Abstract
Algal biomass is a promising feedstock for the environmentally friendly production of a diverse range of high-value products, including bioproducts and biofuels. After extracting the essential macro- and biomolecules, the remaining algae biomass can be used as feedstock and processed into valuable additional goods. Advanced biotechnology techniques and efficient hydrothermal liquefaction (HTL) technologies are used to produce beneficial products such as bioenergy and biochemicals. Carbohydrates, lipids, and proteins are essential biochemical components of algal biomass that can be used to produce biofuel. Hence, algae biomass is gaining popularity as a biorefinery alternative. HTL is a process of converting biomass to a liquid byproduct by intricate chemical reactions. The purpose of this review is to highlight modern biotechnological and hydrothermal liquefaction techniques for extracting biological products from algae. A large number of documents were reviewed and analytically structured to lay the groundwork for the subsequent steps. This review also included information on a simple reaction mechanism for the biomass that algae produce, as well as the impact of process parameters.
Article Highlights
-
Bio compounds derived from algal biomass might be an advantageous approach for human welfare.
-
Algae biomass based renewable products by hydrothermal liquefaction (HL) are more profitable.
-
Biorefinery products derived from algae biomass by HL have biomedical use and can minimize pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The availability of sufficient food as well as energy supply are the three most pressing issues confronting humanity worldwide [1]. The continued use of non-renewable energy sources contribute to deplete the natural supply of petroleum and coal. This renewable energy resource may be depleted within the course of 50 decades. This depletion is putting pressure on many human-caused industries such as agriculture, industry, the extraction of valuable substances for nourishment, and pharmaceuticals, all of which have a direct impact on the global economy because of a lack of energy resources [2]. In these conditions, now is the ideal moment to find and create alternatives, inexpensive, efficient energy sources that are renewable to improve anthropogenic activity sustainably [3]. Because algae have high biomass efficiency, they could be used to generate a large amount of energy. The algae biofuel meets a higher demand for converting the agro-ecosystem beyond nutrition to fuel because these living beings do not require farmland due to their aqueous form.
Microalgae are adaptive organisms that are becoming abundant and widespread; therefore they play an important role in biotechnological as well as environmental studies [4]. Algae are photosynthetic creatures that convert sunlight into biomass and O2. Proteins, lipids, and carbohydrates are the 3 primary macro biomolecules found in algal biomass, along with trace amounts of micronutrients [5]. After the crucial macromolecules are extracted from the algae biomass, the rest of the biomass is able to be fermented to produce additional molecules such as ethanol, biogas, biohydrogen, butanol, and so on. A number of investigations using fermentation by microbial cells to valorize biomass hydrolysate showed the production of bioenergy as well as biochemicals [6]. Numerous researchers are working to identify new renewable energy sources. The use of 1st-generation lignocellulosic algae feedstocks to produce biofuel has sparked a debate between fuel and food. The cost of food is bound to rise as biofuels gain popularity.. As a result, 2nd-generation biofuels derived from both woodland as well as agricultural wastes are being developed [7]. Because of this complicated structure, the sustainability of lignin-rich biomass for biofuel fabrication is limited due to the laborious and pricey pre-treatment needed. Algae are capable of being used as a substrate for producing 3rd-generation biofuels, which might provide an answer for such problems [8].
With the goal to establish an efficient as well as viable organic industry, biological refineries strive to transition from petroleum into readily accessible raw materials that are renewable [9]. In this sense, there are two distinct objectives for the establishment of algae biorefineries: (1) objectives related to production of fuels and other bioproducts (2) objectives related to financial flow or expenditure [10]. Supplying foods as well as biomass, sustaining biomass regrowth as well as variation, reducing ecological impact, reacting to demand swings, as well as transforming different feeds as well as multi-product methods of conversion are all fundamental requirements towards an effective biological refinery [11]. The global energy demand has increased exponentially as employees have multiplied and economies have expanded. Bioenergy is one option that could help the renewable energy sector manage both demand and supply [12]. Bioenergy and other alternative forms of energy outperform traditional energy sources in nearly each usage measure. It also recommends producing supplementary bioenergy from multiple sources in addition to the biological energy generated by particular algae species that thrive on insufficiently utilized water supplies [13]. Because of several advantageous characteristics, bacterial biomass is being given special consideration for biofuels production. Microalgae offer a number of advantages, including rapid development as well as a short harvest period. As a result, they are an ideal source of algae feedstock for the generation of biofuel. It is critical to find an approach to the problem of commercial-scale biomass availability for bioenergy as well as biomolecule production [14].
The algae are able to thrive and develop to become the primary facilitators in water-based environments. The biomass of algae consumes the fewest ingredients in relation to its generation [15]. According to a scientific report, N and P intake for sunflower seeds, rapeseed, and algal cultivation takes about 40 & 25, 45 & 56, as well as 0.003 & 0.002 mg/hectare for the production of 1 kg of biomass per hectare, thus being extremely low for algae growth [16]. The biomass of algae is capable of being used to generate a number of beneficial bioproducts owing to its wide biochemical component. In the past few decades, downstream biological refineries have become interconnected to generate algae-biomass-based biofuels as well as biomolecules at the same time. Because land for farming is constrained, farming algae in marine environments might help fulfill the increasing need for energy worldwide. Microalgal generation of biofuel has exploded and grown more profitable as a result of recent technological advances in photobioreactor technology through combined algae cultivation as well as biorefinery approaches [17]. Before being converted to biofuel, biomass from algae can be used to produce multiple macro biomolecules that include lipids, omega-3 fatty acids, and vital amines. Among the highly valuable goods generated by an algal biorefinery process are biological materials, pharmaceuticals, and biofertilizers [18]. The most valuable compounds extracted from marine algae as well as their possible applications are overviewed in Table 1. Through integrating biofuels as well as natural processing, algal biological refineries have moved closer to commercialization. Microalgae are able to produce high-value goods used in an assortment of industries, including food, nutritional supplements, beauty products, and pharmaceuticals [19]. To accomplish this, a combined biorefinery that produces the majority of its goods using just one biological raw material can be employed. Oil refineries pioneered the concept of biorefinery. Biorefinery generate fuels as well as additional products that are useful to the chemical industry. A variety of strategies can be employed to generate the majority of every single ingredient as well as enhance the quantity of final products [20]. Finding a way to produce such products using an algae-biomass-based biological refinery that is both sustainable and cost-effective is a huge challenge. To transform biomass towards biofuels, various thermal processes, including combustion, hydrothermal liquefaction (HTL), gasification, and pyrolysis, are able to be used. The direct combustion of biomass emits harmful byproducts, including ammonia and NOx, which negate the environmental benefits [21]. Gasification involves a partial process of oxidation that generates syngas at elevated temperatures. Pyrolysis is the process of producing bio-oil, syngas, and biochar from dry algae biomass at moderate to high temperatures without the presence of O2. Nonetheless, the HTL procedure has received a lot of attention because it does not require drying the algae biomass to perform the pre treatment procedures, which saves cost, energy, and time in the entire procedure, as well as additionally generating biocrude, which is the primary byproduct [22]. HTL is an approach that operates in the presence of a solvent under heats ranging from 250 °C to 550 °C as well as pressure ranging from 5–28 Psi whether or not there is the addition of a catalyst over a period of 10 to 60 min [23].
The harsh working conditions cause the biomass to lose water, breaking down into small molecules that are reactive and can re-polymerize into oily compounds. This means that the matter does not need to be dried first. The liquefaction technique produces bioproducts with an O2 level of 10–20 wt.% as well as a boiling point of 30–36 MJ/kg, and that can be further enhanced to fluids that resemble fuel [24]. Several researchers have conducted as overview of the present state of the field as well as potential future of HTL. Microalgae inhabit an unusual environment that allows them to fight for space as well as substances, which means they possess an excellent adaptability approach to living in an assortment of physicochemical parameters [25]. As a result, they have created an excellent defence mechanism as well as novel secondary metabolites. Generally, the generation of such biological products differs from strain to strain, even among identical algae. Environmental factors, seasons, geographic locations, and life cycle stages are just a few that have an impact on the production of bioproducts [26]. Bioactive bioproducts are molecules that are biologically active and have positive effects on humans. People claim that microalgae are genuine organic processors that produce bioactive organic substances. They are also an excellent alternative to chemicals for the production of some bioactive natural compounds, which are important for business. Carotenoids, polyunsaturated compounds, phycocyanins, fatty acids, and polyphenols, for example, have sparked a lot of interest in the research and production of bioactive substances that might have applications as functional constituents [27]. The proteins, lipids, vitamins, and carotenoids of algae can be derived directly from the initial metabolic process or synthesized from additional metabolism. Usually, microalgae acquire bioactive substances in their biomass; nevertheless, in certain instances, these chemical compounds are released into the surrounding environment [28]. A collective information about the updated biotechnological and HTL based approaches to derive the biorefinery products from both micro and macro algae biomass are insufficiently available for the researchers who are working in the algal research. Thus, this review attempts to fill this gap by scientifically compiling the widely discussed limitations and potential solutions to overcome the barriers to obtaining the most valuable biorefinery products from algae biomass using advanced biotechnological and hydrothermal liquefaction (HTL) techniques. This review was prepared by referring more research and review literature from the Scopus database using the following keywords: micro and macro algae biomass, biomolecules, (major and minor), upstream and biomass downstream strategies, advanced technologies for algae cultivation, advancements in HTL process, biotechnological advancements in algae biomass based biorefinery products extractions, and potential catalysts.
