Abstract
Metal pollution of the environment remains a very important topic for scientific discussion. Vanadium (V) is one of the toxic elements with the most extensive distribution in nature. Despite the vast use of V in heavy industries, its presence in the environment can be harmful to living organisms. Soil can be polluted by V released from both natural and anthropogenic sources. The high mobility of V from soil to plants directly affects humans. The current review provides an overview of the impact of certain soil biological–chemical properties on the bioavailability, mobility, and toxicity of V. Although some aspects are well documented, such as pH, Eh, or SOM, there are points that need to be analyzed and described in greater detail. An important aspect that requires further investigation is the effect of vanadium on microorganisms and, more precisely, on the soil processes they carry out. It can be assumed that, analogically to other heavy metals (e.g., Pb, Zn, Ni, Cd), it can impair certain reactions (methanotrophy, methanogenesis), which can have a negative impact on the environment. So far, there are no studies referring to this subject in the soil environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vanadium (V) is one of the most abundant trace elements in the Earth’s crust. In the environment, it occurs in various valence states (− III, − I, 0, + II, + III, + IV, + V), with the most common pentavalent (+ V) and tetravalent (+ IV) states (Gan et al. 2020). The other states are unstable products of physicochemical processes. V (+ V) is the most stable form of vanadium occurring in most environmental conditions as oxyanion vanadate (H2VO4− or HVO42−), while V (+ IV) is stable only at low pH, where it acts as vanadyl (VO2+). Furthermore, the toxicity of V increases with the valence state: pentavalent compounds are more toxic than tetravalent forms (Gan et al. 2020; Llobet and Domingo 1984; Patel et al. 1989; Yu et al. 2020). The pentavalent state is also the most mobile of the V species (Tracey et al. 2007). Moreover, the bioavailability of V is regulated by the changes in the oxidation state (Reijonen et al. 2016). The reduction of V (+ V) to (+ IV) is considered a method to remove the element from the environment (Ortiz-Bernad et al. 2004). It was observed, in the mice model, that the inhalation of vanadium in pentavalent form becomes toxic in concentrations > 0.02 M (Rodríguez-Lara et al. 2016). Moreover, in this form, it causes serious damage to the soil ecosystem.
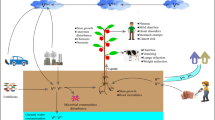
The final concentration of V in the soil is the sum of V from anthropogenic and natural sources. Volcanic activity and weathering of bedrock are regarded as the main natural source of V in soil (Fig. 1) (Altaf et al. 2021; Gustafsson 2019). As presented by Kabata-Pendias and Pendias (2001), soil V concentration varies depending on the parent material classes and is as follows: sandstone and limestone-derived soils (10–91 mg kg−1), shales, and argillaceous sediments (20–150 mg kg−1), and loess (27–110 mg kg−1). Noteworthy, naturally occurring V is never found in a free state; it exists with such natural minerals as patronite, davidite, carnotite, vanadinite, or bravoite (Altaf et al. 2021; Imtiaz et al. 2015). Furthermore, high concentrations of V are released into the soil environment as a result of anthropogenic activities. In recent years, due to the increased demand for V from high-temperature industrial activities, the relevance of anthropogenic V in the environment has increased significantly (Teng et al. 2011). According to reports, about 2.30·108 kg of V are introduced into the environment through human activities every year, of which 1.32 108 kg are deposited on the land, resulting in an increase in the concentration of V in the soil (Hope 1997; Qian et al. 2014). Fertilizers, fossil fuels, municipal sewage sludge, industrial areas, or V products from mine tailings are the main sources connected with human activity (Fig. 1) (Reijonen et al. 2016; Ścibior et al. 2021; Shaheen et al. 2019; Yang et al. 2017).
As reported by Panichev et al. (2006), the average concentration of V in the soil is also regulated by the localization and is approximately 108–150 mg kg−1. The average V concentrations in soil in different countries are presented in Table 1. The V level in the soil varies depending on the soil layer, location, type of soil, and soil use. As reported, the median V concentration in European topsoils is 60.4 mg kg−1, whereas this concentration in subsoils is slightly higher—62.8 mg kg−1. In turn, in American soils, Shacklette and Boerngen (1984) noted vanadium content of 84 mg kg−1. This shows a tendency of the vanadium concentration to increase with increasing soil depth (Gustafsson 2019). Peat soil was characterized by the lowest V concentration (5–22 mg kg−1), while the highest concentration was noted in soils developed from mafic rocks (150–460 mg kg−1) (Kabata-Pendias and Pendias 2001). Areas under the human use are characterized by elevated V concentrations (1510–3600 mg kg−1), while a lower concentration is noted in agricultural soils (Altaf et al. 2021; Imtiaz et al. 2015; Panichev et al. 2006). Studies have revealed that soil near urban areas is characterized by a much higher concentration of V (1510–3600 mg kg−1), while the highest concentrations, up to 5000–9000 mg kg−1, have been observed in soils near vanadium titanomagnetite mines in South Africa, China, Russia, and the USA (Chen et al. 2021; Shaheen et al. 2019). It has been reported that soils in certain areas of China exhibit one of the highest levels of V pollution due to the extensive mining and processing activities. The Panzhihua region in south-western China is given as an example with the V concentration ranging from 49.3 to 4793.6 mg kg−1, which is several times higher than the average concentration in China (82 mg kg−1) (Cao et al. 2017) and the average worldwide value (108 mg kg−1) (Teng et al. 2006, 2011; Yu et al. 2018). China contributes to 57% of world’s vanadium production, which makes this country the largest vanadium consumption/production country in the world (Yang et al. 2017). Furthermore, a strong relationship with total Fe and total Sc concentrations was observed especially in European soils (Gustafsson 2019).
However, numerous reports have demonstrated the toxic and carcinogenic effect of V at higher concentrations, although positive results have also been reported. It has been observed that the presence of V at low concentrations in soil can intensify potassium consumption, nitrogen assimilation, and chlorophyll synthesis (Mandiwana and Panichev 2009; Olness et al. 2005). Nonetheless, as suggested by the available data, the adverse effects of soil V contamination can be observed at several levels. First of all, leads to a reduction in soil quality (Dong et al. 2021) that directly affects plant growth and development (Bonanno 2011). Secondly, the negative effects of soil V on humans are also observed. After being absorbed by the plant roots, soluble forms of V enter the human organism through the food chain (Bonanno 2011; Dong et al. 2021). Moreover, V might enter the human body through the contaminated water from the geological weathering of vanadium‐containing minerals (Zhang et al. 2019). What should also be pointed out is that further studies on the relationships between V and biochemical processes and properties of soil are essential. This is very important due to the potential harmful effect on, e.g., soil microorganisms, which may have an impact on the microbial processes. It is worth paying attention to in the aspect of global warming. There are many reports in the literature on the influence of trace elements on methane oxidation (Walkiewicz et al. 2016; Wnuk et al. 2017, 2020a) and production (Mishra et al. 1999; Wnuk et al. 2020a, b) processes in soil. As for vanadium, this type of research is lacking, opening up the possibility for further analysis.
