Abstract
Protein content and antioxidants in both bee bread and honeybee workers were analyzed investigating the relationships between those two parameters. Results indicated significant differences between both protein and antioxidant content [total antioxidant content (TAX), glutathione (GSH) and lipid peroxidation (LPO) levels] of bee bread types. Generally, workers fed sugar syrup only (control) had significantly lower protein content, superoxide dismutase (SOD), catalase (CAT), and GSH in comparison to bees fed bee bread at all studied ages. On the other hand, LPO levels in control worker bees were significantly higher than those of bees fed fennel, maize, cucumber, clover, and broad bean bee bread. Results also showed a positive relationship between protein content and antioxidants in bee bread and between protein content and the antioxidant system of honeybees, respectively. There was a negative correlation between protein content and antioxidants. In addition, the present study showed that both the protein quantity and quality of bee bread are important for honeybees. To our knowledge, this study is the first to report the relationship between antioxidant content in bee bread and antioxidant system of honeybees. The present study showed a causal link between antioxidant system and protein content in honeybees, and the contents of both antioxidants and protein in bee bread.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For colony growth and development, individual honey bee foragers gather nectar and pollen from their environment. Nectar and pollen provide honey bees with essential nutrients. Nectar serves as a carbohydrate source and primarily consists of monosaccharides and oligosaccharides (Nicolson and Thornburg 2007). Pollen is comprised of varying amounts of protein, amino acids, lipids, vitamins, and minerals, which are essential for growth and development (Brodschneider and Crailsheim 2010; Wright et al. 2018). Over the past years, the rising interest in the extraction and determination of these beneficial bee pollen compounds has been demonstrated by the large number of published research papers dealing with this issue and the large list of countries in which such studies were carried out (Ares et al. 2018). Many studies have been conducted to determine the chemical composition of pollen from many parts of the world (Somerville and Nicol 2006; Tasei and Aupinel 2008; Khider et al. 2013; Corby-Harris et al. 2018; Radev 2019; Telleria et al. 2019; Weglinska et al. 2020). Many of the analyses have been for protein and amino acid content, but others have investigated fat and fatty acid concentrations, sugars and starches, fibrous components, vitamins, and minerals. Bee pollen is not only a source of protein with a wide range of content and it also has great nutrient value, such as antioxidants. Bee pollen possesses a powerful antioxidative activity owing to various compounds contained in it, which has been confirmed in many studies (Komosinska–Vassev et al. 2015; Ares et al. 2018; Kieliszek et al. 2018; Martinello and Mutinelli 2021). Furthermore, bee pollen is a source of hydrophilic antioxidants that protect against oxidative structure damage to the cytoplasm, inside cell organelles, and in the extracellular fluid. Among hydrophilic antioxidants, ascorbate (vitamin C) and phenolic acids can be found. They are hydrophilic to an extent and can also be effective antioxidants in an aqueous phase, e.g., caffeic acid (Rice-Evans et al. 1996). It has also been suggested that the pollen of different botanical origins possesses different antioxidant capacities, which is more related to the specific flavone and phenolic acid profiles (Almaraz-Abarca et al. 2007; LeBlanc et al. 2009; Zilic et al. 2014; Borycka et al. 2015). Overlooked and recent research of pollen protein content shows that protein nutrition of pollen is important for certain aspects of honey bee physiology (Brodschneider and Crailsheim 2010; Wright et al. 2018). But protein alone can’t explain some of the effects of a bee’s diet on their health and how their colonies work. Other nutrients, like antioxidants, are also thought to play a role.
Bee pollen and bee bread contain a wide range of ingredients; therefore, they are foods with good nutritive value, controlling the activity of many internal organs (Mărgăoan et al. 2019). In the hive, bee nutrition comes from two main plant-based sources, such as nectar and pollen. The nectar is brought to the hive and turned into honey, and pollen from flowers is transformed into bee bread which provides bees with carbohydrates and other necessary nutrients. Studies on the chemical composition of bee bread showed that it is usually composed of water, proteins, free amino acids, carbohydrates, fatty acids, and other bioactive molecules; this composition varies from region to region, depending on the melliferous plants of that region, and also depending on the climatic conditions and seasonal variation (Baltrušaitytė et al. 2007; Urcan et al. 2017). Many studies say that bee bread is very good at fighting off free radicals and antioxidants (Urcan et al. 2017; Martinello and Mutinelli 2021). Bee bread is also a good source of antioxidant compounds such as phenolic compounds, coenzyme Q10, and α-tocopherol (Zuluaga et al. 2015; Urcan et al. 2017). Previous reports examined the antioxidant capacities of bee bread and pollen (Urcan et al. 2017; Martinello and Mutinelli 2021). Bee bread’s nutritional value is higher than bee pollen’s and it is more digestible due to the presence of a higher content of free amino acids and easily assimilated sugars (Kieliszek et al. 2018). In comparison with bee pollen, studies on bee bread are scarce and limited, especially concerning their chemical composition (Urcan et al. 2017; Kieliszek et al. 2018; Martinello and Mutinelli 2021). In fact, we know little about the other nutrients in bee bread, including its vitamin composition and antioxidant contents.
An increase in the intracellular generation of reactive oxygen species (ROS), such as superoxide (O2•−), hydroxyl radical (HO¯) and hydrogen peroxide (H2O2), or a defect in the antioxidant defense system, renders the cells oxidatively stressed. Oxidative stress has often been referred to as a harmful state for cell survival and, consistent with that, there is an abundance of literature directly or indirectly implicating ROS in cellular damage (Clément and Pervaiz 2001; Livingstone 2001; Corona and Robinson 2006). ROS are mostly neutralized by enzymatic and non-enzymatic detoxification mechanisms. Under normal conditions, there is a balance between the generation of ROS and antioxidant processes. Oxidative stress results when the dynamic balance between ROS generation exceeds the capacity of antioxidant defenses to remove those toxic species. In many studies, oxidative stress has been linked to stressors influencing honey bee health and colony productivity. Low temperature, flight activity, pathogens in hives, and pesticides used for pest control in a variety of crops all disrupt honey bee oxidative homeostasis (Gregorc et al. 2018; Margotta et al. 2018; Kojić et al. 2019; Li et al. 2020; Mucci et al. 2021). Researchers speculate that the nutritional stress of low pollen quality and, consequently, bee bread quality is one of the contributing factors in the current honeybee colony declines. It is believed that the lack of endogenous antioxidative defense may be compensated by exogenous antioxidants, mainly obtained from foods and natural food supplements. Whether antioxidants in bee bread affect the antioxidant system of honeybees remains unknown. Therefore, this study has been undertaken to examine the effects of different types of bee bread on honeybee physiology (protein content and antioxidant system). The objective of this study was to determine the relationships between protein and antioxidant content in some bee bread, and the protein content and antioxidant system of honeybee workers.
