Abstract
In salt-affected soils, more than one approach should be adopted for minimizing the salinity impacts and enhancing the land productivity. The most effective practices in crop management under saline soil are choosing the plant type and variety and exploiting the best nutrient tactics. Under two soil salinity levels (3.54 and 9.28 dS m−1), representing low and high salinity, respectively), two sugar beet cultivars (Romulus and Francesca) were fertilized with three potassium (K) rates (48, 96, and 144 kg K ha−1), in addition to the check treatment (0 kg K ha−1). During two seasons of 2018/2019 and 2019/2020, treatments were distributed in a split-split plot design based on a randomized complete block arrangement with three replicates. Several physio-biochemical and agronomic traits, as well as leaf mineral contents and juice quality, were assessed. Briefly, findings illustrated that K at a rate of 144 kg ha−1 enhanced cell membrane stability, relative water content, and performance index by 1.17, 1.01, and 2.73 times, respectively, in high salinity soil, compared to low salinity × no K addition. Under high salinity, the addition of 48 and 144 kg K ha−1 recorded the highest values of total phenolic content and total antioxidant activity, respectively. In high salinity soil, K supplying (144 kg ha−1) caused the maximum improvements in gross and white sugar content with a decrease of 42.0% in sodium content and an increase of 35.9% in root yield ha−1. Romulus cultivar fertilized with 144 kg K ha−1 had the maximum relative water content, Fv/Fm, and performance index. Francesca cultivar with 144 kg K ha−1 was the potent combination for increasing total soluble sugars, total phenolic content, total flavonoid content, and total antioxidant activity. Romulus cultivar fertilized with 144 kg K ha−1 was the best practice for improving all agronomic traits of sugar beet. It could be concluded that a high potassium rate, i.e., 144 kg K ha−1, reduced the injury ionic impacts of saline soils along with improving the genetic makeup of sugar beet cultivars, expressed in sugar yield and quality. However, all other attempts for reclamation of the saline soil should be adopted for increasing the potentiality of K fertilizer and enhancing gene expressions of different sugar beet varieties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sucrose, a disaccharide sugar, is one of the main plant-derived foods in the human diet worldwide. Sugar beet (Beta vulgaris L.) is being still one of the major sources for sucrose extraction. Yearly, the total world cultivated acreage of ~ 4.44 Mt produces ~ 253 Mt of sugar beet roots that provides ~ 30% of the gross world’s requirements of white sugar (FAO 2022). Approximately 2.3 million tons (~ 59%) of consumed sucrose in Egypt is produced from beet and ~ 41% being from cane (CCSC 2018). Due to global climate change, sugar beet production in arid and semiarid climates, including Egypt, has frequently faced adverse environmental challenges such as dryness, heat, nutrient deficiency and soil salinization (Abd El-Mageed et al. 2019; Saudy et al. 2020, 2022; Mekdad et al. 2021b; El-Bially et al. 2022a; El-Metwally et al. 2022).
Salinized lands area is about 7% of the total irrigated croplands in the world and the salinization annually causes bringing 1.5 million hectares out of production (Munns and Tester 2008; Semida et al. 2014). Egypt has about 0.9 million hectares (~ 25%) of the total irrigated cultivable croplands suffering from salinization problems (FAO 2016). Sugar beet plants naturally adapt to tolerate salinity up to electric conductivity of 7.0 dS m−1 in growth media without a considerable yield reduction, and each additional EC unit causes a 5.9% yield loss (Grieve et al. 2012). Salinity adversely affects crop growth and survival by instigating osmotic and drought stressors, which results in ionic misbalance due to high accretion of sodium (Na+) and chlorine (Cl−) ions, leading to specific ions cytotoxicity (Wu et al. 2015b; Dadkhah and Rassam 2017; Abd-Elrahman et al. 2022; Makhlouf et al. 2022). When Na+ accretion surpasses the normal level, it turns to be highly deleterious to plant growth owing to cell ionic homeostasis discrepancy by prompting cytosolic K+ flux from cells (Shabala and Pottosin 2014; Semida et al 2015). High osmotic pressure with more Na+ and Cl− influx into root cells create deficiency for essential nutrients uptake, particularly potassium, K+; calcium, Ca2+; magnesium, Mg2+; and nitrate, NO3−, leading to ionic misbalance in plant cells (Wang et al. 2017). On another side, ionic stress drives early leaf senescence, reducing photo assimilates supply for growth-supporting at cost of yield diminution even under low or medium salinity conditions (Negrao et al. 2017). When salinity and phytonutrient deficiency have the same adverse effect on plant growth, a mitigation salinity stress by correcting nutrient deficiency can improve crop returns (Hatam et al. 2020; Shaaban et al. 2022). To counteract the salinity-induced adverse effects on crop plants, various strategies in genetic, chemical, physical, and biological terms have been implemented.
Mineral nutrition plays a beneficial role in developing environmental stress tolerance in crop plants, including salinity (Wu et al. 2015a; Mekdad and Rady 2016; Jan et al. 2017; Mekdad et al. 2021c). Among elements, K+ remains one of the greatest essential phytonutrients for the life cycle of most crop species, including sugar beet (Marschner 2012). K+ is a phyto-beneficial macroelement that performs a pivotal role in organizing physio-biochemical processes to support plant survival against abiotic stresses, including salinity (Merwad 2016; Mekdad et al. 2021a). Keeping a higher ratio of cytosolic K+/Na+ by expelling Na+ from cytosol to sequester into the intracellular vacuoles to limit cellular deterioration is an important adaptive mechanism for cytosolic K+ homeostasis, better crop establishment and survival, and effective CO2 assimilation, even under salinity stress conditions (Wakeel 2013; Pi et al. 2016; Yu et al. 2020). The active compartmentalization of Na+ into the intracellular vacuoles is mediated by the activities of Na+/H+ antiporters localized in tonoplast, which are fundamentally based on the electrochemical H+ gradients produced by the vacuolar H+ pumps (Wu et al. 2019). The proton’s driving energy used by the vacuolar Na+/H+ antiporters is produced by the tonoplast H+-transferring enzymes like plasma membrane-bound H+-ATPase (PM H+-ATPase) being a key mediator for membrane polarization, which is catalyzed by K+ element (Wakeel et al. 2011). When salt-affected soil is poor in available K+ content, its high Na+ content restricts the K+ uptake by root cells and causes a change in membrane polarity to prevent outwardly K+ through cationic efflux channels (Cuin et al. 2008). Thus, adequate K+ nutrition has been shown to mediate PM H+-ATPase activation to increase protons extrusion under abiotic stresses (Weng et al. 2020). Because breeding programs to develop salt-tolerant crop plants have had very limited success (Schubert et al. 2009), as a result, physiological approaches such as maintaining K+/Na+-homeostasis should be given utmost attention through developed field management strategies, e.g., external K+ supplementation for crop plants grown in salt-affected soils (Merwad 2016; Mekdad et al. 2021a). Accordingly, deficit K+ can interrupt many interlinked physio-biochemical activities, plant growth weakness, and premature leaf senescence, resulting in early crop maturity; thus, judicious K+ addition under saline soil conditions could enhance the crop productivity (Salem et al. 2022). However, to the best of the author’s knowledge, relatively little information is available about the beneficial effects of exogenous K+ application for supporting salinity tolerance in sugar beet under semiarid conditions. Accordingly, the current study hypothesized that potassium has different ameliorating influences against salinity impact for tolerant and sensitive sugar beet cultivars. From an agro-physio-biochemical aspect, this investigation was conducted to outstanding how the useful influences of potassium could improve salinity stress tolerance in two sugar beet cultivars?