The main aim of the review was to analyze the available literature for information on the impact of soil properties on the V in this environment. Depending on certain values of factors (e.g., pH, Eh, organic matter), the form and properties of V changed, which could affect its toxicity or availability in soil. The limited literature concerning the V behavior in soil (compared to other heavy metals, i.e., Pb, Cd, Zn, Ni etc.) and its widespread distribution, demonstrates the need for further in-depth studies into the properties of this element and its impact on the environment.
2 Vanadium Response to Soil Properties
The V behavior in the soil is usually linked with soil organic matter, iron and aluminum oxides, elements of soil structure, and its mobility/ bioavailability depending on the physicochemical properties of the soil such as pH and redox conditions.
3 Eh
The soil redox potential (Eh) is an important index of the ability of soil to perform the oxidation–reduction reaction. In natural conditions, the value of Eh varies between − 300 and + 900 mV. Waterlogged soils are characterized by Eh below + 250 mV (value up to − 300 mV), whereas dry and well-aerated soils have Eh above + 400 mV (Husson 2013; Pezeshki 2001). Eh is used as a soil health indicator during the remediation processes (Ugwuegbu et al. 2001). It has an effect on the biogeochemical behavior of metals (V, Cr, or Fe) sensitive to changes in redox conditions in soil (Borch et al. 2010). Furthermore, it indirectly affects soil pH and dissolved organic carbon (DOC) content in soil (Shaheen et al. 2019) and controls the reactivity of Fe and Mn oxides, which have a high capacity for the sorption of heavy metals and pollutants (Husson 2013). As far as V is concerned, the soil Eh regulates the V oxidation state. It was observed that together with the increasing Eh (increasing oxic conditions), the higher mobility of V was observed due to the oxidation of V (+ IV) to (+ V) (Reijonen et al. 2016). Moreover, together with the increasing oxidation state, the highest toxicity was observed. As stated by Haluschak et al. (1998), reducing conditions results in the immobilization of V. On the other hand, analysis of Eh impact on V dynamics in different floodplain soils from the USA (Shaheen et al. 2015) and Germany (Frohne et al. 2015; Shaheen et al. 2014) showed the increased concentration of dissolved V under reducing conditions and decreased under high Eh. Such effect was explained as the oxidation of more soluble V (+ IV) to less soluble V (+ V), together with increasing Eh.
4 pH
pH has a huge influence on biogeochemical processes and biological, physical, and chemical properties of soil. It is regulated by (a) humic residues from the humification of soil organic matter producing high-density carboxyl and phenol groups, which can dissociate and release H+ ions, (b) leaching of alkaline cations (such as Ca, Mg, K, and Na) far beyond their release from weathered minerals, which makes H+ and Al3+ ions the main exchangeable cations, (c) NH4+ nitrification where H+ ions are produced, and (d) inputs from acid rains and plant N uptake (Neina 2019). Moreover, together with DOC, pH regulates the solubility, mobility, and bioavailability of trace elements (Tsadilas and Shaheen 2010). It has been observed that the adsorption of trace elements in the soil increases with the increase in pH (Bradl 2004), as opposed to the mobility, which decreases at higher pH (Rieuwerts et al. 1998). Commonly, trace elements are soluble at low pH due to their high desorption and low adsorption. The higher soil pH is accompanied by increased adsorption of trace elements (Bradl 2004; Neina 2019).
The highest mobility of V in soil was observed mainly in alkaline and neutral conditions, where increased uptake by plants was reported (Chen et al. 2021). Welch (1973) found that V uptake by plants was highly pH-dependent, i.e., it was constant in the range of 5–8 and the highest and lowest values were observed at pH 4 and 10, respectively. Moreover, the solubility of V (+ III) was limited at higher pH values and a sufficient concentration of dissolved V (Gustafsson 2019).
Furthermore, soil pH regulates the bioavailability and mobility of V through mechanisms where pH affects the solubility of SOM and where V absorption by Al and Fe (hydr)oxides is controlled (Chen et al. 2021; Reijonen et al. 2016; Shaheen et al. 2019; Zeng et al. 2011). At low pH’s V form complexes with clay minerals, organic matter and Fe oxides, what directly affect the mobility of the V (Haluschak et al. 1998). Also, Blackmore et al. (1996) observed an increase in V mobility together with a reduction in the capacity for V (+ V) sorption in the soil at higher pH. This is in agreement with the observation of Panichev et al. (2006), Shaheen and Rinklebe (2018), and Brooks (1972) who reported higher V mobility in neutral/alkali soils, which decrease in acidic soils. Moreover, the alkali soils are characterized by higher bioavailability than the acidic ones (Shaheen and Rinklebe 2018). As suggested by Olaniran et al. (2013), the effect of soil pH on V bioavailability may be related to microbial activity. However, such a theory has not been confirmed by any results.
5 SOM
Soil organic matter (SOM) represents the organic constituents in soil. It is identified as a set of humic and non-humic substances. Non-humic substances represent one of the inorganic compounds, such as lipids, carbohydrates, or amino acids. In turn, humic substances (HS) occurring naturally in organic soil are formed during the decomposition and transformation of plant, animal, and microbial residues (Rose et al. 2014). In soil, HS are divided into three classes: fulvic acids (FA), humic acids (HA), and humins. The main difference between FA and HA is the varied molecular weight, i.e., FA are characterized by lower molecular weight than HA. Moreover, the oxygen and carbon content in FA is much higher; in turn, HA are more soluble and can be removed from a solution by precipitation with acids (Rieuwerts et al. 1998). SOM has a strong influence on the retention of heavy metals. As observed by some authors, it plays a crucial role in the governance of V availability, as it can both, decrease or increase the V mobility (Du Laing et al. 2009; Di Giuseppe et al. 2014). It was noted that a high amount of vanadyl cation is mobilized due to the complexation with humic acids (Bloomfield 1981). Moreover, Reijonen et al. (2016) have stated that the bioavailability of V is reduced by its sorption to SOM. On the other hand, they showed the decreased binding capacity of V to SOM together with elevated pH. Furthermore, it was stated that FAs were responsible for V-limited toxicity as they act as adsorbent.
