Abstract
The vision of this study is to find a way for increasing phosphorus release from bone char. An incubation experiment was conducted to study the effect of co-applying different nitrogen fertilizer types with bone char (BC) on the availability and distribution of phosphorus in calcium carbonate-rich soil. The experiment contains the following treatments: soil without any nitrogen fertilizer (BC only), soil + ammonium sulfate (BC + AS), soil + ammonium nitrate (BC + AN), and soil + urea (BC + U). Bone char was added to all treatments at a dose of 4 g kg−1 soil. Co-applying bone char with all nitrogen fertilizers caused a significant decrease in pH and increased significantly phosphorus availability in the soil. The concentrations of soil available phosphorus increased from 8.05 mg kg−1 soil for BC treatment to 8.99, 8.90, and 10.16 mg kg−1 soil for BC + AS, BC + AN, and BC + U treatments, respectively, at the end of incubation. Significant increases in soil available phosphorus were observed with increasing incubation periods in all treatments. The effectiveness of the treatments on the soil available phosphorus increase was in the order of BC + U > BC + AS > BC + AN > BC. Nitrogen fertilization treatments significantly increased the NaHCO3-Pi concentrations compared to the BC treatment. Urea application to the soil increased significantly the NaOH-Pi fraction compared to other treatments at day 10 of incubation. Changes in HCl-Pi and Res-P fractions were non-significant in all treatments under nitrogen fertilization. According to the findings, co-applying bone char with nitrogen fertilizers is an agronomic practice that improves phosphorus availability in calcium carbonate-rich soil, thence it is preferable to add urea rather than other nitrogen fertilizers. This study explores a sustainable management strategy to find cost-effective and environmentally friendly alternatives to phosphate fertilizers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Calcareous soils contain high content of calcium carbonate more than 10% (El Mashad and Ahmed 2016) and are widespread around the world; they represent about 30% of the earth’s land surface (Chevallier et al. 2016). The lack of precipitation in arid and semi-arid regions led to a decrease in the leaching rate which in turn caused calcareous soils to be common in these regions (Hopkins and Ellsworth 2005). These soils in Egypt occupy approximately 25–30% of the total area according to the Ministry of Agriculture and Land Reclamation estimation (Taalab et al. 2019). Moreover, these soils suffer from a lack of nutrient availability in particular phosphorus because of forming insoluble calcium phosphate minerals and its fixation on surfaces of calcium carbonate (Hopkins and Ellsworth 2005). Phosphorus is an essential macronutrient for plant growth, and the application of phosphate fertilizers is also important to provide the global food supply (Torri et al. 2017). More than 40% of the world’s arable land suffers from phosphorus deficiency, which affects crop productivity (Balemi and Negisho 2012; Zhu et al. 2018). Moreover, phosphate fertilizers are considered a non-renewable resource. The continuous excessive use and unsustainable agricultural practices of phosphate fertilizers, to meet the increasing demand for food due to the large population increase, led to soil phosphorus accumulation becoming an economic and environmental problem (Gupta et al. 2014). Predominantly, about 85–90% of the inorganic phosphorus applied via fertilization converted to unavailable form to plants during the year of the addition, this is might be attributed to adsorption and precipitation mechanisms in the soils (Brady and Weil 1999).
It is beneficial to use waste as a source of nutrients; additionally, recycling waste has many benefits such as reducing environmental pollution; at the same time, it is considered to be a rational use of natural resources (Zhan et al. 2020). Globally, the strategies of recycling nutrients in agriculture are promising ways to face the depletion challenges of non-renewable resources as well as to reduce the levels of environmental pollution resulting from their mining and manufacturing processes (Robles et al. 2020). Recycling phosphorus in slaughterhouse wastes such as bone, which is typically rich in phosphorus content, is necessary for sustainable agriculture and global food security (Sun et al. 2018). Transforming animal bones to bone char through the pyrolysis process has many environmental and economic benefits because it is cheap and free of heavy metals in light of environmental pollution and high international prices of phosphate fertilizers (Vassilev et al. 2013). Bone char applications into the soil led to enhancing wheat and potato tuber yield higher than some phosphate fertilizers such as diammonium phosphate and triple superphosphate (Siebers et al. 2014) as well as adding bone char as a phosphate fertilizer in a Pb-contaminated soil improved the shoot of Chinese cabbage and decreased Pb concentrations in the shoot (Chen et al. 2006). The application of bone char to contaminated smelter soil increased the fresh and dry weight of maize plants and decreased the concentrations of Zn and Cd in the roots and shoots of maize (Azeem et al. 2021).
The availability of phosphorus in the soil is greatly affected by different agricultural practices; applications of nitrogen fertilizer, crop residues, manure, biochar, and water (Jiang et al. 2021), as well as types of nitrogen fertilizer (Chien et al. 2011). The frequent use of chemical fertilizers is responsible for the noticeable change in the soil pH, especially nitrogen fertilizers with an acidic effect such as ammonium sulfate, urea, and ammonium nitrate, their addition to the soils in high quantities leads to an increase of soil acidification (Chien et al. 2011; Zhou et al. 2014). The decrease in soil pH is attributed to the production of protons through the nitrification process caused by adding ammonium nitrogen fertilizers (Wang et al. 2020). Soil pH plays a vital role in soil chemistry because it greatly influences numerous reactions in the soil, particularly the solubility of phosphorus, which is greatly affected by pH that controls the bioavailability and mobility of phosphorus in soils (Penn and Camberato 2019). Many important mechanisms that exist in the soil control the availability, fractions, and mobility of phosphorus such as dissolving, leaching, sorption, desorption, mineralization, immobilization, and precipitation (Zhu et al. 2018). Generally, the soluble phosphorus in bone char has low water solubility. So, this study hypothesizes that the application of nitrogen fertilizers into the soil can contribute to increasing soil acidification which in turn improves the dissolving phosphorus from bone char in calcareous soils. The main insight in this study is how to increase phosphorus release from bone char. Our study is designed to evaluate the effects of the co-application of different nitrogen fertilizer types with bone char on the availability and distribution of phosphorus in calcareous sandy soil.