2 Materials and Methods
2.1 Experimental Site
Two experiments were conducted at two different sites, selected in Sinnuris District, El Fayoum region, Egypt, between latitudes 29°02´ and 29°35´ N and longitudes 30°23´ and 31°05´ E (Fig. 1) during two cropping seasons from October to April 2018/2019 and 2019/2020. Salinity levels of selected two sites were measured as the electrical conductivity (ECe) of a saturated-paste extract of the soil of each site. Based on the soil taxonomy (IUSS Working Group WRB 2015), the soil is Typic Torripsamments, siliceous, hyperthermic, and moderately deep. In addition, the main physical and chemical characteristics of the soil of both sites were measured according to Page et al. (1982) and Klute and Dirksen (1986). The experimental soil was sandy loam having sand (78.4–77.6%), silt (11.3–12.4%), clay (10.3–10.0), bulk density (1.5–1.6 g cm−3), pH (7.7–7.6), ECe (3.54–9.28 dS m−1), cation exchange capacity (11.8–11.1 cmol kg−1), calcium carbonate (5.5–4.7%), organic carbon (0.66–0.64%), available N, (58.5–56.3 mg kg−1 soil), available P (5.1–4.7 meq 100–1 g soil), and available K (47.0–45.1.meq 100–1 g soil) for the two sites, respectively. The experimental location is classified as an arid zone with cool winters and nonsignificant precipitation.
The meteorological data collected during the two growing seasons revealed that the averages of minimum air temperature were 9.2–10.9 °C, maximum air temperature were 22.3–23.8 °C, relative humidity were 41.0–41.6%, wind speed were 2.0–1.8 m sec–1, and precipitation were 0.51–0.85 MJ mm day–1, respectively.
2.2 Treatments and Experimental Design
The experimental layout was a split-split plot system based on a randomized complete block design with three replications. Soil salinity (i.e., low = 3.54 and high = 9.28 dS m−1) levels were fallen in the main plots, while cultivars (i.e., Romulus; multigerm and Francesca; monogerm) were allocated in the subplots, and potassium fertilizer (i.e., 0, 48, 96, and 144 kg ha−1) levels were distributed in sub-subplots. Each experimental plot includes five ridges, 0.6 m apart and 6.0 m long, shaping an area of 18.0 m2 (3 m × 6 m) and about 0.20-m interplant distance within the ridge. The K rates in the form of potassium sulphate (K2SO4; 48% K2O) were added as soil application twice at planting and 30 days from planting (DFP).
2.3 Crop Management Practices
Health seeds of multi- and mono-germ sugar beet (Beta vulgaris L. cvs. Romulus and Francesca) were obtained from the Crop Research Institute, Agricultural Research Center, Egypt. These commercial high-production cultivars were chosen based on their widespread in the areas of sugar beet cultivation in Egypt. Seeds were manually planted on 20th and 25th of October, and roots were harvested on 25th and 28th of April in both 2018/2019 and 2019/2020 seasons, respectively. At 4-leaf stage (~ 30 DFP), the thinning process was done to keep one plant hill−1. Based on recommendations of the Egyptian ministry of agriculture and land Reclamation, the tested soil supplied with 90 kg P ha–1 (625-kg calcium monophosphate; 15.5% P2O5) and 290 kg N ha–1 (850 kg ammonium nitrate; 33.5% N). During the growth and development, plants received 4 irrigations through surface irrigation system. The cultural, diseases, fertilization program, and pest management were identical as local commercial sugar beet production.
2.4 Measurements
2.4.1 Physiological Traits
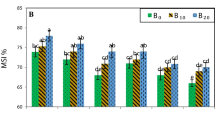
At 95 DFP, 3 sugar beet plants randomly taken, the maximum quantum yield of PSII in a dark-adapted state (variable fluorescence by maximum fluorescence; Fv/Fm and photosynthetic performance index; PPI) was measured using a fluorimeter (Handy PEA, Hansatech Instruments Ltd, Kings Lynn, UK) as shown by Clark et al. (2000); Maxwell and Johnson (2000), respectively. Also, the leaf relative turgidity (LRT%) and membrane stability index (MSI%) of the fully expanded fresh leaves were determined (Premachandra et al. 1990). The relative chlorophyll concentration (SPAD chlorophyll) was determined using a chlorophyll meter (SPAD502, KONICAMINOLTA. Inc., Tokyo).
2.4.2 Biochemical Traits
Total Soluble Sugars (TS\S)
Freshly prepared phenol (1 ml of 5%) was added to 0.1 ml of a carbohydrate solution (samples were prepared at a concentration of 1 mg mL−1) in a test tube. Subsequently, 5 mL of concentrated sulfuric acid is added rapidly to the mixture. After allowing the test tubes to stand for 10 min, they are vortexed for 30 s and placed for 20 min in a water bath at room temperature for color development. Then, the absorbance was measured at 490 nm with VWR 6300 double beam UV–visible spectrophotometer with Hitachi software (Labconco, USA). The TS sugar content in the samples was calculated from a standard curve using pure glucose.
Total Phenolic Content (TPC)
The amount of total phenolic in the plant extract was determined by Folin-Ciocalteu reagent using the procedure of (Yu et al. 2002).A 200 µl of each sample were mixed with 500 µl of the Folin-Ciocalteu reagent and 1.5 ml of 20% sodium carbonate. The mixture was shaken thoroughly and made up to 10 ml using distilled water. After 2 h of incubation at room temperature, the absorbance was measured at 765 nm. The assay was carried out in triplicate and the mean values were calculated. The concentration was determined from the standard curve prepared using serial concentrations of standard gallic acid solution. Total phenolic contents in plant extracts were expressed as mg gallic acid equivalent (GAE) per g plant extract after reading the concentration of phenolic (mg ml−1) from the calibration line and calculated using Eq. 1:
where C is the total content of phenolic compounds in mg GAE per g dry extract; c is the concentration of gallic acid obtained from the calibration curve (mg ml−1); V is the volume of extract (ml); and m is the weight (g) of plant extract.
Total Flavonoid Content (TFC)
Total flavonoid content in the plant extract was determined using the method described by (Lamaison and Carnet, 1990). Briefly, 200 ml of each sample was transferred to a test tube and evaporated to dryness. To the residue, 5 ml of 0.1 M AlCl3 was added and shaken. The absorbance of the samples was measured at 415 nm after keeping the samples for 40 min at room temperature. Total flavonoid content was expressed as mg rutin equivalent (RE) per g plant extract and calculated using Eq. 2:
where × is the total flavonoid content in mg RE per g dry extract; A is the absorption of plant extract solution; A0 is the absorption of standard rutin solution; m is the weight of extract in mg, and mo is the weight of rutin in the solution in mg.
Total Antioxidant Activity
DPPH radical-scavenging activity of all the extracts was measured by the modified method according to (Brand-Williams et al. 1995). A 2.0 ml of ethanolic solution of each extract at a concentration of 50 µg mL−1 was added to a 2.0-ml solution of DPPH (25 mg.l−1) in ethanol. The DPPH solution was prepared freshly every day, and the reaction mixture was shaken vigorously. After incubation at room temperature (28 ± 2 °C) in the dark for half an hour, the absorbance is then determined at 517 nm by VWR 6300 double beam UV–visible spectrophotometer with Hitachi software (Labconco, USA). Ascorbic acid was used as positive control. The percentage inhibition of DPPH free radical-scavenging activity of each extract was calculated using Eq. 3:
where Ac refers to the absorbance of a DPPH solution without extract and As is the absorbance of the tested extract. Triplicate measurements were taken, and mean values calculated. The ascorbic acid calibration curve was prepared for a concentration range from 10 to 200 μg ml−1, and IC50 values were obtained.
2.4.3 Agronomic Traits
At harvest (200 and 210 DFP) in both seasons, respectively, ten individual plants of each experimental plot were sampled randomly. Plants were utilized to measure leaf area plant−1 by digital planometer (Planix 7). The root length was measured by meter-scale from the point where the top was separated to the taproot tip with a diameter of about 1 cm. The root diameter was measured by vernier caliper at the widest area of the root. The root fresh weight plant−1 was measured using digital balance. Moreover, all sugar beet plants in each experimental plot in addition to the ten sugar beet plants, sampled previously, were collected, cleansed, topped, and weighed, for estimate root yield (t ha−1). White sugar yield (t ha−1) was also computed by multiplying root yield by white sugar content (%).