2 Major biomolecules
The polysaccharide constitutes a long-chain carbohydrates composed of simpler carbohydrates known as simple sugars that human beings normally require for nutrition and for assisting with the structure of cells [29]. Polysaccharide worth fluctuates depending to purpose, accessibility, and quality. Algae that synthesise a wide variety of carbohydrates in commercially, Porphyridium cruentum are utilised for the production of carbohydrates [30]. Under appropriate circumstances, polysaccharides are able to be obtained up to 55% of the dried biomass content (dwt) of algae. Cell walls of aquatic green algae (Chlorella strains) contain up to 80% complex carbohydrates (Fig. 1), notably cellulose. Sulphate carbohydrates are among the least significant algal carbohydrates in terms of biological function as well as are a major supplier of bioactive organic substances with biomedical properties such as antitumor, anticoagulant, immunomodulatory, antithrombotic, antimutagenic, antiviral, antimicrobial, and anti-inflammatory properties [31].
The progress of several algal biorefinery products is described in a Scopus data base-based report (a) Quantity of publications by year (b). Image adopted with proper permission [135]
Certain microalgae contain a lot of oil. The quantity and structure of algae lipids, on the other hand, vary depending on algae species, place of origin, period, salinity, temperature, degree of light, and an assortment of all factors [10]. Algae that possess lipids up to 23% dwt. Algae that have an extremely high (60% dwt) lipid percentage in a variety of marine algae like Dunaliella tertiolecta, Porphyridium cruentum, Nannochloropsis sp., and Neochloris oleoabundans, as well as certain freshwater algae like as Chlorella emersonii, Botryococcus braunii, and so on [32]. Moderate level of lipids (60% dwt) had been discovered in a variety of marine algae like Schizochytrium sp., Crypthecodinium cohnii, Nitzschia sp., Dunaliella salina, Nannochloris sp., Isochrysis galbana, Phaeodactylum tricornutum, and Nannochloropsis oculate [33]. The majority of the fat found in algae is composed of polyunsaturated fatty acids (PUFAs) that are vital nutrients for human beings and need to be acquired through food[34]. These also serve as both the functional and structural elements of the membrane of cells. Long-chain ω-3 fatty acids like docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) have received a lot of attention recently because of their significance for development, food, and medicinal and pharmacological advantages [35]. The microalgae S. platensis represent an organic source of DHA, accounting for as much as 9.1% of the overall fatty acid concentration. The EPA value of different microalgae can ranging from 0.7% – 6.1% of the overall lipids. The ω-3 production directly from microalgae has the potential to outperform the fish liver oil [35].
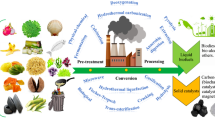
Protein concentrations in microalgae biomass can range between 6 and 52% of their dry mass. The species, pH, temperature, light intensity, salinity, minerals, season, CO2 availability, population density, as well as stage of growth biological status can all affect the quantity of proteins in algae biomass [36]. It contains the total component of various freshwater as well as marine algae. The vital amino acids found in food proteins determine its nutritional value. Microalgae may accumulate substantial quantities of alanine, isoleucine, aspartic acid, glutamic acid, arginine, glycine, valine, leucine, lysine, and threonine are among the most abundant amino acids[37]. Asparagine and glutamine have interesting flavour formation properties. All vitamins produced by larger plants can be synthesised by marine algae. Algae vitamin deposition is higher compared to that of beans as well as grains, however it varies depending on algal species, period, alga phase of growth, as well as conditions in the environment [38]. Researchers discovered that the red-colored Porphyridium cruentum might accumulate an elevated amount of tocopherols [39]. Investigators additionally discovered that the aforementioned microalga contains 55 g/g & 51 g/g dwt of α and γ-tocopherols, correspondingly. Tocopherols represent lipid-soluble anti-oxidants which are important nutrients due to their capability to safeguard lipids in membranes from oxidative damage [40]. Chlorella is the most abundant supply of B12. Vegans and people who are vegetarians may benefit from this alternative source of vitamin B12 [41]. The Fig. 2 and 3 illustrates the process involved in the biorefinery production process and potential applications.
Schematic illustration of the manufacturing process and potential applications. Image adopted with proper permission [135]
Flowchart showing common biorefinery process phases and products derived from algal biomass. Image adopted with proper permission [135]
3 Minorbiochemicals
3.1 Antioxidant
Microalgal biomass contains a high concentration of antioxidants (e.g. Astaxanthin) and has conceivable uses in human consumption, aquatic nutrition, beauty products, and pharmaceuticals [41]. The discovery that reactive oxygen species and oxidation play a role in a variety of physiological processes that contribute to illness has sparked interest in antioxidant therapeutic applications. Algae evolved to withstand harsh conditions over thousands of generations, according to evolutionary theory. They are exposed to significant O2 and radical stressors as a result of their phototrophic lifestyle [42]. Because of this, their bodies have developed many effective defence systems to stop the formation and accumulation of free radicals and reactive oxygen species. This keeps microalgae safe from things that damage cells.
Carotenoids represent a significant group of lipid soluble pigments as well as antioxidants, which have a significant role in oxygen-dependent photosynthesis [43]. They cannot transmit the sunlight's energy through the photosynthesis process; rather, they pass it from one chlorophyll substance to another. Carotenoids content in ranging from 0.1–2% of dry weight in the majority of algae [44]. Although some of the 400 identified carotenoids possess substantial industrial significance, such as astaxanthin and β-carotene, others, such as bixin, lutein, lycopene, and zeaxanthin, have less [45]. The physical and chemical characteristics of aquatic environments can affect the carotenoid content of microalgae biomass. Many environmental properties change based on the seasons and may increase or decrease carotenoids biosynthesis [45]. The finding paved the way for the commercial extraction of natural carotene from this living thing. A variety of investigators have looked into the specific growing circumstances that can enhance β-carotene extraction on a commercial level. In stressful conditions, the marine algae Dunaliella salina and Haematococcus pluvialis can accumulate a great deal of carotenoids (e.g. astaxanthin), as much as 2%–3% by dwt [46].
3.2 Minerals
The presence of minerals in algae is well known. Ashes account for over 6.7% of the total dry weight of microalgae such as Spirulina [46]. Generally, the amount of minerals is heavily reliant on atmospheric growing conditions, including season, temperatures, physiological state, geographical changes, and so on.
3.3 Phenolic substance
Microalgae produce phenols as secondary metabolites, a class of natural products with antioxidant and other biological properties [47]. These substances play a significant role in algal cell protection against stress from both abiotic and biotic stressors. These substances, however, do not play a direct role in algal fundamental functions like the processes of photosynthesis, proliferation of cells, as well as reproduction. Refined phenolic substances have a wide range of operations, including antioxidants, anti-radicals, anti-fouling, UV protection, and chelation of metals. The primary bioactivity associated with phenolic substances, nevertheless, is their antioxidant properties [48]. Many phenolic compounds found in algae, including rutin, epigallocatechin gallate, hesperidin, morin, catechin, caffeic acid, and catechol have been linked to anti-inflammatory activity (Fig. 4) [49]. This phenolic compound may also be able to fight free radicals [47].
The algae biomass biorefinery yields various products with additional value. Image adopted with proper permission [135]
4 Microalgal- biofuels
Autotrophic algae absorb CO2 as well as yield polysaccharides, H2, polypeptides, as well as fats that can be used as substrates for biofuels such as bio-oil, biohydrogen, biodiesel, biochar, biogas, butanol, and bioethanol [50]. A lot of common microalgae can store a lot of biofuels. The biofuels derived from various algae biomass considerably greater than the fuels derived from terrestrial plant biomass. The Table 2 showed the biofuels obtained from algae feedstock were compared with biomass of terrestrial plant. For example, Chlorella protothecoidesis perfect for making biodiesel because it can store 55% of its weight in lipids when grown heterotrophically with few nitrogen [51]. Microalgae-based biodiesel is usually made in two steps: first, lipids are taken out of microalgae cells; then, alcohol is used with catalysts to change the lipid fraction into an ester. A yield of as much as 300 mol H2/mg has been observed in Scenedesmus obliques, making S. obliques appropriate for the production of biohydrogen [51]. The most common routes include an indirect method that first produces biomass through photosynthesis and then converts the polysaccharide-rich biomass into bio-hydrogen through fermentation and/or photofermentation. There is also a direct or indirect water biophotolysis process, which converts water into hydrogen and oxygen. Some types of microalgae have also been shown to produce biogas, with a yield of about 0.70 L biogas g VS-1[52]. These results were obtained by growing a lot of Nannochloropsis salina in photobioreactors at 35 °C. Furthermore, the algae biomass is capable of being fermented to produce biogas, as demonstrated by a concentration of 3.83 g L−1 generated from 10 g L−1 of lipid obtained from Chlorococum sp. biomass while the algae biomass was used as an alternative feedstock via fermentation using yeast [18]. In practice, the incorporation of H has been shown to alter the combustion properties of gas from natural sources. Certain algae, like Spirulina sp., are capable of producing biomethanol via combustion or fermentation in anaerobic conditions [53]. It was additionally found that a micro-algal consortium is able to utilise waste water as an environment for growth in outdoor water bodies, and the recovered biomass can be collected as a type of biochar substrate, yielding 45% dw hard biochar through hydrothermal liquefaction (HTL) [54].
