Abstract
Biodiesel (BD) is one of the efficient alternative fuels for diesel engines (DE) which can be employed sans any modifications. The present study is focused on the extraction of BD from a lemongrass plant and analyzing combustion, efficiency, and emission characteristics of the DE by adding NPs at different concentrations to reduce both hydrocarbon, carbon monoxide, and NOx emissions simultaneously from the DE. The fuel samples were prepared by adding different dosages of zinc oxide nanoparticles (ZnO NPs) with neat lemongrass biodiesel (LGB) such as 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm per liter. From the results, it is found that the properties of BD were improved by the addition of ZnO NPs and it increased oxygen concentration in the sample resulting in better combustion and lower exhaust pollutants. The DE tested with the LGB + 150 ppm sample has registered maximum brake thermal efficiency (BTE) and lower specific fuel combustion (SFC) for all loading conditions compared to other samples. The value of heat release rate (HRR) and in-cylinder pressure are higher for LGB + 150 ppm due to its specific properties compared to other LGB blends. The presence of ZnO NPs in LGB has reduced harmful emissions from the DE such as carbon monoxide (CO), hydrocarbon (HC), oxides of nitrogen (NOx), and smoke by 4.01%, 5.56%, and 19.01%, when compared to neat LGB.
Article Highlights
-
The addition of ZnO NPs improves lemongrass biodiesel combustion, boosting engine efficiency.
-
Nano-biodiesel blend achieves superior BTE and lower fuel consumption.
-
ZnO NPs in LGB decrease CO, HC, NOx, and smoke emissions in diesel engines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Compression ignition engines (CI) have dominated the automobile sector for more than a decade due to their high thermal efficiency and energy density when compared to spark ignition engines (SI). However, the CI engines are still suffering from harmful pollutants like Oxides of nitrogen (NOx), Carbon monoxide (CO), Hydrocarbon (HC), and Particulates (PM) due to poor combustion behavior compared to the SI engine [1, 2]. These exhaust pollutants are further increasing while using biodiesel (BD) as a fuel in a DE due to more heterogeneity of fuel/air mixture [3, 4]. In addition to that, the cost of petroleum products is ever-increasing due to ever-increasing use of automobile. To manage the fuel crisis in the future, the research communities are seeking suitable alternative fuels for DEs [5]. Biodiesel and hydrogen are the proven alternative fuels for DEs that can be derived from various biowastes employing the transesterification process [6,7,8]. The properties of BD are nearer to the properties of fossil fuel except for viscosity, self-ignition temperature, and heating value which reduce the overall combustion efficiency of the DE. At the same time, the BD has the advantages of high cetane number and oxygen concentration compared to petroleum diesel [9,10,11,12]. Baweja et al. tested mustard oil BD and its diesel blends in a DE under different loading conditions and analyzed engine characteristics. The author concluded that the BD is utilized efficiently in a DE with lower exhaust pollutants such as CO and HC, since it contains more oxygen concentration and further improves in-cylinder temperature that causes more NOx formation in the exhaust [13].
Aydin et al. [14] have done similar work on the DE with safflower oil which is extracted via a transesterification process. The extracted fuels were blended with petroleum diesel, methyl proxitol, and ethyl proxitol at different concentrations (50%, 10%, 10%) for effective utilization of BD in a DE. The result revealed that the BD-fueled engine operated with lower SFC and BTE owing to lower calorific value (LCV) and high viscosity. A higher level of NOx emissions due to longer combustion duration was observed. Shrivastava and Verma [15] conducted an experimental study on a DE with roselle oil BD under different injection pressures. From the result, it is observed that the DE reported a higher fuel consumption rate and lower BTE with neat BD owing to its high viscosity and lower LCV. A similar trend of results reported by Man et al. [16] the neat BD-fueled engine emitted minimum HC, CO emission, and higher NOx emission due to high local oxygen concentration and high viscosity that advanced the combustion process. The above studies reveal that BD-fueled CI engines emit higher levels of NOx emission than engines operated with petroleum diesel. The properties of BD are one of the stronger reasons for NOx formation in CI engines. Because of the high viscosity of BD, the injection timing was artificially pushed by 1 to 4 degrees, which accelerated the process of combustion and resulted in increased NOx emissions in the exhaust [17,18,19,20]. Mishra et al. [21] reported that the DE emitted more NOx with neat waste cooking oil. The concentration of NOx increased at a higher WCO concentration in the test fuel blend, thus increasing the viscosity of the sample. The BD has a high cetane number in comparison to petroleum diesel, which helps in shortening the ignition delay and extends the combustion duration. The BD has a cetane number that varies between 58 and 62 based on the source of the fuel extracted [22]. The BD has more oxygen molecules in its chemical composition, about 11% when compared to petroleum diesel. This excess oxygen molecule creates rich local oxygen concentration that promotes the rate of the combustion process in CI engines [23,24,25]. Devarajan et al. [26] and Rajak et al. [27] stated that the reasons for more NOx production from the DE powered with diverse fuels like neem and microalgae BD are due to more oxygen molecules in their chemical composition that advanced combustion.
The above-noted reasons such as artificial advanced injection, high fuel viscosity, shorter delay in ignition, and lengthier combustion duration are factors in raising the concentration of NOx emission in the exhaust of DE operated with BD. In-cylinder reduction methods are being used to control NOx emissions from DEs such as water injection systems and exhaust gas recirculation (EGR) [28,29,30]. EGR is an efficient method to lower NOx emissions by controlling the rate of the combustion process [31]. Even though the EGR reduced the NOx emission from the DE, it increased the concentration of other pollutants such as CO, HC, and PM due to poor oxygen availability resulting in a lower combustion rate [32,33,34]. The EGR has reduced oxygen concentration in the cylinder due to the presence of burnt gas containing CO2 and other combusted products which reduced overall combustion rate and NOx emissions in the exhaust [22]. Pathak et al. [35] in an experimental investigation on DEs reported that by adopting EGR to reduce NOx emission. The DE tested with dual fuel mode with petroleum diesel and natural gas and analyzed their performance and combustion characteristics. This study resulted in ultra-low NOx emission with EGR and reduced NOx emission by up to 15.5% by diluting the oxygen concentration. This study also reported that the addition of EGR affected the combustion process which resulted in lower BTE and higher concentrations of other pollutants like HC, CO, and smoke. A similar trend is observed by Bhowmick et al. [36] and Balasubramanian et al. [37].