Materials and methods
The present study was carried out at the Department of Zoology/Entomology, Faculty of Science, Assiut University, and the Insect Research Laboratory (Assiut), Plant Protection Research Institute. The Carniolan hybrid of honeybee colonies (Apis mellifeara carnica) was used to collect bee bread and as a source for newly emerged workers.
Bee bread source
During the flowering periods, bee bread of common cultivated plants was extracted from honey bee colonies, according to the protocol described by Ahmed et al. (2019). Briefly, bee colonies were placed in five farms containing monocultures of broad bean (Vicia faba), clover (Trifolium alexandrinum), fennel (Faeniculum vulgare), cucumber (Cucumis sativus) and maize (Zea mays). At the end of the flowering period, the bee bread was extracted from the honeybee colonies. Bee bread from each source was stored at -20ºC until using it. Microscopically, pollen grains from bee bread were compared to pollen grains from the anthers of flowers to find out which pollen types they were.
Bee bread analysis
Total protein content, protein fractionation [sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE)] and antioxidants [total antioxidant (TAX), glutathione (GSH) and lipid peroxidation (LPO)]
Homogenates from each bee bread were prepared as follows: 100 mg of each bee bread diet were frozen in liquid nitrogen, ground to powder with a pestle and mortar, and homogenized in 1 mL of ice–cold phosphate buffer (0.1 M) (10% w/v). Crude homogenates were centrifuged at 15,000 rpm, 4 °C for 20 min. The supernatant was kept frozen at -20 °C for the subsequent chemical assays.
Soluble total protein was determined in each sample spectrophotometrically (T60 UV–VIS spectrophotometer) and expressed as mg/dl. The total protein content of bee bread was estimated at 550 nm using a total protein kit (vitro scient perfection for life science, German).
Bee bread samples (100 mg each) were homogenized, 1 ml of lysing buffer was added, and denatured for 5 min at 100 °C with 1% (w/v) SDS and 2% (v/v) 2-mercaptoethanol before centrifugation at 10,000 rpm for 30 min. 10 µl of each extracted sample was used for protein analysis using SDSPAGE. SDSPAGE was performed according to Laemmli (1970), with a 3% stacking gel containing 75 mM Tris–HCl (pH 6.8) followed by a 10% separating gel containing 375 mM Tris–HCl (pH 8.8). Tris–glycine (pH 8.3) with 0.1% SDS was used as the electrode buffer. As reference, SDS protein standard kit (Pierce, USA) was run concurrently. After electrophoresis, protein bands were stained with Coomassie Brilliant Blue R-225, and the protein molecular mass was determined using gel-pro Analyzer packing V 3.1 for Windows XP/NT (Media Cybernetica 1993-97).
The TAX assay of bee bread types was estimated spectrophotometrically at 340 nm using a kit (Biodiagnostic and research reagents) and expressed as mg/mg diet. At 340 nm GSH content was determined using 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) as the substrate (Beutler et al. 1963). The level of LPO was measured according to the method described by Ohkawa et al. (1979) which is based on the principle that malondialdehyde (MDA) i.e., the specific product of lipid peroxidation, reacts with thiobarbituric acid (TBA) to form a colored complex with maximum absorption at 532 nm.
Physiological parameters
Honey bees
Newly emerged honey bee workers (0–24 h old) were obtained by incubating capped worker brood combs at 32 ± 1 °C under standard conditions (Williams et al. 2013). Newly emerging bees from these different brood combs were mixed before being introduced into test cages.
Experimental cages and groups
The experiment was carried out in wooden cages with dimensions of 15 × 15 × 5 cm, covered with black muslin one side and glass on the other side. Each cage was provided with a piece of wax comb attached to one side of the cage as a clustering plat for the bees and a vial of tap water and another vial of sugar syrup 3:1 (w/v). Each cage was provided with one hundred newly emerged honey bee workers.
Bee bread was prepared in dough form as described by Ahmed et al. (2019). Briefly, the bee bread was mixed with a teaspoon of honey until it became homogenous. The diets were introduced to each cage in small plastic feeders. The diets were changed every 3 days. All cages were held in the dark in an incubator at 32 ± 1 °C and 70% RH. Four cages were used for each diet (sex treatment groups as described below).
Group (1) was fed sugar syrup only (control).
Group (2) was fed broad bean bee bread plus sugar syrup.
Group (3) was fed clover bee bread plus sugar syrup.
Group (4) was fed cucumber bee bread plus sugar syrup.
Group (5) was fed fennel bee bread plus sugar syrup.
Group (6) was fed maize bee bread plus sugar syrup.
Tissue preparation and biochemical analyses
Tissue preparation
In order to determine protein content and activity of antioxidative enzymes in whole honey bees (6, 9 & 12 days), homogenization of three pools of nine worker bees was performed for each group and assayed in technical triplicate. Whole bees were frozen in liquid nitrogen, ground to a powder with a pestle and mortar, and subsequently homogenized in ice-cold phosphate buffer, pH 7.4 (10% w/v). Crude homogenates were centrifuged at 15,000 g (4 °C) for 20 min with 1% Triton-X to facilitate cell lysis. The supernatant was collected and stored at -80 °C until biochemical assays were performed.
Activities of superoxide dismutase (SOD) and catalase (CAT), and levels of GSH and LPO
The activities of SOD and CAT, GSH and LPO levels were determined in homogenates made from the entire bodies of honeybee workers and expressed as units (U) of enzyme activity per mg of protein, µg per mg of protein, or nmol per mg of protein, respectively. CAT activity was measured spectrophotometrically at 240 nm with H2O2 as the substrate (Luck 1963), and SOD activity at 550 nm according to the method of Misra and Fridovich (1972) which is based on its ability to inhibit the autoxidation of epinephrine in an alkaline medium. Levels of GSH and LPO were measured according to the methods described above.
Estimation of protein content
The protein content of the samples for the assay of oxidative stress damaged products and antioxidant enzymes was estimated according to the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Statistical analysis
The data is presented as mean ± standard deviation (SD). Changes in protein content, TAX, GSH, SOD, CAT and LPO were compared among groups and within each group using one-way analysis of variance (ANOVA, the data fit the assumptions of this test) followed by Duncan’s new multiple range test. Correlations between parameters (bee bread parameters: protein content, TAX, GSH & LPO and honey bee worker parameters: protein content, SOD, CAT, GSH & LPO) were estimated with Canonical Correlation Analysis (CCA). Differences were considered statistically significant when p < 0.05. For statistical data analysis, the SPSS program version 16 (SPSS Inc., Chicago) was used.