6 Total V Concentration in Soil
So far, only two reports have focused on the effect of the V concentration on its bioavailability in soil. As demonstrated by Tian et al. (2015), the addition of an increased V (+ V) concentration in agricultural soils resulted in a lower proportion of V (+ IV). Furthermore, Reijonen et al. (2016) found that easily soluble V compounds in soil increased together with higher doses of the element added. As suggested by the authors, the increased V (+ V) accessibility may have resulted from (1) a decreased reduction capacity associated with lower binding to SOM, or (2) changes in the V (+ V) sorption behavior affected by the potential formation of polymeric species at higher V concentrations. These polymeric species are also characterized by reduced adsorption to HA.
7 Metal(hydr)oxides
Fe, Mn, and Al (hydr)oxides are considered to be strong primary sorbents of V in soils, even stronger than phosphate and arsenate (Brinza et al. 2008; Larsson et al. 2017; Naeem et al. 2007; Shaheen et al. 2019; Shi et al. 2010). Precisely, iron and aluminum (hydr)oxides are the main compounds determining the mobility of vanadium in soil. This makes V less available and, therefore, less toxic (Wällstedt et al. 2010). It was found that V is closely associated with Fe (hydr)oxides in soil and Fe (hydro)oxides are good V ion adsorbent in soil, because of the presence of this element in clay minerals (Tsadilas and Shaheen 2010; Xiao et al. 2015). As noted by Chen et al. (2019), about 20% of V from the soil of the Panzhihua region was mobilized, where the reaction with Fe and Mn (hydr)oxides was one of the causes. On the other hand, Mn oxides have no strong capacity to bind V compounds (Bing et al. 2020; Wang et al. 2016). Strong sorption of V compounds was also observed by Larsson et al. (2017), where vanadate (+ V) and vanadyl (+ IV) were added to different soils. The analysis showed V (+ V) accumulation to Fe and Al hydrous oxides with marginal complexation of V (+ IV) to organic matter. Moreover, strong V (+ V) sorption to goethite (iron oxyhydroxide) was observed by Peacock and Sherman (2004) and sorption to hematite and magnetite (iron oxides) was reported by Terzano et al. (2007). The strong adsorption of vanadate to Fe (III) (hydr)oxides was observed mostly at low pH, although some reactions at pH 10–11 were noted as well (Blackmore et al. 1996). Frohne et al. (2015) have reported the strong relation between V and Fe(hydr)oxides in soil under reducing conditions which is supposed to be connected with the reduction of Fe(hydr)oxides and the release of the associated metal. Together with increasing Eh value, the immobilization of V was indicated with the mechanism of adsorption on Fe(hydr)oxides surface. Eh As far as Al compounds are concerned, Al hydrous oxides and crystalline aluminosilicates were observed to contribute largely to vanadate sorption (Burke et al. 2012; Larsson et al. 2015). However, there are still not many reports on this subject and further research is needed.
8 Soil Microorganisms and Enzyme Activity
The knowledge of the impact of V on soil microorganisms and enzymatic activity is still limited (Cao et al. 2017; Xiao et al. 2017; Yang et al. 2014). It has been shown that long exposure to high vanadium concentrations results in the inhibition of soil microbiota by inhibition of nitrification and nitrogen mineralization (Gustafsson 2019; Liang and Tabatabai 1978, 1977). Furthermore, as reported by Sun et al. (2018), soil microbiota is much more sensitive to V contamination than other contaminants such as Cu, Cd, Pb, or As. However, as suggested by Wilke (1989), such an effect may decrease due to the adaptation to vanadium and the lower bioavailability of V over time. Moreover, as stated by Cao et al. (2017), V contamination is crucial for bacterial communities in soil. The V contamination caused the changes in the microbial structure of the soil in Panzhihua smelting and mining area. The dominance of Bacteroides and Proteobacteria has been identified as the reason for biogeochemical cycle disturbances in the soil ecosystem.
The enzymatic activity of soil is strongly correlated with the microbial community structure and its activity (Cao et al. 2017; Xiao et al. 2017). As enzyme activity was recommended as a biochemical indicator of the quality of metal-polluted soils, the objective of the research carried out by Xiao et al. (2017) was to evaluate the response of enzyme activity to V stress. The authors noted that V introduced to soil caused significant effects on microbial activity reflected by changes in the activity of soil enzymes (dehydrogenase activity—DHA and urease activity—UA), microbial biomass carbon (MBC), and basal respiration (BR). Authors indicated that BR and DHA are significant indicators of soil V contamination. That was also proved by other authors who stated that inhibition of sulfatase, phenol oxidase (Yang et al. 2014), and other enzymes involved in C-, N-, P-, and S- cycling (urease, arylsulfatase, xylanase, alkaline phosphatase) (Kandeler et al. 2000) was related to reduced enzymes production by microorganisms caused by changes in the microbial community.
9 Soil Texture
As in the case of other metals, the V content and bioavailability are strongly correlated with soil texture. Depending on the source, some soil textural groups connected with changes in the clay content were determined. The presence of clay minerals, SOM, sulfides, and Fe–Mn oxides is supposed to be associated with a high accumulation of metals in the clay fraction (Rieuwerts et al. 1998). Moreover, the mechanisms responsible for metal binding to clay are iron exchange and specific adsorption (Farrah and Pickering 1977). As reported by Haluschak et al. (1998), the mean V concentration increases together with the increasing clay content. The increased concentration was connected with its lower mobility. Within the 5 textural groups, the V concentration increased as follows: coarse < moderately coarse < medium < moderately fine < fine. This was confirmed by Shaheen and Rinklebe (2018) and Wang et al. (2016), who observed that the total V content was higher in soils characterized by high clay content and a high value of CEC (cation exchange capacity). As observed by Reijonen et al. (2016), the V mobility and bioavailability in coarse-textured soils are higher than in fine-textured soils, which is connected with higher adsorption of V onto Fe − and Al − (hydr)oxides. Moreover, in coarse soils, the bioavailability of V is higher with pH above or close to neutral.