Metal oxide additives are an alternative method for EGR to reduce NOx emissions without affecting other pollutants. The metal oxide additives are mixed with the fuels at different concentrations, which enhances the rate of combustion due to the oxidation of metal oxide catalysts and lowers HC, CO, and NOx emissions. A significant drop in the levels of NOx emissions can be observed by controlling the CC temperature to less than 1500 ℃. During the engine operation, the fuel is injected into the cylinder along with metal oxide additives at the end of the compression stroke. The fuel with NPs promoted the combustion process and carried away the heat from the CC while leaving the cylinder during the exhaust stroke, thus maintaining a temperature lower at CC and reducing NOx concentration in the exhaust [38,39,40]. It is also noted from the previous studies that the DE emitted more NOx emission when the engine tested with lemongrass biodiesel due to its better combustible properties compared to other biodiesel [41,42,43]. The objectives of this investigation are structured from the drawbacks of previous studies. This study investigates the impact of ZnONPs on the performance, combustion, and emission characteristics of DE fueled by LGB. During the experimentation, the tests are conducted with higher concentrations of ZnO added to lemongrass oil and are varied as 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm respectively. Based on the engine characteristics, the optimum concentration of ZnO is determined, and the results are compared to the engine powered with neat lemongrass oil.
2 Materials and methodology
2.1 Solvent extraction of bio-oil
Lemongrass oil is extracted from the lemongrass plant through a solvent extraction method as shown in Fig. 1a. The round button flask is held over the electric heater and collects and circulates the hexane solvent in the distillation process. An electric heater with a manual controller is used for heating the hexane solvent to circulate between the distillation flask to the extraction chamber. The solvent is heated at a temperature of 70 °C, then starts to vaporize and flows to the bulb condenser. A bulb-type condenser is placed over the extraction chamber as shown in Fig. 1a for condensing the evaporated hexane solvent and it is collected in the extraction chamber. The specification of the components of the soxhlet apparatus is listed in Table 1.
The condensed hexane is being collected up to the level of Siphon in the extraction chamber. A siphon is a narrow channel used to drain the hexane from the reaction and collect it again in the distillation flask. The hexane solvent extracted organic components from the lemongrass with specific properties listed in Table 2. The process is continued for 5–6 cycles with a set of thimble containing lemongrass leaves. After the extraction process, the oil is collected in the distillation flask with a quantity of 10–15 ml. The same process is repeated to extract the required amount of oil, then it is allowed to do the transesterification process.
2.2 Transesterification process and characterization
After solvent extraction, the lemongrass oil undergoes a transesterification process to produce BD. The laboratory transesterification setup is shown in Fig. 1b. The neat lemongrass oils are high in viscosity, density, and self-ignition temperature owing to the availability of ester molecules. The process of transesterification removed free fatty acids from bio-oil by using methanol and potassium hydroxide as catalysts (KOH) with a ratio of 3:1. These catalysts reacted with triglycerides present in the bio-oil and converted to fatty acid methyl ester (FAME) and glycerol. After adding the catalysts with bio-oil, the process is continued until the deposition of glycerin at the bottom of the container. During the process, the bio-oil is stirred by a DC motor as shown in Fig. 1b. The speed of the DC motor is fixed at a constant of 750 rpm to ensure better mixing catalysts with oil. The manually operated electric heater controlled the reaction chamber temperature and it was maintained by 60 °C for the entire process. The specification of the components used in the transesterification plant is listed in Table 3. The glycerol is separated from the oil at the end of the transesterification process and it is deposited at the bottom of the reaction chamber. The FAME is filtered from the reaction chamber and washed with water to remove the ester fractions from the BD. Finally, the BD is refilled in the reaction chamber and heated to 100° C to remove the water content from it. A 91.7% yield of biodiesel could be achieved in this study. Table 4 shows the properties of the extracted fuel.
2.3 Composition and properties of ZnO nanoparticle
The ZnO NPs were purchased from the market. The size and purity of the purchased ZnO are ensured by using SEM–EDX analysis. The properties of ZnO NPs are listed in Table 5. SEM analysis is used for investigating the morphology of the ZnO NPs. Figure 2a, b shows the SEM images of the pristine ZnO NPs at different magnifications. The morphology of the pure ZnO NPs displayed a hexagonal wurtzite structure as seen from the electron micrographs. Further, it is noted that the size of the NPs is about 200 nm in range. Figure 2c depicts the EDX spectra of the pure ZnO NPs. The EDX spectra revealed the NPs' presence of Zn and O atoms.
2.4 Sample preparation and its composition
For the experimental investigation, the test fuel samples are prepared by adding different quantities of ZnO NPs such as 0 ppm, 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm with LGB and are labeled as LGB, LGB + 50 ppm, LGB + 100 ppm, LGB + 150 ppm, LGB + 200 ppm, and LGB + 250 ppm. The mass of ZnO NPs is measured using an electronic weighing machine to ensure the correct quantity of NPs to be mixed in the sample. Figure 7 depicts the various concentrations of ZnO NPs samples. To further evaluate the fuel properties after adding ZnO NPs, several other physicochemical properties of fuel test samples were also measured according to the ASTM standard method. The compositions of different test LGB blends with ZnO NPs were analyzed using FTIR spectroscopy. The properties of fuel samples are listed in Table 6. According to the properties of different samples, the addition of ZnO NPs was properly mixed with the fuel sample and improved its properties (Fig. 3).
3 Testing setup and process
3.1 Testing setup
A direct-injected DE was employed in the current study and it is connected with a loading device (Eddy current based dynamometer). Test engine has a single-point, multi-hole injector with an electronically controlled system. The schematic diagram of the test engine and supporting components are shown in Fig. 5. The engine is tested with different fuel samples and analyzed. A fuel tank-mounted load sensor is used to measure engine fuel consumption under different loading circumstances. The combustion characteristics of the DEs like HRR and in-cylinder pressure are estimated by employing a combustion analyzer. Emission parameters are recorded employing an AVL make gas analyzer while the smoke was estimated smoke meter, which are adopted with the exhaust pipe. The dynamometer is applied load on the engine for analyzing engine characteristics at different conditions, which is directly coupled with an engine through coupling as depicted in Fig. 4.
3.2 Test procedure
The engine is started first and ought to run for 15–20 min to obtain the saturation temperature of the cooling water. To eliminate errors and ensure the correctness of the values documented during the experimental evaluation, the same technique is followed throughout the testing process. The engine is operated at different loading conditions with different fuel samples and noted engine characteristics. Initially, the engine is tested with petroleum diesel under different loads, and parameters such as time taken for 10 cc fuel consumption, temperature of inlet and exhaust air, temperature of inlet and outlet coolant, combustion, and emission values are noted. These values are used as a reference and compared with the values taken from other fuel samples. The experimental investigation is continued further for testing other fuel samples and noted testing parameters. From the noted readings, the best sample for a DE is identified from the performance, combustion, and emission characteristics.