2 Materials and Methods
2.1 Design of the Incubation Study
Soil samples of the cultivated layer (0 − 30 cm) were collected from the Arab El-Awamer, Assiut, Egypt. The soil samples were air-dried and ground to pass through a 2-mm sieve. The physicochemical soil characteristics are presented in Table 1. The classification of the soil under study is Entisols; Typic Torripsamments according to U.S. Soil Taxonomy. For incubation, each treatment consisted of 80 g of air-dried soil placed in airtight plastic jars (330 mL) with the application of bone char amendment at a level of 0.32 g for each jar and mixed thoroughly with the soil in all treatments. The bone char used in this study was produced from bovine bone and pyrolyzed at 500 °C for 2 h. The main properties of bone char are shown in Table 2 cited from Amin (2023). This experiment contains the following four treatments with three replicates: (1) soil without any nitrogen fertilizer (BC only) (2) soil + ammonium sulfate (BC + AS) (3) soil + ammonium nitrate (BC + AN) (4) soil + urea (BC + U), where the nitrogen fertilizers were added in a solution form at a level of 100 mg N kg−1 soil (equivalent to 240 kg N ha−1) according to the recommended doses of the Egyptian Ministry of Agriculture and Land Reclamation. All treatments were moistened until the field capacity is maintained by distilled water and incubated for 3, 10, 22, and 46 days at 23 °C in the dark as well as placed in a completely randomized design. Usually, the lids of the jars are opened to keep the aerobic conditions and moisture content by weighing the jars; and compensating for the moisture loss using distilled water. After each incubation period, soil samples of all treatments were air-dried, crushed, and prepared for the analysis of the soil’s chemical properties. This incubation experiment and all physicochemical analyses of the soil were established in the Soils and Water Department, Faculty of Agriculture, Assiut University, Assiut, Egypt.
2.2 Soil Chemical Properties
Soil pH was measured in suspension (1:1) and electrical conductivity (EC) was measured in the soil extracts (1:2). Soluble Ca and Mg in the soil extracts (1:2) were determined by titration using Na2EDTA solution (disodium ethylene diamine tetra-acetic acid). The available phosphorus (Olsen-P) within the soil samples was extracted by 0.5 M NaHCO3 at pH 8.5 according to Olsen et al. (1954). Inorganic phosphorus fractions within the soil were extracted by the Hedley sequential fractionation method (Hedley et al. 1982) and modified by Sui et al. (1999). The soil in every tube was extracted by distilled water (H2O-Pi), 0.5 M NaHCO3 at pH 8.5. The extract included inorganic P (NaHCO3-Pi), 0.1 M NaOH; this extract represents the NaOH-Pi, 1 M HCl; this extract represents the HCl-Pi. Each extraction was carried out using a 1:20 soil-solution ratio with shaking for 16 h and centrifuged for 7 min (4000 rpm). The soil residue from the last fraction was digested with concentrated H2SO4, HNO3, and HClO4, this extract represents the residual P. Phosphorus in all extracts was measured by colorimetric analysis using chlorostannous phosphomolybdic acid method in the sulphuric acid system (Jackson 1973).
2.3 Statistical Analysis
Data were statistically analyzed by two-way analysis of variance (ANOVA) and Tukey’s honestly significant difference test (Tukey’s HSD) was used to examine the differences between treatments at p ≤ 0.01, where it was conducted using the MSTAT-C program (version 2.10).
3 Results
3.1 Impacts of Nitrogen Fertilization on Soil Chemical Properties
Our study found that the co-application of nitrogen fertilizers with bone char in calcareous sandy soil has significantly decreased (p ≤ 0.01) soil pH compared to the BC treatment (Fig. 1). But, urea addition led to a significant increase in the pH of calcareous sandy soil on day 3 of incubation compared to other treatments (Fig. 1). After 3 days of incubation, soil pH decreased from 8.31 for the BC treatment to 8.15 and 8.16 for ammonium sulfate and ammonium nitrate treatments, respectively. However, applying urea increased soil pH from 8.31 for the BC treatment to 8.58. The lowest values of soil pH were observed when adding ammonium sulfate fertilizer to the soil under study. The highest soil pH was found using urea fertilizer on day 3. The decrease in soil pH was significant with the application of nitrogen fertilizers and increasing incubation periods. The pH gradually decreased with time. All used nitrogen fertilizers sources have an eventual acidifying effect on the soil. The interaction effect of treatments and incubation periods on changes in soil pH was statistically significant (Fig. 1). In the current study, soil acidity produced from the application of N fertilizers in calcareous sandy soil was in the order of ammonium sulfate > ammonium nitrate > urea (Fig. 1).