10 V Speciation as a Response to Soil Properties
V in the soil can occur in several oxidation states. The form in which it exists determines the mobility and toxicity in the soil–plant system. Very important, from an environmental point of view, is to determine the influence of certain soil properties on V speciation in soil. The analysis of the available literature suggests that the most important factors regulating the form of V in soil are the following Eh, pH, SOM, and the presence of certain soil microorganisms.
Redox potential is a very strong factor which controls the biogeochemical properties of many trace elements including V (Borch et al. 2010). The pentavalent form is easily reduced to the tetravalent form under reducing conditions, especially in the presence of organic compounds that act as an electron donor (Frohne et al. 2015; Gustafsson 2019). Similarly, the oxidation of V (+ IV) to (+ V) is possible together with increasing Eh, which is connected with the presence of air, oxygen, or other oxidizing agents (Frohne et al. 2015). The highest oxidation state of V requires oxygen-rich conditions to exist. Moreover, further reduction to V (+ III) requires strong reductants such as sulfides or organic matter (Shaheen et al. 2019; Wanty and Goldhaber 1992). In reducing conditions, immobile V (+ III) is the dominant form of V in soil; the higher the oxidation state, the higher the solubility of the compound (Imtiaz et al. 2015).
The second factor that governs the chemical speciation of V in the soil is pH. At pH < 5, V (+ IV) is easily converted into the + V state (Imtiaz et al. 2015; Zeng et al. 2011). Furthermore, Reijonen et al. (2016) found that the bioavailability of both V (+ V) and (+ IV) increased at higher pH, which was connected with lower reduction of V (+ V) by soil organic matter (SOM) and enhanced oxidation of V (+ IV) by O2. Figure 2 presents the vanadium speciation as a function of pH-Eh proposed by Gustafsson (2019).
pH-Eh diagram of V speciation in water (Vconc = 0.01 mM; 0.01 M NaCl, 25 C); red lines refer to the transition between oxidation states and blue lines mean the stability limit for water (from Gustafsson’s (2019) publication)
The SOM content determines the potentially available soil V as its significant amounts are bounded to the soil organic fraction (Połedniok and Buhl 2003). Reijonen et al. (2016) have found out that SOM is responsible for V (+ V) reduction to V (+ IV) and, by acting as a sorbent, it reduces the mobility and bioavailability of V in soil. HA have a strong ability to absorb V. This makes V less toxic and contributes to its reduced availability due to immobilization in soil (Chen et al. 2021). This confirms the finding reported by Wilson and Weber (1979), who tested the potential of FA and HA for V (+ V) reduction. Yu et al. (2018) reported that, together with an increasing HA concentration in soil, the adsorption of V on HA increased significantly, while the desorption of vanadium decreased (p < 0.05). This may result in reduced V (+ V) availability and toxicity. Moreover, HA and FA can reduce V (+ V) to V (+ IV) in the common pH conditions in natural systems, resulting in reduced bioavailability and mobility of V in soil (Huang et al. 2015; Reijonen et al. 2016).
Some groups of microorganisms are capable of bioreduction of toxic vanadium compounds, which opened up the possibility of using them as a microbial approach to the removal of toxic V from the environment (Sun et al. 2018; Zhang et al. 2015, 2014). The vanadium-reducing group is represented by, e.g., bacteria from the Pseudomonas strains—Pseudomonas vanadium-reductants (Lyalkova and Yurkova 1992), Thiobacillus thiooxidans (Briand et al. 1996), and Enterobacter cloacae (van Marwijk et al. 2009) with a capability of reduction of V (+ V) to (+ IV) or even (+ III), Shewanella oneidensis (Carpentier et al. 2003, 2005), Geobacter metallireducens capable of growth in an environment where V(+ V) is the only acceptor of electrons (Ortiz-Bernad et al. 2004), and Saccharomyces cerevisiae (Bisconti et al. 1997). Furthermore, the process of biological reduction of V (+ V) to (+ IV) was observed to be carried out by Micrococcus sp., Pichia guillermondii yeast, Acidithiobacillus ferrooxidans, and A. thiooxidans (Bautista and Alexander 1972; Bredberg et al. 2004). Zhang et al. (2014) presented anaerobic vanadium remediation in which pentavalent vanadium was reduced by thermophilic (Methanothermobacter thermautotrophicus) and mesophilic (Methanosarcina mazei) methanogen archaeons, at a concentration of up to 10 mM in the growth medium, while in the non-growth medium, they were not able to reduce even 2 mM of V (+ V).
11 Conclusion
In recent years, increasing attention has been paid to vanadium and its negative effect on the human and the environment. As pointed out by many authors, increasing amounts of V are released to the soil, mainly due to the human activity, as V is a component of fertilizers, fossil fuels, or municipal sewage sludge. Increased V concentrations are also detected near industrial or volcanic areas. V released to the soil is absorbed by plants, and thus may directly affect human health. The toxicity of V in soil depends on a few factors representing the physicochemical and biological properties of soils. The present review summarizes the available knowledge of the impact of soil properties on V availability and mobility. The best described factors are pH and Eh. These two factors determine the oxidation–reduction properties, which influence the speciation of V. The pentavalent form of V, which is the most toxic oxidation state, is available in strongly oxidizing conditions, with pH > 5. An important role in vanadium management in the soil is played by the presence of specific microorganisms, e.g., Pseudomonas or Micrococcus sp., which have the capacity to neutralize V toxicity. However, the literature is rich in numerous studies on the properties of V in soil, its toxicity and impact on the soil environment, but there is still a niche that needs to be supplemented. Further studies concerning the V effect on soil microbial processes influencing the environment.
Data Availability
The author declare that data supporting the findings of this study are available within the article.