3.3 Instruments accuracy and uncertainty analysis
The current experimental investigation used several instruments for observing the DE characteristics operated with different types of fuel samples. The accuracy of the instruments used in this investigation is very important to rectify the errors. The following instruments made a major contribution to the experimental investigation such as smoke meter, gas analyzer, dynamometer controller, crank angle encoder, thermocouples, pressure transducer, and fuel pump. The uncertainty element was quantitatively estimated employing the Kragten spreadsheet approach using Eq. (1) [31]. In case of a single independent variable x1, the measurement uncertainty for y is expressed:
As a consequence, while uncertainty propagation is determined utilizing the root sum of squares, the aggregate standardized uncertainty over a dependent variable is generated using each of the standard uncertainties associated with its independent variables, as shown in Eq. (2) [44]:
The specification and precision of the instruments employed in this study are mentioned in our previous study which used the same engine for testing [45].
4 Result and discussion
4.1 Combustion characteristics
4.1.1 Influence of nanoparticle on combustion process
NPs strongly influenced the combustion process of the DE by accelerating the combustion and retaining the cylinder at a lower temperature. In general, the DEs are operated with higher CC temperature due to a stronger rich fuel region, which is further increasing while using BD as a fuel. It resulted in maximum NOx emission. The blending of NPs with BD is an intelligent approach to control NOx emissions without affecting the combustion process. The rate of combustion is enhanced with NPs concentration in the fuel, since ZnO NPs released oxygen molecules as shown in Fig. 5 are involved in the combustion process and promote it as complete combustion. At the time of combustion, Zn molecules are observed in the heat from the cylinder and left to the atmosphere during the exhaust stroke. The continuous removal of heat from CC by the Zn molecule has drastically reduced the NOx emissions by maintaining the cylinder at a lower temperature.
4.1.2 In-cylinder pressure
Figure 6 illustrates the variation in-cylinder pressure of the DE when fueled with various samples containing varying proportions of ZnO NPs. The analysis reveals that the Diesel Engine (DE) that has a pressure differential has recorded a peak cylinder pressure of 76.53 bar at 3 degrees Crank Angle (CA) following the Top Dead Centre (TDC). This is due to the better fuel properties of diesel such as heating value, evaporation temperature and viscosity compared to BD releases more heat from the combustion. From the result, it is noted that the DE registered lower in-cylinder pressure with BD due to its lower heating value and high viscosity, the addition of ZnO NPs increased cylinder pressure due to better combustion. The sample of LGB + 150ppn operated DE has registered a peak HRR compared to other BD samples due to a better combustion rate. It has registered a pressure value of 70.8 bar at 4 deg CA after TDC. The samples of LGB + 50 ppm and LGB + 100 ppm resulted in pressure values of 68 and 69 bars at 11 deg and 12 deg of CA after TDC. The samples with a high concentration of ZnO NPs like LGB + 200 ppm and LGB + 250 ppm have registered very low-pressure values compared to other samples due to high viscosity resulting in poor combustion rate [46, 47].
4.1.3 Heat release rate
The variation in HRR for the DE operated with different samples having various concentrations of NPs is shown in Fig. 6b. The DE with PD has registered maximum HRR when compared to neat LGB and its samples. From the result, it is noted that the DE with PD has an earlier start of combustion compared to BD samples due to its better properties, which is indicated in Fig. 6b. The PD-fuelled engine has a registered HRR of 65.23 J/deg, which is 11.5% higher than the HRR of neat lemongrass oil. From Fig. 6b, it can be observed that the HRR is improved by increasing ZnO NPs in the BD samples. The sample of LGB + 150 ppm fueled DE registered higher HRR compared to other BD samples since it has an optimum concentration of ZnO NPs. The reason is the addition of ZnO NPs improved oxygen contraction in the BD samples and also improved the average cetane number, in the sample of LGB + 150 ppm. It reduced ignition delay and slightly advanced the combustion process when compared to neat BD. The sample LGB + 150 ppm has a registered HRR value of 60 J/deg, which is 2.56% higher than the HRR of neat BD. The samples LGB + 200 ppm and LGB + 250 ppm have resulted in lower HRR due to the high concentration of ZnO NPs in the samples, which increased the overall self-ignition temperature and viscosity of the sample resulting in poor combustion rate [48,49,50].
4.2 Performance characterization
4.2.1 Specific fuel consumption
The variation in SFC of the DE operated with BD samples at different engine loads is depicted in Fig. 7a. It is noted that the DE with PD has consumed less fuel in comparison with BD samples. It registered SFC values of 0.534, 0.422, 0.341, 0.323, and 0.355 kg/kW-hr for 20%, 40%, 60%, 80%, and 100% loads. From the result, it is observed that the sample of LGB + 150 ppm fueled DE has operated with lower SFC than other BD samples. The reason is ZnO NPs released oxygen during the combustion process, which promoted the combustion further by oxidizing the unburnt fuel. From the experimental study, it is revealed that the value of SFC decreased with the concentration of ZnO in the sample, the same trend is observed up to 150 ppm of NPs further increasing due to more concentration of ZnO. The samples of LGB + 200 ppm and LGB + 250 ppm result with higher SFC values since the samples are in viscus and have high flash points and self-ignition temperatures due to more concentration of NPs [51, 52].
4.2.2 Brake thermal EFFICIENCY
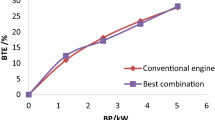
The brake thermal efficiency (BTE) of the DE operated with different BD samples is shown in Fig. 7b. The rate of BTE is increasing concerning the load on the engine for both PD and BD samples. There is a slight reduction in BTE at higher engine load for both samples due to shorter combustion timing and stronger rich fuel region. The engine with PD fuelled has registered higher BTE values as 17.5%, 29.9%, 35.9%, 36.8%, and 35.1% for 20%, 40%, 60%, 80%, and 100% loads, it is better than the values of BD samples. The properties of PD are the reasons for better thermal efficiency in the DE. From the result, it is noted that the BTE of the engine operated with BD was enhanced at higher concentration of ZnO NPs in the sample. The sample of LGB + 150 ppm fuelled DE has registered higher BTE for all operating conditions. The samples LGB + 200 ppm and LGB + 250 ppm have registered lower BTE values even though it has higher cetane numbers and heating values than LGB + 150 ppm. It is due to more concentrations of ZnO NPs that increased overall density, vaporization temperature, and self-ignition temperature of the samples resulted in poor combustion [53,54,55].