Results
Protein and antioxidants of bee bread types
Protein content
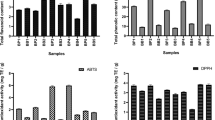
Results indicated significant differences between the protein content of bee bread types (Fig. 1). The highest levels of total protein content were detected in broad bean and clover bee bread (17.73 and 17.63 mg/dl, respectively) followed by cucumber and fennel. The lowest value was 12.60 mg/dl in maize bee bread.
Protein analysis by SDS–PAGE
Electrophoretic analysis of isolated proteins is presented in Fig. 2. SDS–PAGE Broad bean and clover bee bread revealed the presence of 6 fractions with molecular weights of 31.07, 46.18, 51.64, 66, 97.5 & 125 kDa, and 31.18, 40.72, 58.68, 92, 128.86 & 158.43 kDa, respectively. Also, protein fractionation from fennel bee bread showed 6 fractions of molecular weights with 24.21, 44.82, 55.37, 79, 119.5 & 145.57 kDa. The fractionation of cucumber bee bread revealed 7 fractions with molecular weights of 29.9, 41.86, 52.55, 53.48, 71, 94.75 & 134 kDa, whereas, protein profiles from maize bee bread showed 8 fractions of molecular weights of 24.21, 28.5, 35.41, 39.59, 48.91, 64, 76 & 114 kDa.
Determination of TAX, GSH and LPO levels
TAX content, GSH and LPO levels of bee bread types were statistically different (Fig. 3). TAX content, GSH and LPO levels were significantly higher in maize and fennel bee bread compared to other tested bee bread types. Moreover, the TAX content was significantly higher in the maize bee bread than in fennel bee bread. In contrast, LPO level was significantly higher in the fennel bee bread than in maize bee bread.
Effect of different bee bread types on protein content and antioxidant system (SOD, CAT, GSH & LPO) of caged workers.
The total protein content increased throughout the experimental study from 6 to 12 days to reach a maximal level on day 12 in both honeybee workers fed bee bread types and in workers fed sugar syrup only (control) (broad bean: F(2,6) = 6.988, p ˂ 0.03, clover: F(2,6) = 39.071, p ˂ 0.001, cucumber: F(2,6) = 68.113, p ˂ 0.001, fennel: F(2,6) = 61.156, p ˂ 0.001, maize: F(2,6) = 45.687, p ˂ 0.001 and control: F(2,6) = 23.452, p ˂ 0.01) (Fig. 4). Generally, workers fed sugar syrup had significantly lower protein content in comparison to other treatments at all studied ages. On the other hand at all studied ages, workers fed broad bean and clover bee bread had significantly higher concentrations of protein content than bees fed cucumber, fennel, and maize bee bread (Fig. 4).
In honeybee workers who ate bee bread, SOD activity rose steadily over the course of the experiment from 6 to 12 days, reaching its peak on day 12 in honeybee workers fed different bee bread types (broad bean: F(2,6) = 126.5, p ˂ 0.001, clover: F(2,6) = 291.167, p ˂ 0.001, cucumber: F(2,6) = 352.333, p ˂ 0.001, fennel: F(2,6) = 165.125, p ˂ 0.001 and maize: F(2,6) = 228.143, p ˂ 0.001). While, there was a non-significant increase in SOD activity from 6 to 12 days in the control group (Fig. 5a). The activity of SOD in control bees at all studied ages was significantly lower than for other treatments. In worker bees fed maize and fennel bee bread, SOD activities at all studied ages were significantly higher than the control, followed by bees fed clover, cucumber, and then broad bean bee bread (Fig. 5a).
Superoxide dismutase (SOD) activity (U/mg protein min− 1) (a), catalase (CAT) activity (U/mg protein min− 1) (b), glutathione (GSH) level (µg/mg protein) (c), and lipid peroxidation (LPO) level (nmol/mg protein) (d) of caged workers fed on sugar solution (control) and on bee bread types (broad bean, clover, cucumber, fennel, and maize) for different times (6, 9 & 12 days). For each age, bars with different letters indicate statistically significant differences (P < 0.05)
There was a progressive increase in CAT activity throughout the study period from 6 to 12 days to reach a maximal activity on day 12 in honeybee workers fed different bee bread types (broad bean: F(2,6) = 74.273, p ˂ 0.001, clover: F(2,6) = 160.636, p ˂ 0.001, cucumber: F(2,6) = 57.700, p ˂ 0.001, fennel: F(2,6) = 160.800, p ˂ 0.001 and maize: F(2,6) = 15.800, p = 0.004) (Fig. 5b). While, CAT activity decreased gradually but not significantly (F(2,6) = 5.012, p = 0.053) from 6 to 12 days in control bees. CAT activity in control bees at all studied ages was significantly lower than its activity in different treatments. CAT activities at all studied ages were significantly higher in worker bees fed maize and fennel bee bread compared to other treatments (bees fed cucumber, clover and broad bean bee bread) (Fig. 5b).
Honeybee workers who ate bee bread types like broad bean, clover, cucumber, and fennel had the highest GSH levels on day 12 of the experiment. This was because the bee bread types had a lot of GSH in them, which is why the GSH level rose over the course of the experiment from 6 to 12 days to reach its highest level on day 12 (Fig. 5c). While, in control bees the level of GSH decreased gradually from 6 to 12 days (F(2,6) = 6.000, p = 0.037). GSH levels in control bees at all studied ages were significantly lower than treatments. The highest levels of GSH were detected in worker bees fed on maize and fennel bee bread at all studied ages, followed by bees fed cucumber, clover, and then broad bean bee bread (Fig. 5c).
There were no significant differences in LPO values throughout the study period from 6 to 12 days (F(2,6) = 0.273, p = 0.770, F(2,6) = 2.111, p = 0.202, F(2,6) = 2.714, p = 0.145 and F(2,6) = 4.429, p = 0.066) in workers fed broad bean, clover, cucumber and fennel bee bread, respectively (Fig. 5d), whereas, LPO level increased gradually throughout the study from 6 to 12 days to reach maximum on day 12 (F(2,6) = 28.500, p = 0.001) in honey bee workers fed maize bee bread. In contrast, in workers fed sugar syrup only (control) LPO level decreased gradually throughout study period to reach its minimum on day 12 (F(2,6) = 30.333, p = 0.001) (Fig. 5d). Generally, LOP levels in control worker bees at all ages were significantly higher than bees fed fennel, maize, cucumber, clover, and broad bean bee bread (Fig. 5d).
In general, SOD and CAT activities, and GSH levels at all studied ages honeybee workers fed on maize and fennel bee bread were significantly higher followed by bees fed on clover, cucumber, broad bean bee bread, and then control bees. Moreover, LPO levels in honey bee workers fed broad bean and clover bee bread at all studied ages were significantly lower than in bees fed cucumber, fennel, and maize bee bread (Figs. 1, 3 and 5a, b, c, amp and d).