Abbreviations
- BR:
-
Basal respiration
- CEC:
-
Cation exchange capacity
- DHA:
-
Dehydrogenase activity
- DOC:
-
Dissolved organic carbon
- Eh:
-
Soil redox potential
- FA:
-
Fulvic acids
- HA:
-
Humic acids
- HS:
-
Humic substances
- MBC:
-
Microbial biomass carbon
- SOM:
-
Soil organic matter
- UA:
-
Urease activity
- V:
-
Vanadium
References
Altaf MM, Diao X, Shakoor A, Imtiaz M, Atique-ur-Rehman AMA, Khan LU (2021) Delineating vanadium (V) ecological distribution, its toxicant potential, and effective remediation strategies from contaminated soils. J Soil Sci Plant Nutr 22:121–139. https://doi.org/10.1007/s42729-021-00638-2
Angelone M, Bini C (1992) Trace Elements Concentrations in Soils and Plants of Western Europe. In: Adriano DC (ed) Biochemistry of Trace Metals, 1st Editio. Lewis Publisher, pp 19–59
Baken S, Larsson MA, Gustafsson JP, Cubadda F, Smolders E (2012) Ageing of vanadium in soils and consequences for bioavailability. European Journal of Soil Science 63(6):839–847. https://doi.org/10.1111/j.1365-2389.2012.01491.x
Bautista EM, Alexander M (1972) Reduction of inorganic compounds by soil microorganisms. Soil Sci Soc Am J 36:918–920. https://doi.org/10.2136/sssaj1972.03615995003600060024x
Bing H, Zhong Z, Wang X, Zhu H, Wu Y (2020) Spatiotemporal distribution of vanadium in the flooding soils mediated by entrained-sediment flow and altitude in the Three Gorges Reservoir. Sci Total Environ 724:138246. https://doi.org/10.1016/j.scitotenv.2020.138246
Bisconti L, Pepi M, Mangani S, Baldi F (1997) Reduction of vanadate to vanadyl by a strain of Saccharomyces cerevisiae. Biometals 10:239–246. https://doi.org/10.1023/a:1018360029898
Blackmore DPT, Ellis J, Riley PJ (1996) Treatment of a vanadium-containing effluent by adsorption/coprecipitation with iron oxyhydroxide. Water Res 30:2512–2516. https://doi.org/10.1016/0043-1354(96)00080-2
Bloomfield C (1981) The translocation of metals in soils. In: Greenland DJ and Hayes MHB (eds.)The chemistry of soil processes. John Wiley and Sons, New York, NY
Bonanno G (2011) Trace element accumulation and distribution in the organs of Phragmites australis (common reed) and biomonitoring applications. Ecotoxicol Environ Saf 74:1057–1064. https://doi.org/10.1016/j.ecoenv.2011.01.018
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23. https://doi.org/10.1021/es9026248
Bradl HB (2004) Adsorption of heavy metal ions on soils and soils constituents. J Colloid Interface Sci 277:1–18. https://doi.org/10.1016/j.jcis.2004.04.005
Bredberg K, Karlsson HT, Holst O (2004) Reduction of vanadium(V) with Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. Bioresour Technol 92:93–96. https://doi.org/10.1016/j.biortech.2003.08.004
Briand L, Thomas H, Donati E (1996) Vanadium(V) reduction in Thiobacillus thiooxidans cultures on elemental sulfur. Biotechnol Lett 18:505–508. https://doi.org/10.1007/BF00140192
Brinza L, Benning LG, Statham PJ (2008) Adsorption studies of Mo and V onto ferrihydrite. Mineral Mag 72:385–388. https://doi.org/10.1180/minmag.2008.072.1.385
Brooks RR (1972) Geobotany and biogeochemistry ofmineral exploration. Harper and Row Publishers, New York, NY
Burke IT, Mayes WM, Peacock CL, Brown AP, Jarvis AP, Gruiz K (2012) Speciation of arsenic, chromium, and vanadium in red mud samples from the Ajka Spill Site, Hungary. Environ Sci Technol 46:3085–3092. https://doi.org/10.1021/es3003475
Cao X, Diao M, Zhang B, Liu H, Wang S, Yang M (2017) Spatial distribution of vanadium and microbial community responses in surface soil of Panzhihua mining and smelting area, China. Chemosphere 183:9–17. https://doi.org/10.1016/j.chemosphere.2017.05.092
Carpentier W, De Smet L, Van Beeumen J, Brigé A (2005) Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301. https://doi.org/10.1128/JB.187.10.3293-3301.2005
Carpentier W, Sandra K, De Smet I, Brigé A, De Smet L, Van Beeumen J (2003) Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639. https://doi.org/10.1128/AEM.69.6.3636-3639.2003
Chen L, Liu J, Gao J, Hu W, Yang J (2021) Vanadium in soil-plant system: source, fate, toxicity, and bioremediation. J Hazard Mater 405:124200. https://doi.org/10.1016/j.jhazmat.2020.124200
Chen L, Wang K-P, Yang J-Y (2019) Evaluate the potential bioavailability of vanadium in soil and vanadium titano-magnetite tailing in a mining area using BCR sequential and single extraction: a case study in Panzhihua, China. Soil Sediment Contam an Int J 29:232–245. https://doi.org/10.1080/15320383.2019.1702623
Di Giuseppe D, Vittori Antisari L, Ferronato C, Bianchini G (2014) New insights on mobility and bioavailability of heavy metals in soils of the Padanian alluvial plain (Ferrara Province, northern Italy). Chem Erde 74:615–623. https://doi.org/10.1016/j.chemer.2014.02.004
Dong Y, Lin H, Zhao Y, GueretYadiberetMenzembere ER (2021) Remediation of vanadium-contaminated soils by the combination of natural clay mineral and humic acid. J Clean Prod 279:123874. https://doi.org/10.1016/j.jclepro.2020.123874
Dudka S, Markert B (1992) Baseline concentrations of As, Ba, Be, Li, Nb, Sr and V in surface soils of Poland. Science of The Total Environment 122(3):279–290. https://doi.org/10.1016/0048-9697(92)90046-U
Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Trace metal behaviour in estuarine and riverine floodplain soils and sediments : a review. Sci Total Environ 407:3972–3985. https://doi.org/10.1016/j.scitotenv.2008.07.025
Eriksson J, Sveriges lantbruksuniversitet., Sverige. Naturvårdsverket. (2001) Concentrations of 61 trace elements in sewage sludge, farmyard manure, mineral fertiliser, precipitation and in oil and crops. Swedish Environmental Protection Agency
Farrah H, Pickering WF (1977) Influence of clay-solute interactions on aqueous heavy metal ion levels. Water Air Soil Pollut 8:189–197. https://doi.org/10.1007/BF00294042