Dynamics of soil pH in calcium carbonate-rich soil as a function of the nitrogen fertilizers application in the presence of bone char during different incubation periods. The vertical bars represent the standard error of the means (n = 3). BC only or control: soil without any nitrogen fertilizer in the presence of bone char; BC + AS: soil + ammonium sulfate in the presence of bone char; BC + AN: soil + ammonium nitrate in the presence of bone char; BC + U soil + urea in the presence of bone char. Different letters within the same column indicate that the mean significantly differs according to Tukey’s honestly significant difference test (Tukey’s HSD) at p < 0.01. **significant at p < 0.01 (F-test)
Electrical conductivity increased significantly with the co-application of nitrogen fertilizers with bone char in calcareous sandy soil compared to the BC treatment (soil without any nitrogen fertilizer). The values of electrical conductivity increased significantly with increasing incubation time in all treatments. The interaction effect of treatments and incubation periods on electrical conductivity was statistically significant (Fig. 2). The applications of nitrogen fertilizers in calcareous sandy soil increased the electrical conductivity from 0.19 dS m−1 for control treatment to 0.55, 0.41, and 0.27 dS m−1 for ammonium sulfate, ammonium nitrate, and urea, respectively, after 3 days of the incubation. At the end of the incubation period, the lowest values of EC were obtained in the BC treatment at all incubation periods, while the highest values of EC were found in ammonium sulfate treatment (Fig. 2).
Electrical conductivity variability in calcium carbonate-rich soil as a function of the nitrogen fertilizers application in the presence of bone char during different incubation periods. The vertical bars represent the standard error of the means (n = 3). BC only or control: soil without any nitrogen fertilizer in the presence of bone char; BC + AS: soil + ammonium sulfate in the presence of bone char; BC + AN: soil + ammonium nitrate in the presence of bone char; BC + U soil + urea in the presence of bone char. Different letters within the same column indicates that the mean significantly differs according to Tukey’s honestly significant difference test (Tukey’s HSD) at p < 0.01. **significant at p < 0.01 (F-test)
Compared to the BC treatment, the concentrations of soluble calcium and magnesium in the soil solution increased significantly under co-applying nitrogen fertilizers with bone char (Fig. 3 and Table 3). The concentrations of soluble calcium and magnesium increased significantly with increasing incubation time in nitrogen fertilizers treatments. The interaction effects of treatments and incubation periods on the soluble calcium and magnesium were significant (Fig. 3). Adding nitrogen fertilizers in the presence of bone char increased the soluble calcium in the order of BC + AS > BC + AN > BC + U > BC (Fig. 3). The highest concentrations of soluble calcium were recorded in the ammonium sulfate treatment. After 3 days of incubation, the soluble calcium increased from 1.99 mmol kg−1 soil for the BC to 3.28, 2.79, and 2.05 mmol kg−1 soil for BC + AS, BC + AN, and BC + U, respectively. At the end of day 46 of incubation, the soluble calcium increased from 2.17 mmol kg−1 soil at the control to 6.26, 4.59, and 4.37 mmol kg−1 soil for BC + AS, BC + AN, and BC + U, respectively (Fig. 3).
Soluble calcium variability in calcium carbonate-rich soil fertilized with different nitrogen fertilizers in the presence of bone char during different incubation periods. The vertical bars represent the standard error of the means (n = 3). BC only or control: soil without any nitrogen fertilizer in the presence of bone char; BC + AS: soil + ammonium sulfate in the presence of bone char; BC + AN: soil + ammonium nitrate in the presence of bone char; BC + U soil + urea in the presence of bone char. Different letters within the same column indicates that the mean significantly differs according to Tukey’s honestly significant difference test (Tukey’s HSD) at p < 0.01. **significant at p < 0.01 (F-test)
3.2 Impacts of Nitrogen Fertilization on Phosphorus Availability
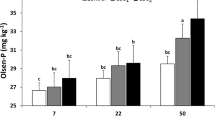
Co-applying nitrogen fertilizers with bone char increased significantly the phosphorus availability in calcareous sandy soil compared to the BC treatment (soil without any nitrogen fertilizer). The available phosphorus increased significantly with increasing incubation time in all treatments (Fig. 4). The interaction effect of (treatments X incubation periods) on phosphorus availability was statistically significant (Fig. 4). The lowest concentration of available phosphorus was observed in the BC treatment at 3 days of incubation. The highest increase in soil’s available phosphorus was in the soil treated with urea fertilizer in the presence of bone char at the end of incubation. The concentrations of available phosphorus in this study were 6.78, 6.79, 7.06, and 7.02 mg kg−1 soil for BC, BC + AS, BC + AN, and BC + U treatments, respectively, at the beginning of incubation. Whereas, these values increased to 8.05, 8.99, 8.90, and 10.16 mg kg−1 soil for BC, BC + AS, BC + AN, and BC + U treatments, respectively, at the end of incubation (Fig. 4). The effectiveness of the treatments in this incubation study on the available phosphorus increase was in the order of BC + U > BC + AS > BC + AN > BC (Fig. 4).
