Abstract
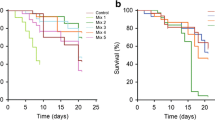
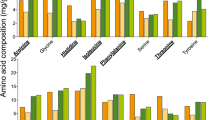
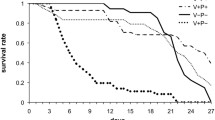
The effects of different levels of dietary crude protein on the development, antioxidant enzymatic activity, and total midgut protease activity of honey bees were investigated in the study. A total of 30 colonies of bees with sister queens were used in the test. Dietary treatments were pure rape pollen (Control) and pollen substitutes (PS) with crude protein (CP) levels at 15%, 20%, 25%, 30%, and 35% (designated as PS15, PS20, PS25, PS30, and PS35), respectively. We compared the effects of these diets on honey bees by measuring diet consumption, bee development (egg hatch, pupation success, and pupal weight), and the protein content of emergent adult bees, their antioxidant status and the activity of their midgut digestive proteases. Bees consumed significantly more (P < 0.001) natural pollen than any PS, and bees fed PS had similar diet consumption over the entire experimental period. However, the total protein intake was varied (P < 0.05). PS with a protein level about 30% was recognized as excellent quality diet for maximum body weight, highest protein content and antioxidant enzymatic activity, and PS with a protein rate about 35% exerted the greatest effect on increasing percentage of hatch and percentage of pupation. All these results indicate that PS appeared to be a valuable proteinaceous food approximated to the pollen, and 30∼35% of dietary protein level was optimal to maintain the colony development.



Similar content being viewed by others
References
Ahmad, S., Duval, D.L., Weinhold, L.C., Pardini, R.S. (1991) Cabbage looper antioxidant enzymes: tissue specificity. Insect Biochem. 21, 563–572
Alaux, C., Ducloz, F., Crauser, D., Le Conte, Y. (2010) Diet effects on honeybee immunocompetence. Biol. Lett. 6, 562–565
Arking, R., Burde, V., Graves, K., Hari, R., Feldman, E., Zeevi, A. (2000) Forward and reverse selection for longevity in Drosophila is characterized by alternation of antioxidant gene expression and oxidative damage patterns. Exp. Gerontol. 35, 167–185
Boch, R. (1982) Relative attractiveness of different pollens to honeybees when foraging in a flight room and when fed in the hive. J. Apic. Res. 21, 104–106
Burgess, E.P.J., Malone, L.A., Christeller, J.T. (1996) Effects of two proteinase inhibitors on the digestive enzyme. J. Insect Physiol. 42, 823–828
Campbell, F.L. (1929) The detection and estimation of insect chitin; and the relation of "chitinization" to hardness and pigmentation of the cuticula of the American cockroach, P. americana. Ann. Entomol. Soc. Am. 22, 401–440
Chen, S.L. (2001) The apicultural science in china. China Agricultural Press, Beijing
Cremonez, T.M., De Jong, D., Bitondi, M.M.G. (1998) Quantification of hemolymph protein as a fast method for testing protein diets for honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 91, 1284–1289
De Jong, D., da Silva, E.J., Kevan, P.G., Atkinson, J.L. (2009) Pollen substitutes increase honey bee haemolymph protein levels as much as or more than does pollen. J. Apic. Res. 48, 34–37
DeGrandi-Hoffman, G., Wardell, G., Ahumada-Segura, F., Rinderer, T., Danka, R., Pettis, J. (2008) Comparisons of pollen substitute diets for honey bees: consumption rates by colonies and effects on brood and adult populations. J. Apic. Res. 47, 265–270
DeGrandi-Hoffman, G., Chen, Y.P., Huang, E., Huang, M.H. (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J. Insect Physiol. 56, 1184–1191
Dietz, A. (1969) Initiation of pollen consumption and pollen movement through the alimentary canal of newly emerged honeybees. Ann. Entomol. Soc. Am. 62, 43–46
Dobson, H.E.M. (1988) Survey of pollen and pollenkitt lipids-chemical cues to flower visitors. Am. J. Bot. 75, 170–182
Doull, K.M. (1973) Relationship between pollen, brood rearing and consumption of pollen supplements by honeybees. Apidologie 4, 285–293