Ferreira A, Inácio MM, Morgado P, Batista MJ, Ferreira L, Pereira V, Pinto MS (2001) Low-density geochemical mapping in Portugal. Applied Geochemistry 16(11–12):1323–1331. https://doi.org/10.1016/S0883-2927(01)00037-3
Frohne T, Diaz-Bone RA, Du Laing G, Rinklebe J (2015) Impact of systematic change of redox potential on the leaching of Ba, Cr, Sr, and V from a riverine soil into water. J Soils Sediments 15:623–633. https://doi.org/10.1007/s11368-014-1036-8
Gan C, Chen T, Yang J (2020) Remediation of vanadium contaminated soil by alfalfa (Medicago sativa L.) combined with vanadium-resistant bacterial strain. Environ Technol Innov 20:101090. https://doi.org/10.1016/j.eti.2020.101090
Govindaraju K (1994) Compilation of working values and sample description for 383 geostandards. Geostandards and Geoanalytical Research 18(1):1–158. https://doi.org/10.1111/j.1751-908X.1994.tb00502.x
Granero S, Domingo JL (2002) Levels of metals in soils of Alcalá de Henares, Spain: Human health risks. Environ Int 28:159–164. https://doi.org/10.1016/S0160-4120(02)00024-7
Gustafsson JP (2019) Vanadium geochemistry in the biogeosphere –speciation, solid-solution interactions, and ecotoxicity. Appl Geochemistry 102:1–25. https://doi.org/10.1016/j.apgeochem.2018.12.027
Haluschak P, Eilers R, Mills G, Grift S (1998) Status of selected trace elements in agricultural soils of Southern Manitoba. Technical Report 1998–6E. Land Resource Unit, Brandon Research Centre, Research Branch, Agriculture and Agri-Food Canada 80
Hope BK (1997) An assessment of the global impact of anthropogenic vanadium. Biogeochemistry 37:1–13. https://doi.org/10.1023/A:1005761904149
Huang J-H, Huang F, Evans L, Glasauer S (2015) Vanadium: global (bio)geochemistry. Chem Geol 417:68–89. https://doi.org/10.1016/j.chemgeo.2015.09.019
Husson O (2013) Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362:389–417. https://doi.org/10.1007/s11104-012-1429-7
Imtiaz M, Rizwan MS, Xiong S, Li H, Ashraf M, Shahzad SM, Shahzad M, Rizwan M, Tu S (2015) Vanadium, recent advancements and research prospects: a review. Environ Int 80:79–88. https://doi.org/10.1016/j.envint.2015.03.018
Kabata-Pendias A, Pendias H (2001) Trace Elements in Soils and Plants, 3rd edn. CRC Press, Boca Raton
Kandeler E, Tscherko D, Bruce KD, Stemmer M, Hobbs PJ, Bardgett RD, Amelung W (2000) Structure and function of the soil microbial community in microhabitats of a heavy metal polluted soil. Biol Fertil Soils 32:390–400. https://doi.org/10.1007/s003740000268
Larsson MA, D’Amato M, Cubadda F, Raggi A, Öborn I, Kleja DB, Gustafsson JP (2015) Long-term fate and transformations of vanadium in a pine forest soil with added converter lime. Geoderma 259–260:271–278. https://doi.org/10.1016/j.geoderma.2015.06.012
Larsson MA, Hadialhejazi G, Gustafsson JP (2017) Vanadium sorption by mineral soils: development of a predictive model. Chemosphere 168:925–932. https://doi.org/10.1016/j.chemosphere.2016.10.117
Liang CN, Tabatabai MA (1978) Effects of trace elements on nitrification in soils. J Environ Qual 7:291–293. https://doi.org/10.2134/jeq1978.00472425000700020028x
Liang CN, Tabatabai MA (1977) Effects of trace elements on nitrogen mineralisation in soils. Environ Pollut 12:141–147
Llobet JM, Domingo JL (1984) Acute toxicity of vanadium compounds in rats and mice. Toxicol Lett 23:227–231. https://doi.org/10.1016/0378-4274(84)90131-0
Lyalkova NN, Yurkova NA (1992) Role of microorganisms in vanadium concentration and dispersion. Geomicrobiol J 10:15–26. https://doi.org/10.1080/01490459209377901
Mandiwana KL, Panichev N (2009) The leaching of vanadium(V) in soil due to the presence of atmospheric carbon dioxide and ammonia. J Hazard Mater 170:1260–1263. https://doi.org/10.1016/j.jhazmat.2009.05.061
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243. https://doi.org/10.1016/S0048-9697(02)00273-5
Mishra SR, Bharati K, Sethunathan N, Adhya TK (1999) Effects of heavy metals on methane production in tropical rice soils. Ecotoxicol Environ Saf 44:129–136. https://doi.org/10.1006/eesa.1999.1809
Naeem A, Westerhoff P, Mustafa S (2007) Vanadium removal by metal (hydr)oxide adsorbents. Water Res 41:1596–1602. https://doi.org/10.1016/j.watres.2007.01.002
Neina D (2019) The role of soil pH in plant nutrition and soil remediation. Appl Environ Soil Sci 2019:1–9. https://doi.org/10.1155/2019/5794869
Olaniran AO, Balgobind A, Pillay B (2013) Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci 14:10197–10228. https://doi.org/10.3390/ijms140510197
Olness A, Gesch R, Forcella F, Archer D, Rinke J (2005) Importance of vanadium and nutrient ionic ratios on the development of hydroponically grown cuphea. Ind Crops Prod 21:165–171. https://doi.org/10.1016/j.indcrop.2004.02.005
Ortiz-Bernad I, Anderson RT, Vrionis HA, Lovley DR (2004) Vanadium respiration by geobacter metallireducens: novel strategy for in situ removal of vanadium from groundwater. Appl Environ Microbiol 70:3091–3095. https://doi.org/10.1128/AEM.70.5.3091-3095.2004
Óvári M, Csukás M, Záray G (2001) Speciation of beryllium, nickel, and vanadium in soil samples from Csepel Island, Hungary. Fresenius J Anal Chem 370:768–775. https://doi.org/10.1007/s002160100877
Panichev N, Mandiwana K, Moema D, Molatlhegi R, Ngobeni P (2006) Distribution of vanadium(V) species between soil and plants in the vicinity of vanadium mine. J Hazard Mater 137:649–653. https://doi.org/10.1016/j.jhazmat.2006.03.006
Patel B, Haswell SJ, Grzeskowiak R (1989) Flow injection flame atomic absorption spectrometry system for the pre-concentration of vanadium(V) and characterisation of vanadium(1V) and -(V) species. J Anal at Spectrom 4:195–198. https://doi.org/10.1039/JA9890400195