Dynamics of phosphorus availability in calcium carbonate-rich soil as affected by different nitrogen fertilizers in the presence of bone char during different incubation periods. The vertical bars represent the standard error of the means (n = 3). BC only or control: soil without any nitrogen fertilizer in the presence of bone char; BC + AS: soil + ammonium sulfate in the presence of bone char; BC + AN: soil + ammonium nitrate in the presence of bone char; BC + U soil + urea in the presence of bone char. Different letters within the same column indicates that the mean significantly differs according to Tukey’s honestly significant difference test (Tukey’s HSD) at p < 0.01. **significant at p < 0.01 (F-test)
3.3 Impacts of Nitrogen Fertilization on Inorganic Phosphorus Fractions
The fraction of soluble phosphorus (H2O-P) in water decreased significantly with the application of nitrogen fertilizers in the existence of bone char compared to the BC at all incubation periods, except for the application of urea at 3 days of incubation caused a significant increase in H2O-P fraction (Table 3). The application of urea increased the H2O-P fraction from 4.59 mg kg−1 soil for the BC to 7.16 mg kg−1 soil after 3 days of incubation, while, at the end of the incubation, the nitrogen fertilizer addition decreased the concentration of soluble phosphorus from 5.46 mg kg−1 soil for the BC treatment to 2.48, 3.24, and 3.16 mg kg−1 soil for BC + AS, BC + AN, and BC + U, respectively. The interaction effect of (treatments × incubation periods) on the H2O-P fraction was significant (Table 3). Fertilizing treatments increased the concentrations of NaHCO3-Pi significantly compared to the BC treatment. The incubation periods caused a significant effect on the NaHCO3-Pi fraction in all treatments. After 3 days of incubation, the concentrations of NaHCO3-Pi increased with fertilizing treatments from 20.66 mg kg−1 soil (BC) to 22.93, 23.10, and 22.01 mg kg−1 soil for BC + AS, BC + AN, and BC + U, respectively. At the end of incubation, the addition of nitrogen fertilizers to alkaline soils increased the content of NaHCO3-Pi fraction from 20.47 mg kg−1 soil (BC treatment) to 28.04, 27.47, and 23.58 mg kg−1 soil for BC + AS, BC + AN, and BC + U treatments, respectively (Table 3). The interaction effect of treatments with the incubation periods on the NaHCO3-Pi fraction was statistically significant. After 10 days of incubation, the application of urea into calcareous sandy soil in the presence of bone char lead to a significant increase in the NaOH-Pi fraction compared to the other treatments. As for the rest of the treatments, the effect was not significant. Compared to the BC treatment, fertilization by urea increased the content of soil moderately NaOH-Pi from 8.40 to 9.39 mg kg−1 soil. The interaction effect of treatments and incubation periods on the NaOH-Pi fraction was significant. The concentrations of HCl-Pi and Res-P fractions varied among all the treatments under nitrogen fertilization in the existence of bone char but these differences were not significant. Interaction effects of (treatments × incubation periods) on HCl-Pi and Res-P fractions were non-significant (Table 3).
4 Discussion
Nitrogen fertilization plays a vital role in affecting soil chemical properties, where adding nitrogen fertilizers promotes the activity of microorganisms and enzymes. Furthermore, the activity of microorganisms and enzymes in the soil is highly related to the availability of nutrients (Sun et al. 2020). Our results revealed that the effects of applying different nitrogen fertilizers on the soil pH varied with the type of nitrogen fertilizer in the presence of bone char were consistent with numerous researchers who found that the applications of several nitrogen fertilizers in different soils lead to a decrease in soil pH (Malhi et al. 2000; Zhang et al. 2021a, b). Actually, in the beginning, soil pH decreased after the application of ammonium sulfate, at the same time soil pH increased with urea application compared to the control treatment, then it decreased (Zhou et al. 2014). In the present study, the decrease in soil pH is attributed to applying ammoniacal nitrogen fertilizers such as ammonium sulfate and ammonium nitrate which causes soil acidification as a result of the transformation of ammonium to nitrate during the nitrification process, which in turn releases acidifying hydrogen ions (Chien et al. 2008, 2011), while the increases in soil pH, in the beginning, are because of hydrolyzing urea where two hydrogen ions are consumed for each urea molecule, and this tends to increase soil pH. Then, two ammonium molecules are produced and the nitrification process occurs to them by producing four hydrogen ions into the soil solution causing eventual acidification of the soil (Sposito 2008). Our study demonstrated that all sources of nitrogen fertilizers used to have an ultimately acidic effect on the soil, these results were in agreement with Apthorp et al. (1987) who reported that the effectiveness of nitrogen fertilizers in decreasing soil pH was in the order of ammonium sulfate > ammonium nitrate > urea > control. Moreover, the rate of soil pH decrease mainly relies on the physical, chemical, and biological soil properties (Wang et al. 2021; Zhou et al. 2014) as well as the level and type of fertilizer added to the soils (Schroder et al. 2011). The results obtained from this study were compatible with (Chien et al. 2011) who found that the soluble calcium in the soil solution increased with applying nitrogen fertilizers because soil acidification accelerates the dissolution of calcium carbonate, which in turn increases the electrical conductivity.
