Abstract
Wood extractives usually do not exceed five percent of dry wood mass but can be a serious issue for pulping as well as for the pulp itself. They cause contamination and damages to process equipment and negatively influence pulp quality. This paper addresses not only the extractives-related problems but also different solutions for these issues. It is an extensive review of different technologies for removing wood extractives, starting with methods prior to pulping. Several wood yard operations like debarking, knot separation, and wood seasoning are known to significantly decreasing the amount of wood extractives. Biological treatment has also been proven as a feasible method for reducing the extractives content before pulping, but quite hard to handle. During pulping, the extractives reduction efficiency depends on the pulping method. Mechanical pulping removes the accessory compounds of wood just slightly, but chemical pulping, on the other hand, removes them to a large extent. Organosolv pulping even allows almost complete removal of wood extractives. The residual extractives content can be significantly reduced by pulp bleaching. Nevertheless, different extraction-based methods have been developed for removing wood extractives before pulping or bleaching. They range from organic-solvent-based extractions to novel processes like supercritical fluid extractions, ionic liquids extractions, microwave technology, and ultrasonic-assisted extraction. Although these methods deliver promising results and allow utilization of wood extractives in most cases, they suffer from many drawbacks towards an economically viable industrial-scale design, concluding that further research has to be done on these topics.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 General introduction
Wood has been used from prehistory on and therefore rated among the oldest materials of humanity. In the beginning, wood was just used as fuel wood for simple cooking and heating purposes. Over time, wood has become more important as a building material, and since the industrial revolution, wood has also been intensively used for different industrial purposes [1, 2]. Nowadays, there is still a broad range of wood usages even in highly developed countries—from the initial use as fuel wood, i.e., wood pellets, through to high-performance materials for various applications, like wood polymer composites (WPC) as a special structural material, xylose as base chemical and lignin as biopolymer [3,4,5,6]. One important application is wood pulp—used for paper and cardboard—with a global production of 184 million tons in 2017, excluding recovered paper [7].
For producing paper, wood must be defibered, which is known as pulping. Unfortunately, this defibration is quite challenging because of the complex structure of wood with the lignin matrix. Therefore mechanical (including thermomechanical and semi-chemical) or chemical methods are applied [8, 9]. In mechanical pulping, on the one hand, the grinding and refining processes increase the temperature, which leads to softening the lignin and breaking the bonds between the fibers. On the other hand, chemical pulping achieves defibration by removing the two cellulose-fiber-surrounding components, lignin, and hemicelluloses [10]. Besides these three main components, wood also contains many organic compounds. Since those organic compounds can be extracted with organic solvents or hot water, they are called accessory compounds or extractives [11]. Björklund Jansson and Nilvebrant [12] define wood extractives as the chemical compounds that are extractable from wood with various neutral solvents. With this definition, the accessory components of wood can be clearly differentiated from the major structural components (cellulose, hemicelluloses, and lignin), because neutral solvents, in general, do not dissolve any of the main wood components. Cellulose can only be dissolved by special solvents that break the intermolecular hydrogen bridge bonds [13, 14]. Hemicelluloses must be hydrolysed under acidic (sulfite pulping) or alkaline (Kraft pulping) conditions. Apart from novel methods, in conventional pulping lignin has to be cleaved and dissolved under alkaline conditions (Kraft pulping), or sulfonated first (sulfite process) to make it soluble [15].
Although wood extractives usually do not exceed five percent of dry wood mass on average (heartwood and sapwood)—except some tropical and subtropical woods, which contain significantly higher amounts of extractives (up to 20 wt% based on dry wood)—they can be a serious issue for pulping as well as for the pulp itself. Therefore, the removal of these wood extractives should be considered as pulp (pre-)treatment—not only for avoiding any problems during pulping but also for ensuring satisfying pulp quality [11, 16, 17].
After a short introduction to wood extractives, including an overview on analytical basics, this paper describes the effects of wood extractives on pulp and paper quality and production. The removal of wood extractives as solution for these problems is addressed in the main part of this paper later on. Beginning with wood yard operations and biological treatment, this paper is an extensive review of different technologies for removing wood extractives, from conventional pulping and bleaching processes through different organic-solvent-based extractions to several promising novel methods, including the utilization of extracted wood extractives.
2 Introduction to wood extractives
The so-called wood extractives or accessory compounds are a mixture of many low- and high-molecular-weight compounds that can be extracted from wood with hot water and organic solvents [11, 12, 18]. In living tress, wood extractives play different important roles, which is a reason for the complex extractive’s composition. They protect living trees against biotic attacks like insects and fungi, and play an important role for the metabolism of trees (particularly the parenchyma resin which is described later on) [11, 12, 19,20,21].
2.1 Content and classification
The extractives content of wood, as well as the exact extractives composition, depends on various factors. On the one hand, the extractives vary not only between the two groups, softwoods (Gymnospermae, SW) and hardwoods (Angiospermae, HW) but also among species and even single trees, as shown by Ekeberg et al. [22]. On the other hand, there are differences in the accessory component’s content depending on the location inside the tree and the growth conditions like the geographical site, age, and cutting season [11, 21, 23, 24]. Thus, universal statements about the extractives content in woods are almost impossible.