4.2.3 Exhaust gas temperature
The variation in EGT of the DE operated under different loading conditions is shown in Fig. 7c. From the result, it is noted that the BD samples emitted exhaust gas at higher temperatures than the DE operated with PD due to its advanced combustion. The earlier start of combustion shortened the combustion duration and released more heat during the combustion process which increased the temperature of the exhaust gas. The EGT is further increased due to the addition of ZnO NPs with BD since Zn molecules carried the heat from CC and left along with exhaust gas during the exhaust stroke. From Fig. 7c, it is understood that the rate of EGT is increased with ZnO concentration in the samples. The samples of LGB + 200 ppm and LGB + 250 ppm release more heat through exhaust gas at lower loading due to more concentration of ZnO NPs. At higher loading conditions the rate of heat released by the DE with the same sample is decreased due to poor combustion process [56,57,58].
4.3 Emission characterization
4.3.1 Hydrocarbon
The variation in hydrocarbon (HC) emission from the DE operated with different BD samples is shown in Fig. 7d. The DE with PD has emitted more HC emission at 40 ppm, 58 ppm, 63 ppm, 73 ppm and 89 ppm for different loading conditions. The reason is PD is a lighter fuel and has a lower vaporization temperature than LGB and its sample. It created an ultra-lean mixture near the cylinder wall which resulted in the absence of combustion. It is noted that the HC emissions are increased with increasing the load on the engine due to stronger lean regions at higher load conditions. From the result, it came to know that LGB and its blends have operated with low HC emissions due to its high viscosity and more oxygen molecules, which eliminates the lean fuel region near the cylinder wall as created with PD. By analyzing Fig. 7d, it could be illustrated that the emission of HC from DEs decreases as the concentration of additives in the sample increases. The sample of LGB + 150 ppm resulted in lower HC emissions as 27 ppm, 31 ppm, 38 ppm, 43 ppm, and 52 ppm for 20%, 40%, 60%, 80%, and 100% loads, which are less than the values of other samples. The reason is the sample of LGB + 150 ppm has average viscosity and more oxygen concentration compared to LGB + 50 ppm and LGB + 100 ppm samples. The above factors improved the combustion rate by advancing the combustion and minimizing the lean fuel region. At the same time, the samples LGB + 200 ppm and LGB + 250 ppm registered more HC emission due to the high concentration of ZnO NPs in the sample which rapidly increased the viscosity [59,60,61].
4.3.2 Carbon monoxide
Figure 7e shows variation in CO emissions from a DE operated with different BD samples having various ZnO NP concentrations. The engine operated with BD has registered lower CO emissions and it is further decreasing by adding the ZnO NPs in the test blend. The inclusion of ZnO NPs enhanced the oxygen concentration in the test blend, resulting in better combustion efficiency and decreased CO emission in the exhaust. The LGB + 200 ppm and LGB + 250 ppm samples have exhibited reduced CO emissions under minimal loading conditions of up to 60%. Nevertheless, beyond this point, the emissions increase as a result of higher ZnO concentration. The LGB + 150 ppm sample exhibits reduced carbon monoxide (CO) emissions under higher loads in comparison to other BD samples, owing to its optimal viscosity [62, 63].
4.3.3 Carbon dioxide
The variation in carbon dioxide (CO2) emission from the DE operated with different BD samples containing various concentrations of ZnO NPs is shown in Fig. 7f. From the result, it is noted that the CO2 emissions varied based on the load on the engine for all the samples. The DE emitted lower CO2 emission at 20% load and gradually increased with increasing load up to 60% for all the samples tested in the engine. It happened due to increasing the combustion rate at mid-loading conditions and there is a slight reduction in CO2 emission at 80% load due to a reduction in combustion rate caused by incomplete combustion. From Fig. 7f, it is observed that the DE with PD has emitted lesser CO2 emission than BD samples. It is also noted that the rate of CO2 emissions in the exhaust is increased with increasing the concentration of ZnO in BD blends due to the availability of oxygen. The sample LGB + 250 ppm registered higher CO2 emissions compared to other samples since it has more oxygen availability.
4.3.4 Oxides of nitrogen
The variation in NOx from the DE operated with different BD blends having various concentrations of ZnO NPs is shown in Fig. 7g. From the result, it could be noted that the neat BD-powered engine has registered higher NOx emissions owing to its high viscosity and cetane number. The BD with high viscosity and cetane number has artificially advanced the injection timing and started the combustion earlier when compared with an engine that operated with PD, it is visible clearly in Fig. 7g. Improved combustion resulted in higher in-cylinder temperatures, creating ideal conditions for the generation of NOx. NOx emissions are known to increase with engine load, owing to the accompanying rise in combustion temperatures. This phenomenon can be described by the Zeldovich mechanism, which details how NOx is formed at high temperatures by the reaction of nitrogen and oxygen in the combustion air [64, 65]. Experimental results show that incorporating ZnO NPs into BD greatly decreases NOx emissions. The fundamental mechanism involves ZnO NPs' catalytic characteristics, which improve the combustion process, resulting in improved combustion as well as lower peak temperatures inside the cylinder [66, 67]. As a result, NOx emissions are lowered. The nanoparticles increase combustion efficiency by suppressing NOx production pathways. Thus, adding ZnO NPs to biodiesel improves fuel combustion efficiency while simultaneously contributing to a cleaner emissions profile.
From the result, it is noted that the NOx emission is decreased with the addition of ZnO NPs in the BD sample. The DE with LGB + 250 ppm has registered ultra-lower NOx emissions as 21 ppm, 98 ppm, 168 ppm, 254 ppm, and 302 ppm compared to other BD samples. The reason is the sample LGB + 250 ppm has a higher concentration of ZnO NPs, which observed more heat from CC and maintained it at a lower temperature compared to other BD samples and emitted lower NOx emission in the exhaust [68, 69].
4.3.5 Smoke
Figure 7h shows the changes in the levels of smoke emission from the DE operated with different BD samples containing various concentrations of ZnO NPs. The results show that the smoke emission is increased with the addition of load for all the samples tested in the engine. The DE emitted lesser smoke emission when the engine tested with PD and the values are 28 HSU, 35HSU, 43HSU, 56HSU, and 64HSU for 20%, 40%, 60%, 80%, and 100% of loads compared to BD samples. The properties of PD are the reason for lower smoke emission, it created an improved air–fuel mixture that resulted in enhanced engine efficiency [70]. From Fig. 7h, it is noted that the smoke emission from the test engine is varied for the concentration of ZnO in the BD samples. The smoke emission is reduced at higher ZnO concentration in the sample up to 150 ppm, further, it is increasing the smoke emissions from the DE for 200 ppm and 250 ppm concentrations. It happened due to the increasing viscosity of BD by adding more concentration of NPs in the samples. The sample of LGB + 150 ppm has registered lower smoke emission than the neat LGB due to slight improvement in the properties by adding the nanoparticle. The samples of LGB + 200 ppm and LGB + 250 ppm have resulted in higher smoke emissions compared with other samples tested in a DE [39, 71].