Peacock CL, Sherman DM (2004) Vanadium(V) adsorption onto goethite (α-FeOOH) at pH 1.5 to 12: a surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta 68:1723–1733. https://doi.org/10.1016/j.gca.2003.10.018
Pezeshki S (2001) Wetland plant responses to soil flooding. Environ Exp Bot 46:299–312. https://doi.org/10.1016/S0098-8472(01)00107-1
Połedniok J, Buhl F (2003) Speciation of vanadium in soil. Talanta 59:1–8. https://doi.org/10.1016/S0039-9140(02)00322-3
Protasova NA, Kopayeva MT (1985) Trace and dispersed elements in soils of Russian Plateau. Pochvovedeniye 1:29–37
Qian Y, Gallagher FJ, Feng H, Wu M, Zhu Q (2014) Vanadium uptake and translocation in dominant plant species on an urban coastal brownfield site. Sci Total Environ 476–477:696–704. https://doi.org/10.1016/j.scitotenv.2014.01.049
Rawlins BG, Lister TR, Mackenzie AC (2002) Trace-metal pollution of soils in northern England. Environ Geol 42:612–620. https://doi.org/10.1007/s00254-002-0564-5
Reijonen I, Metzler M, Hartikainen H (2016) Impact of soil pH and organic matter on the chemical bioavailability of vanadium species: the underlying basis for risk assessment. Environ Pollut 210:371–379. https://doi.org/10.1016/j.envpol.2015.12.046
Rieuwerts JS, Thornton I, Farago ME, Ashmore MR (1998) Factors influencing metal bioavailability in soils: preliminary investigations for the development of a critical loads approach for metals. Chem Speciat Bioavailab 10:61–75. https://doi.org/10.3184/095422998782775835
Rodríguez-Lara V, Morales-Rivero A, Rivera-Cambas AM, Fortoul TI (2016) Vanadium inhalation induces actin changes in mice testicular cells. Toxicol Ind Health 32:367–374. https://doi.org/10.1177/0748233713501364
Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR (2014) A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv Agron 124:37–89. https://doi.org/10.1016/B978-0-12-800138-7.00002-4
Salminen R, Gregorauskiene V (2000) Considerations regarding the definition of a geochemical baseline of elements in the surficial materials in areas differing in basic geology. Appl Geochemistry 15:647–653. https://doi.org/10.1016/S0883-2927(99)00077-3
Ścibior A, Wnuk E, Gołębiowska D (2021) Wild animals in studies on vanadium bioaccumulation - potential animal models of environmental vanadium contamination: a comprehensive overview with a Polish accent. Sci Total Environ 785:1–37. https://doi.org/10.1016/j.scitotenv.2021.147205
Shacklette HT, Boerngen JG (1984) Element concentrations in soils and other surficial materials of the conterminous United States. U.S. Geological Survey Professional Paper 1270, U.S. Department of the Interior, Washington, D.C., United States Government Printing Office
Shaheen SM, Alessi DS, Tack FMG, Ok YS, Kim K-H, Gustafsson JP, Sparks DL, Rinklebe J (2019) Redox chemistry of vanadium in soils and sediments: Interactions with colloidal materials, mobilization, speciation, and relevant environmental implications - a review. Adv Colloid Interface Sci 265:1–13. https://doi.org/10.1016/j.cis.2019.01.002
Shaheen SM, Rinklebe J (2018) Vanadium in thirteen different soil profiles originating from Germany and Egypt: geochemical fractionation and potential mobilization. Appl Geochemistry 88:288–301. https://doi.org/10.1016/j.apgeochem.2017.02.010
Shaheen SM, Rinklebe J, Frohne T, White JR, DeLaune RD (2015) Redox effects on release kinetics of arsenic, cadmium, cobalt, and vanadium in Wax Lake Deltaic freshwater marsh soils. Chemosphere 150:740–748. https://doi.org/10.1016/j.chemosphere.2015.12.043
Shaheen SM, Rinklebe J, Rupp H, Meissner R (2014) Lysimeter trials to assess the impact of different flood-dry-cycles on the dynamics of pore water concentrations of As, Cr, Mo and V in a contaminated floodplain soil. Geoderma 228–229:5–13. https://doi.org/10.1016/j.geoderma.2013.12.030
Shi H, Witt EC, Shu S, Su T, Wang J, Adams C (2010) Toxic trace element assessment for soils/sediments deposited during Hurricanes Katrina and Rita from Southern L ouisiana, USA: a sequential extraction analysis. Environ Toxicol Chem 29:1419–1428. https://doi.org/10.1002/etc.218
Sun W, Xiao E, Krumins V, Häggblom MM, Dong Y, Pu Z, Li B, Wang Q, Xiao T, Li F (2018) Rhizosphere microbial response to multiple metal(loid)s in different contaminated arable soils indicates crop-specific metal-microbe interactions. Appl Environ Microbiol 84:e00701-e718. https://doi.org/10.1128/AEM.00701-18
Takeda A, Kimura K, Yamasaki S (2004) Analysis of 57 elements in Japanese soils, with special reference to soil group and agricultural use. Geoderma 119:291–307. https://doi.org/10.1016/j.geoderma.2003.08.006
Teng Y, Ni S, Zhang C, Wang J, Lin X, Huang Y (2006) Environmental geochemistry and ecological risk of vanadium pollution in Panzhihua mining and smelting area, Sichuan, China. Chinese J Geochemistry 25:379–385. https://doi.org/10.1007/s11631-006-0378-3
Teng Y, Yang J, Sun Z, Wang J, Zuo R, Zheng J (2011) Environmental vanadium distribution, mobility and bioaccumulation in different land-use Districts in Panzhihua Region, SW China. Environ Monit Assess 176:605–620. https://doi.org/10.1007/s10661-010-1607-0
Terzano R, Spagnuolo M, Vekemans B, De Nolf W, Janssens K, Falkenberg G, Fiore S, Ruggiero P (2007) Assessing the origin and fate of Cr, Ni, Cu, Zn, Pb, and V in industrial polluted soil by combined microspectroscopic techniques and bulk extraction methods. Environ Sci Technol 41:6762–6769. https://doi.org/10.1021/es070260h
Tian L-Y, Yang J-Y, Huang J-H (2015) Uptake and speciation of vanadium in the rhizosphere soils of rape (Brassica juncea L.). Environ Sci Pollut Res 22:9215–9223. https://doi.org/10.1007/s11356-014-4031-0
Tracey AS, Willsky GR, Takeuchi ES (2007) Vanadium: chemistry, biochemistry, pharmacology and practical applications. CRC Press