4.1 Upstream strategies progress
Generally, the upstream strategies suitable for microalgae are divided into three categories. In the beginning, it is preferable to choose some appropriate isolates from the wild that are characterized by durability, rapid growth, as well as lipid richness [55]. Second, sophisticated genetic manipulation can be employed to alter to generate strains that have elevated yields of lipids as well as rapid growth rates. Third, additional techniques including genetic engineering may be employed to increase lipid along with additional fuel component deposition. Microalgae genetic engineering could potentially be employed to, for instance, remove photosaturation as well as photoinhibition, that will substantially boost outside culture yield while enhancing the financial viability of algal oil generation [56]. To circumvent the present limitations on the distribution of genetically engineered organisms, nevertheless long-term investigations and investment will be required [57]. As a result, for the time being, it seems sensible to constrain expectations to what has been accomplished with wild-type varieties.
4.2 Strain selection
According to estimates, there are approximately ten million algae species on the planet, with more than forty thousand species documented [58]. Microalgae are known for their fast expansion rate as well as their elevated lipid level; however, not all of them are considered the best lipid producers; this depends on the strain. In this context, identifying appropriate variants with the ideal ratio of algae biomass yield and lipid composition in external cultivation is an essential need for algae biomass-based biofuels [59]. It would be interesting, for example, if a strain could accumulate fats despite a lack of nutrients. The strains should be able to withstand the breaking stress caused by mixing with wild-type strains or other types of microbes. Furthermore, they are adaptable to changes in the physical and chemical properties of their growing environment. A specific strain of sea or freshwater algae can be extracted using a tiny pipette beneath a magnifying glass, followed by cell dilution and propagation on a fluid medium or an agar plate [60]. The Winkler test screening protocol included a new, quick way to choose mutant strains and study how algae use hydrogen without putting them under nutritional stress [61]. This method was suggested as a way to separate the Chlamydomonas reinhardtii strains that produce hydrogen. A total of four strains exhibiting elevated biomass yield and lipid content have been identified among 30 algae strains, two of them marine and two of them freshwater. During nitrogen deprivation, the Nannochloropsis sp. acquired as much as sixty percent of the lipids [62]. Furthermore, some regionally isolated varieties are better adapted to the highly unpredictable setting of outside cultures, yet they may struggle to dominate year-round in the fluctuating cultivation parameters [62].
4.3 Genetic engineering
Genetic engineering can be described as the use of biotechnology to directly alter the DNA of an organism [63]. Some researchers have previously used a critical systemic approach to obtain high concentrations of microalgal biomass for long-term industrial applications, as well as to alter the metabolic pathway to produce more expected high-value products. There are many ways to change marine algae, such as using glass beads, electroporation, the artificial transposon method, biolistic transformation, recombinant eukaryotic algal viruses, the silicon carbon whiskers method, microinjection, and also changing the algae on its own [64]. Regarding the approaches, transferring genes by electroporation is distinguished because of its ease of use and high effectiveness with only a tiny amount of DNA. It has been used for more than three decades in different cells, including bacteria [65]. A voltage field (1–1.5 kV, or 250–750 V/cm) is able to be applied to cells to improve the permeability of cell membranes, followed by chemical compounds or DNA that are able to enter the cells [66]. The technique works on both bacterial cells as well as eukaryotic algae. For instance, the marine algae Nannochloropsis sp., which has the ability to produce biofuels, has been efficiently genetically modified by inserting multiple knockout genes that regulate the metabolism of nitrogen [67]. Nonetheless, the limitations of genetic engineering on algae are due to immature protoplast processing as well as juvenile regenerating methods. For many types of diatoms, direct gene transfer through biolistic transformation (micro-particle bombardment) has been shown to work best [68]. This method is also frequently used to alter the expression systems in the nucleus and chloroplasts. The approach has some advantages, such as being the only effective method for continuously transforming chloroplasts, mitochondria, and other organelles. It has the ability to deliver external DNA to a wide variety of cells. Various endogenous vectors can also be used to change living things via a mature and controllable manipulation process [69]. This method, on the other hand, is highly reproducible and works with specialized and costly gadgets. DNA, in particular, is usually covered with gold atoms and delivered inside the cell through pressurised helium gas [70]. Over 20 marine algae strains were successfully modified using the methods described above. Scientists have shown that marine cyanobacteria can change their genes using the trans-conjugation approach on five strains of Pseudanabaena sp. and Synechococystis sp. [64]. There are five types of acceptor cells in brown algae that can change in response to particles. These are young sporophytes, male and female gametophytes, tissue remnants from sporophytes, and parthenogenetic sporophytes [71]. Foreign DNA can also be introduced into the marine microalgae Dunaliella salina and the freshwater microalgaeC. reinhardtii employing glass-bead agitation [72].
4.4 Downstream strategies progress
Microalgae downstream technologies mostly include growing a lot of the right microalgae strains, coming up with efficient cultivation systems and different ways to grow them to avoid conflicts between producing biomass and lipids, finding energy-efficient ways to harvest and dewater them, and coming up with efficient extraction and conversion technologies.
5 Mass cultivation
Mass cultivation is essential for commercializing microalgal-derived biofuels because substantial biomass allows for the production of biofuel [73]. In general, factors like the right strain, cultivation, process control, and other parameters limit it. A suitable strain ensures a substantial biomass yield. As previously stated, it is critical to choose viable algae species that have substantial oil content along with the ability to develop rapidly in culture, since this constitutes one of the critical keys to producing biodiesel, biocrude, drop-in fuels, and establishing sustainable projects [74]. According to Algae 2020, five key tactics, including algae kinds that have elevated oil content, establish more swiftly, less expensively, simpler, and fragmentation marketing approaches, are being recognised as critical factors in the industrialization of microalgae-biomass-based bioenergy [75]. Strains that have elevated lipid levels have potential as species biomass for biodiesel, while this could serve as the foundation for profitable commercial microalgae cultivation. Triglycerides (TAG) are found in huge quantities in diatoms, while Dunaliella sp. and Tetraselmis sp. have lipids that contain up to 56% as well as 50% of their dry cell weight, respectively [76].
5.1 Scale‑up process
The industrialization of algae-based biofuel depends upon the elevated concentration of biomass as well as amount of lipids obtained by massive production of microalgae [77]. Massive farming, conversely, presents enormous obstacles, the most significant of which is its elevated manufacturing cost, which is due to the intense consumption of energy needed for the intricate methods of algal production such as sterilisation, blending, air circulation, light, exchange of gases, as well as others [78]. Techniques that include sterilisation for huge volumes of cultures and maintaining sterility throughout the production system, appropriate illumination as well as deoxygenation approaches, along with additional processes for improved procedure control, for instance, remain difficult and costly [56]. Hence, it is essential to reduce the expenses associated with farming microalgal that may be triggered by the aforementioned processes whilst preserving substantial biomass concentrations as well as lipid yield. Offsetting the total expenses primarily consists of collaborating on products with added value, optimising algal production methods, and reducing expenses for cultivation through the use of effluent or flue gas as carbon and nutrient sources [79]. Several effective instances of large-scale algae cultivation have been carried out by enterprises as a result of ongoing efforts to enhance and advance techniques. Algenol is a well-known excellent team equipped with cutting-edge technology and unique algae production techniques. The organisation uses photobioreactors with outputs 2–3 times those of natural ponds, as well as VIPER manufacture in its 40,000-square-foot plant. Besides producing some valuable goods including organic colourants, protein, Spirulina, cosmetic components biofertilizers, as well as biological stimulants, Algenol additionally gives attention to renewable energy sources such as ethanol from biomass as well as green oil that are produced using HTL technological advances for crude oil production [80]. Optimised extraction and conversion methods increase the potential for harvesting microalgal biomass. Most extraction techniques work well in a research setting but are difficult to apply on an industrial level due to their size and complexity. Earlier studies, for example, discovered that large amounts of usage of solvent typically the most expensive when harvesting lipid from moist algae employing sustainable solvents like cyclopentyl methyl ether and 2-methyltetrahydrofuran [81]. They additionally investigated various lipids extraction techniques and found that the Hara &Radin technique extracted the greatest amount of lipids from Chlorella pyrenoidosa (66% moisture) employing 3:2 (v/v) hexane/isopropanol [82, 83]. Nevertheless, the costs of green solvents cannot be comparable with those of fossil-based chemicals. Furthermore, a without solvents osmotic shock prior to treatment approach has been employed to obtain lipid in order to generate methane from Chaetoceros muelleri, and D. salina yielding a fatty acid extraction effectiveness of 21% as well as 72%, correspondingly [84]. In addition, for inexpensive lipids extraction, several modern techniques such as supercritical fluid extraction, microwave-assisted extraction, switchable hydrophilicity solvents, and the application of ionic fluids are suggested [85].
