Abstract
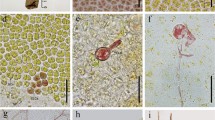
Pyropia haitanensis is one of the most economically important seaweeds growing in the coastal regions of south China. Previous studies revealed that parthenogenesis could emerge in P. haitanensis under certain culture conditions. In the present study, detailed cytological observations were made of morphogenesis and chromosomal behaviors of female gametophytes of P. haitanensis during parthenogenesis. Unisexual cultures of female gametophytes, derived from isolated somatic cells of wild gametophytes or obtained from cloned sporophytes, ultimately led to their development into parthenosporophytes. Based on the changes in shape and color of the cellular chromatophore, as well as the metamorphosis and condition of the released cells, the parthenogenesis of P. haitanensis was divided into six developmental stages: vegetative cells (VCs) with multiple stellate chromatophores, carpogonium-like cells (CLCs) with dispersed chromatophores, carpogonium-like protoplasts (CLPs) released post-cell wall dissolution, carpospore-like cells (CCs) with regenerated cell wall, carpospore-like germlings (CGs), and parthenosporophytes. Chromosomal observations showed that VCs were haploid (n = 5), small proportions of CLCs, and CLPs and CCs were diploid (2n = 10), while all the CGs, filamentous conchocelis cells, and conchosporangial branch cells of parthenosporophytes were diploid (2n = 10). Haploid, aneuploid, and polyploidy were not observed in the latter group of cells. Spontaneous chromosome doubling occurred during the parthenogenesis of P. haitanensis, first in highly differentiated CLCs. Furthermore, pit connections could be seen throughout the parthenosporophyte development process similar to that in heterozygous sporophytes. The chromosomes were seen undergoing meiosis during the germination of conchospores released from mature parthenosporophytes, leading to the generation of tetrad cells, which developed into haploid female gametophytes. These results provide evidence for better understanding the mechanism underlying parthenogenesis in Pyropia.






Similar content being viewed by others
References
Asensi A, Gall EA, Marie D, Billot C, Dion P, Kloareg B (2001) Clonal propagation of Laminaria digitata (Phaeophyceae) sporophytes through a diploid cell–filament suspension. J Phycol 37:411–417
Aylon Y, Kupiec M (2004) DSB repair: the yeast paradigm. DNA Repair (Amst) 3:797–815
Belcher BJH (1960) Culture studies of Bangia atropurpurea (Roth) Ag. New Phytol 59:367–373
Bharathan G, Shinmura I (1986) A note on parthenogenesis in four species of Laminariales. Jap J Phycol 34:13–18
Bonneau ER (1978) Asexual reproductive capabilities in Ulva lactuca L. (Chlorophyceae). Bot Mar 21:117–122
Bothwell JH, Marie D, Peters AF, Cock JM, Coelho SM (2010) Role of reduplication and apomeiosis during parthenogenetic reproduction in the model brown alga Ectocarpus. New Phytol 188:111–121
Cole KM, Hymes BJ, Sheath RG (1983) Karyotypes and reproductive seasonality of the genus Bangia in British Columbia, Canada. J Phycol 19:136–145
Fang TC, Dai JX, Chen DQ (1982) Parthenogenesis and the genetic properties of parthenosporophytes of Undaria pinnatifida. Acta Oceanol Sin 1:107–111
Fang TC, Dai JX, Oü YL, Tsuei CC, Chen TC (1978) Some genetic observations on the monoploid breeding of Laminaria japonica. Sci Sinica 21:401–408
Freshwater DW, Kapraun DF (1986) Field, culture and cytological studies of Porphyra carolinensis Coll et Cox (Rhodophyta, Bangiales) from North Carolina. Jap J Phycol 34:251–262
Garbary DJ, Clarke B (2002) Intraplant variation in nuclear DNA content in Laminaria saccharina and Alaria esculenta (Phaeophyceae). Bot Mar 45:211–216
Garbary DJ, Mcdonald AR (1998) Molecules, organelles and cells: fluorescence microscopy and red algal development. In: Cooksey K (ed) Molecular approaches to the study of the ocean. Chapman and Hall, London, pp 409–422
Gall EA, Asensi A, Marie D, Kloareg B (1996) Parthenogenesis and apospory in the Laminariales: a flow cytometry analysis. Eur J Phycol 31:369–380
Goff LJ, Coleman AW (1990) DNA: microspectrofluorometric studies. In: Cole KM, Sheath RG (eds) Biology of the red algae. Cambridge Univ Press, New York, pp 43–71
Hawkes MW (1978) Sexual reproduction in Porphyra gardneri (Smith et Hollenberg) Hawkes (Bangiales, Rhodophyta). Phycologia 17:329–353
Hinson TK, Kapraun DF (1991) Karyology and nuclear DNA quantification of four species of Chaetomorpha (Cladophorales, Chlorophyta) from the western Atlantic. Helgol Meeresunters 45:273–285
Hiraoka M, Shimada S, Ohno M, Serisawa Y (2003) Asexual life history by quadriflagellate swarmers in Ulva spinulosa (Ulvales, Ulvophyceae). Phycol Res 51:29–34
Inze D, De Veylder L (2006) Cell cycle regulation in plant development. Annu Rev Genet 40:77–105
Jensen CJ (1974) Chromosome doubling techniques in haploids. In: Kasha KJ (ed) Haploids in higher plants, advances and potential. Guelph University Guelph, pp 153–190
Kamiya M, West JA (2008) Origin of apomictic red algae: outcrossing studies of different strain in Caloglossa monosticha (Ceramiales, Rhodophyta). J Phycol 44:977–984