Duff, S.R., Furgala, B. (1986) Pollen trapping honey bee colonies in Minnesota: part II: effect on foraging activity, honey production, honey moisture content, and nitrogen content of adult workers. Am. Bee J. 126, 755–758
Felton, G.W., Summers, C.B. (1995) Antioxidant systems in insects. Arch. Insect Biochem. Physiol. 29, 187–197
Flores, J.M., Gutiérrez, I., Espejo, R. (2005) The role of pollen in chalkbrood disease in Apis mellifera: transmission and predisposing conditions. Mycologia 97, 1171–1176
Gilliam, M., Taber, S., Richardson, G. (1983) Hygienic behavior of honey bee in relation to chalkbrood disease. Apidologie 14, 29–39
Hagedorn, H.H., Moeller, F.E. (1967) The rate of pollen consumption by newly emerged honeybees. J. Apic. Res. 6, 159–162
Haydak, M.H. (1970) Honey bee nutrition. Ann. Rev. Entomol. 15, 143–156
Herbert, E.W., Shimanuki, H. (1982) Effect of population density and available diet on the rate of brood rearing by honey bees offered a pollen substitute. Apidologie 13, 21–28
Herbert, E.W., Shimanuki, H., Caron, D. (1977) Optimum protein levels required by honey bees (Hymenoptera: Apidae) to initiate and maintain brood rearing. Apidologie 8, 141–146
Hornitzky, M. (2010) Honey bee diseases, Australia and New Zealand Standard Diagnostic Procedure 2003. Available at: www.scahls.org.au/procedures/anzsdp
Jay, S.C. (1963) The development of honeybees in their cells. J. Apic. Res. 2, 117–134
Jay, S.C. (1964) Starvation studied of larval honey bees. Can. J. Zool. 42, 455–462
Joanisse, D.R., Storey, K.B. (1996) Oxidative stress and antioxidants in overwintering larvae of cold-hardy goldenrod gall insects. J. Exp. Biol. 199, 1483–1491
Knox, D.A., Shimanuki, H., Herbert, E.W. (1971) Diet and the longevity of adult honey bees. J. Econ. Entomol. 64, 1415–1416
Levin, M.D., Haydak, M.H. (1951) Seasonal variation in weight and ovarian development in the worker honeybee. J. Econ. Entomol. 44, 54–57
Lowry, O., Rosebrough, N., Farr, A., Randall, R. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 365–275
Malone, L.A., Burgess, E.P.J., Christeller, J.T., Gatehouse, H.S. (1998) In vivo responses of honey bee midgut proteases to two protease inhibitors from potato. J. Insect Physiol. 44, 141–147
Malone, L.A., Burgess, E.P.J., Stefanovic, D., Gatehouse, H.S. (2000) Effects of four protease inhibitors on the survival of worker bumblebees (Bombus terrestris L.). Apidologie 31, 25–38
Mattila, H.R., Otis, G.W. (2006) Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 99, 604–613
Michiels, C., Raes, M., Toussaint, O., Remacle, J. (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radical Biol. Med. 17, 235–248
Moritz, B., Crailsheim, K. (1987) Physiology of protein digestion in the midgut of the honeybee (Apis mellifera L.). J. Insect Physiol 33, 923–931
Nguyen, V.N. (1999) Effect of protein nutrition and pollen supplementation of honeybee, Apis mellifera L. colonies on characteristics of drones with particular reference to sexual maturity. Aust. Beekeeper (Mar) 101, 374–376
Oyanagui, Y. (1984) Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 142, 290–296
Pernal, S.F., Currie, R.W. (2000) Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31, 387–409
Phillips, J.P., Campbell, S.D., Michaud, D., Charbonneau, M., Hilliker, A.J. (1989) Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. (USA) 86, 2761–2765
Placer, Z.A., Cushman, L.L., Johnson, B.C. (1966) Estimation of production of lipid peroxidation, malindialdehyde in biochemical system. Anal. Biochem. 16, 359–367
Ribeiro, M. (1994) Growth in bumble bee larvae: relation between development time, mass, and amount of pollen ingested. Can. J. Zool. 72, 1978–1985
Rothenbuhler, W. (1964) Behavior genetics of nest cleaning in honey bees. I. Response of four inbred lines to disease-killed brood. Anim. Behav. 12, 578–583
Roulston, T.H., Cane, J.H. (2002) The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16, 49–65
SAS Institute. 2003. SAS/STAT User’s Guide: version 9.1th edn