The chemical composition of animal bone char is similar to phosphate rock in that both of them contain mainly apatite (Vassilev et al. 2013). Recently, many studies suggested utilizing bone char as a renewable substitutional phosphate fertilizer (Amin 2020, 2021; Glæsner et al. 2019). This is attributed to its high content of available and total phosphorus in comparison with rock phosphate (Warren et al. 2009). Many factors controlling the concentrations of dissolving phosphorus from bone char depend on some soluble ions in the soil solution such as hydrogen, calcium, and orthophosphate (Warren et al. 2009) as well as sulfate ions (Amin 2020; Jaggi et al. 2005). In the present study, the incorporation of bone char with nitrogen fertilizers enhanced phosphorus availability in the calcareous sandy soil because of increasing soil acidity resulting from the addition of nitrogen fertilizers, which in turn leads to increasing the solubility of insoluble phosphate compounds which existed in bone char. Also, several studies found that the co-application of nitrogen fertilizers with insoluble phosphate fertilizers increases phosphorus release in different soils which is attributed to acidifying soil caused by nitrogen fertilizers (Agyarko et al. 2017; Apthorp et al. 1987; McDowell et al. 2002). The availability of phosphorus in the present study tends to increase with increasing incubation period for all treatments. These results were compatible with previous studies which found that amending soil with bone char caused a gradual increase of the phosphorus availability in calcareous sandy soil with an increasing incubation period (Amin 2023; Amin and Mihoub 2021). Our results showed that the treatments efficiency of nitrogen fertilizers on the phosphorus availability increase was in the order of urea > ammonium sulfate > ammonium nitrate. On the other hand, the efficacy of ammonium sulfate to increase rock phosphate dissolution was higher than any other type of nitrogen fertilizers such as ammonium nitrate and urea, respectively. Although urea has less effect than ammonium sulfate in improving phosphate rock dissolution, it has the privilege of being the cheapest and the most available among N fertilizers (Apthorp et al. 1987). Applying ammoniacal nitrogen fertilizers such as ammonium nitrate and ammonium sulfate incremented initially the dissolution of rock phosphate after that it declined quietly. Furthermore, urea fertilization reduced the dissolution of rock phosphate and then increased it. All changes in phosphorus release are attributed to the differences in acidification time (He et al. 1999). It is also possible that phosphorus anions are desorbed to the soil solution by sulfate anions which take their place on the binding sites (Jaggi et al. 2005). The application of nitrogen fertilizers into the soils enhances the activity of microorganisms which increases excreting of organic acids leading to improve the dissolution of insoluble phosphate compounds (Jing et al. 2021) as well as the production of phosphatase enzyme which in turn increases phosphorus release resulting from phosphorus mineralization (Horner 2008). The quantity of phosphorus solubility in the presence of ammoniacal nitrogen fertilizers is high in comparison with nitrate nitrogen fertilizers (Sharan et al. 2008). Also, the high activity of calcium ions in calcareous soils plays an important role in the mobility and availability of phosphorus, due to the increased chances of forming low-soluble calcium phosphate (Jalali and Jalali 2016; Pizzeghello et al. 2014). The potentiality for insoluble calcium phosphate formation increases with increasing the concentrations of orthophosphate and calcium ions in the soil solution in the presence of high pH values. The sources of soluble calcium in the soil are numerous and include many minerals, the most important of them is calcium carbonate (Penn and Camberato 2019). The phosphorus availability for plants is closely related to the concentrations of various P fractions in soils especially the concentration of soluble phosphorus (Zhang et al. 2021a, b).
Some important processes control the dynamics of phosphorous in the soil such as dissolution, sorption, desorption, precipitation, and transformation of organic and inorganic phosphorus fractions (Rawat et al. 2021). Inorganic phosphorus existing in the soil is the result of weathering primary minerals or through the use of mineral phosphate fertilizers and organic manure (Johan et al. 2021). The distribution of inorganic phosphorus forms is greatly affected by several physical and chemical properties of the soil such as pH, texture, organic matter, calcium carbonate, competing ions, moisture content, and redox potential (Shao et al. 2019). Nitrogen applications can have a considerable impact on the transformations and turnover of phosphorus in the soils (Chen et al. 2018; Jing et al. 2021). The application of nitrogen fertilizer had a greater effect on phosphorus fractions in soil than tillage practices (Zhang et al. 2021a, b). The concentrations of H2O-P and NaHCO3-P fractions were mainly affected by fertilizer application and land use (Tian et al. 2020). The results obtained from this study showed that there were obvious changes in the inorganic phosphorus fractions noticed when co-applying nitrogen fertilizers with bone char compared to without nitrogen fertilizer treatment. Our results revealed that the concentrations of H2O-P fraction decreased with increasing incubation period because of increasing soluble calcium in the soil solution. In addition, many previous studies have illustrated that increasing soluble calcium and phosphorus in soil solution led to decreasing soluble phosphorus because of forming insoluble Ca-phosphates (Penn and Camberato 2019; Tunesi et al. 1999). In this work, treatments of nitrogen fertilizers increased the NaHCO3-Pi content significantly as well as decreased HCl-Pi and Res-P fractions in this soil with increasing incubation period, which may be caused by the dissolution of insoluble phosphate minerals which existed in bone char. These results were in line with Zhang et al. (2021a, b) who demonstrated that the application of nitrogen fertilizer caused a significant increase in the inorganic P fraction extracted by NaHCO3 (NaHCO3-Pi) and declined HCl-Pi fraction; this may be attributed to the dissolution of calcium phosphate minerals because of soil acidification produced from the added nitrogen fertilizer. Moreover, bone char addition leads to non-significant differences in NaOH-Pi, while the addition of bone char with sulfur leads to a significant increase in NaOH-Pi compared to unamended soil because of the dissolving insoluble calcium phosphate in bone char (Morshedizad et al. 2018). However, ammonium nitrate applications to the soil decreased the content of NaHCO3-Pi fraction significantly, as well as causing a significant increment of NaOH-Pi fraction because of changing soil pH, while non-significant changes were observed in the concentrations of H2O-Pi, HCl-Pi, and residual-P fractions (Yang et al. 2015). This study revealed that the addition of nitrogen fertilizers did not cause any significant effects on HCl-Pi and Res-P fractions; these results agree with Chen et al. (2018) who suggested that the non-labile P fractions were non-significantly affected by adding nitrogen fertilizer into the soil. The concentrations of labile and moderately labile P fractions in the soils are mainly dependent on the source and amount of the applied phosphate fertilizer (Frazão et al. 2019). In this study, the highest concentrations of phosphorus were found in the form of HCl-Pi fraction, this is consistent with many researchers who found that HCl-Pi is the largest P fraction in some calcareous sandy soils in Egypt, and the proportion of HCl-Pi always represents about 70–83% of the total inorganic phosphorus (Amin and Mihoub 2021). The phosphorus availability for uptake by plants depends on the concentrations of phosphorus fractions in the soils, where the phosphorus present in inorganic fractions such as H2O-Pi and NaHCO3-Pi were largely available for plants (Tian et al. 2020; Zhang et al. 2021a, b). NaOH-Pi plays an important role in biological transformations, on the other hand, HCl-Pi has more stability and it is difficult for microorganisms to utilize it compared to all other fractions (Tian et al. 2020).