2.4.4 Leaf Mineral Contents
For assessment of the macronutrient (i.e., nitrogen, N; phosphorus, P; potassium, K; calcium, Ca; and sodium, Na) concentrations in plant tissues, sugar beet leaves (n = 10) were dried and milled into powder before chemical analysis. Using a micro-Kjeldahl apparatus (Ningbo Medical Instruments Co., Ningbo, China), the N content was determined following the methods of (AOAC, 2012). The P content was assessed by quantification according to (Jackson, 1973) using standard reagents of H2MoO7S, molybdenum blue, diluted H2MoO7S, and 8% (w/v) NaHSO3–H2SO4. Additionally, the Ca2+ content was assessed using an atomic absorption spectrophotometer (Perkin–Elmer, Model 3300) described by Chapman and Pratt (1961). Finally, K+ and Na+ contents were assessed according to Lachica et al. (1973) in a 50-mg freeze-dried leaf powder suspension and centrifuged at 3.000 × g for 10 min at 25 °C.
2.4.5 Beet Juice Quality
The beet juice quality attributes, i.e., gross sugar content (%) (i.e., polarity %), was estimated as outlined in McGinnus (1971). The non-sucrose impurities, i.e., sodium and potassium contents, were measured using a flame photometer, while alpha-amino-nitrogen was colorimetrically determined by a spectrophotometer. Moreover, the true sugar (TS %) was computed as follows:
2.5 Statistical Analysis
Microsoft Excel 2016 was used to compute means ± standard error and prepare the figures. Also, pre-running the variance analysis for both seasons and error variance homogeneity for all variables was tested. The analysis for the two seasons was performed based on a split-split plot in RCBD using GenStat statistical package (version 12) (VSN International Ltd, Oxford, UK). Means for all variables were separated using Fisher’s least-significant difference test at p ≤ 0.05 (GENSTAT 2007).
3 Results
3.1 Main Effects
3.1.1 Physiological Changes
Distinctive declines in cell membrane stability, relative water content, Fv/Fm, performance index, and SPAD-reading were obtained with high soil salinity than low soil salinity (Table 1). Romulus cultivar had values of membrane stability, relative water content, Fv/Fm, performance index, and SPAD-reading greater than Francesca cultivar with increases of 12.2, 9.4, 2.5, 18.3, and 18.6% respectively. Progressive increase in K levels showed increases in sugar beet physiological traits. In this respect, K at a rate of 144 kg ha−1 achieved 19.8, 12.9, 6.3, 218.9, and 57.9% increases in cell membrane stability, relative water content, Fv/Fm, performance index, and SPAD-reading, respectively, compared to no K application.
3.1.2 Biochemical Changes
Total soluble sugars, total phenolic content, total flavonoid content, and total antioxidant activity were higher under high salinity than low salinity (Table 1). Variation the two sugar beet cultivars in biochemical constituents was only obtained for total soluble sugars. In this respect, total soluble sugars value of Francesca cultivar was greater by 1.13 times than those of Romulus cultivar. Application of K at a rate of 144 kg ha−1 (for all assessed biochemical constituents), in addition to K at a rate of 96 kg ha−1 (for total flavonoid content, and total antioxidant activity), possessed the maximum increases.
3.1.3 Leaf Mineral Contents
Sugar beet leaf N (Fig. 2a), P (Fig. 2b), K+ (Fig. 2c), Na+ (Fig. 3a), Ca+2 (Fig. 3b), and Na+/K+ (Fig. 3c) were significantly influenced by salinity level, cultivar, and K rate except Ca2+ with salinity level and K+:Na+ with cultivar. Owing to high salinity, decreases in N, P, K+, and K+:Na+ were approximately 21.4, 11.0, 2.8, and 6.6%, respectively, while Na+ was higher (12.3%) compared to low level of soil salinity. The leaves of Romulus cultivar contained higher values of N, P, K+, and K+ and lower values of Na+ and Ca2+ as compared to Francesca one. Application of K at a rate of 144 kg ha−1 possessed the maximum increases in N, P, K+, Ca2+, and K+:Na+ surpassing the other K rates, except the rate of 48 kg K ha−1 for Ca2+. Unlike, the maximal Na+ was obtained with no K addition (0 K kg ha−1).
Leaf N (a), P (b), and K+ (c) contents of sugar beet as influenced by salinity, cultivar, and potassium (K) rate (kg ha−1). Low and high soil salinity refer to an electrical conductivity; ECe = 3.54 and 9.28 dS m−1, respectively. Means not sharing the common letters for each factor, in each bar, differ significantly at p ≤ 0.05 based on student–Newman–Keuls test. N, nitrogen; P, phosphorus; and K.+, potassium
Leaf Na+ (a), Ca+2 (b), and Na+/K+ (c) contents of sugar beet as influenced by salinity, cultivar, and potassium (K) rate (kg ha−1). Low and high soil salinity refer to an electrical conductivity; ECe = 3.54 and 9.28 dS m−1, respectively. Means not sharing the common letters for each factor, in each bar, differ significantly at p ≤ 0.05 based on student–Newman–Keuls test. Na+, sodium; Ca+2, calcium; and K.+, potassium
3.1.4 Agronomic Traits
All agronomic traits of sugar beet markedly responded to salinity level, cultivar, and K rate (Table 2). As expected, the values of root length, root diameter, root fresh weight, leaf area plant−1, root yield ha−1, and white sugar yield ha−1 were lower with high salinity than low salinity. Romulus cultivar was more efficient for recording improved agronomic traits higher than Francesca cultivar. Romulus cultivar exceeded Francesca cultivar by about 15.6, 19.5, 47.5, 62.1, 19.2, and 24.5% for root length, root diameter, root fresh weight, leaf area plant−1, root yield ha−1, and white sugar yield ha−1, respectively. Increases in all crop traits of sugar beet due progressive increase the K level supply were observed. Herein, application of K at a rate of 144 ha−1 caused 1.33, 1.27, 2.36, 2.13, 1.70, and 2.26 folds in root length, root diameter, root fresh weight, leaf area plant−1, root yield ha−1, and white sugar yield ha−1, respectively, comparing to no K application.
3.1.5 Beet Juice Quality
Gross sugar content (Fig. 4a), sodium (Fig. 4b), potassium (Fig. 4c), alpha-amino-nitrogen (Fig. 5a), and white sugar content (Fig. 5b) of sugar beet were statistically affected by salinity, cultivar, and K application. Compared to low soil salinity (ECe of 3.54 dS m−1), soil with ECe of 9.28 dS m−1 caused 1.5, 17.9, 9.2, and 1.2% increases in sugar content, sodium, alpha-amino-nitrogen, and white sugar content, respectively, as well as 0.8% decrease in potassium. Romulus cultivar produced higher gross sugar content and white sugar content as well as lower sodium, potassium, and alpha-amino-nitrogen than that of Francesca cultivar. More gross and white sugar content, in addition to more potassium, were accumulated in roots of sugar beet plants fertilized with 144 kg K ha−1. Sodium and alpha-amino-nitrogen were more pronounced in K-unfertilized plants.