Schäfer, M.O., Dietemann, V., Pirk, C.W.W., Neumann, P., Crewe, R.M., Hepburn, H.R., Tautz, J., Crailsheim, K. (2006) Individual versus social pathway to honeybee worker reproduction (Apis mellifera): pollen or jelly as protein source for oogenesis. J. Comp. Physiol. A 192, 761–768
Schmickl, T., Crailsheim, K. (2001) Cannibalism and early capping: strategy of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol. A 187, 541–547
Schmickl, T., Blaschon, B., Gurmann, B., Crailsheim, K. (2003) Collective and individual nursing investment in the queen and in young and old honeybee larvae during foraging and non-foraging period. Insect Soc. 50, 174–184
Schmidt, J.O., Johnson, B.E. (1984) Pollen feeding preference of Apis mellifera, a polylectic bee. Southwest. Entomol. 9, 41–47
Schmidt, J.O., Thoenes, S.C., Levin, M.D. (1987) Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Ann. Entomol. Soc. Am. 80, 176–183
Schmidt, L.S., Schmidt, J.O., Hima, R., Wang, W.Y., Xu, L.G. (1995) Feeding preference and survival of young worker honey bees (Hymenoptera: Apidae) fed rape, sesame, and sunflower pollen. J. Econ. Entomol. 88, 1591–1595
Somerville, D. (2000) Honey bee nutrition and supplementary feeding, Agnote DAI/178, NSW Agriculture
Somerville, D. (2005) Fat bees skinny bees—a manual on honey bee nutrition for beekeepers. Publication no. 05/054. A report for the Rural Industries Research and Development Corporation, Barton
Somerville, D.C., Nicol, H.I. (2006) Crude protein and amino acid composition of honey bee-collected pollen pellets from south-east Australia and a note on laboratory disparity. Aust. J. Exp. Agr. 46, 141–149
Spivak, M., Reuter, G.S. (2001) Resistance to American foulbrood diseases by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 32, 555–565
Stanley, R.G., Linskens, H.F. (1974) Pollen: biology, biochemistry, management. Springer, Berlin
Sumida, S., Tanaka, K., Kitao, H., Nakadomo, F. (1989) Exercise induced lipid peroxidation and leakage of enzyme before and after vitamin E supplementation. Int. J. Biochem. 21, 835–838
Szymaś, B., Jędruszuk, A. (2003) The influence of different diets on haemocytes of adult worker honey bees, Apis mellifera. Apidologie 34, 97–102
Wigglesworth, V.B. (1933) The physiology of the cuticle and of ecdysis in Rhodnius prolixus (Triatomidae, Hemiptera); with special reference to the function of the oenocytes and of the dermal glands. Quart. J. Microsc. Sci. 76, 269–318
Winston, M.L. (1987) The biology of the honey bee. Harvard University Press, Cambridge
Acknowledgments
We thank staff apiarist G. L. Zhang and students F. Liu, B. L. Zheng, Z. Jiao, G. Y. Wang, Y. J. Li, Y. Wang, L. T. Ma, Z. F. Wu, Y. D. Zhang, G. Zhang, and X. B. Hang for assistance with data collection. We express our cordial thanks to Y. Wang and P. Liu for the linguistic correction of our paper. This research was financially supported by the earmarked fund for Modern Agro-industry Technology Research System (CARS-45) and Special Fund for Agro-scientific Research in the Public Interest (no. 200903006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Klaus Hartfelder
Effets de différents niveaux de protéines d’origine alimentaire sur le développement, le statut antioxidant et l’activité protéasique de l’intestin moyen des abeilles ( Apis mellifera ligustica )
Apis mellifera ligustica / protéine d’origine alimentaire / développement/antioxydant / activité protéasique / intestin moyen
Effekte des Gesamptproteingehalts in der Nahrung auf die Entwicklung, den Antioxidanz-Status und die Gesamtproteaseaktivität im Mitteldarm von Honigbienen ( Apis mellifera ligustica )
Apis mellifera ligustica / Nahrungsgesamtproteingehalt / Entwicklung / Antioxidanz-Status / Mitteldarmgesamtproteaseaktivität
Rights and permissions
About this article
Cite this article
Li, C., Xu, B., Wang, Y. et al. Effects of dietary crude protein levels on development, antioxidant status, and total midgut protease activity of honey bee (Apis mellifera ligustica). Apidologie 43, 576–586 (2012). https://doi.org/10.1007/s13592-012-0126-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-012-0126-0