Kapraun DF, Lemus AJ (1987) Field and culture studies of Porphyra spiralis var. amplifolia Oliveira Filho et Coll (Bangiales, Rhodophyta) from Isla de Margarita, Venezuela. Bot Mar 30:483–490
Kornmann P (1994) Life histories of monostromatic Porphyra species as a basis for taxonomy and classification. Eur J Phycol 29:69–71
Kornmann P, Sahling PH (1991) The Porphyra species of Helgoland (Bangiales, Rhodophyta). Helgol Meeresunters 45:1–38
Krueger–Hadfield SA, Küber JE, Dudgeon SR (2013) Reproductive effort of Mastocarpus papillatus (Rhodophyta) along the California coast. J Phycol 49:271–281
Lewis RJ, Jiang BY, Neushul M, Fei XG (1993) Haploid parthenogenetic sporophytes of Laminaria japonica (Phaeophyceae). J Phycol 29:363–369
Lǿvlie A, Bryhni E (1978) On the relation between sexual and parthenogenetic reproduction in haplo–diplontic algae. Bot Mar 21:155–163
Nakahara H (1984) Alternation of generations of some brown algae in unialgal and axenic cultures. Sci Pap Inst Algol Res Fac Sci, Hokkaido Univ 7:77–194
Nakahara H, Nakamura Y (1973) Parthenogenesis, apogamy and apospory in Alaria crassifolia (Laminariales). Mar Biol 18:327–332
Nelson WA, Brodie J, Guiry MD (1999) Terminology used to describe reproduction and life history stages in the genus Porphyra (Bangiales, Rhodophyta). J Appl Phycol 11:407–410
Notoya M, Aruga Y (1992) Tissues culture of parthenogenetic and normal young sporophytes of Ecklonia cava Kjellman (Laminariales, Phaeophyta). Jap J Phycol 40:53–55
Notoya M, Iijima N (2003) Life history and sexuality of archeospore and apogamy of Bangia atropurpurea (Roth) Lyngbye (Bangiales, Rhodophyta) from Fukaura and Enoshima. Fish Sci 69:799–805
Oppliger LV, Correa JA, Peters AF (2007) Parthenogenesis in the brown alga Lessonia nigrescens (Laminariales, Phaeophyceae) from Central Chile. J Phycol 43:1295–1301
Sugimoto–Shirasu K, Roberts K (2003) ‘Big it up’: endoreduplication and cell–size control in plants. Curr Opin Plant Biol 6:544–553
Tom Dieck (Bartsch) I. (1992) North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 31: 147–163
Tseng CK, Sun AS (1989) Studies on the alternation of the nuclear phases and chromosome numbers in the life history of some species of Porphyra from China. Bot Mar 32:1–8
Wang SJ, Zhang XP, Xu ZD, Sun YL (1986) A study on the cultivation of the vegetative cells and protoplasts of Porphyra haitanensis. Oceanol Limnol Sinica 17:217–221
West JA, Zuccarello GC, Kamiya M (2001) Reproductive patterns of Caloglossa species (Delesseriaceae, Rhodophyta) from Australia and New Zealand: multiple origins of asexuality in C. leprieurii. Literature review on apomixis, mixed-phase, bisexuality and sexual compatibility. Phycol Res 49:183–200
Wittmann W (1965) Acto–iron–haematoxylin–chloral hydrate for chromosome staining. Stain Technol 40:161–164
Yan XH, He LH, Aruga Y (2007a) Karyological observation on the occurrence of meiosis in the life cycle of Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). Bot Mar 50:257–263
Yan XH, Li L, Chen JH, Aruga Y (2007b) Parthenogenesis and isolation of genetic pure strains in Porphyra haitanensis (Bangiales, Rhodophyta). High Technol Lett 17:205–210
Yan XH, Liu XS (2007) Comparison on cell differentiation of male and female blades in Porphyra haitanensis (Bangiales, Rhodophyta). J Fish China 31:184–192
Yan XH, He LH, Huang J, Song WL, Ma P, Aruga Y (2008) Cytological studies on Porphyra haitanensis Chang et Zheng (Bangiales, Rhodophyta). J Fish China 32:131–137
Zeng QG, Liu BQ, Yang R, Luo QJ, Wang YJ (2004) Morphogeny of conchocelis thalli from single somatic cell clone cultivation of Porphyra haitanensis. J Fish Sci China 11:549–552
Zhang Y, Yan XH (2013) Observation on tetrad development and formation of sex phenotype of Pyropia haitanensis blades in natural conditions. J Fish China 37:871–883
Zhang Y, Yan XH, Aruga Y (2013) The sex and sex determination in Pyropia haitanensis (Bangiales, Rhodophyta). PLoS One 8:e73414
Zhou W, Zhu JY, Shen SD, Lu S, Wang JF, Xu JR, Xu P (2008) Observations on the division characterization of diploid nuclear in Porphyra (Bangiales, Rhodophyta). J Appl Phycol 20:991–999
Acknowledgements
The authors would like to acknowledge Prof. Qi Lin (Fisheries Research Institute of Fujian Province, China) for reading the manuscript.
Funding
The study was supported in part by the National Key Research and Development Program of China (2018YFD0900606), National Natural Science Foundation of China (31072208), Major Science and Technology Specific Program of Zhejiang Province (2016C02055-6), and Science and Tecnology Planning Project of Jiangsu Province, China (BE2018335).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhong, C., Aruga, Y. & Yan, X. Morphogenesis and spontaneous chromosome doubling during the parthenogenetic development of haploid female gametophytes in Pyropia haitanensis (Bangiales, Rhodophyta). J Appl Phycol 31, 2729–2741 (2019). https://doi.org/10.1007/s10811-019-01769-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01769-x