Canonical correlation analysis (CCA) showed that bee bread samples are separated into samples of maize, fennel, and cucumber in one group and samples of clover and broad bean in another group. In addition, both the protein content and antioxidants of bee bread had an impact on the protein content and antioxidant system of honeybee workers (Fig. 6). Results also revealed a positive relationship between protein content and antioxidants in bee bread and between protein and antioxidant system of honeybees, respectively. However there was a negative correlation between protein content and the antioxidants. Protein content of bee bread had higher impact on honey bees (bee protein: r = 0.796, p = 0.107; bee GSH: r = -0.784, p = 0.117; bee SOD: r = -0.796, p = 0.107; bee CAT: r = -0.727, p = 0.164 & bee LPO: r = -0.965, p = 0.008) followed by GSH (bee protein: r = -0.789, p = 0.113; bee GSH: r = 0.818, p = 0.091; bee SOD: r = 0.834, p = 0.079; bee CAT: r = 0.747, p = 0.147 & bee LPO: r = 0.977, p = 0.004), TAX (bee protein: r = -0.617, p = 0.268; bee GSH: r = 0.883, p = 0.047; bee SOD: r = 0.980, p = 0.003; bee CAT: r = 0.853, p = 0.066 & bee LPO: r = 0.753, p = 0.142) and then LPO (bee protein: r = -0.381, p = 0.527; bee GSH: r = 0.624, p = 0.260; bee SOD: r = 0.773, p = 0.126; bee CAT: r = 0.522, p = 0.367 & bee LPO: r = 0.718, p = 0.172).
Correlations between bee bread parameters [protein content, total antioxidant content (TAX), glutathione (GSH) and lipid peroxidation (LPO)] and honey bee worker parameters [protein content, superoxide dismutase (SOD), catalase (CAT), GSH & LPO] using Canonical Correlation Analysis (CCA). CCA showing that bee bread samples are separated to samples of maize, fennel and cucumber in one group and samples of clover and broad bean in another group
Discussion
The present study showed that the highest levels of total protein content were detected in broad bean, and clover bee bread followed by cucumber, fennel, and then maize bee bread. Pollen is the only source of protein that bees collect from nature. Bee bread is a fermented mixture of plant pollen, honey, and bee saliva that worker bees use as food for the larvae, and for young bees to produce royal jelly. Pollen collected by bees is mixed with a small amount of honey and saliva and packed into the cells of the honey comb, where it undergoes a chemical change to form a product called bee bread. During lactic fermentation, the chemical composition of bee pollen changes (Vásquez and Olofsson 2009). The bursting of pollen grains is of great importance for the easier assimilation of pollen by bees. Consequently, bee bread contains more vitamins and reduced sugars. Anđelković et al. (2012) reported that the content of crude protein was increased in bee bread (29.93%) when compared to pollen (23.97%). Kaškoniene et al. (2020) compared natural with “fermented” pollen to determine the different antioxidant potential of pollen and bee bread, showing that fermentation had a positive effect on antioxidant properties. The increase in biologically active compounds is assumed to be the consequence of the partial destruction of the pollen cell walls by bacteria added during fermentation, as occurs naturally in the fermentation of bee bread in the hive. Pollen from different plant species and, consequently, bee bread made from it, differ in their nutritional value. For instance, high quality pollen is produced by diverse clover species (Trifolium sp.), oilseed rape (Brassica napus), pear (Pyrus communis), almond (Prunus dulcis), Populus sp. or lupin (Lupinus angustifolius) (Schmidt et al. 1987; Pernal and Currie 2001; Somerville and Nicol 2006). Pollen of lower quality can come from sunflower (Helianthus annuus), blueberry (Vaccinium sp.), or Typha sp. (Cat-tail, Typhaceae) (Schmidt et al. 1987). Moreover, Radev (2018) reported that sunflower (Helianthus annuus) and maize (Zea mays) produce pollen with a rather low protein content, findings that agree with the results of the present study and with other previous studies (Schmidt et al. 1987; Pernal and Currie 2001). Similarly, maize pollen had a lower total protein content than that of clover (Kihder et al. 2013; Liolios et al. 2016). Also, Somerville and Nicol (2006) reported that the crude protein content of honeybee collected pollen of white clover, balansa clover, and faba beans (25.9, 25.3 & 24.4%, respectively) was higher than that of maize (14.9%). In contrast, for maize, Vaissiere and Vinson (1994) and Höcherl et al. (2012) found higher protein contents (23.9% and 26% per fresh weight, respectively). Indeed, Radev (2019) showed that the pollen protein content of clover (Trifolum repens, 21.5%) was higher than the protein content of bean (Vicia sp., 16.2%). Kaplan et al. (2016) examined the chemical composition of eight bee bread samples of different botanical origins. They reported that clover bee bread samples had the highest protein content (22.6–24.2%) and cotton appeared to have the lowest content of protein (14.8–15%). Another study found that the total protein content of bee bread samples ranged between 17.5% and 21.3% (Mayda et al. 2020; Bleha et al. 2019) reported that the protein contents in bee bread were ~ 14–20% in wet matter and ~ 15–28% in dry matter. Also, Zuluaga et al. (2015) found that bee bread protein content ranged from 19.1 to 27.3%. De grandi-Hoffman et al. (2013), on the other hand, compared bee bread made by Africanized and European honeybees (Apis mellifera), and discovered that protein concentration in bee bread was similar for both subspecies (577 µg/ml and 567 µg/ml, respectively). Bobis et al. (2017) reported that total protein content of bee bread from Apis mellifera and Apis dorsata situated between 16.86 and 19.19%. Bovi et al. (2020) showed that the percentage of crude protein in bee bread did not change with the seasons (% for autumn, winter, spring, and summer were 19.6, 17.5, 21.5 and 17.8, respectively).
Results of the present study confirmed our previous study that both broad bean and clover bee bread contain significantly higher total protein content than cucumber, fennel and maize bee bread (Ahmed et al. 2019). The protein content of investigated bee bread ranged from 12.60 to 17.73 mg/dl in maize and broad bean, respectively. Bakour et al. (2019) demonstrated that carbohydrates (74.82 g/100 g) followed by protein (19.96 g/100 g) were the main macronutrients in bee bread. Nutritional analysis of 576 bee bread samples, on the other hand, found that the main source of protein in the bread was 569.2 mg/g wet weight (Donkersley et al. 2014).