5 Conclusion
Agronomic practices especially nitrogen fertilization affect the transformations of phosphorus in the soil. Hence the main insight in this study is how to increase phosphorus release from bone char. In this study, nitrogen fertilization in every form led to an increase in the acidity of the soil, which in turn plays a vital role in influencing many chemical properties of soil as well as many processes in the soil. One of the most important results obtained from this study is that the addition of nitrogen fertilizers with bone char led to the improvement of phosphorus release in calcium carbonate-rich soil. The addition of nitrogen fertilizers in the presence of bone char had the main effect on the distribution of phosphorus fractions in the soil. Our results suggest that the majority of phosphorus in this soil exists as an HCl-Pi fraction. Based on the results obtained from this study, bone char can be used as a promising alternative to chemical phosphate fertilizers. Moreover, some agronomic practices may affect phosphorus transformations in the soils, such as adding nitrogen fertilizers which is one of the essential strategies to increase releasing phosphorus from bone char. Thence, it is preferable to add urea to other fertilizers in calcareous sandy soil.
References
Agyarko K, Frimpong KA, Abunyewa AA (2017) Phosphorus release dynamics under phosphate rock and ammonium sulphate in soil amendment. Eurasian J Soil Sci 6:312–318. https://doi.org/10.18393/ejss.306535
Amin AA (2020) Sulfur, Na2-EDTA and their mixture effects on phosphorus release from cow bone char in P-poor sandy soil. Environ Technol Innov 17:100636. https://doi.org/10.1016/j.eti.2020.100636
Amin AA (2021) Enhancement of releasing phosphorus from bone char in calcareous sandy soil under applying different levels of water salinity. J Soil Sci Plant Nutr 21:476–486. https://doi.org/10.1007/s42729-020-00376-x
Amin AA (2023) Effects of pyrolysis temperatures on bone char characterization and its releasing phosphorus in sandy soil. Arch Agron Soil Sci 69:304–313. https://doi.org/10.1080/03650340.2021.1988940
Amin AA, Mihoub A (2021) Effect of sulfur-enriched biochar in combination with sulfur-oxidizing bacterium (Thiobacillus Spp.) on release and distribution of phosphorus in high calcareous P-fixing soils. J Soil Sci Plant Nutr 21:2041–2047. https://doi.org/10.1007/s42729-021-00500-5
Apthorp JN, Hedley MJ, Tillman RW (1987) Effects of nitrogen fertilizer form on the plant availability of phosphate from soil, phosphate rock and mono-calcium phosphate. Fertil Res 12:269–284. https://doi.org/10.1007/BF01315111
Azeem M, Ali A, Jeyasundar PGSA, Bashir S, Hussain Q, Wahid F, Ali EF, Abdelrahman H, Li R, Antoniadis V, Rinklebe J, Shaheen SM, Li G, Zhang Z (2021) Effects of sheep bone biochar on soil quality, maize growth, and fractionation and phytoavailability of Cd and Zn in a mining-contaminated soil. Chemosphere 282:131016. https://doi.org/10.1016/j.chemosphere.2021.131016
Balemi T, Negisho K (2012) Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J Soil Sci Plant Nutr 12:547–561. https://doi.org/10.4067/S0718-95162012005000015
Brady NC, Weil RR (1999) The nature and properties of soils, 11th edn. Prentice-Hall International Inc, Upper Saddle River
Chen S-B, Zhu Y-G, Ma Y-B, McKay G (2006) Effect of bone char application on Pb bioavailability in a Pb-contaminated soil. Environ Pollut 139:433–439. https://doi.org/10.1016/j.envpol.2005.06.007
Chen H, Chen M, Li D, Mao Q, Zhang W, Mo J (2018) Responses of soil phosphorus bioavailability to nitrogen addition in a legume and a non-legume plantation. Geoderma 322:12–18. https://doi.org/10.1016/j.geoderma.2018.02.017
Chevallier T, Cournac L, Hamdi S, Gallali T, Bernoux M (2016) Temperature dependence of CO2 emissions rates and isotopic signature from a calcareous soil. J Arid Environ 135:132–139. https://doi.org/10.1016/j.jaridenv.2016.08.002
Chien SH, Gearhart MM, Collamer DJ (2008) The effect of different ammonical nitrogen sources on soil acidification. Soil Sci 173:544–551. https://doi.org/10.1097/SS.0b013e31817d9d17
Chien SH, Gearhart MM, Villagarc S (2011) Comparison of ammonium sulfate with other nitrogen and sulfur fertilizers in increasing crop production and minimizing environmental impact: a review. Soil Sci 176:327–335. https://doi.org/10.1097/SS.0b013e31821f0816
Frazão JJ, Benites VM, Ribeiro JVS, Pierobon VM, Lavres J (2019) Agronomic effectiveness of a granular poultry litter-derived organomineral phosphate fertilizer in tropical soils: soil phosphorus fractionation and plant responses. Geoderma 337:582–593. https://doi.org/10.1016/j.geoderma.2018.10.003