Gross sugar content (a), Na (b), and K (c) contents of sugar beet juice as influenced by salinity, cultivar, and potassium (K) rate (kg ha−1). Low and high soil salinity refer to an electrical conductivity; ECe = 3.54 and 9.28 dS m.−1, respectively. Means not sharing the common letters for each factor, in each bar, differ significantly at p ≤ 0.05 based on student–Newman–Keuls test. Na, sodium; K, potassium
Alpha-amino nitrogen, α-amino-N (a), and white sugar (b) content of sugar beet juice as influenced by salinity, cultivar, and potassium (K) rate (kg ha−1). Low and high soil salinity refer to an electrical conductivity; ECe = 3.54 and 9.28 dS m.−1, respectively. Means not sharing the common letters for each factor, in each bar, differ significantly at p ≤ 0.05 based on student–Newman–Keuls test
3.2 Interactions
3.2.1 Physiological Changes
Salinity level × cultivar interaction had significant effects on performance index and SPAD-reading, while cell membrane stability, relative water content, and Fv/Fm did not affect (Table 3). Romulus cultivar recorded the highest values of performance index and SPAD-reading in low salinity soil. K application enhanced the physiological traits of sugar beet whether with or without salt stress. Generally, K addition at a rate of 144 kg ha−1 was the effective practice for enhancing cell membrane stability, relative water content, and performance index under both low and high salinity. Especially under high salinity, K at a rate of 144 kg ha−1 enhanced cell membrane stability, relative water content, and performance index by 1.17, 1.01, and 2.73 times, respectively, compared to low salinity × no K addition as well as 1.21, 1.17, and 3.07 times, respectively, compared to high salinity × no K addition. The interaction of cultivar × K application revealed that Romulus plants fertilized with 144 kg K ha−1 had the maximum relative water content, Fv/Fm and performance index, while the highest SPAD-reading was obtained with Francesca plants fertilized with 96 kg K ha−1.
3.2.2 Biochemical Changes
Under high salinity, Francesca cultivar showed the highest amounts of total soluble sugars and total phenolic content, while Romulus cultivar recorded the maximum increase in total antioxidant activity (Table 3). Fertilizing sugar beet grown in low salinity soil by 144 kg K ha−1 gave the maximum total soluble sugars. Under high salinity addition of 48 and 144 kg K ha−1 recorded, the highest values of total phenolic content and total antioxidant activity, respectively. Application of 96 or 144 kg K ha−1 whether with low or high salinity showed similar values of total flavonoid content. The interaction of sugar beet cultivar and potassium fertilization cleared that fertilizing Francesca cultivar with 144 kg K ha−1 was the potent combination for increasing the total soluble sugars, total phenolic content, total flavonoid content, and total antioxidant activity.
3.2.3 Leaf Mineral Contents
Only N, K+, and K+:Na+ responded to the different salinity level × cultivar, while P, Na+, and Ca2+ did not affect (Table 4). Romulus plants grown in low salinity conditions showed the highest N, K+, and K+:Na+ content. Overall, interaction between salinity level and K fertilization revealed that sugar beet plants fertilized with 144 kg K ha−1 under low salinity level recorded the maximum N, K+, Ca2+, and K+:Na+. The highest leaf Na+ content was obtained with no K application under high salinity. Fertilizing Romulus cultivar by 144 kg K ha−1 achieved the maximum values of N, K+, Ca2+, and K+:Na+ significantly equaling Romulus × 96 kg K ha−1 (for N) as well as Romulus × 48 kg K ha−1 and Francesca × 48 kg K ha−1, 96 kg K ha−1, or 144 kg K ha−1 (for Ca2+).
3.2.4 Agronomic Traits
The potent combination of salinity level and cultivar for enhancing root diameter, root fresh weight, and leaf area plant−1 was low salinity × Romulus (Table 5). Under low salinity, application of 144 kg K ha−1 produced the maximum increases in root length, root yield ha−1, and white sugar yield ha−1. Without K application high salinity caused reduction in root yield by about 11.8 t ha−1 (− 21.7% decrease) compared to low salinity (Table 5). On the contrary, K supplying (144 kg ha−1) compensated such reduction with higher increase by about 19.5 t ha−1 (35.9% increase). Romulus cultivar fertilized with 144 kg K ha−1 was the best practice for improving all agronomic traits of sugar beet.
3.2.5 Beet Juice Quality
The maximum sodium content in sugar juice was obtained under high salinity with Francesca cultivar. Under high salinity, gross sugar content, and white sugar content (with 144 kg K ha−1) and sodium content (without K fertilizer) gave the maximum values (Table 6). In K-unfertilized plots cultivated by Francesca cultivar, sodium, and alpha-amino-nitrogen showed the highest values, while plots of Francesca cultivar receiving 144 kg K ha−1 recorded the maximum potassium content in sugar juice.
4 Discussion
It has been documented that growing various crop plants in adverse edaphic of climatic environments undoubtedly leads to exposure to several stresses and ultimately poor yield (El-Metwally et al. 2021; El-Metwally and Saudy 2021a; Saudy et al. 2021a; El-Bially et al. 2022b), which requires confronting such conditions to ensure food (El-Metwally and Saudy 2021b; Mubarak et al. 2021; Salem et al. 2021; Saudy et al. 2021b). Under salinity stress, it is the accumulation of salts around the plant root system causes reduction in water movement from soil to the plant with reducing the plant’s internal water potential and ceasing nutrients transport towards the plant. Accordingly, the current findings proved that soil salinity caused perturbations in the physiological state of sugar beet plant. Consequently, leaf nutritional status, growth and yield, and juice quality were affected. In this regard, inside the plant cells, the excess soluble salts damage the leaves and thereby reduce the process of photosynthesis (Arraouadi et al. 2011). Under salinity, the excess chloride and sodium ions cause ionic imbalance with reducing the uptake of several ions, i.e., Ca, K, and P (Abbasi et al. 2015). Thus, salinity adversely affects all plant growth phases including germination, seedlings, vegetative, and maturity (Nawaz and Ashraf 2010; Abbasi et al. 2015). Disturbance in different physiological pathways i.e., respiration, photosynthesis, and carbohydrates metabolism could be occurred owing to ionic toxicity and osmotic stress of salinity (Abbasi et al. 2016). Under high level of salts, the water potential of root surrounding environment is reduced that makes the plant unable to absorb water from the soil solution by roots (Abbasi et al. 2014). As salt stress minimizes the soil solution’s osmotic potential, it disrupts water and phytonutrients uptake from the soil, thus directly impairs crop growth and productivity (Abd El-Mageed et al. 2021). Salinity-induced osmotic and ionic phases prompted oxidative stress, ending up reactive oxygen species (ROS) overaccumulation (Liu et al. 2020). ROS, being highly reactive under the absence of any defensive mechanism, causes cell structure disintegration by lipids peroxidation, DNA and protein deterioration, and enzyme deactivation (Wang et al. 2017).
Salt tolerance is a complex mechanism, including tolerance to osmotic and ionic stresses caused by high soil salinity (Chiconato et al. 2019). An increase in cellular concentrations of Na+ and Cl− and a decrease of K+ concentrations are the distinctive outputs of high salt concentration in plant growth media (Deinlein et al. 2014; Munns and Gilliham 2015; Abbasi et al. 2016). Accordingly, the regulation of Na+ and Cl− uptake is so important for avoiding toxicity of accumulated Na+ in leaves with maintaining a high K+/Na+ which is important for the activity of K+-dependent enzymes (Shabala and Pottosin 2014). On the other hand, salt stress can induce the accumulation of ROS, which could highly influence plant photosynthesis, metabolism, signal transduction, and other physio-biochemical processes. Increased ROS production greater than the cell’s tolerance capacity will cause plant damage by spoiling the cell membrane structure or interposing apoptosis (Xue et al. 2013). Therefore, a decrease in leaf expansion, stomatal closure, inhibition of photosynthesis, and reduced biomass associated salt stress (Zhang and Shi 2013).