DS–PAGE Significant differences in protein fractionation were revealed between different diets. Protein fractionation from broad bean, clover and fennel bee bread revealed the presence of 6 fractions (31.07, 46.18, 51.64, 66, 97.5, & 125 kDa, 31.18, 40.72, 58.68, 92, 128.86, & 158.43 kDa and 24.21, 44.82, 55.37, 79, 119.5, & 145.57 kDa, respectively). Protein profiles from cucumber and maize bee bread showed 7 and 8 fractions (29.9, 41.86, 52.55, 53.48, 71, 94.75, & 134 kDa and 24.21, 28.5, 35.41, 39.59, 48.91, 64, 76, & 114 kDa, respectively). Omar (1989) used polyacrylamide disc electrophoreses to determine the proteinaceous fractions in pollen and bee bread, and reported that, pollen and bee bread showed 6 and 8 fractions, respectively. Using SDSPAGE, Nagai et al. (2004) studied the protein pattern of the water-soluble fraction of bee bread. They reported that bee bread fractionation showed the main protein band of about 50 kDa and the minor bands of ~ 90, 80, 37, 34, and 26 kDa. On the other hand, Treigytė et al. (2014) performed a comprehensive analysis of proteomes characteristic of pollen specific to different clover species (Trifolium pratense, T. alexandrinum and T. repens). They found that the proteins identified in pollen differed in quantity and quality between the three clover species. Proteomic analysis of red clover pollen identified abundant groups of proteins with a molecular weight of about 12, 15, 30, and 50 kDa (Čeksterytė et al. 2016). It is important to perform a comprehensive analysis of the protein profile characteristic of each type of bee bread. Future analysis is required in the separation and identification of the protein fractions in bee bread, in order to elucidate the differences between various samples and their impact on honeybee physiology.
The present study showed that TAX content, GSH and LPO levels were significantly higher in maize and fennel bee bread than in broad bean, clover, and cucumber bee bread. In addition, the TAX content was significantly higher in maize bee bread than in fennel bee bread. Contrary to this LPO level was significantly higher in fennel bee bread than in maize bee bread. Several publications have recorded that there is a significant antioxidant activity in pollen and bee bread, primarily depending on its botanical and geographical origin (Urcan et al. 2017; Martinello and Mutinelli 2021). Most of these studies have been conducted to evaluate the nutritional values and antioxidant capacities or activities. However, very few studies have addressed the antioxidant content of bee pollen or bee bread (Zhang et al. 2014; Aslmoshtaghi and Shahsavar 2015; Chantarudee et al. 2012) compared the free radical scavenging activity of corn pollen collected from Apis mellifera hive compared to floral corn pollen and they reported that the free radical scavenging activity of bee pollen derived from corn (Zea mays) was better than floral corn pollen. Also, Khider et al. (2013) found that maize pollen extract was more effective as an antioxidant than date, clover, or palm pollen.
Results of the present study showed that workers fed on sugar solution only (control) had significantly lower concentrations of protein content than the workers fed bee bread at all studied ages. Workers fed on broad bean, and clover bee breads had significantly higher concentrations of protein content than bees fed on fennel and maize bee breads. Ahmed et al. (2019) showed that the thorax weight, hypopharyngeal gland (HG) development, total number of wax-built cells, and longevity of bees fed on bee bread were significantly higher than for bees fed on sugar syrup alone. Moreover, they reported that the highest values of thorax weight, HG development, total number of wax-built cells, and longevity were recorded in bees fed on broad bean or clover bee bread compared to cucumber, fennel, and maize bee bread. Protein is probably one of the most important constituents of pollen, and is essential for adult honey bees and brood rearing. There is a positive correlation between the necessary amino acids and the amount of protein present in the pollen of one plant species. High protein contents often provide necessary amino acids in high concentrations (Pamminger et al. 2019). Therefore, many studies had shown that the pollen of various plant species and consequently bee bread contains different amounts of protein and, hence, they differ in their value to honey bees. Li et al. (2012) reported that protein content of newly emerged workers increased linearly as dietary protein increased. Dietary protein and amino acid concentrations influence some aspects of immune function in honey bees (Alaux et al. 2010; De Grandi-Hoffman et al. 2010; Di Pasquale et al. 2013) as well as learning and memory ability during their development (Wright et al. 2007, 2009). In addition, previous studies have shown that abiotic stressors, such as malnutrition, could decrease the activities and transcript levels of the antioxidant enzymes in honey bees. Li et al. (2014) showed that there was a clear effect of dietary protein level on antioxidant gene expression of honey bees. These antioxidant genes make enzymes that help break down ROS (Radyuk et al. 2001; Missirlis et al. 2003), and the mRNA levels of these genes have been shown to be linked to how well these enzymes work in bees (Collins et al. 2004).
GST, CAT, SOD, and GSH are the major ROS scavenging and antioxidant enzymes in honey bees; they play important roles in antioxidant defense in honey bees exposed to biotic and abiotic stressors (Weirich et al. 2002; Li et al. 2020; Tawfik et al. 2020). Results of the present study showed that levels of TAX, GSH and LPO in maize and fennel bee bread are high, which resulted in high activities of SOD and CAT and high levels of GSH and LPO in worker bees fed on maize and fennel bee bread. On the other hand, low levels of TAX, GSH and LPO in broad bean and clover bee bread resulted in low activities of SOD and CAT and low levels of GSH and LPO in worker bees fed on broad bean and clover bee bread. Thus, the antioxidant content in bee bread may exert a very subtle influence on honey bee physiology. Nagai et al. (2004) found that bee bread extracts have high antioxidant abilities and scavenging abilities against superoxide anion radical and hydroxyl radical. Borawska et al. (2013) studied the composition of ethanol extracts from three different samples of bee bread, and they found that the total antioxidant status consisting of all the antioxidants was present in bee bread samples. Also, in bee bread samples, Hryniewicka et al. (2016) identified lipophilic antioxidants such as α-tocopherol and coenzyme Q10, which play essential regulatory and metabolic functions in each cell of living organisms. Furthermore, bee bread has high bioactive properties, which can be expressed as antioxidant and/or antibacterial activity (Bobis et al. 2017; Di Pasquale et al. 2013) showed that the activities of GST, ALP (alkaline phosphatase) and PO (phenoloxidase) changed according to the pollen diets. To our knowledge, this study is the first analysis of antioxidant contents in bee bread, showing the relationship between bee bread antioxidant contents and antioxidant system in honeybees. The data gathered from this study provides new information regarding relationship between the antioxidant contents in bee bread and the antioxidant system of honeybee workers.