Glæsner N, Hansen HCB, Hu Y, Bekiaris G, Bruun S (2019) Low crystalline apatite in bone char produced at low temperature ameliorates phosphorus-deficient soils. Chemosphere 223:723–730. https://doi.org/10.1016/j.chemosphere.2019.02.048
Gupta DK, Chatterjee S, Datta S, Veer V, Walther C (2014) Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 108:134–144. https://doi.org/10.1016/j.chemosphere.2014.01.030
He ZL, Baligar VC, Martens DC, Ritchey KD, Elrashidi M (1999) Effect of byproduct, nitrogen fertilizer, and zeolite on phosphate rock dissolution and extractable phosphorus in acid soil. Plant Soil 208:199–207. https://doi.org/10.1023/A:1004545115290
Hedley MJ, Stewart JWB, Chauhan BS (1982) Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am J 46:970–976. https://doi.org/10.2136/sssaj1982.03615995004600050017x
Hopkins B, Ellsworth J (2005) Phosphorus availability with alkaline/calcareous soil. Western nutrient management conference, 6. Salt Lake City, UT, 88–93
Horner ER (2008) The effect of nitrogen application timing on plant available phosphorus. M.Sc. Thesis. Graduate School of The Ohio State University
Jackson ML (1973) Soil chemical analysis. Prentice-Hall of India Private Limited, New Delhi
Jaggi RC, Aulakh MS, Sharma AR (2005) Impacts of elemental S applied under various temperature and moisture regimes on pH and available P in acidic, neutral and alkaline soils. Biol Fertil Soils 41:52–58. https://doi.org/10.1007/s00374-004-0792-9
Jalali M, Jalali M (2016) Relation between various soil phosphorus extraction methods and sorption parameters in calcareous soils with different texture. Sci Total Environ 566–567:1080–1093. https://doi.org/10.1016/j.scitotenv.2016.05.133
Jiang B, Shen J, Sun M, Hu Y, Jiang W, Wang J, Li Y, Wu J (2021) Soil phosphorus availability and rice phosphorus uptake in paddy fields under various agronomic practices. Pedosphere 31:103–115. https://doi.org/10.1016/S1002-0160(20)60053-4
Jing D, Yan Y, Ren T, Lu J, Wang X, Chen J, Tan W, Liu F, Jaisi DP, Feng X (2021) Effects of nitrogen application rate on phosphorus transformation in an Alfisol: results from phosphate-oxygen isotope ratios. Appl Geochem 134:105094. https://doi.org/10.1016/j.apgeochem.2021.105094
Johan PD, Ahmed OH, Omar L, Hasbullah NA (2021) Phosphorus transformation in soils following co application of charcoal and wood ash. Agron 11:2010. https://doi.org/10.3390/agronomy11102010
Malhi SS, Harapiak JT, Nyborg M, Gill KS (2000) Effects of long-term applications of various nitrogen sources on chemical soil properties and composition of bromegrass hay. J Plant Nutr 23:903–912. https://doi.org/10.1080/01904160009382069
El Mashad M, Ahmed MA (2016) Improvement of calcareous soil using bentonite. First International Conference on Research and Technology Development for Sustainable Water Resources Management. Cairo – Egypt. 4–6
McDowell RW, Brooks PC, Mahieu N, Poulton PR, Johnston AE, Sharpley AN (2002) The effect of soil acidity on potentially mobile phosphorus in a grassland soil. J Agric Sci 139:27–36. https://doi.org/10.1017/S0021859602002307
Morshedizad M, Panten K, Klysubun W, Leinweber P (2018) Bone char effects on soil: sequential fractionations and XANES spectroscopy. Soil 4:23–35. https://doi.org/10.5194/soil-4-23-2018
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular/United States Department of Agriculture (no. 939)
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agric 9:120. https://doi.org/10.3390/agriculture9060120
Pizzeghello D, Berti A, Nardi S, Morari F (2014) Phosphorus-related properties in the profiles of three Italian soils after long-term mineral and manure applications. Agric Ecosyst Environ 189:216–228. https://doi.org/10.1016/j.agee.2014.03.047
Rawat P, Das S, Shankhdhar D, Shankhdhar SC (2021) Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nutr 21:49–68. https://doi.org/10.1007/s42729-020-00342-7
Robles Á, Aguado D, Barat R, Borrás L, Bouzas A, Giménez JB, Martí N, Ribes J, Ruano MV, Serralta J, Ferrer J, Seco A (2020) New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour Technol 300:122673. https://doi.org/10.1016/j.biortech.2019.122673
Schroder JL, Zhang H, Girma K, Raun WR, Penn CJ, Payton ME (2011) Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci Soc Am J 75:957–964. https://doi.org/10.2136/sssaj2010.0187
Shao W, Zhu J, Teng Z, Zhang K, Liu S, Li M (2019) Distribution of inorganic phosphorus and its response to the physicochemical characteristics of soil in Yeyahu Wetland, China. Process Saf Environ Prot 125:1–8. https://doi.org/10.1016/j.psep.2019.02.025