Metabolic turbulences in several physiological and biochemical processes such as nutrition, respiration, organic solutes/osmolyte synthesis, enzyme activities, and photosynthesis could be occurred due to salinity (Siddiqui et al. 2010; Wu et al. 2013). Nevertheless, plants have developed various levels of adaptability and defensive mechanisms, based on plant species and cultivar, to survive in salt conditions (Manaa et al. 2011). Therefore, different performance to soil salinity between Romulus and Francesca cultivars was obtained. Herein, Romulus had higher adaptability to salt stress, since its values of cell membrane stability index, relative water content, Fv/Fm, and performance index were greater than Francesca. Adaptive mechanisms of plant genotypes to salt stress tolerance occur at molecular level (Yuan et al. 2016) and the physio-biochemical levels (Kong et al. 2016; Leng et al. 2018). Gantang7’ cultivar maintained the lower shoot Na+/K+ and root Na+/Ca+2 ratios than the other tested cultivars (Wu et al. et al. 2013). The differential expressions of salt-related tolerance genes, i.e., OsCCC1 (Kong et al. 2011), AtZFP1 (Han et al. 2014), and PcAPX (Cao et al. 2017), drive the salt-stress responses at the physiological and biochemical levels (Cui et al. 2018). In this context, presence of apoplastic barriers via Casparian bands and suberin lamellae could prevent Na+ transport to plant shoots (Krishnamurthy et al. 2011). Salt-tolerance mechanism could be attributed to maintaining a high shoot sugar content which is enabled by protecting photosystems structures, stimulating photosynthetic rate and sucrose synthetase activity, and inhibiting sucrose degradation (Yang et al. 2020). Genotypic variations clarified strength association between salt tolerance and high activities of superoxide dismutase, catalase and glutathione peroxidase enzymes, and proline, total soluble sugars, and glycine betaine contents under salt stress conditions (Gholipor et al. 2022). Additionally, salinity damage can be mitigated if the plant tissue effectively accumulates compatible solutes (Sun et al. 2015). The presence of these compounds relieves the osmotic pressure induced by soil salinity and thus allows the plant to continue taking up water, stabilize the photosystem II complex, protect the structure of enzymes and proteins, maintain membrane integrity, and protect cells against oxidative damage by scavenging ROS (Szabados and Savouré 2010; Gupta and Huang 2014). Many physio-adaptation strategies to overcome salinity have been reported in sugar beet plants, including selective uptake and transportation of salt ions, compartmentalization and sequestration of salt ions, and enhancement of enzymatic and nonenzymatic capacities (Wang et al. 2017; Mari et al. 2018). Boosting enzymatic (e.g., peroxidase, catalase, superoxide dismutase, ascorbate peroxidase, etc.) and nonenzymatic (e.g., total proteins, total soluble sugars, free proline, etc.) antioxidants are necessitated for ROS scavenging resulting from oxidative stress (Liu et al. 2020). Recently, proteomic profiles study of Wang et al. (2019) revealed that the tricarboxylic acid cycle, cell wall synthesis, and ROS scavenging showed differential changes between the sensitive and tolerant cultivars, indicating that these pathways may participate in the salt tolerance of sugar beet. Salt-tolerant genotypes respond to salinity by increasing anti-oxidative defense systems for detoxification of ROS (Sun et al. 2011). Hence, the varietal variations between Romulus and Francesca cultivars led to discrepancy in agronomic traits and juice quality.
As suppression of plant growth is the prevailing influence of salinity stress, physiological and growth parameters of sugar beet were declined with increasing salt concentration. For instance, by comparing the effect of low and high salinity on sugar beet physiological traits under no K application, it should be noted that the damage impact of salinity on cell membrane stability, relative water content, and performance index were –2.9, –13.6, –11.1%, respectively. Contrariwise, K supply (144 kg ha−1) alleviated such effect by 21.5, 17.3, and 207.5%, respectively. One approach to minimize effects of salinity is use of K application to increase tolerance of plant to salinity by alleviating Na+ and Cl− injury (Gul et al. 2019). Keeping adequate potassium levels is great of importance for survival of plants in saline medium (Abbasi et al. 2014). Despite the findings of the present research revealed that salinity induced reduction in agronomic and quality traits of sugar beet, potassium counteracted the salinity effect. Addition of potassium under salinity increased root length, shoot fresh weight, and root fresh weight (Mehmood et al. 2020). Salt tolerance in plants increased by increasing K+ uptake which leads to increasing K/Na ratio in plant cells (Ali et al. 2019). In this respect several kinds of K+ and Na+ transport systems, such as inward-rectifier shaker K+ channel (AKT1), high-affinity K+ transporter 5 (HAK5), shaker-like K+ outward rectifying channel (SKOR), high-affinity K+ transporter 1;5 (HKT1;5), tonoplast Na+/H+ antiporter 1 (NHX1), and plasma membrane Na+/H+ antiporter 1 (SOS1) have been documented to synergistically regulate ion (K+ and Na+) homeostasis in sugar beet under saline condition (Li et al. 2022). Moreover, application of potassium induced salt tolerance as the production of malondialdehyde content and electrolyte leakage decreased, while growth parameters, chlorophyll contents, antioxidant enzymes, gas exchange characteristics, and sugar contents were improved (Parveen et al. 2021). Potassium also played an important role in plant water relation under salinity stress and helped the plants to absorb more water to attain turgidity and membrane stability (Abbasi et al. 2016). K is a key element for crop growth and productivity (Hasanuzzaman et al. 2018). It is an essential nutrient for photosynthesis and the transport of assimilates (Wang et al. 2015). Potassium affects the osmotic adjustment of the plant and by enhancing the translocation of assimilates and maintaining osmotic charge (Marschner 2012; Mubarak et al. 2016). Since the transport of Na+ from roots to leaves can also be restricted by a high-affinity K+ transporter (Byrt et al. 2014), K supply is a crucial act for crop protection in saline soils.
Complementary effect between K addition and genotypic performance of sugar beet exhibited that K positively affected almost physiological and agronomic traits and mitigated the negative impact of salinity on growth with superiority of Romulus cultivar. Accordingly, gene expression response could be improved with K application under saline soil conditions. In consistence with the current research, fertilizing sugar beet, whether highly or moderately tolerant genotype to salt stress, with adequate K fertilizer raise the plant potentiality to counteract the salinity hazards. In this regard, Deinlein et al. (2014) stated that potassium is considered a major osmotically active solute of plant cell, where it enhances water uptake and root permeability and acts as a guard cell controller, beside its role in increasing water use efficiency.
5 Conclusions
The findings of the current work could summarize the significance of complementary effect of potassium and varietal differences for improving the physio-biochemical, yield, and quality traits of sugar beet under saline soils. In this connection, Romulus cultivar fertilized by potassium at a rate of 144 kg per hectare exhibited high membrane stability index, relative water content, efficiency of photosynthesis, leaf nutrient contents, sugar yield with low sodium, and alpha-amino-nitrogen in beet sugar juice. Hence, providing Romulus cultivar with 144-kg potassium per hectare regarded as a promising practice for sugar beet production under soil electric conductivity of 9.28 dS m−1. As a new approach, sugar beet breeders should focus not only on yield and its components but also plant physiological properties for achieving distinctive progress in sugar beet genotypes that have high tolerant degrees to salinity. Furthermore, since the highest potassium rate showed the best performance of sugar beet cultivars against salinity, further studies could be implemented using higher potassium rates than 144 kg ha−1.