The nutritional status of a colony has a marked effect on the growth and development of individual bees, their lifespan, foraging capacity, brood rearing and resistance to diseases (Brodschneider and Crailsheim 2010; De Grandi-Hoffman and Chen 2015; Glavinic et al. 2017; Ahmed et al. 2019). Without proper nutrition, colonies may experience stress that negatively influences other important health factors (Brodschneider and Crailsheim 2010). Poor nutrition results in a weakened immune system in honey bees (Alaux et al. 2010). Also, nutritional deficiency predisposes honey bees to higher susceptibility to disease and sensitivity to pesticide exposure (Eischen et al. 2008; Foley et al. 2012; Di Pasquale et al. 2013; Crone and Grozinger 2021). Bee bread is characterized by a higher nutritional value than pollen and a richer chemical composition (Anđelković et al. 2012; Borycka et al. 2015). Further, bee bread is more biologically active and easily digestible due to the high content of easily digestible sugars, fat, mineral components, and a higher proportion of free amino acids when compared to pollen (Nagai et al. 2004; De Grandi-Hoffman et al. 2013). Overall, the data showed that the investigated bee bread differed in their total protein content, protein quality and antioxidant content. The results of this study showed how chemical composition of bee bread (protein quantity and quality and antioxidant content) impacts the protein content and antioxidant system of honey bee workers. Broad bean and clover bee bread are characterized by a higher nutritional value than cucumber, fennel, and maize bee bread. This large variability in chemical composition causes an equally large variability in nutritional value for bees. In conclusion, this study reports that the quality bee bread influences bee physiology. Therefore, the consumption of protein (quantity and quality) and antioxidants influences honey bee worker physiology (protein content and antioxidant system), and certain bee bread types are more detrimental than others. Therefore, diets intended for feeding should be optimized for the nutritional requirements of honey bee workers. However, additional studies on other bee bread are important to better understand the relationships between protein and antioxidant content in bee bread and the protein content and antioxidant system of honeybees. Further laboratory experiments will be needed to establish such a causal link.
References
Ahmed ZH, Tawfik AI, Abdel-Rahman MF, Moustafa AM (2019) Nutritional value and physiological effects of some proteinaceous diets on honey bee workers (Apis mellifera L.). Bee World 97(1):26–31. https://doi.org/10.1080/0005772X.2019.1672983
Alaux C, Ducloz F, Crauser D, Le Conte Y (2010) Diet effects on honeybee immunocompetence. Biol Lett 6(4):562–565. https://doi.org/10.1098/rsbl.2009.0986PMID:20089536
Almaraz-Abarca N, Campos MG, Ávila-Reyes JA, Naranjo-Jiménez N, Corral JH, González-Valdez LS (2007) Antioxidant activity of polyphenolic extract of monofloral honeybee-collected pollen from mesquite (Prosopis juliflora, Leguminosae). J Food Compos Anal 20(2):119–124
Anđelković B, Jevtić G, Mladenović M, Marković J, Petrović M, Nedić N (2012) Quality of pollen and honey bee bread collected in spring.J Hyg Engin Des275–277
Ares AM, Valverde S, Bernal JL, Nozal MJ, Bernal J (2018) Extraction and determination of bioactive compounds from bee pollen. J Pharm Biomed Anal 147:110–124
Aslmoshtaghi E, Shahsavar RS (2015) Study on some chemical composition of five olive pollens. Agric Consp Sci 80(2):119–123
Bakoura M, Fernandesb A, Barrosb L, Sokovicc M, Ferreirab ICFR, lyoussia B (2019) Bee bread as a functional product: Chemical composition and bioactive properties. LWT - Food Sci Technol 109:276–282. https://doi.org/10.1016/j.lwt.2019.02.008
Baltrušaitytė V, Venskutonis PR, Čeksterytė V (2007) Radical scavenging activity of different floral origin honey and beebread phenolic extracts. Food Chem 101(2):502–514. 10.1016/j. foodchem.2006.02.007
Beutler E, Duron O, Kelly BM (1963) Improved method for the determination of blood glutathione. Lab Clin Med 61:882–888
Bleha R, Shevtsova T, Kružik V, Šorpilová T, Saloň I, Erban E, Brindza J, Brovarskyi V, Sinica A (2019) Bee breads from two regions of Eastern Ukraine: composition, physical properties and biological activities. Czech J Food Sci 37:9–20
Bobis O, Dezmirean D, Marghitas LA, Bonta V, Margaona R, Pasca C, Urcan A, Singh Bandhar P (2017) Bee bread from Apis mellifera and Apis dorsata. Comparative Chemical Composition and Bioactivity. Bull UASVM Anim Sci Biotechnol 74(1): 43–50
Borawska MH, Markiewicz-Zukowska R, Naliwajko SK, Bartosiuk E, Moskwa J, Isidorov VA, Soroczyńska J (2013) Chemical composition and antioxidant activity of beebread, and its influence on the glioblastoma cell line (U87MG). J Apic Sci 57(2):147–157
Borycka K, Grabek-Lejko D, Kasprzyk I (2015) Antioxidant and antibacterial properties of commercial bee pollen products. J Apic Res 54(5):491–502. DOI: https://doi.org/10.1080/00218839.2016.1185309. https://doi.org/10.1080/00218839.2016.1185309
Bovi TdS, Caeiro A, Santos SAAd, Zaluski R, Shinohara AJ, Lima GPP, Campos MdGR, Junior LAJ, Orsi RdO (2020) Seasonal variation of flavonoid content in bee bread: Potential impact on hypopharyngeal gland development in Apis mellifera honey bees. J Apic Res 59(2):170–177. DOI:https://doi.org/10.1080/00218839.2019.1702321. https://doi.org/10.1080/00218839.2019.1702321
Brodschneider R, Crailsheim K (2010) Nutrition and health in honey bees. Apidologie 41(3):278–294. https://doi.org/10.1051/apido/2010012
Čeksteryte V, Navakauskiene R, Treigyte G, Jansen E, Kurtinaitiene B, Dabkevičiene G, Balžekas J (2016) Fatty acid profiles of monofloral clover beebread and pollen and proteomics of red clover (Trifolium pratense) pollen. Biosci Biotechnol Biochem 80:2100–2108
Chantarudee A, Phuwapraisirisan P, Kimura K, Okuyama M, Mori H, Kimura A, Chanchao C (2012) Chemical constituents and free radical scavenging activity of corn pollen collected from Apis mellifera hives compared to floral corn pollen at Nan, Thailand. BMC Complement Altern Med 12:1–12
Clément M-V, Pervaiz S (2001) Intracellular superoxide and hydrogen peroxide concentrations: a critical balance that determines survival or death. Redox Rep 6(4):211–214. DOI: https://doi.org/10.1179/135100001101536346. https://doi.org/10.1179/135100001101536346