6 Progress in algae biomass conversion to biofuels
Algae biomass is capable of being transformed to sustainable fuels as well as energy sources using a variety of techniques such as (a) thermochemical conversion (gasification, combustion, and pyrolysis) (b) transesterification, as well as (c) bio conversion (photobiological hydrogen fabrication, anaerobic digestion, and fermentation) [86]. In a supply of O2, furnace (combustion chamber), boiler, and turbine of steam at approximately 1000 °C, burning directly is employed to transform algae biomass towards hot gases for the generation of energy, and this can operate a turbine as well as turn the generator to generate power [87]. For instance, using biomass for electricity as well as heat could be accomplished by burning direct-firing within a furnace, at which high-pressured steam is generated as well as established towards a turbine powered by steam, and then moves over a number of blades on the turbine, causing the engine as well as electric generator to spin thereby producing electricity[87, 88]. The combustion of biomass requires a restricted quantity of O2 to generate CO2 and power, and this promotes another response, the transformation of more biological substance to H2 as well as more CO2. While the final reaction takes place in the form of CO as well as leftover water, certain CH4 as well as CO2 are generated. Direct as well as traditional transesterification can generate methyl esters (FAME), as well as the chemical formula indicates that triglyceride as well as methanol act as catalysts to yield glycerol as well as methyl esters [89]. In contrast, the hydrotreatment as well as gasification procedure for turning algal oil generating jet fuel includes the stages of gasification, dehydration, combustion, and pyrolysis shift reactions. Pyrolysis involves the thermal breakdown of biomass with the lack of O2, as well as the technique can be categorized into traditional pyrolysis, rapid pyrolysis, and flashing pyrolysis in order to generate liquid fuel, biochar, (solid fuel), as well as gas fuel molecules (H2, and CH4) [90, 91]. Anaerobic breakdown is the technique that produces methane using delipidized algae biomass containing C and N through a series of phases of acetogenesis, hydrolysis, methanogenesis, and fermentation. The technique transform organic matter produce biogases, as well as tiny digesters for biogas are currently used in a number of developing nations[92]. Fermenting seeks at transforming the cellulose-based or starch components of algae biomass towards bioethanol through a series of processes including dehydrating, grinding, liquefaction, and ultimately when the bioethanol is produced [93]. HTL has become known as a highly feasible method for converting algal biomass into liquid fuels and other products with additional value. The process produces bio-oil at lower temperatures (typically 300 °C -350 °C) with elevated pressures (5–20 MPa) using catalysts as well as hydrogen [94]. The process efficiently converts algal biomass with water-related activity towards simpler molecular elements with substantial amounts of energy [95]. The disadvantage of the traditional HTL approach opens up opportunities for a two-phase sequential hydrothermal liquefaction (SEQHTL) approach, which overcomes the constraints of the traditional approach in retrieving bioactive substances [95, 96].
6.1 Dry thermochemical process
This encompasses multiple processes such as pyrolysis, gasification, torrefaction, carbonization, and liquefaction, with the main requirement that the biomass be moisture-free [97]. Carbonization is a lengthy procedure in which biomass is subjected to heat in a vacuum to generate carbon-based goods, pyrolysis is a procedure in which biomass is ignited in a fatal inactive environment primarily using N2 at temperatures ranging from 300 °C to 900 °C [98], liquefaction can be separated towards both direct and indirect steps, in which the biomass becomes liquid at approximately 160 °C to 280 °C in an atmosphere consisting of glycerol; and gasification is a process in which biomass has become heated over 1000 K [99].
6.1.1 Pyrolysis
When dry biomass is heated with nitrogen, it can go through flash or fast pyrolysis, slow pyrolysis based on heating rate, microwave-assisted pyrolysis based on heating medium, and hydropyrolysis, which takes place in a high-pressure hydrogen environment [100]. The correct sort of conversion technique must be chosen in order to achieve the intended outcomes. The slow process of pyrolysis usually takes place at an extremely modest heating speed of 5–10 °C/min, and as examined by the investigation team, this approach generates an estimated 43% by quantity of oil [101]. Rapid pyrolysis, on the other hand, requires a greater heating rate ranging from 100 °C—200 °C per second as well as a brief time to dwell over the reaction; greater heating temperatures of up to 500 °C were also examined by researchers in order to generate bio-oil [102]. The identification of the product revealed that fast pyrolysis yields bio-oil with less O2 than slow pyrolysis.
6.1.2 Torrefaction
The technique involves heating algae biomass at temperatures ranging from 200 °C to 300 °C. The occurrence of N2 at the level of the atmosphere generates solid char as the primary outcome and liquids as well as gases as leftovers. A combination of temperatures as well as residency time, the conversion technique is divided into two different groups: both dry and wet torrefaction [103]. Both of them have different benefits and drawbacks when employed for the process of converting algae to solid fuels. Because dry (200–300°C) torrefaction consists of moderate pyrolysis, the water content in the algae biomass must be decreased to 10% to achieve outstanding results [103]. Wet torrefaction (180–260°C) necessitates an intricate reactor method to sustain elevated pressure, which raises the total expense of the method. The main benefit of both methods is that the biochar obtained has superior properties and can be processed, dried, and employed directly solid fuel without additional preparation [104].
6.2 Wet thermochemical conversion
There are three kinds of wet thermochemical conversions: hydrothermal carbonisation, hydrothermal gasification, and hydrothermal liquefaction. Hydrothermal carbonisation is primarily concerned with the generation of solid substances like biochar, and it takes place at moderate to medium temperature ranges (180–275 °C) as well as pressures less than 2 MPa [105]. Hydrothermal liquefaction emphasises the generation of a fluid substance known as bio-crude, which is subsequently transformed into bio-oil following treatment. The procedure occurs at moderate to elevated temperatures (280–370 °C) as well as pressures that vary from 10 to 25 MPa. The main outcome of the hydrothermal gasification process is combustion, with high calorie values. This gasification procedure occurs at temperatures ranging from 400 °C to 700 °C and pressures ranging from 25 to 30 MPa [106].
6.2.1 Hydrothermal liquefaction (HL)
The HL is also referred to as a method that converts biomass into biocrude in either the presence or absence of a catalyst [107]. The operating temperature ranges from 280 °C to 370 °C, and the pressure ranges from 10 to 25 MPa during hydrothermal liquefaction. Processing conditions like temperature, pressure, biomass loading, and residence time affect the process's yield and efficiency [108]. The transformation of biomass into valuable products is additionally influenced by the algae strain employed, and numerous attempts have been made with different processing circumstances to generate a liquid product according to the data available. The process of depolymerization of algae biomass occurs during hydrothermal liquefaction, thereby helping in the generation of biorefinery. The biorefinery obtained has an increased value of energy compared to pyrolysis, which uses less energy [109]. If the generated biorefinery is destined to be employed as fuel, the greater temperature coefficient achieved by hydrothermal liquefaction seems critical.
6.2.2 Non-Catalytic HL (NCHL)
Liquid is important for both catalysts and solvents for non-catalytic hydrothermal liquefaction (NCHL). Water can be employed as a catalyst, but a catalyst isn't employed in the HL method [110]. The properties of water at subcritical pressure are important for figuring out how biomass breaks down and how it is processed hydrothermally. This is because at subcritical and critical temperatures, water, a gas like CO2, and O2 can mix [110]. In essential as well as subcritical illnesses, organic substances are dissolved in water. In contrast, inorganic substances that use solvents are going to decrease under extreme circumstances.
6.2.3 Catalytic HL (CHL)
It has been extensively shown that the existence of a catalyst promotes the transformation of algae biomass into biorefinery products, resulting in a greater quantity of products [111]. Various catalysts are employed in the HL process. In HL, two distinct kinds of catalysts are employed: uniform catalysts as well as heterogeneous catalysts. KOH, K2CO3, NaOH, Na2CO3, and LiOH among the most frequently employed catalysts in the HL of alkaline solutions [112]. It is very important for catalysts to break down the existing polysaccharides (like cellulose and hemicellulose) into small pieces that are unstable, reactive, and can re-polymerize into oil-based compounds. The biorefinery output differs based on the type of microalgae. The different lipid content of the algae is to blame for this. Catalytic NaOH substantially boosts the final yield of spirulina [113].
6.2.4 Homogenous and heterogeneous catalyst
Typically, HL employs water-soluble catalysts such as HCOOH, Na2CO3, CH3COOH, and KOH. Thus, through enhancing the hydrothermal procedure, they often cause algae to dissolve [107]. In the HL process, sodium carbonate is a very frequently employed homogeneous catalyst. In hydrothermal processes, carboxylic acid, carbonate, and hydroxide homogeneous catalysts do not appear to have become extremely prevalent [114]. The homogeneous catalyst is employed to a certain degree over the reaction, however, the restoration expense is elevated, so it can be challenging to choose it for long-term cyclic application [115]. The heterogeneous catalyst system has the benefit of separating liquefied substances from solid leftovers and can be recycled after a thorough cleaning. Because of their greater catalytic effectiveness, low rust, as well as increased biorefinery reimbursement, mixed catalysts are extensively utilized. Among the more common drawbacks of homogeneous catalysts is the harm caused by chemical reactions, which do not exist in heterogeneous types of catalysts [116]. Supporting metallic catalysts like Ni, Ru, Pd, and so on have been thoroughly studied for HL. Metallic catalysts had an intricate impact on biorefinery yield in algal biomass HL, yet not every catalyst can substantially boost yield [107].
7 Influence of various parameters on the Biorefineries
7.1 Temperature
The temperatures of the hydrothermal method are critical in the development as well as the yield of bio-crudes; the incorporation of catalysts merely enhances output but also improves the properties of the biorefinery produced [117]. A temperature rise typically results in faster depolymerization, resulting in increased biorefinery result in as well as gas products, nevertheless, this additionally relies on different procedure conditions such as catalysts quantity, residence time, and so on, so biorefinery production can differ at the identical temperature owing to variations among different process illnesses or catalysts kind [118]. The existence of bonding peptides is among the primary explanations that the HL production of algal feedstock exceeds that with other resources; at reduced temperatures, the bonds of peptides remain firm, causing lower biorefinery production [119]. However, at greater temperatures ranging from 300 to 400 °C, the bonds of peptides become unstable and promptly hydrolyze, resulting in an elevated biorefinery yield [120].