Sharan A, Shikha, Darmwal NS (2008) Efficient phosphorus solubilization by mutant strain of Xanthomonas campestris using different carbon, nitrogen and phosphorus sources. World J Microbiol Biotechnol 24:3087–3090. https://doi.org/10.1007/s11274-008-9807-2
Siebers N, Godlinski F, Leinweber P (2014) Bone char as phosphorus fertilizer involved in cadmium immobilization in lettuce, wheat, and potato cropping. J Plant Nutr Soil Sci 177:75–83. https://doi.org/10.1002/jpln.201300113
Sposito G (2008) The Chemistry of Soils, 2nd edn. Oxford University Press, New York
Sui Y, Thompson ML, Shang C (1999) Fractionation of phosphorus in a Mollisol amended with biosolids. Soil Sci Soc Am J 63:1174–1180. https://doi.org/10.2136/sssaj1999.6351174x
Sun D, Hale L, Kar G, Soolanayakanahally R, Adl S (2018) Phosphorus recovery and reuse by pyrolysis: applications for agriculture and environment. Chemosphere 194:682–691. https://doi.org/10.1016/j.chemosphere.2017.12.035
Sun J, Li W, Li C, Chang W, Zhang S, Zeng Y, Zeng C, Peng M (2020) Effect of different rates of nitrogen fertilization on crop yield, soil properties and leaf physiological attributes in banana under subtropical regions of China. Front Plant Sci 11:613760. https://doi.org/10.3389/fpls.2020.613760
Taalab AS, Ageeb GW, Hanan, SS, Safaa AM (2019) Some characteristics of calcareous soils. A review. Middle East J Agric Res 8:96–105. https://www.curresweb.com/mejar/mejar/2019/96-105.pdf
Tian L, Guo Q, Yu G, Zhu Y, Lang Y, Wei R, Hu J, Yang X, Ge T (2020) Phosphorus fractions and oxygen isotope composition of inorganic phosphate in typical agricultural soils. Chemosphere 239:124622. https://doi.org/10.1016/j.chemosphere.2019.124622
Torri SI, Correa RS, Renella G (2017) Biosolid application to agricultural land–a contribution to global phosphorus recycle: A review. Pedosphere 27:1–16. https://doi.org/10.1016/S1002-0160(15)60106-0
Tunesi S, Poggi V, Gessa C (1999) Phosphate adsorption and precipitation in calcareous soils: the role of calcium ions in solution and carbonate minerals. Nutr Cycling Agroecosyst 53:219–227. https://doi.org/10.1023/A:1009709005147
Vassilev N, Martos E, Mendes G, Martos V, Vassileva M (2013) Biochar of animal origin: a sustainable solution to the global problem of high-grade rock phosphate scarcity? J Sci Food Agric 93:1799–1804. https://doi.org/10.1002/jsfa.6130
Wang J, Tu X, Zhang H, Cui J, Ni K, Chen J, Cheng Y, Zhang J, Chang SX (2020) Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Sci Total Environ 747:141340. https://doi.org/10.1016/j.scitotenv.2020.141340
Wang J, Cui W, Che Z, Liang F, Wen Y, Zhan M, Dong X, Jin W, Dong Z, Song H (2021) Effects of synthetic nitrogen fertilizer and manure on fungal and bacterial contributions to N2O production along a soil acidity gradient. Sci Total Environ 753:142011. https://doi.org/10.1016/j.scitotenv.2020.142011
Warren GP, Robinson JS, Someus E (2009) Dissolution of phosphorus from animal bone char in 12 soils. Nutr Cycl Agroecosys 84:167–178. https://doi.org/10.1007/s10705-008-9235-6
Yang K, Zhu J, Gu J, Yu L, Wang Z (2015) Changes in soil phosphorus fractions after 9 years of continuous nitrogen addition in a Larix gmelinii plantation. Ann for Sci 72:435–442. https://doi.org/10.1007/s13595-014-0444-7
Zhan L, Jiang L, Zhang Y, Gao B, Xu Z (2020) Reduction, detoxification and recycling of solid waste by hydrothermal technology: a review. Chem Eng J 390:124651. https://doi.org/10.1016/j.cej.2020.124651
Zhang Y, Dalal RC, Bhattacharyya R, Meyer G, Wang P, Menzies NW, Kopittke PM (2021) Effect of long-term no-tillage and nitrogen fertilization on phosphorus distribution in bulk soil and aggregates of a Vertisol. Soil Tillage Res 205:104760. https://doi.org/10.1016/j.still.2020.104760
Zhang Y, Li Y, Wang S, Umbreen S, Zhou C (2021) Soil phosphorus fractionation and its association with soil phosphate-solubilizing bacteria in a chronosequence of vegetation restoration. Ecol Eng 164:106208. https://doi.org/10.1016/j.ecoleng.2021.106208
Zhou J, Xia F, Liu X, He Y, Xu J, Brookes PC (2014) Effects of nitrogen fertilizer on the acidification of two typical acid soils in South China. J Soils Sediments 14:415–422. https://doi.org/10.1007/s11368-013-0695-1
Zhu J, Li M, Whelan M (2018) Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci Total Environ 612:522–537. https://doi.org/10.1016/j.scitotenv.2017.08.095
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amin, A.EE.A.Z. Effect of Co-applying Different Nitrogen Fertilizers with Bone Char on Enhancing Phosphorus Release in Calcium Carbonate-Rich Soil: an Incubation Study. J Soil Sci Plant Nutr 23, 1565–1575 (2023). https://doi.org/10.1007/s42729-023-01217-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01217-3