plant tissues. This is also seen in PCA3, where NOCU and COCU were positively correlated to total K and N content. In contrast, NOCU and COCU were inversely correlated to total Na and Cl content, suggesting that the oil-coated ureas stimulated the accumulation of the former two elements while reducing the absorption of the latter two elements.
The rest of the beneficial effects are consistent with the above picture of better nutrient homeostasis associated with oil coated vs. normal urea. In fact, the improved leaf water status under salinity was at least partially due to improved nutrient uptake supporting root growth, which in turn improves water uptake and leaf hydration, i.e., RWC (Docimo et al. 2020). In parallel, EL and MSI were significantly restrained and augmented, respectively, due to a substantial increase in the two oxidative stress markers (MDA and H2O2). However, the application of coated urea (N) reduced their accumulation by increasing the antioxidant activities and osmolyte accumulation. Since MDA is a product of lipid peroxidation, MDA reduction with oil-coated urea is consistent with the maintenance of cell membrane functionality, which in turn is the reason for EL reduction under salinity (Mumtaz et al. 2018).
In the case of osmo-regulating compounds, salinity exerted a dual effect by depressing TSP and Free a.a., while enhancing TSS and proline. Once more, the decrease in compounds such as TSP and Free a.a., which are directly linked to the metabolism of nitrogen, is consistent with insufficient N uptake and explains why N sources that are more efficient than NU, such as NOCU and COCU, could counterbalance TSP and Free a.a. decrease. More to this, oil-coated urea increased the accumulation of TSS and proline. These two compounds are potential osmolytes ready to face salinity, owing to the fact that TSS is the carbon source for other organic solutes and plays a key role in protecting enzymes, while proline is the most water-soluble amino acid regulating cytoplasmic osmotic potential and protecting protein molecules (El-Saidi 1997; Feng et al. 2023). All this is evident in PCA1, where it appears that NOCU, and secondly COCU, were more effective than NU in stimulating the production of osmoregulating compounds, which contributed to preserving the plant hydration and the pool of photosynthetic pigments, with an overall positive effect on plant growth and yield traits.
Lastly, the four antioxidant enzymes (CAT, POD, APX, and AsA) were significantly increased under salinity and further increased by applying oil-coated urea. More specifically, PC3 showed that, among the three urea treatments, NOCU mostly stimulated plant enzymatic response and reduced the production of oxidative stress markers, conferring a greater ability to scavenge the ROS and contain the oxidative stress processes. Nitrogen is assumed to be the first defence line against abiotic stresses (Khan et al. 2017; Singh et al. 2019). Besides, N positive effects might be due to N being an effective component of antioxidant enzymes (Singh et al. 2019). This is consistent with the results of oil-coated urea in our experiment, showing a decrease in the two oxidative stress markers (MDA and H2O2) in parallel to the aforementioned increase in the four antioxidant enzymes.
5 Conclusions
Soil salinity significantly reduced the growth and final yield of wheat plants, which could be ascribed to salinity-induced oxidative, ionic, and osmotic stresses that damage the photosynthetic apparatus and alter wheat physiological activities. Under such circumstances, the application of oil-coated urea significantly improved all growth and physiological traits, osmo-regulating compounds, oxidative stress markers, antioxidant activities, and favourable (N and K) vs. unfavourable (Na and Cl) elements. This, in turn, positively influenced final yield and the related traits with respect to normal urea, under both normal and saline conditions.
However, the two vegetable oils (neem and castor oil) did not exhibit the same effectiveness. Neem oil–coated urea always performed significantly better than castor oil–coated urea, but even the former oil could only mitigate the adverse effects of high soil salinity (12 dS m-1). In this respect, it is sensed that oil choice for urea coating depends on several factors besides their effectiveness: among them, oil availability, alternative uses, and market price.
While the beneficial effects of oil-coated urea in terms of slow nitrogen release have already been proved in previous works, the advantages under salinity constitute a novelty requiring to be supported by further evidence. It is, therefore, hoped that additional experiments will be set up in order to validate our results or suggest changes in the use of vegetable oil–coated urea as a means to cope with salinity.
Data Availability
Not applicable.