7.2 Pressure
When it relates to degradation and hydrolysis, HL additionally gets affected by pressure. Although greater enthalpies aren't needed in phase shifts, a boost in pressure can be employed throughout the HL method [121]. To determine the influence of pressure in the method, every other parameter was held steady whilst the applied pressure was changed; this revealed that there would be a substantial rise in biofuel as the pressure rises. The stresses beyond the critical value can be sustained to regulate the speed of hydrolysis as well as biomass disintegration, increasing biorefinery yield via enhanced thermodynamics [122]. While the stress is raised, the quantity of oil generated by a catalyst decreases. This can be owing to the blockage on the catalyst's active location via higher-density solvents. This emphasizes the importance of catalytic selection when elevated-pressure liquefaction.
7.3 Residence time
The period needed for the hydrothermal process to take place once the ideal temperature has been attained, excluding both heating as well as cooling time, is known as resident time [123]. This residence period is critical to acquiring more valuable biorefinery along with additional beneficial products. Inadequate conversion can happen from an absence of residence time, whereas excess residence time could lead to a breakdown of desired goods [124]. Because constituent breakdown, as well as hydrolysis, occurs quickly, shorter residence times have been favored over extended residence times in HL at greater temperatures. The incorporation of the catalyst during the moment could enhance the conversion rate because it allows the response to take place much faster compared to what it could in identical process circumstances [125].
7.4 pH
The substrate of the reaction is typically pH-dependent throughout the hydrothermal procedure, investigators have discovered that the HL of feedstock generates greater quantities in alkaline as well as acidic environments based on the sort of catalysts as well as solvents used [126]. Under acidic environments, substances such as levulinic acids, as well as 5-(Hydroxymethyl) furfural (5-HMF), are unable to disintegrate. Because of the self-destruction of water to H+ as well as OH− in extremely elevated temperatures, the resulting biomass generates carboxylic acid functional groups including lactic acids and acetic acids. When the process's environment remains slightly alkaline at the start, its pH will rise to acidity as the process continues. The byproducts of HL consist mainly of liquid as well as the pH level of the reactant environment is critical [127]. When the biorefinery tested the pH of a solution, it discovered that the medium used for the reaction should be acidic to ensure greater quantities at low temperatures, but alkaline to generate higher yields at low to high temperatures. However, it is important to recognise that there can be variations in output at identical conditions while utilising various catalysts because they typically influence the reaction's medium; thus, catalytic selection is critical in the hydrothermal procedure [128].
7.5 Biomass loading
Biomass loading plays an important role in assessing the procedure and the quantity of product that can be acquired; during the HL procedure, the feedstock-to-water proportion is expected to be lower than during carbonization for easier hydrolysis as well as the production of more liquid compounds [129]. Retaining optimal loading is critical, and it has been stated that increasing biomass load increases bio-crude yield, however after a certain level, output decreases owing to feedstock degradation via cyclic development, polymerization, as well as condensation processes [130]. According to researchers the biomass input of 10–20%, the production rate of biofuel improved and achieved an optimal level of 30% at the critical temperature. The catalytic transformation of lipid-high microalgae biomass as well as discovered an optimal output of 28% for a feedstock load of nearly 9% as well as associated catalyst concentration [131]. Other researchers investigated sustained HL of algae as well as discovered that at 350 °C with a 10% loading generated an output of 41%. Researchers also demonstrated that increasing the 5–35% of Nannochloropsis sp. feedstock loading for the HL produced a 36–46% rise in biorefinery yield [132]. As a result, numerous investigations have found that increasing the biomass dosage enhances biorefinery production, but this is dependent upon the type of biomass as well as the process parameters [129].
7.6 Dosage of Catalyst
While investigating, the catalyst quantity is critical. When there exists too little or excessive catalysts in the overall system, the resulting yield could be suboptimal [133]. A research team examined the catalytic HL of Nannochloropsis sp. biomass employing a metal-based TiO2 as a catalyst; their findings demonstrated that the final volume of biorefinery climbed from 30–42% while Ni-based titanium dioxide was employed, but decreased to 29% while Fe was employed; every other process-related factors remained a constant [107]. The two types of catalysts, HCOOH, and Na2CO3 are homogeneous, which results in less production when juxtaposed with heterogeneous catalysts such as Co/Al2O3 and Ni/Al2O3 in chlorella vulgaris through the HL process [107]. While Ni/Al2O3 has been employed as the catalyst of choice for the process, the biofuel yield corresponds to approximately 30% at 350 °C, while when Co/Al2O3 is employed as the catalyst for the process, the biofuel production is approximately 39% at 350 °C [134].
8 Conclusion and recommendations
Algae-based biorefinery and biofuel have been identified as one of the most promising substrates for the production of highly valuable goods and the development of new biofuels. However, the most significant impediment to its industrialization is its high manufacturing cost, which is the result of substantial investments and operating costs in terms of sophisticated arrangement, light, cooling, blending, deoxygenation, and other operational requirements. Many resources have been spent to investigate significant breakthroughs in the development of upstream and downstream methods to reduce the costs of acquiring large biomass concentrations as well as the increased levels of expected biorefineries and biofuel products. Several suitable microalgal strains have been investigated or genetically engineered to ensure excessive microalgal biomass production, and win–win approaches such as two-stage farming have demonstrated the potential for acquiring both a large amount of biomass and elevated levels of lipids or alternative fuels. Furthermore, improved HL harvesting and algae biomass-to-bioproducts-biofuels conversion methods make algae-based biorefineries, specifically polysaccharides, lipids, fuels, and so on, more viable. The production of catalysts with increased activity and equilibrium, longer lifespan, and lower costs remains dependent on a thorough understanding of the catalytic process. Furthermore, it appears that gathering and cleaning water-insoluble and soluble biorefinery separately is a better strategy than processing them all at once. Nonetheless, significant obstacles remain in scaling up algae biomass generation and the HL procedure, which should be addressed in the coming years. Although genetic engineering and the HL process produce fine biorefinery at a lower cost than traditional methods, there is still a long way to go in terms of effectiveness and cost-effectiveness. The hydrothermal technique is still largely a lab-scale procedure with small results that will suffice if scaled up to a commercial level, so much research is needed to improve the procedure and scale it up to meet future demands.
Data availability
All data is available under request.
References
Owusu PA, Asumadu-Sarkodie S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Engineering. 2016;3:1167990.
Maximillian J, Brusseau M, Glenn E, Matthias AD, Pollution and environmental perturbations in the global system, In: Environmental and pollution science, Elsevier, 2019, pp. 457–476.
Oyedepo SO. Towards achieving energy for sustainable development in Nigeria. Renew Sustain Energy Rev. 2014;34:255–72.
Beacham TA, Sweet JB, Allen MJ. Large scale cultivation of genetically modified microalgae: a new era for environmental risk assessment. Algal Res. 2017;25:90–100.
D.K. Yadav, A. Singh, V. Agrawal, N. Yadav, Algal biomass: a natural resource of high‐value biomolecules, bioprospecting of plant biodiversity for industrial molecules, 2021:303–334.
Bhatia SK, Ahuja V, Chandel N, Gurav R, Bhatia RK, Govarthanan M, Tyagi VK, Kumar V, Pugazendhi A, Banu JR. Advances in algal biomass pretreatment and its valorisation into biochemical and bioenergy by the microbial processes. Biores Technol. 2022;358: 127437.
Mungodla SG, Linganiso LZ, Mlambo S, Motaung T. Economic and technical feasibility studies: technologies for second generation biofuels. J Eng Design Technol. 2019;17:670–704.
Thanigaivel S, Priya A, Dutta K, Rajendran S, Vasseghian Y. Engineering strategies and opportunities of next generation biofuel from microalgae: a perspective review on the potential bioenergy feedstock. Fuel. 2022;312: 122827.
DwiPrasetyo W, Putra ZA, Bilad MR, Mahlia TMI, Wibisono Y, Nordin NAH, Wirzal MDH. Insight into the sustainable integration of bio-and petroleum refineries for the production of fuels and chemicals. Polymers. 2020;12:1091.
Bhattacharya M, Goswami S. Microalgae–a green multi-product biorefinery for future industrial prospects. Biocatal Agric Biotechnol. 2020;25: 101580.
Dutta N, Kundu P, Lee JTE, Bhattacharya S. Implementation and optimization of algal biomass in value-added products recovery: a step towards algae-based green economy. Hydrobiology. 2023;2:326–46.
Souza GM, Ballester MVR, de Brito Cruz CH, Chum H, Dale B, Dale VH, Fernandes EC, Foust T, Karp A, Lynd L. The role of bioenergy in a climate-changing world. Environ Dev. 2017;23:57–64.
Zhang T-Y, Hu H-Y, Wu Y-H, Zhuang L-L, Xu X-Q, Wang X-X, Dao G-H. Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew Sustain Energy Rev. 2016;60:1602–14.
Hoang AT, Sirohi R, Pandey A, Nižetić S, Lam SS, Chen W-H, Luque R, Thomas S, Arıcı M, Pham VV. Biofuel production from microalgae: challenges and chances. Phytochem Rev. 2023;22:1089–126.
Bharathiraja B, Chakravarthy M, Kumar RR, Yogendran D, Yuvaraj D, Jayamuthunagai J, Kumar RP, Palani S. Aquatic biomass (algae) as a future feed stock for bio-refineries: a review on cultivation, processing and products. Renew Sustain Energy Rev. 2015;47:634–53.