5 Conclusion
This study experimentally investigated the performance, emission, and combustion characteristics of the DE operated with lemongrass oil added with different concentrations of ZnO NPs. For this study, the BD was extracted from the lemongrass leaf by both solvent extraction and transesterification process. In addition, the ZnO NPs were added with the extracted LGB at different concentrations such as 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 250 ppm, and tested in a single cylinder DE. The following observations were concluded from the test results,
-
The sample of LGB + 150 ppm fuelled DE registered a higher in-cylinder pressure of about 70.8 bar, which is 10.9% higher than the pressure value of the engine tested with neat BD.
-
The sample of LGB + 150 ppm was effectively utilized in the DE with higher HRR for all loading conditions compared to neat LGB. It registered an HRR of 60 J/deg for 80% load, which is 6.7% higher than the values of HRR registered by the engine operated with neat LGB.
-
The sample of LGB + 250 ppm operated engine resulted in higher EGT values compared to other samples. The sample of the LGB + 150 ppm operated engine registered lower SFC values compared to neat LGB, which is 7.7% lower than the values of SFC registered by the engine tested with neat LGB.
-
The sample LGB + 150 ppm powered DE registered higher BTE compared to other LGB samples, it improved about 5.9% when compared with the values of BTE of neat LGB.
-
The DE registered lower HC emission with LGB for all loading conditions than PD. The sample of LGB + 150 ppm registered lower HC emissions compared to other samples. The sample of LGB + 150 ppm operated engine registered lower CO emission for all the loading conditions, which is 14.36% lower than the values of CO of DE operated with neat LGB.
-
It is concluded that the sample LGB + 250 ppm has emitted more CO2 emission when compared with other samples tested in the engine due to more concentration of ZnO.
-
The DE powered by neat LGB has resulted in higher NOx emissions than PD. The sample of LGB + 250 ppm drastically reduced the NOx emission by 41.66% when compared NOx emissions from neat LGB. The sample LGB + 150 ppm emitted lower smoke emissions, which is 11.2% lower than the values of smoke emitted by neat LGB.
Future research can investigate the possibility of adjusting the concentrations of ZnO nanoparticles in order to achieve even lower emissions and higher efficiency. In addition, the investigation of various metal oxide nanoparticles and the synergistic effects that they have with lemongrass biodiesel could further improve engine performance and emission characteristics, thereby offering useful insights for the development of sustainable fuels for diesel engines.
Availability of data and materials
The data used to support the findings of this study are included in the article. Should further data or information be required, these are available from the corresponding author upon request.
Abbreviations
- BD:
-
Biodiesel
- DE:
-
Diesel engine
- ZnO:
-
Zinc oxide
- NPs:
-
Nanoparticles
- SFC:
-
Specific fuel consumption
- ZnO NPs:
-
Zinc oxide nanoparticles
- LGB:
-
Lemongrass biodiesel
- LGB + 50 ppm:
-
Lemongrass biodiesel with 50 ppm of ZnO nanoparticle
- LGB + 100 ppm-:
-
Lemongrass biodiesel with 100 ppm of ZnO nanoparticle
- LGB + 150 ppm-:
-
Lemongrass biodiesel with 150 ppm of ZnO nanoparticle
- LGB + 200 ppm-:
-
Lemongrass biodiesel with 200 ppm of ZnO nanoparticle
- LGB + 250 ppm-:
-
Lemongrass biodiesel with 250 ppm of ZnO nanoparticle
- HRR:
-
Heat release rate
- BTE:
-
Brake thermal efficiency
- CO:
-
Carbon monoxide
- CO2 :
-
Carbon dioxide
- NOx:
-
Oxides of nitrogen
- PM:
-
Particulate matters
- PD:
-
Petroleum diesel
- HC:
-
Hydrocarbon
- EGT:
-
Exhaust gas temperature
References
Sharma P, Sharma AKR. Statistical and continuous wavelet transformation-based analysis of combustion instabilities in a biodiesel-fueled compression ignition engine. J Energy Resour Technol. 2022;144: 032304. https://doi.org/10.1115/1.4051340.
Antony Casmir Jayaseelan G, Anderson A, Manigandan S, Elfasakhany A, Dhinakaran V. Effect of engine parameters, combustion and emission characteristics of diesel engine with dual fuel operation. Fuel. 2021;302: 121152. https://doi.org/10.1016/j.fuel.2021.121152.
Alenezi RA, Erdiwansyah, Mamat R, Norkhizan AM, Najafi G. The effect of fusel-biodiesel blends on the emissions and performance of a single cylinder diesel engine. Fuel 2020;279:118438. https://doi.org/10.1016/j.fuel.2020.118438.
Simsek S. Effects of biodiesel obtained from Canola, sefflower oils and waste oils on the engine performance and exhaust emissions. Fuel. 2020;265: 117026. https://doi.org/10.1016/j.fuel.2020.117026.
Elkelawy M, Etaiw SEH, Bastawissi HAE, Marie H, Radwan AM, Dawood MM, et al. WCO biodiesel production by heterogeneous catalyst and using cadmium (II)-based supramolecular coordination polymer additives to improve diesel/biodiesel fueled engine performance and emissions. J Therm Anal Calorim. 2022;147:6375. https://doi.org/10.1007/s10973-021-10920-1.
Elkelawy M, El Shenawy EA, Mohamed SA, Elarabi MM, Bastawissi HAE. Impacts of using EGR and different DI-fuels on RCCI engine emissions, performance, and combustion characteristics. Energy Convers Manage. 2022;15: 100236. https://doi.org/10.1016/j.ecmx.2022.100236.
Elkelawy M, Bastawissi H, Sathyamurthy R, Chandra Sekar S, Karuppasamy K, Vedaraman N, et al. Numerical and experimental investigation of ethyl alcohol as oxygenator on the combustion, performance, and emission characteristics of diesel/cotton seed oil blends in homogenous charge compression ignition engine. SAE Technical Papers, vol. 2018, 2018. https://doi.org/10.4271/2018-01-1680.
Elkelawy M, Zhang YS, El-Din HA, Yu JZ. A comprehensive modeling study of natural gas (HCCI) engine combustion enhancement by using hydrogen addition. SAE Tech Papers. 2008. https://doi.org/10.4271/2008-01-1706.
Yesilyurt MK, Aydin M, Yilbasi Z, Arslan M. Investigation on the structural effects of the addition of alcohols having various chain lengths into the vegetable oil-biodiesel-diesel fuel blends: an attempt for improving the performance, combustion, and exhaust emission characteristics of a compression ignition engine. Fuel. 2020;269:117455. https://doi.org/10.1016/j.fuel.2020.117455.