References
Aebi H (1984) Catalase in vitro. In Methods Enzymol; Academic press. Elsevier 105:121-126. https://doi.org/10.1016/s0076-6879(84)05016-3
Affendi NM, Yusop MK, Othman R (2018) Efficiency of coated urea on nutrient uptake and maize production. Commun Soil Sci Plant Anal 49:1394–1400. https://doi.org/10.1080/00103624.2018.1464182
Aggarwal RK, De AB (2013) Production of neem coated urea: an urgent necessity for India. Indian J Fert 9:22–26
Ahanger MA, Tomar NS, Tittal M, Argal S, Agarwal R (2017) Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol Mol Biol Plant 23:731–744. https://doi.org/10.1007/s12298-017-0462-7
Ain NU, Naveed M, Hussain A, Mumtaz MZ, Rafique M, Bashir MA, Alamri S, Siddiqui MH (2020) Impact of coating of urea with Bacillus-augmented zinc oxide on wheat grown under salinity stress. Plants 9:1375. https://doi.org/10.3390/plants9101375
Altaf A, Zhu X, Zhu M, Quan M, Irshad S, Xu D, Aleem M, Zhang X, Gull S, Li F, Shah AZ (2021) Effects of environmental stresses (heat, salt, waterlogging) on grain yield and associated traits of wheat under application of sulfur-coated urea. Agron 11:2340. https://doi.org/10.3390/agronomy11112340
Al-Yasi H, Attia H, Alamer K, Hassan F, Ali E, Elshazly S, Siddique KH, Hessini K (2020) Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol Biochem 1(150):133–139. https://doi.org/10.1016/j.plaphy.2020.02.038
Asghar MG, Ikram RM, Hashim S, Hussain S, Irfan M, Mubeen K, Ali M, Alam M, Ali M, Haider I, Shakir M (2022) Sulphur coated urea improves morphological and yield characteristics of transplanted rice (Oryza sativa L.) through enhanced nitrogen uptake. J King Saud Uni Sci 34:101664. https://doi.org/10.1016/j.jksus.2021.101664
Athar HUR, Zulfiqar F, Moosa A, Ashraf M, Zafar ZU, Zhang L, Ahmed N, Kalaji HM, Nafees M, Hossain MA, Islam MS, El Sabagh A, Siddique KHM (2022) Salt stress proteins in plants: an overview. Front Plant Sci 13:999058. https://doi.org/10.3389/fpls.2022.999058
Ayers RS, Westcot DW (1985) Water quality for agriculture. Rome: Food and Agriculture Organization of the United Nations. FAO Irrigation and Drainage Paper 29, Rev. 1
Baghbani A, Namdari A, Kadkhodaie A (2012) Effects of salinity and nitrogen supply on nitrogen-fixation nodules and nitrogen, sodium and potassium concentration of alfalfa cultivars. J Basic Appl Sci Res 2:9978–9984
Bailey-Serres J, Parker JE, Ainsworth EA, Oldroyd GE, Schroeder JI (2019) Genetic strategies for improving crop yields. Nat 575:109–118. https://doi.org/10.1038/s41586-019-1679-0
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bortoletto-Santos R, Ribeiro C, Polito WL (2016) Controlled release of nitrogen-source fertilizers by natural-oil-based poly (urethane) coatings: The kinetic aspects of urea release. J Appl Polym Sci 133:33–41. https://doi.org/10.1002/app.43790
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein sutilising the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Carpici EB, Celik N, Bayram G (2010) The effects of salt stress on the growth, biochemical parameter and mineral element content of some maize (Zea mays L.) cultivars. Afr J Biotechnol 9:6937–6942. https://doi.org/10.5897/AJB10.780
Chen S, Xing J, Lan H (2012) Comparative effects of neutral salt and alkaline salt stress on seed germination, early seedling growth and physiological response of a halophyte species Chenopodium glaucum. Afr J Biotechnol 11:9572–9581. https://doi.org/10.5897/AJB12.320
Denaxa NK, Nomikou A, Malamos N, Liveri E, Roussos PA, Papasotiropoulos V (2022) Salinity effect on plant growth parameters and fruit bioactive compounds of two strawberry cultivars, coupled with environmental conditions monitoring. Agron 12:2279. https://doi.org/10.3390/agronomy12102279