Bastianoni S, Coppola F, Tiezzi E, Colacevich A, Borghini F, Focardi S. Biofuel potential production from the Orbetello lagoon macroalgae: a comparison with sunflower feedstock. Biomass Bioenerg. 2008;32:619–28.
Wicker RJ, Kumar G, Khan E, Bhatnagar A. Emergent green technologies for cost-effective valorization of microalgal biomass to renewable fuel products under a biorefinery scheme. Chem Eng J. 2021;415: 128932.
Siddiki SYA, Mofijur M, Kumar PS, Ahmed SF, Inayat A, Kusumo F, Badruddin IA, Khan TY, Nghiem L, Ong HC. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: an integrated biorefinery concept. Fuel. 2022;307: 121782.
Mehta P, Singh D, Saxena R, Rani R, Gupta RP, Puri SK, Mathur AS, High-value coproducts from algae—an innovational way to deal with advance algal industry, Waste to Wealth 2018:343–363.
A. Dossey, J. Tatum, W. McGill, Modern insect-based food industry: current status, insect processing technology, and recommendations moving forward, in: Insects as sustainable food ingredients, Elsevier, 2016, pp. 113–152.
Tomlin AS. Air quality and climate impacts of biomass use as an energy source: a review. Energy Fuels. 2021;35:14213–40.
Okolie JA, Epelle EI, Tabat ME, Orivri U, Amenaghawon AN, Okoye PU, Gunes B. Waste biomass valorization for the production of biofuels and value-added products: a comprehensive review of thermochemical, biological and integrated processes. Process Saf Environ Prot. 2022;159:323–44.
Mathanker A, Das S, Pudasainee D, Khan M, Kumar A, Gupta R. A review of hydrothermal liquefaction of biomass for biofuels production with a special focus on the effect of process parameters, co-solvents, and extraction solvents. Energies. 2021;14:4916.
Perkins G, Batalha N, Kumar A, Bhaskar T, Konarova M. Recent advances in liquefaction technologies for production of liquid hydrocarbon fuels from biomass and carbonaceous wastes. Renew Sustain Energy Rev. 2019;115: 109400.
An B, Wang Y, Huang Y, Wang X, Liu Y, Xun D, Church GM, Dai Z, Yi X, Tang T-C. Engineered living materials for sustainability. Chem Rev. 2022;123:2349–419.
Usmani Z, Sharma M, Awasthi AK, Sivakumar N, Lukk T, Pecoraro L, Thakur VK, Roberts D, Newbold J, Gupta VK. Bioprocessing of waste biomass for sustainable product development and minimizing environmental impact. Biores Technol. 2021;322: 124548.
Bortolini DG, Maciel GM, Fernandes IDAA, Pedro AC, Rubio FTV, Branco IG, Haminiuk CWI. Functional properties of bioactive compounds from Spirulina spp.: current status and future trends. Food Chem Mol Sci. 2022;5:100134.
Dolganyuk V, Belova D, Babich O, Prosekov A, Ivanova S, Katserov D, Patyukov N, Sukhikh S. Microalgae: a promising source of valuable bioproducts. Biomolecules. 2020;10:1153.
S. Chaudhary, V.P. Jain, G. Jaiswar, The composition of polysaccharides: monosaccharides and binding, group decorating, polysaccharides chains, in: Innovation in Nano-Polysaccharides for Eco-sustainability, Elsevier, 2022, pp. 83–118.
Li S, Ji L, Shi Q, Wu H, Fan J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Biores Technol. 2019;292: 122048.
Costa LEC, Brito TV, Damasceno ROS, Sousa WM, Barros FCN, Sombra VG, Júnior JSC, Magalhaes DA, Souza MH, Medeiros J-VR. Chemical structure, anti-inflammatory and antinociceptive activities of a sulfated polysaccharide from Gracilaria intermedia algae. Int J Biol Macromol. 2020;159:966–75.
Kowthaman C, Kumar PS, Selvan VAM, Ganesh D. A comprehensive insight from microalgae production process to characterization of biofuel for the sustainable energy. Fuel. 2022;310: 122320.
Tazikeh S, Zendehboudi S, Ghafoori S, Lohi A, Mahinpey N. Algal bioenergy production and utilization: Technologies, challenges, and prospects. J Environ Chem Eng. 2022;10: 107863.
Rocha CP, Pacheco D, Cotas J, Marques JC, Pereira L, Gonçalves AM. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int J Environ Res Public Health. 2021;18:4968.
Saini RK, Prasad P, Sreedhar RV, Akhilender Naidu K, Shang X, Keum Y-S. Omega− 3 polyunsaturated fatty acids (PUFAs): Emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—a review. Antioxidants. 2021;10:1627.
Chia SR, Ong HC, Chew KW, Show PL, Phang S-M, Ling TC, Nagarajan D, Lee D-J, Chang J-S. Sustainable approaches for algae utilisation in bioenergy production. Renewable Energy. 2018;129:838–52.
Nateghpour B, Kavoosi G, Mirakhorli N. Amino acid profile of the peel of three citrus species and its effect on the combination of amino acids and fatty acids Chlorella vulgaris. J Food Compos Anal. 2021;98: 103808.
Ammar EE, Aioub AA, Elesawy AE, Karkour AM, Mouhamed MS, Amer AA, El-Shershaby NA. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J Biol Sci. 2022;29:3083–96.
Stoyneva-Gärtner M, Uzunov B, Gärtner G. Enigmatic microalgae from aeroterrestrial and extreme habitats in cosmetics: The potential of the untapped natural sources. Cosmetics. 2020;7:27.
Shahidi F, Ambigaipalan P. Antioxidants in oxidation control, Measurement of antioxidant activity & capacity: recent trends and applications, 2018:287–320.
Durdakova M, Kolackova M, Janova A, Krystofova O, Adam V, Huska D, Microalgae/cyanobacteria: the potential green future of vitamin B12 production, Crit Rev Food Sci Nutr 2022:1–12.
Guleri S, Tiwari A, Algae and ageing, Microalgae biotechnology for food, health and high value products, 2020:267–293.
Sandmann G. Antioxidant protection from UV-and light-stress related to carotenoid structures. Antioxidants. 2019;8:219.
Osório C, Machado S, Peixoto J, Bessada S, Pimentel FB, Alves RC, Oliveira MBP. Pigments content (chlorophylls, fucoxanthin and phycobiliproteins) of different commercial dried algae. Separations. 2020;7:33.
Saini RK, Prasad P, Lokesh V, Shang X, Shin J, Keum Y-S, Lee J-H. Carotenoids: Dietary sources, extraction, encapsulation, bioavailability, and health benefits—a review of recent advancements. Antioxidants. 2022;11:795.
Fernandes T, Cordeiro N. Microalgae as sustainable biofactories to produce high-value lipids: Biodiversity, exploitation, and biotechnological applications. Mar Drugs. 2021;19:573.
Del Mondo A, Smerilli A, Ambrosino L, Albini A, Noonan DM, Sansone C, Brunet C. Insights into phenolic compounds from microalgae: structural variety and complex beneficial activities from health to nutraceutics. Crit Rev Biotechnol. 2021;41:155–71.
M.M. Vuolo, V.S. Lima, M.R.M. Junior, Phenolic compounds: Structure, classification, and antioxidant power, in: Bioactive compounds, Elsevier, 2019, pp. 33–50.
Fernando IS, Nah J-W, Jeon Y-J. Potential anti-inflammatory natural products from marine algae. Environ Toxicol Pharmacol. 2016;48:22–30.
K. Iqbal, N. Sharma, S. Takkar, S. Shukla, K. Shukla, A. Varma, A. Mishra, Integrated CO2 sequestration, wastewater treatment, and biofuel production by microalgae culturing: needs and limitations, Integrated Environmental Technologies for Wastewater Treatment and Sustainable Development 2022:217–240.
Patel A, Matsakas L, Rova U, Christakopoulos P. Heterotrophic cultivation of Auxenochlorella protothecoides using forest biomass as a feedstock for sustainable biodiesel production. Biotechnol Biofuels. 2018;11:1–16.
González-González LM, Astals S, Pratt S, Jensen PD, Schenk PM. Osmotic shock pre-treatment of Chaetoceros muelleri wet biomass enhanced solvent-free lipid extraction and biogas production. Algal Res. 2021;54: 102177.
Liu S, Cao L, Xu F, Yang L, Li Y, Inalegwu OS, Integration of algae cultivation to anaerobic digestion for biofuel and bioenergy production, in: Advances in Bioenergy, Elsevier, 2021, pp. 199–300.
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T. Integrating micro-algae into wastewater treatment: a review. Sci Total Environ. 2021;752: 142168.
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F. Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sustain Energy Rev. 2016;55:1–16.
Peng L, Fu D, Chu H, Wang Z, Qi H. Biofuel production from microalgae: a review. Environ Chem Lett. 2020;18:285–97.
Séralini G-E, Clair E, Mesnage R, Gress S, Defarge N, Malatesta M, Hennequin D, de Vendômois JS. Republished study: long-term toxicity of a Roundup herbicide and a Roundup-tolerantgenetically modified maize, Environmental Sciences. Europe. 2014;26:1–17.
A. Muller‐Feuga, Microalgae for aquaculture: the current global situation and future trends, Handbook of microalgal culture: applied phycology and biotechnology, 2013. 613–627.
dos Santos MGB, Duarte RL, Maciel AM, Abreu M, Reis A, de Mendonça HV. Microalgae biomass production for biofuels in Brazilian scenario: a critical review. BioEnergy Res. 2021;14:23–42.