Sharma P, Sivaramakrishnaiah M, Deepanraj B, Saravanan R, Reddy MV. A novel optimization approach for biohydrogen production using algal biomass. Int J Hydrogen Energy. 2022. https://doi.org/10.1016/j.ijhydene.2022.09.274.
Huang J, Xiao H, Yang X, Guo F, Hu X. Effects of methanol blending on combustion characteristics and various emissions of a diesel engine fueled with soybean biodiesel. Fuel. 2020;282: 118734. https://doi.org/10.1016/j.fuel.2020.118734.
Sharma P, Sharma AK. AI-based prognostic modeling and performance optimization of CI engine using biodiesel-diesel blends. Int J Renew Energy Res. 2021;11:701–8. https://doi.org/10.20508/ijrer.v11i2.11854.g8191.
Baweja S, Trehan A, Kumar R. Combustion, performance, and emission analysis of a CI engine fueled with mustard oil biodiesel blended in diesel fuel. Fuel. 2021;292: 120346. https://doi.org/10.1016/j.fuel.2021.120346.
Aydın S. Detailed evaluation of combustion, performance and emissions of ethyl proxitol and methyl proxitol-safflower biodiesel blends in a power generator diesel engine. Fuel. 2020;270: 117492. https://doi.org/10.1016/j.fuel.2020.117492.
Shrivastava P, Verma TN. Effect of fuel injection pressure on the characteristics of CI engine fuelled with biodiesel from Roselle oil. Fuel. 2020;265: 117005. https://doi.org/10.1016/j.fuel.2019.117005.
Man XJ, Cheung CS, Ning Z, Wei L, Huang ZH. Influence of engine load and speed on regulated and unregulated emissions of a diesel engine fueled with diesel fuel blended with waste cooking oil biodiesel. Fuel. 2016;180:41–9. https://doi.org/10.1016/j.fuel.2016.04.007.
Kanth S, Ananad T, Debbarma S, Das B. Effect of fuel opening injection pressure and injection timing of hydrogen enriched rice bran biodiesel fuelled in CI engine. Int J Hydrogen Energy. 2021;46:28789. https://doi.org/10.1016/j.ijhydene.2021.06.087.
Babu D, Karvembu R, Anand R. Impact of split injection strategy on combustion, performance and emissions characteristics of biodiesel fuelled common rail direct injection assisted diesel engine. Energy. 2018;165:577. https://doi.org/10.1016/j.energy.2018.09.193.
Ge JC, Kim HY, Yoon SK, Choi NJ. Optimization of palm oil biodiesel blends and engine operating parameters to improve performance and PM morphology in a common rail direct injection diesel engine. Fuel. 2020;260: 116326. https://doi.org/10.1016/j.fuel.2019.116326.
Ryu K. Effects of pilot injection pressure on the combustion and emissions characteristics in a diesel engine using biodiesel-CNG dual fuel. Energy Convers Manag. 2013. https://doi.org/10.1016/j.enconman.2013.07.085.
Mishra S, Chauhan A, Mishra KB. Role of binary and ternary blends of WCO biodiesel on emission reduction in diesel engine. Fuel. 2020;262: 116604. https://doi.org/10.1016/j.fuel.2019.116604.
Biswakarma K, Sarmah P, Paramasivam P, Dhanasekaran S, Kumar Yadav S, Kumar V. Experimental probe into an automative engine run on waste cooking oil biodiesel blend at varying engine speeds. Fuel. 2023;346: 128364. https://doi.org/10.1016/j.fuel.2023.128364.
Yesilyurt MK. The effects of the fuel injection pressure on the performance and emission characteristics of a diesel engine fuelled with waste cooking oil biodiesel-diesel blends. Renew Energy. 2019;132:649. https://doi.org/10.1016/j.renene.2018.08.024.
Ghadikolaei MA, Wei L, Cheung CS, Yung KF, Ning Z. Particulate emission and physical properties of particulate matter emitted from a diesel engine fueled with ternary fuel (diesel-biodiesel-ethanol) in blended and fumigation modes. Fuel. 2020;263:16665. https://doi.org/10.1016/j.fuel.2019.116665.
Allami HAR, Nayebzadeh H. The assessment of the engine performance and emissions of a diesel engine fueled by biodiesel produced using different types of catalyst. Fuel. 2021;305:121525. https://doi.org/10.1016/j.fuel.2021.121525.
Devarajan Y, Munuswamy DB, Nagappan B, Choubey G. Study on the effect on combining long-chain additive with neat bio-diesel fueled engine to examine its ignition characteristics. Fuel. 2020;279:118400. https://doi.org/10.1016/j.fuel.2020.118400.
Rajak U, Nashine P, Dasore A, Verma TN. Utilization of renewable and sustainable microalgae biodiesel for reducing the engine emissions in a diesel engine. Fuel. 2022;311:122498. https://doi.org/10.1016/j.fuel.2021.122498.
Fayad MA, Ibrahim SI, Omran SH, Martos FJ, Badawy T, Al Jubori AM, et al. Experimental effect of CuO2 nanoparticles into the RME and EGR rates on NOX and morphological characteristics of soot nanoparticles. Fuel. 2023;331: 125549. https://doi.org/10.1016/J.FUEL.2022.125549.
Fayad MA, Radhi AA, Omran SH, Mohammed FM. Influence of environment-friendly fuel additives and fuel injection pressure on soot nanoparticles characteristics and engine performance, and NOX emissions in CI diesel engine. J Adv Res Fluid Mech Therm Sci. 2021;88:58–70. https://doi.org/10.37934/arfmts.88.1.5870.
Fayad MA, Chaichan MT, Dhahad HA, Al-Amiery AA, Wan Isahak WNR. Reducing the effect of high sulfur content in diesel fuel on NOxEmissions and PM characteristics using a PPCI mode engine and gasoline-diesel blends. ACS Omega. 2022;7:37328. https://doi.org/10.1021/acsomega.2c03878.
Raja E, Premjeyakumar M. Potent effect of prosopis juliflora (biodiesel + isopropanol + diesel) fueled with diesel engine and egr alteration. Clean Eng Technol. 2021;4:100205. https://doi.org/10.1016/j.clet.2021.100205.
Jayabal R, Thangavelu L, Subramani S. Combined effect of oxygenated additives, injection timing and EGR on combustion, performance and emission characteristics of a CRDi diesel engine powered by sapota biodiesel/diesel blends. Fuel. 2020;276:118020. https://doi.org/10.1016/j.fuel.2020.118020.
Rami Reddy S, Murali G, Ahamad Shaik A, Dhana Raju V, Sreekara Reddy MBS. Experimental evaluation of diesel engine powered with waste mango seed biodiesel at different injection timings and EGR rates. Fuel. 2021;285:119047. https://doi.org/10.1016/j.fuel.2020.119047.