Collins AM, Williams V, Evans JD (2004) Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Mol Biol 13(2):141–146
Corby-Harris V, Snyder L, Meador C, Ayotte T (2018) ) Honey bee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS ONE 13(1):e0191050. https://doi.org/10.1371/journal.pone.0191050
Corona M, Robinson GE (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mol Biol 15(5):687–701
Crone MK, Grozinger CM (2021) Pollen protein and lipid content influence resilience to insecticides in honey bees (Apis mellifera). J Exp Biol 224:jeb242040. Doi:https://doi.org/10.1242/jeb.242040
De Grandi-Hoffman G, Chen Y (2015) Nutrition, immunity and viral infections in honey bees. Curr Opin Insect Sci 10:170–176
De Grandi-Hoffman G, Chen E, Huang MH (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J Insect Physiol 56:1184–1191
De Grandi-Hoffman G, Eckholm BJ, Huang MH (2013) A comparison of bee bread made by Africaized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie 44(1):52–63
Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet J-L, Alaux C (2013) Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 8(8):e72016. Doi:https://doi.org/10.1371/journal.pone.0072016
Donkersley P, Rhodes G, Pickup RW, Jones KC, Wilson K (2014) Honeybee nutrition is linked to landscape composition. Ecol Evol 4:4195–4206. Doi: https://doi.org/10.1002/ece3.1293
Eischen FA, Grahamj RH, Riveraj R (2008) Overwintering nutritionally stressed honey bee colonies infested with Varroa destructor. Am Bee J 148:555–556
Foley K, Fazio G, Jensen AB, Hughes WHO (2012) Nutritional limitation and resistance to opportunistic Aspergillus parasites in honeybee larvae. J Invertebr Pathol 111:68–73. Doi:https://doi.org/10.1016/j.jip.2012.06.006. PubMed: 22750047
Glavinic U, Stankovic B, Draskovic V, Stevanovic J, Petrovic T, Lakic N, Stanimirovic Z (2017) Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 12(11):e0187726. https://doi.org/10.1371/journal.pone.0187726
Gregorc A, Alburaki M, Rinderer N, Sampson B, Kinight PR, Karim S, Adamczyk J (2018) Effects of coumaphos and imidacloprid on honey bee (Hymenoptera: Apidae) lifespan and antioxidant gene regulations in laboratory experiments. Sci Rep 8:1–13. https://doi.org/10.1038/s41598-018-33348-4
Höcherl N, Siede R, Illies I, Gatschenberger H, Tautz J (2012) Evaluation of the nutritive value of maize for honey bees. J Insect Physiol 58:278–285
Hryniewicka M, Karpinska A, Kijewska M, Turkowicz MJ, Karpinska J (2016) LC/MS/MS analysis of α-tocopherol and coenzyme Q10 content in lyophilized royal jelly, beebread and drone homogenate. J Mass Spectrometry 51(11):1023–1029
Kaplan M, Karaoglu O, Eroglu N, Silici S (2016) Fatty acids and proximate composition of bee bread. Food Technol Biotechnol 54(4):497–504. Doi:https://doi.org/10.17113/ftb.54.04.16.4635
Kaškoniene V, Adaškeviciute V, Kaškonas P, Mickiene R, Maruška A (2020) Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci 34:100532. https://doi.org/10.1016/j.fbio.2020.100532
Khider M, Elbanna K, Mahmoud A, Owayss AA (2013) Egyptian honeybee pollen as antimicrobial, antioxidant agents, and dietary food supplements. Food Sci Biotechnol 22(5):1461–1469
Kieliszek M, Piwowarek K, Kot AM, Błażejak S, Chlebowska-Śmigiel A, Wolska I (2018) ) Pollen and bee bread as new health-oriented products: A review. Trends Food Sci Technol 71:170–180. DOI https://doi.org/10.1016/j.tifs.2017.10.021. https://doi.org/10.1016/j.tifs.2017.10.021
Kojić DK, Purać JS, Nikolić TV, Orčić SM, Vujanović D, Ilijević K, Vukašinović EL, Blagojević D (2019) Oxidative stress and the activity of antioxidative defense enzymes in overwintering honey bees. Entomol Gen 39:33–44. https://doi.org/10.1127/entomologia/2019/0743
Komosinska–Vassev K, Olczyk P, Kaźmierczak J, Mencner L, Olczyk K (2015) Bee pollen: Chemical composition and therapeutic application. Evidence–based Complement Altern Med 6. https://doi.org/10.1155/2015/297425 Article ID 297425
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature 227:680–685
LeBlanc BW, Davis OK, Boue S, DeLucca A, Deeby T (2009) Antioxidant activity of Sonoran Desert bee pollen. Food Chem 115:1299–1305
Li CC, Xu BH, Wang YX, Feng Q, Yang W (2012) Effects of dietary crude protein levels on development, antioxidant status, and total midgut protease activity of honey bee (Apis mellifera ligustica). Apidologie 43:576–586
Li CC, Xu BH, Wang YX, Yang ZB, Yang WR (2014) Protein content in larval diet affects adult longevity and antioxidant gene expression in honey bee workers. Entomol Exp et Appl 151(1):19–26. Doi:https://doi.org/10.1111/eea.12167
Li Z, Hou M, Qiu Y, Zhao B, Nie H, Su S (2020) Changes in antioxidant enzymes activity and metabolomic profiles in the guts of honey bee (Apis mellifera) larvae infected with Ascosphaera apis. Insects 11:1–12. https://doi.org/10.3390/insects11070419
Liolios V, Tananaki C, Dimou M, Kanelis D, Goras G, Karazafiris E, Thrasyvoulou A (2016) Ranking pollen from bee plants according to their protein contribution to honey bees. J Apic Res DOI. https://doi.org/10.1080/00218839.2016.1173353. http://dx.doi.org/10.1080/00218839.2016.1173353
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666. https://doi.org/10.1016/S0025-326X(01)00060-1
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. Biol Chem 41:1863–1870
Luck H (1963) Catalasein. In: Bergmeyer H-U (ed) Methods of enzymatic analysis. Academic Press, pp 885–888
Mărgăoan R, Strant M, Varadi A, Topal E, Yücel B, Cornea-Cipcigan M, Campos MG, Vodnar DC (2019) Bee collected pollen and bee bread: Bioactive constituents and health benefits. Antioxid 8:568. Doi:https://doi.org/10.3390/antiox8120568
Margotta JW, Roberts SP, Elekonich MM (2018) Effects of flight activity and age on oxidative damage in the honey bee, Apis mellifera. J Exp Biol. https://doi.org/10.1242/jeb.183228. 221;jeb183228
Martinello M, Mutinelli F (2021) Antioxidant Activity in Bee Products: A Review. Antioxid 10:71. https://doi.org/10.3390/antiox10010071
Mayda N, Ozkok A, Bayram NE, Gerçek YC, Sorkun K (2020) Bee bread and bee pollen of different plant sources: determination of phenolic content, antioxidant activity, fatty acid and element profiles. J Food Meas Charact 14:1795–1809