S. L’Haridon, G.H. Markx, C.J. Ingham, L. Paterson, F. Duthoit, G. Le Blay, New approaches for bringing the uncultured into culture, The marine microbiome: an untapped source of biodiversity and biotechnological potential, 2016:401–434.
Limongi AR, Viviano E, De Luca M, Radice RP, Bianco G, Martelli G. Biohydrogen from microalgae: production and applications. Appl Sci. 2021;11:1616.
Liang J, Wen F, Liu J. Transcriptomic and lipidomic analysis of an EPA-containing Nannochloropsis sp. PJ12 in response to nitrogen deprivation. Sci Rep. 2019;9:4540.
Ishino Y. Studies on DNA-related enzymes to elucidate molecular mechanisms underlying genetic information processing and their application in genetic engineering. Biosci Biotechnol Biochem. 2020;84:1749–66.
Banerjee C, Singh PK, Shukla P. Microalgal bioengineering for sustainable energy development: recent transgenesis and metabolic engineering strategies. Biotechnol J. 2016;11:303–14.
Young JL, Dean DA. Electroporation-mediated gene delivery. Adv Genet. 2015;89:49–88.
Zhang KS, Nadkarni AV, Paul R, Martin AM, Tang SK. Microfluidic surgery in single cells and multicellular systems. Chem Rev. 2022;122:7097–141.
Wang Q, Lu Y, Xin Y, Wei L, Huang S, Xu J. Genome editing of model oleaginous microalgae Nannochloropsis spp. by CRISPR/Cas9. Plant J. 2016;88:1071–81.
M. Moosburner, A.E. Allen, F. Daboussi, Genetic Engineering in Marine Diatoms: Current Practices and Emerging Technologies, The Molecular Life of Diatoms, (2022) 743–773.
Ricci I, Valzano M, Ulissi U, Epis S, Cappelli A, Favia G. Symbiotic control of mosquito borne disease. Pathog Glob Health. 2012;106:380–5.
Stewart MP, Langer R, Jensen KF. Intracellular delivery by membrane disruption: mechanisms, strategies, and concepts. Chem Rev. 2018;118:7409–531.
Zhong C, Aruga Y, Yan X. Morphogenesis and spontaneous chromosome doubling during the parthenogenetic development of haploid female gametophytes in Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol. 2019;31:2729–41.
A.A.Z. Abidin, M. Suntarajh, Z.N.B. Yusof, Microalgae as a vaccine delivery system to aquatic organisms, Microalgae biotechnology for food, health and high value products, 2020:353–372.
Shuba ES, Kifle D. Microalgae to biofuels:‘Promising’alternative and renewable energy, review. Renew Sustain Energy Rev. 2018;81:743–55.
Su Y, Song K, Zhang P, Su Y, Cheng J, Chen X. Progress of microalgae biofuel’s commercialization. Renew Sustain Energy Rev. 2017;74:402–11.
Ogbonna CN, Nwoba EG. Bio-based flocculants for sustainable harvesting of microalgae for biofuel production A review. Renew Sustain Energy Rev. 2021;139: 110690.
Morales M, Aflalo C, Bernard O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenerg. 2021;150: 106108.
Raheem A, Prinsen P, Vuppaladadiyam AK, Zhao M, Luque R. A review on sustainable microalgae based biofuel and bioenergy production: Recent developments. J Clean Prod. 2018;181:42–59.
Khan S, Naushad M, Iqbal J, Bathula C, Sharma G. Production and harvesting of microalgae and an efficient operational approach to biofuel production for a sustainable environment. Fuel. 2022;311: 122543.
Arora K, Kaur P, Kumar P, Singh A, Patel SKS, Li X, Yang Y-H, Bhatia SK, Kulshrestha S. Valorization of wastewater resources into biofuel and value-added products using microalgal system. Front Energy Res. 2021;9: 646571.
AlProl AE, Elkatory MR, The current status of various algal industries, Handbook of Algal Biofuels, 2022:123–147.
de Jesus SS, Ferreira GF, Moreira LS, Maciel Filho R. Biodiesel production from microalgae by direct transesterification using green solvents, Renew. Energy. 2020;160:1283–94.
de Jesus SS, Ferreira GF, Moreira LS, Maciel MRW, Maciel Filho R. Comparison of several methods for effective lipid extraction from wet microalgae using green solvents, Renew. Energy. 2019;143:130–41.
Sati H, Mitra M, Mishra S, Baredar P. Microalgal lipid extraction strategies for biodiesel production: a review. Algal Res. 2019;38: 101413.
Tzima S, Georgiopoulou I, Louli V, Magoulas K. Recent advances in supercritical CO2 extraction of pigments lipids and bioactive compounds from microalgae. Molecules. 2023;28:1410.
Lee SY, Khoiroh I, Vo DVN, SenthilKumar P, Show PL. Techniques of lipid extraction from microalgae for biofuel production: a review. Environ Chem Lett. 2021;19:231–51.
Lee SY, Sankaran R, Chew KW, Tan CH, Krishnamoorthy R, Chu D-T, Show P-L. Waste to bioenergy: a review on the recent conversion technologies. Bmc Energy. 2019;1:1–22.
Beyene A, Kothari D, Subbarao P, Power generation, Springer Handbook of Mechanical Engineering 2021:1223–1271.
Barot S, Biomass and bioenergy: resources, conversion and application. Renew Energy Sustain Growth Assess 2022:243–262.
Salaheldeen M, Mariod AA, Aroua MK, Rahman SA, Soudagar MEM, Fattah IR. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts. 2021;11:1121.
Amenaghawon AN, Anyalewechi CL, Okieimen CO, Kusuma HS, Biomass pyrolysis technologies for value-added products: a state-of-the-art review, Environ Dev Sustain 2021:1–55.
Yogalakshmi K, Sivashanmugam P, Kavitha S, Kannah Y, Varjani S, AdishKumar S, Kumar G. Lignocellulosic biomass-based pyrolysis: a comprehensive review. Chemosphere. 2022;286: 131824.
Nguyen LN, Kumar J, Vu MT, Mohammed JA, Pathak N, Commault AS, Sutherland D, Zdarta J, Tyagi VK, Nghiem LD. Biomethane production from anaerobic co-digestion at wastewater treatment plants: a critical review on development and innovations in biogas upgrading techniques. Sci Total Environ. 2021;765: 142753.
Devi A, Bajar S, Sihag P, Sheikh ZUD, Singh A, Kaur J, Bishnoi NR, Pant D. A panoramic view of technological landscape for bioethanol production from various generations of feedstocks. Bioengineered. 2023;14:81–112.
Behera B, Selvam M, Paramasivan B. Research trends and market opportunities of microalgal biorefinery technologies from circular bioeconomy perspectives. Bioresour Technol. 2022;351:127038.
Behera B, Mari Selvam S, Balasubramanian P. Hydrothermal processing of microalgal biomass: Circular bio-economy perspectives for addressing food-water-energy nexus. Bioresour Technol. 2022;359:127443.
Gu X, Martinez-Fernandez J, Pang N, Fu X, Chen S. Recent development of hydrothermal liquefaction for algal biorefinery. Renew Sustain Energy Rev. 2020;121: 109707.
Patra BR, Mohapatro RN, Routray S, Swain R, Nanda S, Dalai AK, Thermochemical conversion of organic waste: New horizons for production of green energy, In: Innovations in thermochemical technologies for biofuel processing, Elsevier, 2022, pp. 1–21.
Gupta M, Savla N, Pandit C, Pandit S, Gupta PK, Pant M, Khilari S, Kumar Y, Agarwal D, Nair RR. Use of biomass-derived biochar in wastewater treatment and power production: a promising solution for a sustainable environment. Sci Total Environ. 2022;825: 153892.
Kumar R, Ghosh AK, Pal P. Synergy of biofuel production with waste remediation along with value-added co-products recovery through microalgae cultivation: a review of membrane-integrated green approach. Sci Total Environ. 2020;698: 134169.
Asomaning J, Haupt S, Chae M, Bressler DC. Recent developments in microwave-assisted thermal conversion of biomass for fuels and chemicals. Renew Sustain Energy Rev. 2018;92:642–57.
Varma AK, Lal N, Rathore AK, Katiyar R, Thakur LS, Shankar R, Mondal P. Thermal, kinetic and thermodynamic study for co-pyrolysis of pine needles and styrofoam using thermogravimetric analysis. Energy. 2021;218: 119404.
Ansari KB, Arora JS, Chew JW, Dauenhauer PJ, Mushrif SH. Fast pyrolysis of cellulose, hemicellulose, and lignin: effect of operating temperature on bio-oil yield and composition and insights into the intrinsic pyrolysis chemistry. Ind Eng Chem Res. 2019;58:15838–52.
Thengane SK, Kung KS, Gomez-Barea A, Ghoniem AF. Advances in biomass torrefaction: Parameters, models, reactors, applications, deployment, and market. Prog Energy Combust Sci. 2022;93: 101040.
Sahoo K, Bilek E, Bergman R, Mani S. Techno-economic analysis of producing solid biofuels and biochar from forest residues using portable systems. Appl Energy. 2019;235:578–90.
Maniscalco MP, Volpe M, Messineo A. Hydrothermal carbonization as a valuable tool for energy and environmental applications: a review. Energies. 2020;13:4098.