Naresh Kumar A, Kishore PS, Brahma Raju K, Nanthagopal K, Ashok B. Experimental study on engine parameters variation in CRDI engine fuelled with palm biodiesel. Fuel. 2020;276:118076. https://doi.org/10.1016/j.fuel.2020.118076.
Pathak SK, Nayyar A, Goel V. Optimization of EGR effects on performance and emission parameters of a dual fuel (Diesel + CNG) CI engine: an experimental investigation. Fuel. 2021;291: 120183. https://doi.org/10.1016/j.fuel.2021.120183.
Bhowmick P, Jeevanantham AK, Ashok B, Nanthagopal K, Perumal DA, Karthickeyan V, et al. Effect of fuel injection strategies and EGR on biodiesel blend in a CRDI engine. Energy. 2019;181:1094. https://doi.org/10.1016/j.energy.2019.06.014.
Balasubramanian D, Hoang AT, Papla Venugopal I, Shanmugam A, Gao J, Wongwuttanasatian T. Numerical and experimental evaluation on the pooled effect of waste cooking oil biodiesel/diesel blends and exhaust gas recirculation in a twin-cylinder diesel engine. Fuel. 2021;287: 119815. https://doi.org/10.1016/j.fuel.2020.119815.
Janakiraman S, Lakshmanan T, Raghu P. Experimental investigative analysis of ternary (diesel + biodiesel + bio-ethanol) fuel blended with metal-doped titanium oxide nanoadditives tested on a diesel engine. Energy. 2021;235: 121148. https://doi.org/10.1016/j.energy.2021.121148.
Gad MS, Jayaraj S. A comparative study on the effect of nano-additives on the performance and emissions of a diesel engine run on Jatropha biodiesel. Fuel. 2020;267: 117168. https://doi.org/10.1016/j.fuel.2020.117168.
Nega T, Tesfaye A, Paramasivam P. Design and CFD modeling of gasifier stove combined with heat exchanger for water heating application. AIP Adv. 2022. https://doi.org/10.1063/5.0081001.
Manivasagam V, Narayanan P, Kuma Gupta N, Shinde T, Panchal H, Thangavel R, et al. Investigation on 1-Propanol Electronic mode of fumigation on diesel engine performance and emission fuelled with diesel and lemongrass biodiesel blend using AHP-COPRAS. Energy Convers Manage. 2023;20:1400468. https://doi.org/10.1016/j.ecmx.2023.100468.
Kumar P, Kumar D, Shankar R, Kumar S, Saini P, Kumar N. Effect of synthesized lemongrass biodiesel on the performance and emission characteristics of a CI engine. Sustain Energy Technol Assess. 2023;57:103221. https://doi.org/10.1016/j.seta.2023.103221.
Asokan MA, Prabu SS, Prathiba S, Popli R, Bakshi R, Teja EV. Emission and performance behaviour of blends of diesel/lemongrass oil in di diesel engine. Mater Today Proc. 2021;46:8080. https://doi.org/10.1016/j.matpr.2021.03.038.
Kline JS. Describing uncertainties in single sample experiments. Mech Eng. 1953;75:3–8.
Gowthaman S, Thangavel K. Performance, emission and combustion characteristics of a diesel engine fuelled with diesel/coconut shell oil blends. Fuel. 2022;322:124293. https://doi.org/10.1016/j.fuel.2022.124293.
Jain A, Bora BJ, Kumar R, Sharma P, Deepanraj B, Irshad K, et al. Application of hybrid Taguchi L16 and desirability for model prediction and optimization in assessment of the performance of a novel Water Hyacinth biodiesel run diesel engine. Fuel. 2023;339: 127377. https://doi.org/10.1016/J.FUEL.2022.127377.
Said Z, Sharma P, Bora BJ, Nguyen VN, Bui TAE, Nguyen DT, et al. Modeling-optimization of performance and emission characteristics of dual-fuel engine powered with pilot diesel and agricultural-food waste-derived biogas. Int J Hydrogen Energy. 2022. https://doi.org/10.1016/j.ijhydene.2022.07.150.
Murugesan P, Hoang AT, Perumal Venkatesan E, Santosh Kumar D, Balasubramanian D, Le AT, et al. Role of hydrogen in improving performance and emission characteristics of homogeneous charge compression ignition engine fueled with graphite oxide nanoparticle-added microalgae biodiesel/diesel blends. Int J Hydrogen Energy. 2021. https://doi.org/10.1016/j.ijhydene.2021.08.107.
Sharma P, Said Z, Kumar A, Nižetić S, Pandey A, Hoang AT, et al. Recent advances in machine learning research for nanofluid-based heat transfer in renewable energy system. Energy Fuels. 2022;36:6626–58. https://doi.org/10.1021/acs.energyfuels.2c01006.
Hoang AT. Combustion behavior, performance and emission characteristics of diesel engine fuelled with biodiesel containing cerium oxide nanoparticles: a review. Fuel Process Technol. 2021;218: 106840. https://doi.org/10.1016/j.fuproc.2021.106840.
Sathish T, Ağbulut Ü, George SM, Ramesh K, Saravanan R, Roberts KL, et al. Waste to fuel: Synergetic effect of hybrid nanoparticle usage for the improvement of CI engine characteristics fuelled with waste fish oils. Energy. 2023;275: 127397. https://doi.org/10.1016/J.ENERGY.2023.127397.
Jit Sarma C, Sharma P, Bora BJ, Bora DK, Senthilkumar N, Balakrishnan D, et al. Improving the combustion and emission performance of a diesel engine powered with mahua biodiesel and TiO2 nanoparticles additive. Alex Eng J. 2023;72:387–98. https://doi.org/10.1016/J.AEJ.2023.03.070.
Padmanabhan S, Selvamuthukumar M, Gopi Krishna B, Kumar M, Sudheer K, Baskar S, et al. Enhancement of engine performance by nano-coated pistons fuelled with nano-additive biodiesel blends. Mater Today Proc. 2023. https://doi.org/10.1016/j.matpr.2023.02.231.
Rangabashiam D, Jayaprakash V, Ganesan S, Nagaraj M, Rameshbabu A. Emission, performance, and combustion study on nanoparticle-biodiesel fueled diesel engine. Energy Sour Part A Recov Util Environ Effects. 2023;45:8396–407. https://doi.org/10.1080/15567036.2019.1677821.
Pullagura G, Vanthala VSP, Vadapalli S, Bikkavolu JR, Barik D, Sharma P, et al. Enhancing performance characteristics of biodiesel-alcohol/diesel blends with hydrogen and graphene nanoplatelets in a diesel engine. Int J Hydrogen Energy. 2024;50:1020–34. https://doi.org/10.1016/j.ijhydene.2023.09.313.