References
Abbasi GH, Akhtar J, Haq MA, Ali S, Chen ZH, Malik W (2014) Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak J Bot 46:135–146
Abbasi GH, Akhtar J, Ahmad R, Jamil M, Haq MA, Ali S, Ijaz M (2015) Potassium application mitigates salt stress differentially at different growth stages in tolerant and sensitive maize hybrids. Plant Growth Regul 76:111–125. https://doi.org/10.1007/s10725-015-0050-1
Abbasi H, Jamil M, Haq A, Ali S, Ahmad R, Malik Z, Parveen, (2016) Salt stress manifestation on plants, Mechanism of salt tolerance and potassium role in alleviating it: a review. Zemdirbyste-Agric 103:229–238. https://doi.org/10.13080/z-a.2016.103.030
Abd El-Mageed TA, El-Sherif AM, El-Mageed SAA, Abdou NMA (2019) A novel compost alleviate drought stress for sugar beet production grown in Cd-contaminated saline soil. Agric Water Manage 226:105831. https://doi.org/10.1016/j.agwat.2019.105831
Abd El-Mageed TA, Rady MO, Semida WM, Shaaban A, Mekdad AA (2021) Exogenous micronutrients modulate morpho-physiological attributes, yield, and sugar quality in two salt-stressed sugar beet cultivars. J Soil Sci Plant Nutr 21:1421–1436. https://doi.org/10.1007/s42729-021-00450-y
Abd-Elrahman SH, Saudy HS, Abd El-Fattah DA, Hashem FA (2022) Effect of irrigation water and organic fertilizer on reducing nitrate accumulation and boosting lettuce productivity. J Soil Sci Plant Nutr 22:2144–2155. https://doi.org/10.1007/s42729-022-00799-8
Ali A, Maggio A, Bressan RA, Yun D-J (2019) Role and functional differences of HKT1-type transporters in plants under salt stress. Int J Mol Sci 20:1059. https://doi.org/10.3390/2Fijms20051059
AOAC (2012) Association of Official Agriculture Chemists, Official Method of Analysis: Association of Analytical Chemists. 19th Ed, Washington DC, pp 121–130
Arraouadi S, Badri M, Taamalli W, Huguet T, Aouani ME (2011) Variability salt stress response analysis of Tunisian natural populations of Medicago truncatula (Fabaceae) using salt response index (SRI) ratio. Afr J Biotechnol 10:10636–10647. https://doi.org/10.5897/AJB10.1784
Brand-William W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Byrt CS, Xu B, Krishnan M, Lightfoot DJ, Athman A, Jacobs AK, Watson-Haigh NS, Plett D, Munns R, Tester M, Gilliham M (2014) The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J Cell Mol Biol 80:516–526. https://doi.org/10.1111/tpj.12651
Cao S, Du XH, Li LH, Liu YD, Zhang L, Pan X, Ll Y, Li H, Lu H (2017) Overexpression of Populus tomentosa cytosolic ascorbate peroxidase enhances abiotic stress tolerance in tobacco plants. Russ J Plant Physiol 64:224–234. https://doi.org/10.1134/s1021443717020029
CCSC (2018) Central Council for Sugar Crops annual report, Ministry of Agriculture and Land Reclamation, Cairo
Chapman HD, Pratt PF (1961) Methods of analysis for soils, plants and waters. University of California, Los Angeles
Chiconato DA, Cruz FJR, da Silva SJG, dos Santos DMM, Munns R (2019) Adaptation of sugarcane (Saccharum officinarum L.) plants to saline soil. Environ Exp Bot 162:201–211. https://doi.org/10.1016/j.envexpbot.2019.02.021
Clark AJ, Landolt W, Bucher JB, Strasser RJ (2000) Beech (Fagus sylvatica) response to ozone exposure assessed with a chlorophyll a fluorescence performance index. Environ Pollut 109:501–507. https://doi.org/10.1016/S0269-7491(00)00053-1
Cui F, Sui N, Duan G, Liu Y, Han Y, Liu S, Wan S, Li G (2018) Identification of metabolites and transcripts involved in salt stress and recovery in peanut. Front Plant Sci 9:217. https://doi.org/10.3389/fpls.2018.00217
Cuin TA, Betts SA, Chalmandrier R, Shabala S (2008) A root’s ability to retain K+ correlates with salt tolerance in wheat. J Exp Bot 59:2697–2706. https://doi.org/10.1093/2Fjxb/2Fern128
Dadkhah A, Rassam G (2017) Effect of short-term salinity on photosynthesis and ion relations in two sugar beet cultivars. Iran J Plant Physiol 7:1983–1989. https://doi.org/10.22034/ijpp.2017.532387
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trend. Plant Sci 19:371–379. https://doi.org/10.1016/j.tplants.2014.02.001
El-Bially MA, Saudy HS, El-Metwally IM, Shahin MG (2022a) Sunflower response to application of L–ascorbate under thermal stress associated with different sowing dates. Gesun Pflan 74:87–96. https://doi.org/10.1007/s10343-021-00590-2
El-Bially MA, Saudy HS, Hashem FA, El–Gabry YA, Shahin MG (2022b) Salicylic acid as a tolerance inducer of drought stress on sunflower grown in sandy soil. Gesun Pflan. https://doi.org/10.1007/s10343-022-00635-0
El-Metwally IM, Saudy HS (2021) Interactional impacts of drought and weed stresses on nutritional status of seeds and water use efficiency of peanut plants grown in arid conditions. Gesun Pflan 73:407–416. https://doi.org/10.1007/s10343-021-00557-3
El-Metwally IM, Saudy HS (2021) Interactive application of zinc and herbicides affects broad–leaved weeds, nutrient uptake, and yield in rice. J Soil Sci Plant Nutr 21:238–248. https://doi.org/10.1007/s42729-020-00356-1
El-Metwally IM, Saudy HS, Abdelhamid MT (2021) Efficacy of benzyladenine for compensating the reduction in soybean productivity under low water supply. Ital J Agromet 2:81–90. https://doi.org/10.36253/ijam-872
El-Metwally IM, Geries L, Saudy HS (2022) Interactive effect of soil mulching and irrigation regime on yield, irrigation water use efficiency and weeds of trickle–irrigated onion. Archiv Agron Soil Sci 68:1103–1116. https://doi.org/10.1080/03650340.2020.1869723
FAO (2016) AQUASTAT Country Profil-Egypt; Food and Agriculture Organization of the United Nations: Rome, Italy. http://www.fao.org/3/i9729en/I9729EN.pdf. Accessed 10 June 2021
FAO (2022) Food and agriculture organization. World Food and Agriculture - Statistical Pocketbook, FAO: Rome, Italy. https://doi.org/10.4060/cb1521en. Accessed 15 June 2022
GENSTAT (2007) Genstat statistical packages GenStat Release 9.2 (PC/Windows) (9th ed.). Lawes Agriculture Trust, Rothamsted Experimental Station, UK
Gholipor B, Mozaffari A, Maleki A, Mirzaei Heydari M, Babaii F (2022) Antioxidant and biochemical alterations in sea beet (Beta vulgaris subsp. maritime (L.) Arcang.) and sugar beet (Beta vulgaris L.) exposed to salt stress. J Agric Sci Tech 24:123–138
Grieve CM, Grattan SR, Maas EV (2012) Plant salt tolerance. In: Wallender WW, Tanji KK (eds) Agricultural salinity assessment and management. ASCE Manual and Reports on Engineering Practice No. 71, 2nd ed. Reston, VA, US: ASCE, pp 405–459
Gul M, Wakeel A, Steffens D, Lindberg S (2019) Potassium-induced decrease in cytosolic Na+ alleviates deleterious effects of salt stress on wheat (Triticum aestivum L.). Plant Biol 21:825–831. https://doi.org/10.1111/plb.12999
Gupta B, Huang B (2014) Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int J Genomics 701596. https://doi.org/10.1155/2014/701596
Han G, Wang M, Yuan F, Sui N, Song J, Wang B (2014) The CCCH zinc finger protein gene AtZFP1 improves salt resistance in Arabidopsis thaliana. Plant Mol Biol 86:237–253. https://doi.org/10.1007/s11103-014-0226-5
Hasanuzzaman M, Bhuyan MHMB, Nahar K, Hossain MS, Mahmud JA, Hossen MS, Masud AAC, Moumita Fujita M (2018) Potassium: a vital regulator of plant responses and tolerance to abiotic stresses. Agron 8:31. https://doi.org/10.3390/agronomy8030031