Okolie JA, Nanda S, Dalai AK, Berruti F, Kozinski JA. A review on subcritical and supercritical water gasification of biogenic, polymeric and petroleum wastes to hydrogen-rich synthesis gas. Renew Sustain Energy Rev. 2020;119: 109546.
Shah AA, Sharma K, Haider MS, Toor SS, Rosendahl LA, Pedersen TH, Castello D. The role of catalysts in biomass hydrothermal liquefaction and biocrude upgrading. Processes. 2022;10:207.
Das P, Chandramohan V, Mathimani T, Pugazhendhi A. A comprehensive review on the factors affecting thermochemical conversion efficiency of algal biomass to energy. Sci Total Environ. 2021;766: 144213.
Brindhadevi K, Anto S, Rene ER, Sekar M, Mathimani T, Chi NTL, Pugazhendhi A. Effect of reaction temperature on the conversion of algal biomass to bio-oil and biochar through pyrolysis and hydrothermal liquefaction. Fuel. 2021;285: 119106.
Liu D, Chen X, Xu G, Guan J, Cao Q, Dong B, Qi Y, Li C, Mu X. Iridium nanoparticles supported on hierarchical porous N-doped carbon: an efficient water-tolerant catalyst for bio-alcohol condensation in water. Sci Rep. 2016;6:21365.
Fan L, Zhang H, Li J, Wang Y, Leng L, Li J, Yao Y, Lu Q, Yuan W, Zhou W. Algal biorefinery to value-added products by using combined processes based on thermochemical conversion: a review. Algal Res. 2020;47: 101819.
Gai W-Z, Wang L-Y, Lu M-Y, Deng Z-Y. Effect of low concentration hydroxides on Al hydrolysis for hydrogen production. Energy. 2023;268: 126731.
Saravanan A, Kumar PS, Badawi M, Mohanakrishna G, Aminabhavi TM. Valorization of micro-algae biomass for the development of green biorefinery: perspectives on techno-economic analysis and the way towards sustainability. Chem Eng J. 2023;453: 139754.
Scarsella M, de Caprariis B, Damizia M, De Filippis P. Heterogeneous catalysts for hydrothermal liquefaction of lignocellulosic biomass: a review. Biomass Bioenerg. 2020;140: 105662.
Oh W-D, Lim T-T. Design and application of heterogeneous catalysts as peroxydisulfate activator for organics removal: an overview. Chem Eng J. 2019;358:110–33.
Dibenedetto A, Angelini A, Stufano P. Use of carbon dioxide as feedstock for chemicals and fuels: homogeneous and heterogeneous catalysis. J Chem Technol Biotechnol. 2014;89:334–53.
Nallasivam J, Prashanth PF, Vinu R, Hydrothermal liquefaction of biomass for the generation of value-added products, Biomass Biofuels Biochem 2022:65–107.
Almomani F, Hosseinzadeh-Bandbafha H, Aghbashlo M, Omar A, Joo S-W, Vasseghian Y, Karimi-Maleh H, Lam SS, Tabatabaei M, Rezania S. Comprehensive insights into conversion of microalgae to feed, food, and biofuels: current status and key challenges towards implementation of sustainable biorefineries. Chem Eng J. 2023;455: 140588.
Chauhan PS, Agrawal R, Kumar R, Gupta RP, Ramakumar S. Next generation applications of lignin derived commodity products, their life cycle, techno-economics and societal analysis. Int J Biol Macromol. 2022;197:179–200.
De Schouwer F, Claes L, Vandekerkhove A, Verduyckt J, De Vos DE. Protein-rich biomass waste as a resource for future biorefineries: state of the art, challenges, and opportunities. Chemsuschem. 2019;12:1272–303.
Ramasubramanian B, Sundarrajan S, Rao RP, Reddy M, Chellappan V, Ramakrishna S. Novel low-carbon energy solutions for powering emerging wearables, smart textiles, and medical devices. Energy Environ Sci. 2022;15:4928–81.
Sun C, Meng X, Sun F, Zhang J, Tu M, Chang J-S, Reungsang A, Xia A, Ragauskas AJ. Advances and perspectives on mass transfer and enzymatic hydrolysis in the enzyme-mediated lignocellulosic biorefinery: a review. Biotechnol Adv. 2023;62: 108059.
Basso D, Patuzzi F, Castello D, Baratieri M, Rada EC, Weiss-Hortala E, Fiori L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manage. 2016;47:114–21.
Garcia-Ochoa F, Vergara P, Wojtusik M, Gutiérrez S, Santos VE, Ladero M, Villar JC. Multi-feedstock lignocellulosic biorefineries based on biological processes: an overview. Ind Crops Prod. 2021;172: 114062.
Katsounaros I, Cherevko S, Zeradjanin AR, Mayrhofer KJ. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew Chem Int Ed. 2014;53:102–21.
Kumar A, Sharma G, Naushad M, Ala’a H, García-Peñas A, Mola GT, Si C, Stadler FJ. Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: a review. Chem Eng J. 2020;382: 122937.
Zhang S, Dai S, Finkelman RB, Graham IT, French D, Hower JC, Li X. Leaching characteristics of alkaline coal combustion by-products: a case study from a coal-fired power plant Hebei Province, China. Fuel. 2019;255: 115710.
Zhao Y, Lu K, Xu H, Zhu L, Wang S. A critical review of recent advances in the production of furfural and 5-hydroxymethylfurfural from lignocellulosic biomass through homogeneous catalytic hydrothermal conversion. Renew Sustain Energy Rev. 2021;139: 110706.
Kang K, Nanda S, Hu Y. Current trends in biochar application for catalytic conversion of biomass to biofuels. Catal Today. 2022. https://doi.org/10.1016/j.cattod.2022.06.033.
Mathimani T, Mallick N. A review on the hydrothermal processing of microalgal biomass to bio-oil-Knowledge gaps and recent advances. J Clean Prod. 2019;217:69–84.
Kandasamy S, Zhang B, He Z, Bhuvanendran N, El-Seesy AI, Wang Q, Narayanan M, Thangavel P, Dar MA. Microalgae as a multipotential role in commercial applications: current scenario and future perspectives. Fuel. 2022;308:122053.
EswaryDevi T, Parthiban R, Arun J, Gopinath KP. Processing of marine microalgae biomass via hydrothermal liquefaction for bio-oil production: study on algae cultivation, harvesting, and process parameters. Biomass Convers Biorefin. 2022. https://doi.org/10.1007/s13399-022-03446-5.
Bligaard T, Bullock RM, Campbell CT, Chen JG, Gates BC, Gorte RJ, Jones CW, Jones WD, Kitchin JR, Scott SL. Toward benchmarking in catalysis science: best practices, challenges, and opportunities. ACS Catal. 2016;6:2590–602.
Aker V, Ayas N. Boosting hydrogen production by ethanol steam reforming on cobalt-modified Ni–Al2O3 catalyst. Int J Hydr Energy. 2023. https://doi.org/10.1016/j.ijhydene.2022.12.310.
Narayanan M. Promising biorefinery products from marine macro and microalgal biomass: a review. Renew Sustain Energy Rev. 2024;190: 114081.
Sudhakar K, Mamat R, Samykano M, Azmi W, Ishak W, Yusaf T. An overview of marine macroalgae as bioresource. Renew Sustain Energy Rev. 2018;91:165–79.
Olabi A, Shehata N, Sayed ET, Rodriguez C, Anyanwu RC, Russell C, Abdelkareem MA. Role of microalgae in achieving sustainable development goals and circular economy. Sci Total Environ. 2023;854: 158689.
Ciancia M, Fernández PV, Leliaert F. Diversity of sulfated polysaccharides from cell walls of coenocytic green algae and their structural relationships in view of green algal evolution. Front Plant Sci. 2020;11: 554585.
Wang X, Zhang Y, Xia C, Alqahtani A, Sharma A, Pugazhendhi A. A review on optimistic biorefinery products: Biofuel and bioproducts from algae biomass. Fuel. 2023;338: 127378.
Ko JK, Lee JH, Jung JH, Lee S-M. Recent advances and future directions in plant and yeast engineering to improve lignocellulosic biofuel production. Renew Sustain Energy Rev. 2020;134: 110390.
Bilal M, Iqbal HM. Marine seaweed polysaccharides-based engineered cues for the modern biomedical sector. Mar Drugs. 2019;18:7.
Pfeifer L, Shafee T, Johnson KL, Bacic A, Classen B. Arabinogalactan-proteins of Zostera marina L. contain unique glycan structures and provide insight into adaption processes to saline environments. Sci Rep. 2020;10:8232.
Hossain N, Bhuiyan MA, Pramanik BK, Nizamuddin S, Griffin G. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: a critical review. J Clean Prod. 2020;255: 120261.
Loh JM, Gourich W, Chew CL, Song CP, Chan E-S. Improved biodiesel production from sludge palm oil catalyzed by a low-cost liquid lipase under low-input process conditions. Renew Energy. 2021;177:348–58.
Olaniran AO, Balgobind A, Pillay B. Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. Int J Mol Sci. 2013;14:10197–228.
Acknowledgements
The authors would like to thank the Center for Research and Innovations, Department of Biotechnology, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Science, Chennai-602 105, Tamil Nadu, India for their constant support for this work.
Author information
Authors and Affiliations
Contributions
MN: Conceptualization, Analysis, Investigation, Project administration, Writing—Original Draft, Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Narayanan, M. Biorefinery products from algal biomass by advanced biotechnological and hydrothermal liquefaction approaches. Discov Appl Sci 6, 146 (2024). https://doi.org/10.1007/s42452-024-05777-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05777-6