Dimkpa CO, Andrews J, Fugice J, Singh U, Bindraban PS, Elmer WH, Gardea-Torresdey JL, White JC (2020) Facile coating of urea with low-dose ZnO nanoparticles promotes wheat performance and enhances Zn uptake under drought stress. Front Plant Sci 11:168. https://doi.org/10.3389/fpls.2020.00168
Docimo T, De Stefano R, Cappetta E, Piccinelli AL, Celano R, De Palma M, Tucci M (2020) Physiological, biochemical, and metabolic responses to short and prolonged saline stress in two cultivated cardoon genotypes. Plant 9:554. https://doi.org/10.3390/plants9050554
Dong FG, Ma Y, Zhang M, You JP, Hong HL, Guo LC, Hong ZY (2019) Polyurethane-coated urea using fully vegetable oil-based polyols: Design, nutrient release and degradation. Prog Org Coat 133:267–275. https://doi.org/10.1016/j.porgcoat.2019.04.053
El-Sabagh A, Hossain A, Barutçular C, Iqbal MA, Islam MS, Fahad S, Sytar O, Çiğ F, Meena RS, Erman M (2020) Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: an outlook of arid and semi-arid regions. In: Environment, climate, plant and vegetation growth Springer, Cham, p 503-533
El-Saidi MT (1997) Salinity and its effect on growth, yield and some physiological processes of crop plants. In: strategies for improving salt tolerance in higher plants. Science Publishers: Enfield, NH, USA, p 111-127
Etesami H, Noori F (2019) Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants. In: saline soil-based agriculture by halotolerant microorganisms. Springer, Singapore, p 1-22
FAO (2015) Status of the World’s Soil Resources (SWSR): Main Report; Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome, Italy
FAOSTAT (2022) Available online: https://www.fao.org/faostat/en/#data . Accessed 22 Mar 2023
Feng D, Gao Q, Liu J, Tang J, Hua Z, Sun X (2023) Categories of exogenous substances and their effect on alleviation of plant salt stress. Eur J Agron 142:126656. https://doi.org/10.1016/j.eja.2022.126656
Fox J, Weisberg S (2018) An R companion to applied regression 3rd ed. Sage publications. Inc: Newburry Park, California, p 18-42
Ghafoor I, Habib-ur-Rahman M, Ali M, Afzal M, Ahmed W, Gaiser T, Ghaffar A (2021) Slow-release nitrogen fertilizers enhance growth, yield, NUE in wheat crop and reduce nitrogen losses under an arid environment. Environ Sci Pollut Res 28:43528–43543. https://doi.org/10.1007/s11356-021-13700-4
Giambalvo D, Amato G, Borgia D, Ingraffia R, Librici C, Lo Porto A, Puccio G, Ruisi P, Frenda AS (2022) Nitrogen availability drives mycorrhizal effects on wheat growth, nitrogen uptake and recovery under salt stress. Agron 12:2823. https://doi.org/10.3390/agronomy12112823
Giraldo P, Benavente E, Manzano-Agugliaro F, Gimenez E (2019) Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agron 9:352. https://doi.org/10.3390/agronomy9070352
Guan YJ, Hu J, Wang XJ, Shao CX (2009) Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J Zhejiang Univ Sci B 10:427–433. https://doi.org/10.1631/jzus.B0820373
Gupta DK, Bhatia A, Kumar A, Das TK, Jain N, Tomer R, Malyan SK, Fagodiya RK, Dubey R, Pathak H (2016) Mitigation of greenhouse gas emission from rice–wheat system of the Indo-Gangetic plains: through tillage, irrigation and sfertiliser management. Agric Ecosyst Environ 230:1–9. https://doi.org/10.1016/j.agee.2016.05.023
Gutteridge JMC, Halliwell B (1990) The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci 15:129–135. https://doi.org/10.1016/0968-0004(90)90206-q
Hamilton PB, Van Slyke DD (1943) Amino acid determination with ninhydrin. J Biol Chem 150:231–250
Hassan F, Ali E (2014) Effects of salt stress on growth, antioxidant enzyme activity and some other physiological parameters in jojoba [Simmondsia chinensis (Link) Schneider] plant. Aust J Crop Sci 8:1615–1624
Isayenkov SV, Maathuis FJ (2019) Plant salinity stress: many unanswered questions remain. Front Plant Sci 10:80. https://doi.org/10.3389/fpls.2019.00080