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. Biol Chem 247:3170–3175
Missirlis F, Rahlfs S, Dimopoulos N, Bauer H, Becker K, Hilliker A, Phillips JP, Jäckle1 H (2003) A putative glutathione peroxidase of Drosophila encodes a thioredoxin peroxidase that provides resistance against oxidative stress but fails to complement a lack of catalase activity. Biol Chem 384:463–472
Mucci CA, Ramirez L, Giffoni RS, Lamattina L (2021) Cold stress induces specific antioxidant responses in honey bee brood. Apidologie 52:596–607. DOI: https://doi.org/10.1007/s13592-021-00846-w
Nagai T, Nagashima T, Myoda T, Inoue R (2004) Preparation and functional properties of extracts from beebread. Mol Nut Food Res 48(3):226–229. Doi:https://doi.org/10.1002/food.200300421
Nicolson SW, Thornburg RW (2007) Nectar chemistry. In: Nicolson SW, Nepi M, Pacini E (eds) Nectar and Nectaries. Dordrecht, Netherland, Springer, pp 215–264
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Omar MOM (1989) The protein quality of bee bread during active season in Assiut area. Assiut J Agric Sci 20(3):339–350
Pamminger T, Becker R, Himmelreich S, Schneider CW, Bergtold M (2019) Pollen report: quantitative review of pollen crude protein concentrations offered by bee pollinated flowers in agricultural and non-agricultural landscapes. PeerJ 7:e7394. https://doi.org/10.7717/peerj.7394
Pernal SP, Currie RW (2001) The influence of pollen quality on foraging behaviour in honey bees (Apis mellifera L.). Behav Ecol Sociobiol 51(1):53–68
Radev Z (2018) Variety in protein content of pollen from 50 plants from Bulgaria. Bee World 95(3):81–83. Doi:https://doi.org/10.1080/0005772X.2018.1486276
Radev Z (2019) Pollen protein content from different regions in Bulgaria suggests low variability. Bee World 96(4):108–110. DOI: https://doi.org/10.1080/0005772X.2019.1654593. https://doi.org/10.1080/0005772X.2019.1654593
Radyuk SN, Klichko VI, Spinola B, Sohal RS, Orr WC (2001) The peroxiredoxin gene family in Drosophila melanogaster. Free Radic Biol Med 31:1000–1009
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Schmidt JO, Thoenes SC, Levin MD (1987) Survival of honeybees, Apis mellifera (Hymenoptera: Apidae) fed various pollen sources. Ann Entomol Soc America 80:176–183
Somerville DC, Nicol HI (2006) Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Australian J Exp Agric 46:141–149
Tasei JN, Aupinel P (2008) Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39(4):397–409
Tawfik AI, Ahmed ZH, Abdel-Rahman MF, Moustafa AM (2020) Influence of winter feeding on colony development and the antioxidant system of the honey bee, Apis mellifera. J Apic Res 1–12. https://doi.org/10.1080/00218839.2020.1752456
Telleria MC, Salgado-Laurenti CR, Marinozzi LA, Apóstolo N, Pérez B (2019) Protein content of pollen of Asteraceae collected by honey bees. Bee World 964:104–107. DOI: https://doi.org/10.1080/0005772X.2019.1634958. https://doi.org/10.1080/0005772X.2019.1634958
Treigytė G, Zaikova I, Matuzevičius D, Ceksterytė V, Dabkevičienė G, kurtinaitienė B, Navakauskienė R (2014) Comparative proteomic analysis of pollen of Trifolium pratense, T. alexandrinum and T. repens. Zemdirbyste-Agriculture 101:453–460. DOI https://doi.org/10.13080/z-a.2014.101.058
Urcan A, Marghitas L, Dezmirean DS, Bobis O, Bonta V, Muresan C, Margaoan R (2017) Chemical composition and biological activities of beebread – Review. Bull University Agric Sci Vet Cluj-Napoca. Anim Sci Biotechnol 74:6–14. Doi:https://doi.org/10.15835/buasvmcn-asb:12646
Vaissière BE, Vinson SB (1994) Pollen morphology and its collection effectiveness by honey bees, Apis mellifera L. (Hymenoptera: Apidae), with special reference to upland cotton, Gossypium hirsutum L. (Malvaceae). Grana 33: 128–138
Vásquez A, Olofsson TC (2009) The lactic acid bacteria involved in the production of bee pollen and bee bread. J Apic Res Bee World 48(3):189–195. https://doi.org/10.3896/IBRA.1.48.3.07
Weglinska M, Szostak R, Kita A, Nems A, Mazurek S (2020) Determination of nutritional parameters of bee pollen by Raman and infrared spectroscopy. Talanta 212:120790. https://doi.org/10.1016/j.talanta.2020.120790
Weirich GF, Collins AM, Williams VP (2002) Antioxidant enzymes in the honey bee, Apis mellifera. Apidologie 33(1):3–14
Williams GR, Alaux C, Costa C, Csaki T, Doublet V, Eisenhardt D, Brodschneider R (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J Apic Res 52(1):1–36. Doi:https://doi.org/10.3896/IBRA.1.52.1.04
Wright GA, Choudhary AF, Bentley MA (2009) Reward quality influences the development of learned olfactory biases in honeybees. Proc R Soc B Biol Sci 276:2597–2604. https://doi.org/10.1098/rspb.2009.0040
Wright GA, Mustard JA, Kottcamp SM, Smith BH (2007) Olfactory memory formation and the influence of reward pathway during appetitive learning by honey bees. J Exp Biol 210:4024–4033
Wright GA, Nicolson SW, Shafir S (2018) Nutritional physiology and ecology of honey bees. Annu Rev Entomol 63:327–344. ento.annualreviews.orghttps://doi.org/10.1146/annurev-ento-020117-043423
Zhang XY, Piao Y-L, Cui S-Y, Lee Y-Ill (2014) Determination of reduced glutathione, cysteine and total thiols in pine pollen powder by in situ derivatization. Microchem J 112:1–6
Žilić S, Vancetovic J, Jankovic M, Maksimovic V (2014) Chemical composition, bioactive compounds, antioxidant capacity and stability of floral maize (Zea mays L.) pollen. J Funct Foods 10:65–74
Zuluaga CM, Serratob JC, Quicazana MC (2015) Chemical, nutritional and bioactive characterization of Colombian bee-bread. Chem Eng 43:175–180
Acknowledgements
The authors wish to thank the anonymous referees for constructive comments on manuscript. The manuscript was improved by critical readings from Prof. K. Hoffmann, Animal Ecology 1, Bayreuth University, Germany. Thanks to Prof. K. El Wakel, Assiut University, Egypt, for helping with the statistical analysis.
Funding
No funds, grants, or other support was received.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tawfik, A.I., Ahmed, Z.H., Abdel-Rahman, M.F. et al. Effect of some bee bread quality on protein content and antioxidant system of honeybee workers. Int J Trop Insect Sci 43, 93–105 (2023). https://doi.org/10.1007/s42690-022-00888-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00888-2