Tamil Selvam N, Devaraj A. Emission study on MgO2 nano-additive doped biodiesel on immobile diesel engine. Int J Amb Energy. 2021;42:1595. https://doi.org/10.1080/01430750.2019.1611654.
Mofijur M, Ahmed SF, Ahmed B, Mehnaz T, Mehejabin F, Shome S, et al. Impact of nanoparticle-based fuel additives on biodiesel combustion: an analysis of fuel properties, engine performance, emissions, and combustion characteristics. Energy Convers Manage. 2024;21: 100515. https://doi.org/10.1016/j.ecmx.2023.100515.
Ramakrishnan G, Krishnan P, Rathinam S, Thiyagu R, Devarajan Y. Role of nano-additive blended biodiesel on emission characteristics of the research diesel engine. Int J Green Energy. 2019;16:435. https://doi.org/10.1080/15435075.2019.1577742.
Kalaimurugan K, Karthikeyan S, Periyasamy M, Mahendran G, Dharmaprabhakaran T. Experimental studies on the influence of copper oxide nanoparticle on biodiesel-diesel fuel blend in CI engine. Energy Sour Part A Recov Util Environ Effects. 2023;45:8997. https://doi.org/10.1080/15567036.2019.1679290.
Hoseini SS, Najafi G, Ghobadian B, Ebadi MT, Mamat R, Yusaf T. Biodiesels from three feedstock: the effect of graphene oxide (GO) nanoparticles diesel engine parameters fuelled with biodiesel. Renew Energy. 2020;145:190. https://doi.org/10.1016/j.renene.2019.06.020.
Sateesh KA, Yaliwal VS, Soudagar MEM, Banapurmath NR, Fayaz H, Safaei MR, et al. Utilization of biodiesel/Al2O3 nanoparticles for combustion behavior enhancement of a diesel engine operated on dual fuel mode. J Therm Anal Calorim. 2022;147:5897–911. https://doi.org/10.1007/s10973-021-10928-7.
Gavhane RS, Kate AM, Soudagar MEM, Wakchaure VD, Balgude S, Rizwanul Fattah IM, et al. Influence of silica nano-additives on performance and emission characteristics of soybean biodiesel fuelled diesel engine. Energies (Basel). 2021;14:1489. https://doi.org/10.3390/en14051489.
Hosseini SH, Taghizadeh-Alisaraei A, Ghobadian B, Abbaszadeh-Mayvan A. Artificial neural network modeling of performance, emission, and vibration of a CI engine using alumina nano-catalyst added to diesel-biodiesel blends. Renew Energy. 2020;149:951–61. https://doi.org/10.1016/j.renene.2019.10.080.
Rao A, Liu Y, Ma F. Study of NOx emission for hydrogen enriched compressed natural along with exhaust gas recirculation in spark ignition engine by Zeldovich’ mechanism, support vector machine and regression correlation. Fuel. 2022;318: 123577. https://doi.org/10.1016/J.FUEL.2022.123577.
Fayad MA, Sobhi M, Chaichan MT, Badawy T, Abdul-Lateef WE, Dhahad HA, et al. Reducing soot nanoparticles and NOX emissions in CRDI diesel engine by incorporating TiO2 nano-additives into biodiesel blends and using high rate of EGR. Energies (Basel). 2023;16:3921. https://doi.org/10.3390/en16093921.
Fayad MA, Aljuwaya TM, Alhuzaymi TM, Majdi HS, Sultan AJ, Chaichan MT, et al. Incorporating of TiO2 with oxygenated fuel and post-injection strategy in CRDI diesel engine equipped with EGR: a step towards lower NOX, PM and enhance soot oxidation reactivity. Case Stud Therm Eng. 2024;53: 103894. https://doi.org/10.1016/j.csite.2023.103894.
Fayad MA, Alani WK, Dhahad HA, Zheng J. Diminution of air pollution from nox and smoke/soot emitted from alcohols/diesel blends in diesel engine and influence of the exhaust gas recirculation (EGR). J Environ Eng Landsc Manage. 2023;31:103. https://doi.org/10.3846/jeelm.2023.17410.
Shelare SD, Belkhode PN, Nikam KC, Jathar LD, Shahapurkar K, Soudagar MEM, et al. Biofuels for a sustainable future: examining the role of nano-additives, economics, policy, internet of things, artificial intelligence and machine learning technology in biodiesel production. Energy. 2023;282:128874. https://doi.org/10.1016/j.energy.2023.128874.
Moorthi M, Murugesan A, Alagumalai A. Effect of nanoparticles on DI-CI engine characteristics fueled with biodiesel–diesel blends—a critical review. J Therm Anal Calorim. 2022;147:9163–79. https://doi.org/10.1007/s10973-022-11234-6.
Fayad MA, Abed AM, Omran SH, Jaber AA, Radhi AA, Dhahad HA, et al. Influence of renewable fuels and nanoparticles additives on engine performance and soot nanoparticles characteristics. Int J Renew Energy Dev. 2022;11:1068. https://doi.org/10.14710/ijred.2022.45294.
Ahmadi MA, Mostafaei M, Najafi G, Rabbani H. Effect of nano-additives blended diesel-biodiesel on performance and emissions of CI engine in the presence of magnetic field. Energy Sour Part A Recov Util Environ Effects. 2022;44:5048. https://doi.org/10.1080/15567036.2019.1657523.
Acknowledgements
The authors thank their respective institutions for their kind support. The authors extend their appreciation to Mattu University, Ethiopia in this publication process.
Funding
The authors declared that no funding was received for this Research and Publication.
Author information
Authors and Affiliations
Contributions
SG, AIAKS, KT: Data collection, carried out research, data analysis/interpretation, produced drafted manuscript, and scientifically discussed results. LM, PP: Supervised project, provision of materials, and contributed to scientific discussion. SG, PP: Co-supervised the project and contributed to the interpretation and scientific discussion of results.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research conducted in this study adhered to the ethical guidelines of the Journal. All participants provided informed consent to participate in this research. Informed consent was obtained in writing from all participants, and they were informed about the purpose of the study, potential risks, and their rights to withdraw their participation at any time without consequence.
Competing interests
The authors declare that they have no competing interests relevant to the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gowthaman, S., Anu Karthi Swaghatha, A.I., Thangavel, K. et al. Effect of ZnO nanoparticle on combustion and emission characteristics of a diesel engine powered by lemongrass biodiesel: an experimental approach. Discov Appl Sci 6, 344 (2024). https://doi.org/10.1007/s42452-024-06045-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06045-3