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30:435–458. https://doi.org/10.1080/07352689.2011.605739
Joshi S, Nath J, Singh AK, Pareek A, Joshi R (2022) Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiol Plant 7:e13702. https://doi.org/10.1111/ppl.13702
Khan A, Tan DK, Afridi MZ, Luo H, Tung SA, Ajab M, Fahad S (2017) Nitrogen fertility and abiotic stresses management in cotton crop: a review. Environ Sci Pollut Res 24:14551–14566. https://doi.org/10.1007/s11356-017-8920-x
Kim Y, Mun BG, Khan AL, Waqas M, Kim HH, Shahzad R, Imran M, Yun BW, Lee IJ (2018) Regulation of reactive oxygen and nitrogen species by salicylic acid in rice plants under salinity stress conditions. PLoS One 13:0192650
Kumar M, Etesami H, Kumar V (2019) Saline soil-based agriculture by halotolerant microorganisms. Springer Singapore, pp 1-253. https://doi.org/10.1371/journal.pone.0192650
Langridge P, Alaux M, Almeida NF, Ammar K, Baum M, Bekkaoui F, Bentley AR, Beres BL, Berger B, Braun HJ, Brown-Guedira G (2022) Meeting the challenges facing wheat production: the strategic research agenda of the global wheat initiative. Agron 12:2767. https://doi.org/10.3390/agronomy12112767
Lê S, Josse J, Husson F (2008) FactoMineR: An R Package for Multivariate Analysis. J Stat Softw 25:1–18. https://doi.org/10.18637/jss.v025.i01
Lenth R, Singmann H, Love J, Buerkner P, Herve M (2018) Emmeans: Estimated marginal means, aka least-squares means. R Pack Version 1:3. Available online: https://cran.r-project.org/web/packages/emmeans/index.html . Accessed 28 Dec 2022
Li G, Zhao B, Dong S, Zhang J, Liu P, Vyn T (2017) Impact of controlled release urea on maize yield and nitrogen use efficiency under different water conditions. PLoS One 12:e0181774. https://doi.org/10.1371/journal.pone.0181774
Li P, Lu J, Wang Y, Wang S, Hussain S, Ren T, Cong R, Li X (2018) Nitrogen losses, use efficiency, and productivity of early rice under controlled-release urea. Agric Ecosyst Environ 251:78–87. https://doi.org/10.1016/j.agee.2017.09.020
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Methods Enzymol, vol 148. Academic Press, pp 350–382
Martinez C, Clarke D, Dang YP, Janke C, Bell MJ (2021) Integrated field assessment of nitrogen release dynamics and crop recovery of band-applied controlled-release fertilizers. Plant Soil 466:257–273. https://doi.org/10.1007/s11104-021-05043-3
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicol 22:959–973. https://doi.org/10.1007/s10646-013-1073-x
Mukherjee SP, Choudhuri MA (1983) Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166–170. https://doi.org/10.1111/j.1399-3054.1983.tb04162.x
Mumtaz MZ, Saqib M, Abbas G, Akhtar J, Qamar ZU (2018) Genotypic variation in rice for grain yield and quality as affected by salt-affected field conditions. J Plant Nutr 41:233–242. https://doi.org/10.1080/01904167.2017.1385796
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nazir F, Mahajan M, Khatoon S, Albaqami M, Ashfaque F, Chhillar H, Chopra P, Khan MIR (2023) Sustaining nitrogen dynamics: A critical aspect for improving salt tolerance in plants. Front Plant Sci 14:1087946. https://doi.org/10.3389/fpls.2023.1087946
Noor S, Yaseen M, Naveed M, Ahmad R (2017) Effectiveness of diammonium phosphate impregnated with Pseudomonas putida for improving maize growth and phosphorus use efficiency. J Anim Plant Sci 27:1588–1595
Perri S, Molini A, Hedin LO, Porporato A (2022) Contrasting effects of aridity and seasonality on global salinisation. Nat Geosci 15:375–381. https://doi.org/10.1038/s41561-022-00931-4
Rao KM, Sresty TV (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128. https://doi.org/10.1016/S0168-9452(00)00273-9