Hatam Z, Sabet MS, Malakouti MJ, Mokhtassi-Bidgoli A, Homaee M (2020) Zinc and potassium fertilizer recommendation for cotton seedlings under salinity stress based on gas exchange and chlorophyll fluorescence responses. S Afr J Bot 130:155–164. https://doi.org/10.1016/j.sajb.2019.11.032
IUSS Working Group WRB (2015) World Reference Base for Soil Resources 2014, update 2015 International soil classification system for naming soils and creating legends for soil maps. World Soil Resources Reports No. 106. FAO, Rome, p 192
Jackson ML (1973) Soil chemical analysis. Prentice Hall India Pvt. Ltd., New Delhi, p 498
Jan AU, Hadi F, Nawaz MA, Rahman K (2017) Potassium and zinc increase tolerance to salt stress in wheat (Triticum aestivum L.). Plant Physiol Biochem 116:139–149. https://doi.org/10.1016/j.plaphy.2017.05.008
Klute A, Dirksen C (1986) Hydraulic conductivity and diffusivity: laboratory methods, in methods of soil analysis: Part 1-physical and mineralogical methods (Soil Science Society of America), Amer Soc Agron 687–734.https://doi.org/10.2136/sssabookser5.1.2ed.c28
Kong XQ, Gao XH, Sun W, An J, Zhao YX, Zhang H (2011) Cloning and functional characterization of a cation-chloride cotransporter gene OsCCC1. Plant Mol Biol 75:567–578. https://doi.org/10.1007/s11103-011-7446
Kong XQ, Wang T, Li WJ, Tang W, Zhang DM, Dong HZ (2016) Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol Plantar 38:61. https://doi.org/10.1007/s11738-016-2079-9
Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011) Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). J Exp Bot 62:4215–4228. https://doi.org/10.1093/jxb/err135
Lachica M, Aguilar A, Yanez J (1973) Analisis foliar: Métodos utilizados enla estaciln experimental del zaidin. An Edafol Agrobiol 32:1033–1047
Lamaison JLC, Carnet A (1990) Teneurs en principaux flavonoides des fleurs de Crataegus monogyna Jacq et de Crataegus Laevigata (Poiret DC) en fonction de la vegetation pharmaceut. Acta Helve 65:315–320
Leng BY, Yuan F, Dong XX, Wang J, Wang BS (2018) Distribution pattern and salt excretion rate of salt glands in two recretohalophyte species of Limonium (Plumbaginaceae). South Afr J Bot 115:74–80. https://doi.org/10.1016/j.sajb.2018.01.002
Li SJ, Wu GQ, Lin LY (2022) AKT1, HAK5, SKOR, HKT1;5, SOS1 and NHX1 synergistically control Na+ and K+ homeostasis in sugar beet (Beta vulgaris L.) seedlings under saline conditions. J Plant Biochem Biotech 31:71–84. https://doi.org/10.1007/s13562-021-00656-2
Liu L, Wang B, Liu D, Zou C, Wu P, Wang Z, Wang Y, Li C (2020) Transcriptomic and metabolomic analyses reveal mechanisms of adaptation to salinity in which carbon and nitrogen metabolism is altered in sugar beet roots. BMC Plant Biol 20:1–21. https://doi.org/10.1186/s12870-020-02349-9
Makhlouf BSI, Khalil Soha RA, Saudy HS (2022) Efficacy of humic acids and chitosan for enhancing yield and sugar quality of sugar beet under moderate and severe drought. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-022-00762-7
Manaa A, Ben Ahmed H, Valot B, Bouchet JP, Aschi-Smiti S, Causse M, Faurobert M (2011) Salt and genotype impact on plant physiology and root proteome variations in tomato. J Exp Bot 62:2797–2813. https://doi.org/10.1093/jxb/erq460
Mari AH, Rajpar I, Tunio S, Ahmad S (2018) Ions accumulation, proline content and juice quality of sugar beet genotypes as affected by water salinity. J Anim Plant Sci 28:1405–1412
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic press, London, UK, Waltham, MA, USA; San Diego, CA, USA
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence- a practical guide. J Exp Bot 51:659–668. https://doi.org/10.1093/jxb/51.345.659
McGinnus RA (1971) Sugar beet technology, 2nd ed. Color, Sugar Beet Development Foundation, pp 330–333
Mehmood H, Abbasi GH, Jamil M, Malik Z, Ali M, Iqbal R, Ahmed S, Akhtar MW, Irfan M, Afzal S (2020) Potential of applied potassium in improving salt tolerance of Maize (Zea mays L.) hybrids. J Biol Environ Sci 16:35–43
Mekdad AAA, Rady MM (2016) Response of Beta vulgaris L. to nitrogen and micronutrients in dry environment. Plant Soil Environ 62:23–29. https://doi.org/10.17221/631/2015-PSE
Mekdad AA, El-Enin MMA, Rady MM, Hassan FA, Ali EF, Shaaban A (2021) Impact of level of nitrogen fertilization and critical period for weed control in peanut (Arachis hypogaea L.). Agron 11:909. https://doi.org/10.3390/agronomy11050909
Mekdad AA, Rady MM, Ali EF, Hassan FA (2021) Early sowing combined with adequate potassium and sulfur fertilization: Promoting Beta vulgaris (L.) yield, yield quality, and K-and S-use efficiency in a dry saline environment. Agron 11:806. https://doi.org/10.3390/agronomy11040806
Mekdad AA, Shaaban A, Rady MM, Ali EF, Hassan FA (2021) Integrated application of K and Zn as an avenue to promote sugar beet yield, industrial sugar quality, and K-use efficiency in a salty semi-arid agro-ecosystem. Agron 11:780. https://doi.org/10.3390/agronomy11040780
Merwad AMA (2016) Efficiency of potassium fertilization and salicylic acid on yield and nutrient accumulation of sugar beet grown on saline soil. Commun Soil Sci Plant Anal 47:1184–1192. https://doi.org/10.1080/00103624.2016.1166242
Mubarak MU, Zahir M, Ahmad S, Wakeel A (2016) Sugar beet yield and industrial sugar contents improved by potassium fertilization under scarce and adequate moisture conditions. J Integ Agric 15:2620–2626. https://doi.org/10.1016/S2095-3119(15)61252-7
Mubarak M, Salem EMM, Kenawey MKM, Saudy HS (2021) Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J Soil Sci Plant Nutr 21:2020–2031. https://doi.org/10.1007/s42729-021-00498-w
Munns R, Gilliham M (2015) Salinity tolerance of crops-what is the cost? New Phytol 208:668–673. https://doi.org/10.1111/nph.13519
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Bio l59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nawaz K, Ashraf M (2010) Exogenous application of glycine betaine modulates activities of antioxidants in maize plants subjected to salt stress. J Agron Crop Sci 196:28–37. https://doi.org/10.1111/j.1439-037X.2009.00385.x
Negrao S, Schmockel SM, Tester M (2017) Evaluating physiological responses of plants to salinity stress. Ann Bot 119:1–11. https://doi.org/10.1093/2Faob/2Fmcw191
Page AI, Miller RH, Keeny, DR (1982) Methods of soil analysis, in Part II. Chemical and Microbiological Methods, 2nd ed. Amer Soc Agron, Madison, pp 225–246
Parveen A-U-H, Aziz T, Aziz O, Maqsood L (2021) Potassium induces carbohydrates accumulation by enhancing morpho-physiological and biochemical attributes in soybean under salinity. Arch Agron Soil Sci 67:946–959. https://doi.org/10.1080/03650340.2020.1769075
Pi Z, Stevanato P, Sun F, Yang Y, Sun X, Zhao H, Geng G, Yu L (2016) Proteomic changes induced by potassium deficiency and potassium substitution by sodium in sugar beet. J Plant Res 129:527–538. https://doi.org/10.1007/s10265-016-0800-9
Premachandra G, Saneoka H, Ogata S (1990) Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soybean. J Agric Sci 115:63–66. https://doi.org/10.1017/S0021859600073925
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2021) Soil mulching and deficit irrigation effect on sustainability of nutrients availability and uptake, and productivity of maize grown in calcareous soils. Comm Soil Sci Plant Anal 52:1745–1761. https://doi.org/10.1080/00103624.2021.1892733