Tsadilas CD, Shaheen SM (2010) Distribution of total and ammonium bicarbonate-DTPA-extractable soil vanadium from Greece and Egypt and their correlation to soil properties. Soil Sci 175:535–543. https://doi.org/10.1097/SS.0b013e3181fba8fe
Tume P, Bech J, Longan L, Tume L, Reverter F, Sepulveda B (2006) Trace elements in natural surface soils in Sant Climent (Catalonia, Spain). Ecol Eng 27:145–152. https://doi.org/10.1016/j.ecoleng.2006.01.004
Ugwuegbu BU, Prasher SO, Ahmad D, Dutilleul P (2001) Bioremediation of residual fertilizer nitrate: II. Soil redox potential and soluble iron as indicators of soil health during treatment. J Environ Qual 30:11–18. https://doi.org/10.2134/jeq2001.30111x
van Marwijk J, Opperman DJ, Piater LA, van Heerden E (2009) Reduction of vanadium(V) by Enterobacter cloacae EV-SA01 isolated from a South African deep gold mine. Biotechnol Lett 31:845–849. https://doi.org/10.1007/s10529-009-9946-z
Walkiewicz A, Bulak P, Brzezińska M, Wnuk E, Bieganowski A (2016) Methane oxidation in heavy metal contaminated Mollic Gleysol under oxic and hypoxic conditions. Environ Pollut 213:403–411. https://doi.org/10.1016/j.envpol.2016.02.048
Wällstedt T, Björkvald L, Gustafsson JP (2010) Increasing concentrations of arsenic and vanadium in (southern) Swedish streams. Appl Geochemistry 25:1162–1175. https://doi.org/10.1016/j.apgeochem.2010.05.002
Wang Y, Jiao JJ, Zhang K, Zhou Y (2016) Enrichment and mechanisms of heavy metal mobility in a coastal quaternary groundwater system of the Pearl River Delta, China. Sci Total Environ 545–546:493–502. https://doi.org/10.1016/j.scitotenv.2015.12.019
Wanty RB, Goldhaber MB (1992) Thermodynamics and kinetics of reactions involving vanadium in natural systems: accumulation of vanadium in sedimentary rocks. Geochim Cosmochim Acta 56:1471–1483. https://doi.org/10.1016/0016-7037(92)90217-7
Welch RM (1973) Vanadium uptake by plants: absorption kinetics and the effects of pH, metabolic inhibitors, and other anions and cations. Plant Physiol 51:828–832. https://doi.org/10.1104/pp.51.5.828
Wilke B-M (1989) Long-term effects of different inorganic pollutants on nitrogen transformations in a sandy cambisol. Biol Fertil Soils 7:254–258. https://doi.org/10.1007/BF00709657
Wilson SA, Weber JH (1979) An EPR study of the reduction of vanadium(V) to vanadium(IV) by fulvic acid. Chem Geol 26:345–354. https://doi.org/10.1016/0009-2541(79)90056-1
Wnuk E, Walkiewicz A, Bieganowski A (2017) Methane oxidation in lead-contaminated mineral soils under different moisture levels. Environ Sci Pollut Res 24:25346–25354. https://doi.org/10.1007/s11356-017-0195-8
Wnuk E, Walkiewicz A, Bieganowski A (2020) Methanogenesis and aerobic methanotrophy in arable soils contaminated with cadmium. Catena 189:104480. https://doi.org/10.1016/j.catena.2020.104480
Wnuk E, Walkiewicz A, Bieganowski A (2020) Effect of lead and chloride ions on methane production in arable soils. Int Agrophysics 3:185–193. https://doi.org/10.31545/INTAGR/118096
Xiao X, Wang M, Zhu H, Guo Z, Han X, Zeng P (2017) Response of soil microbial activities and microbial community structure to vanadium stress. Ecotoxicol Environ Saf 142:200–206. https://doi.org/10.1016/j.ecoenv.2017.03.047
Xiao XY, Yang M, Guo ZH, Jiang ZC, Liu YN, Cao X (2015) Soil vanadium pollution and microbial response characteristics from stone coal smelting district. Trans Nonferrous Met Soc China 25:1271–1278. https://doi.org/10.1016/S1003-6326(15)63727-X
Yang J, Huang JH, Lazzaro A, Tang Y, Zeyer J (2014) Response of soil enzyme activity and microbial community in vanadium-loaded soil. Water Air Soil Pollut 225:1–10. https://doi.org/10.1007/s11270-014-2012-z
Yang J, Wang M, Jia Y, Gou M, Zeyer J (2017) Toxicity of vanadium in soil on soybean at different growth stages. Environ Pollut 231:48–58. https://doi.org/10.1016/j.envpol.2017.07.075
Yay OD, Alagha O, Tuncel G (2008) Multivariate statistics to investigate metal contamination in surface soil. J Environ Manage 86:581–594. https://doi.org/10.1016/j.jenvman.2006.12.032
Yu Y, Liu M, Yang J (2018) Characteristics of vanadium adsorption on and desorption from humic acid. Chem Ecol 34:548–564. https://doi.org/10.1080/02757540.2018.1452915
Yu Y, Luo H, Tang W, Yu C, Lu L, Li J, Yang J (2020) Mechanism of vanadium(IV) resistance of the strains isolated from a vanadium titanomagnetite mining region. Ecotoxicol Environ Saf 195:110463. https://doi.org/10.1016/j.ecoenv.2020.110463
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Zhang B, Hao L, Tian C, Yuan S, Feng C, Ni J, Borthwick AGL (2015) Microbial reduction and precipitation of vanadium (V) in groundwater by immobilized mixed anaerobic culture. Bioresour Technol 192:410–417. https://doi.org/10.1016/j.biortech.2015.05.102
Zhang B, Wang S, Diao M, Fu J, Xie M, Shi J, Liu Z, Jiang Y, Cao X, Borthwick AGL (2019) Microbial community responses to vanadium distributions in mining geological environments and bioremediation assessment. J Geophys Res Biogeosciences 124:601–615. https://doi.org/10.1029/2018JG004670
Zhang C (2006) Using multivariate analyses and GIS to identify pollutants and their spatial patterns in urban soils in Galway, Ireland. Environ Pollut 142:501–511. https://doi.org/10.1016/j.envpol.2005.10.028
Zhang J, Dong H, Zhao L, McCarrick R, Agrawal A (2014) Microbial reduction and precipitation of vanadium by mesophilic and thermophilic methanogens. Chem Geol 370:29–39. https://doi.org/10.1016/j.chemgeo.2014.01.014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wnuk, E. Mobility, Bioavailability, and Toxicity of Vanadium Regulated by Physicochemical and Biological Properties of the Soil. J Soil Sci Plant Nutr 23, 1386–1396 (2023). https://doi.org/10.1007/s42729-023-01130-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01130-9