Rehman A, Nawaz M, Chattha MU, Khan I, Chattha MB, Hussain F, Ayub MA, Iqbal MM, Ahmed F, Aslam MT, Khan FA, Kharal M, Hassan MU (2021) Neem coated urea improves the productivity, nitrogen use efficiency and economic return of wheat crop. Int J Agric Biol 26:450–460. https://doi.org/10.17957/IJAB/15.1856
RStudio Team (2020) RStudio: Integrated Development for R. RStudio, PBC, Boston, MA. https://www.rstudio.com/
Sairam RK (1994) Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Ind J Exp Biol 32:594–597
Shrivastava P, Kumar R (2015) Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Singh M, Singh TA (1989) Comparison of neem (Azadirachta indica) oil coated urea with some other coated urea fertilizer on an alkaline calcareous soil. J Indian Soc Soil Sci 37:314–318
Singh M, Singh VP, Prasad SM (2019) Nitrogen alleviates salinity toxicity in Solanum lycopersicum seedlings by regulating ROS homeostasis. Plant Physiol Biochem 14:466–476
Sofy MR, Elhawat N, Alshaal T (2002) Glycine betaine counters salinity stress by maintaining high K+/Na+ ratio and antioxidant defense via limiting Na+ uptake in common bean (Phaseolus vulgaris L.). Ecotoxicol Environ Saf 200:110732. https://doi.org/10.1016/j.ecoenv.2020.110732
Stocker TF, Qin D, Plattner GK, Tignor MM, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (2013) Climate change: the physical science basis. Contribution of working group I to the fifth assessment report of IPCC the intergovernmental panel on climate change. Cambridge Univ Press, Cambridge, UK, New York, NY, USA
Suri IK (1995) Coating of urea with neem. Fert News 40:55–59
Tarighaleslami M, Zarghami R, Boojar MMA, Oveysi M (2012) Effects of drought stress and different nitrogen levels on morphological traits of proline in leaf and protein of corn seed (Zea mays L.). Am Eurasian J Agric Environ Sci 12:49–56
Van Zelm E, Zhang Y, Testerink C (2020) Salt tolerance mechanisms of plants. Ann Review Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Voutsinos-Frantzis O, Loannis K, Dimitrios P, Georgios Z, Dimitrios F, Theodora N, Ropokis A, Karkanis A, Leo S, Dimitrios S, Georgia N (2023) Effects of NaCl and CaCl2 as eustress factors on growth, yield, and mineral composition of hydroponically grown Valerianella locusta. Plants 12:1454. https://doi.org/10.3390/plants12071454
Yaseen M, Aziz MZ, Manzoor A, Naveed M, Hamid Y, Noor S, Khalid MA (2017) Promoting growth, yield, and phosphorus-use efficiency of crops in maize–wheat cropping system by using polymer-coated diammonium phosphate. Commu Soil Sci Plant Anal 48:646–655. https://doi.org/10.1080/00103624.2017.1282510
Yuan S, Cheng L, Tan Z (2022) Characteristics and preparation of oil-coated fertilizers: A review. J Controlled Release 345:675–684. https://doi.org/10.1016/j.jconrel.2022.03.040
Zhu J, Sun L, Ju F, Wang Z, Xiong C, Yu H, Yu K, Huo Y, Khattak WA, Hu W, Wang S (2022) Potassium application increases cotton (Gossypium hirsutum L.) fiber length by improving K+/Na+ homeostasis and potassium transport capacity in the boll-leaf system under moderate salinity. Agron 12:2962. https://doi.org/10.3390/agronomy12122962
Zörb C, Ludewig U, Hawkesford MJ (2018) Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci 23:1029–1037. https://doi.org/10.1016/j.tplants.2018.08.012
Acknowledgments
The authors are thankful to the laboratory staff for their support during lab analysis. The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code (22UQU4281560DSR13).
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 130 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, I., Ali, S.M., Chattha, M.U. et al. Neem and Castor Oil–Coated Urea Mitigates Salinity Effects in Wheat by Improving Physiological Responses and Plant Homeostasis. J Soil Sci Plant Nutr 23, 3915–3931 (2023). https://doi.org/10.1007/s42729-023-01311-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01311-6