Salem EMM, Kenawey MKM, Saudy HS, Mubarak M (2022) Influence of silicon forms on nutrient accumulation and grain yield of wheat under water deficit conditions. Gesun Pflan. https://doi.org/10.1007/s10343-022-00629-y\
Saudy HS, Noureldin NA, Mubarak M, Fares W, Elsayed M (2020) Cultivar selection as a tool for managing soil phosphorus and faba bean yield sustainability. Archiv Agron Soil Sci 66:414–425. https://doi.org/10.1080/03650340.2019.1619078
Saudy HS, El-Bially MA, El-Metwally IM, Shahin MG (2021) Physio–biochemical and agronomic response of ascorbic acid–treated sunflower (Helianthus annuus) grown at different sowing dates and under various irrigation regimes. Gesun Pflan 73:169–179. https://doi.org/10.1007/s10343-020-00535-1
Saudy HS, El-Metwally IM, Shahin MG (2021) Co–application effect of herbicides and micronutrients on weeds and nutrient uptake in flooded irrigated rice: Does it have a synergistic or an antagonistic effect? Crop Prot 149:105755. https://doi.org/10.1016/j.cropro.2021.105755
Saudy HS, Abd El-Samad GA, El-Temsah ME, El-Gabry YA (2022) Effect of iron, zinc and manganese nano-form mixture on the micronutrient recovery efficiency and seed yield response index of sesame genotypes. J Soil Sci Plant Nutr 22:732–742. https://doi.org/10.1007/s42729-021-00681-z
Schubert S, Neubert A, Schierholt A, Sümer A, Zörb C (2009) Development of salt-resistant maize hybrids: the combination of physiological strategies using conventional breeding methods. Plant Sci 177:196e202. https://doi.org/10.1016/J.PLANTSCI.2009.05.011
Semida WM, Abd El-Mageed TA, Howladar SM (2014) A novel organo-mineral fertilizer can alleviate negative effects of salinity stress for eggplant production on reclaimed saline calcareous soil. Acta Hort 493–500. https://doi.org/10.17660/ActaHortic.2014.1034.61
Semida WM, Abd El-Mageed TA, Howladar SM, Mohamed GF, Rady MM (2015) Response of Solanum melongena L. seedlings grown under saline calcareous soil conditions to a new organo-mineral fertilizer. J Anim Plant Sci 25:485-493
Shaaban A, Al-Elwany OAAI, Abdou NM, Hemida KA, El-Sherif AMA, Abdel-Razek MA, Semida WM, Mohamed GF, Abd El-Mageed TA (2022) Filter mud enhanced yield and soil properties of water-stressed Lupinus termis L. in saline calcareous Soil. J Soil Sci Plant Nutr 22:1572–1588. https://doi.org/10.1007/s42729-021-00755-y
Shabala S, Pottosin I (2014) Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol Plantar 151:257–279. https://doi.org/10.1111/ppl.12165
Siddiqui M, Mohammad F, Khan MN, Al-Whaib MH, Bahkali AHA (2010) Nitrogen in relation to photosynthetic capacity and accumulation of osmoprotectant and nutrients in Brassica genotypes grown under salt stress. Agric Sci China 9:671–680. https://doi.org/10.1016/S1671-2927(09)60142-5
Sun JK, Li T, Xia JB, Tian JY, Lu ZH, Wang RT (2011) Influence of salt stress on ecophysiological parameters of Periploca sepium bunge. Plant Soil Environ 57:139–144. https://doi.org/10.17221/227/2010-PSE
Sun Y, Kong X, Li C, Liu Y, Ding Z (2015) Potassium retention under salt stress is associated with natural variation in salinity tolerance among Arabidopsis accessions. PLoS ONE 10:e0124032. https://doi.org/10.1371/journal.pone.0124032
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trend Plant Sci 15:89–97. https://doi.org/10.1016/j.tplants.2009.11.009
Wakeel A (2013) Potassium–sodium interactions in soil and plant under saline-sodic conditions. J Plant Nutr Soil Sci 176:344–354. https://doi.org/10.1002/jpln.201200417
Wakeel A, Sümer A, Hanstein S, Yan F, Schubert S (2011) In vitro effect of different Na+/K+ ratios on plasma membrane H+–ATPase activity in maize and sugar beet shoot. Plant Physiol Biochem. 49:341e345. https://doi.org/10.1016/j.plaphy.2011.01.006
Wang XG, Zhao HZX, Jiang JC, Li HC, Cong S, Wu D, Chen YQ, Yu HQ, Wang CY (2015) Effects of potassium deficiency on photosynthesis and photoprotection mechanisms in soybean (Glycine max (L.) Merr.). J Integ Agric 14:856–863. https://doi.org/10.1016/S2095-3119(14)60848-0
Wang Y, Stevanato P, Yu L, Zhao H, Sun X, Sun F, Li J, Geng G (2017) The physiological and metabolic changes in sugar beet seedlings under different levels of salt stress. J Plant Res 130:1079–1093. https://doi.org/10.1007/s10265-017-0964-y
Wang Y, Stevanato P, Lv C, Li R, Geng G (2019) Comparative physiological and proteomic analysis of two sugar beet genotypes with contrasting salt tolerance. J Agric Food Chem 67:6056–6073. https://doi.org/10.1021/acs.jafc.9b00244
Weng L, Zhang M, Wang K, Chen G, Ding M, Yuan W, Zhu Y, Xu W, Xu F (2020) Potassium alleviates ammonium toxicity in rice by reducing its uptake through activation of plasma membrane H+–ATPase to enhance proton extrusion. Plant Physiol Biochem 151:429–437. https://doi.org/10.1016/j.plaphy.2020.03.040
Wu GQ, Liang N, Feng RJ, Zhang JJ (2013) Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol Plantar 35:2665–2674. https://doi.org/10.1007/s11738-013-1298-6
Wu GQ, Feng RJ, Liang N, Yuan HJ, Sun WB (2015) Sodium chloride stimulates growth and alleviates sorbitol-induced osmotic stress in sugar beet seedlings. Plant Growth Regul 75:307–316. https://doi.org/10.1007/s10725-014-9954-4
Wu GQ, Shui QZ, Wang CM, Zhang JL, Yuan HJ, Li SJ, Liu ZJ (2015) Characteristics of Na+ uptake in sugar beet (Beta vulgaris L.) seedlings under mild salt conditions. Acta Physiol Plant 37:70. https://doi.org/10.1007/s11738-015-1816-9
Wu GQ, Wang JL, Li SJ (2019) Genome-wide identification of Na+/H+ antiporter (NHX) genes in sugar beet (Beta vulgaris L.) and their regulated expression under salt stress. Genes 10:401. https://doi.org/10.3390/2Fgenes10050401
Xue X, Zhang Q, Wu J (2013) Research of reactive oxygen species in plants and its application on stress tolerance. Biotechnol Bull 36:6–11. https://doi.org/10.13560/j.cnki.biotech.bull.1985.2013.10.033
Yang Z, Li J-L, Liu L-N, Xie Q, Sui N (2020) Photosynthetic regulation under salt stress and salt-tolerance mechanism of sweet sorghum. Front Plant Sci 10:1722. https://doi.org/10.3389/fpls.2019.01722
Yu L, Haley S, Perret J, Harris M, Wilson J, Ming Q (2002) Free radical scavenging properties of wheat extracts. J Agric Food Chem 50:1619–1624. https://doi.org/10.1021/jf010964p
Yu B, Chen M, Grin I, Ma C (2020) Mechanisms of sugar beet response to biotic and abiotic stresses. Adv Exp Med Biol 1241:167–194. https://doi.org/10.1007/978-3-030-41283-8-10
Yuan F, Lyu MJA, Leng BY, Zhu XG, Wang BS (2016) The transcriptome of NaCl-treated Limonium bicolor leaves reveals the genes controlling salt secretion of salt gland. Plant Mol Biol 91:241–256. https://doi.org/10.1007/s11103-016-0460-0
Zhang JL, Shi H (2013) Physiological and molecular mechanisms of plant salt tolerance. Photosyn Res 115:1–22. https://doi.org/10.1007/s11120-013-9813-6
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests concerning the current research publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Mageed, T.A.A., Mekdad, A.A.A., Rady, M.O.A. et al. Physio-biochemical and Agronomic Changes of Two Sugar Beet Cultivars Grown in Saline Soil as Influenced by Potassium Fertilizer. J Soil Sci Plant Nutr 22, 3636–3654 (2022). https://doi.org/10.1007/s42729-022-00916-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-022-00916-7