Table 1 clearly shows the broad range of extractives contents published by different authors. Besides different genera and species, the determined extractives content is also influenced by several other factors, i.e., the sampling locations inside the tree, which are often not included in literature (like in the references of Table 1). One significant variable is the extraction itself because different solvents with different polarities result in different contents and compositions of determined accessory compounds [41]. Two wood-related factors important for pulping, since they can easily be influenced, are listed by Nisula [21]: tree age and the part of the tree. Older trees contain more extractives than younger ones; especially, the extractives content of heartwood is higher in older trees than in younger ones [23, 42,43,44]. Experiments of Miranda et al. [45], Morais and Pereira [46], and Tasooji et al. [47] showed that heartwood, in general, contains significantly more extractives than the surrounding sapwood. Other parts like knots (the part of the branches inside the stem) or the bark contain even more and different accessory compounds [48,49,50]. Björklund Jansson and Nilvebrant [12] categorized the different wood extractives depending on the chemical composition and structure into aliphatic compounds, terpenes, and phenols (presented in Table 2), since many important properties, like dissolution behavior and volatility, are similar within each of these groups.
The aliphatic compounds and the terpenes can be grouped to the so-called resin or pitch, including terpenes, resin acids, fatty acids and esters, various alcohols, hydrocarbons, and other neutral compounds [51]. There are significant differences in the resin composition depending on the location of the resin inside the wood, inside parenchyma cells, or in resin canals. Parenchyma resin, located in the parenchyma cells, serves as a reserve nutrient and therefore contains mainly fatty acids [52]. In growing trees, fatty acids mainly occur as esters (as fats when esterified with glycerol in the form of mono-, di- and triglycerides, as waxes when esterified with fatty alcohols, and as steryl esters when esterified with sterols) [12]. When cells die during the transition from sapwood to the inner heartwood or when the tree is felled, esters are enzymatically hydrolyzed into free fatty acids [21, 51]. The fatty acids in wood have lengths of 16–24 carbon atoms, with the unsaturated fatty acids oleic, linoleic, and linolenic acid (each with 18 carbon atoms) as the main components [12]. On the other hand, the resin inside resin canals, canal resin, or oleoresin differs from the parenchyma resin in its composition. Oleoresin mainly consists of terpenoids, mainly resin acids, dissolved in terpenes to ensure flowability. The function of canal resin is to seal wounds of the tree through evaporation of the volatile terpenes leaving the solid resin acids (called rosin) as a hydrophobic mechanical seal back on the tree surface [53]. Although wood does not contain phenol, compounds with one or more phenol units occur in wood as structural phenolic components (i.e., lignin) and non-structural ones [54]. The non-structural phenolic components are the phenolic extractives, one major group of wood extractives. They are essential for the durability of wood since they protect the tree from different biotic attacks [20]. Björklund Jansson and Nilvebrant [12] grouped the many different phenolic extractives to lignans, stilbenes, flavonoids, tannins, and tropolones.
Since extractives are, as already mentioned, the accessory compounds of wood that can be extracted with hot water or organic solvents, they can also be categorized with their dissolution behavior. Depending on the solvent, they can be divided into hydrophilic and lipophilic extractives, which are also presented in Table 2 [55].
The classification of wood extractives based on their dissolution behavior rates among the most used categorization strategies, but this method also has problems. Both lipophilicity and hydrophilicity are just parameters—depending on various factors—not states [56, 57]. The polarity of solvents also depends on many parameters and is rather a spectrum than just two absolute conditions “polar” and “non-polar” [58]. Therefore, the classification of the accessory compounds based on their dissolution behavior is just an approximate simplification. However, it provides an opportunity for predicting dissolution behaviors and choosing the proper solvent(s) or mixture(s) for the extraction of the desired accessory compound(s), rather polar solvents for hydrophilic extractives, and rather non-polar solvents for lipophilic extractives. For comparing determined extractives contents, a more reproducible way to group wood extractives is the classification depending on the solvent itself, i.e. (hot-)water extractives, acetone extractives, or cyclohexane extractives [59, 60].
2.2 Determination and analysis
Wood can be analyzed for extractives directly with different spectroscopy methods. This fast and non-destructive measurement allows classifying and comparing wood species and wood treatments in terms of different wood compounds, i.e., certain extractives. Wagner et al. [61] successfully applied Fourier-transform RAMAN spectroscopy (FT-RAMAN), Fourier-transform infrared spectroscopy (FT-IR), and Fourier-transform near-infrared spectroscopy (FT-NIR) for this purpose. Similar qualitative analyses were carried out by Schnabel et al. [62] with FT-NIR for larch wood. However, spectroscopy methods are still not the right choice for quantifying wood extractives and require further research [61,62,63].
The two standards T204 [59] and NREL/TP-510–42,619 [60] describe how to determine the extractives content quantitatively. Wood shall be extracted with one or more solvents (depending on the desired group(s) of extractives). These solvents shall be evaporated afterward so that the residue can be weighed for calculating the extractives content. Even though the evaporation shall be done under vacuum and low temperature, the volatile components, particularly (mono)terpenes, are mostly also evaporated and therefore usually not included in the extract weight [59, 60]. Due to their volatility, terpenes, in general, do not play an essential role for pulps anyway. Thus, neglecting their contribution to the extractives content is mainly accepted, especially when analyzing woods for (developing) pulping (methods) [12].
However, wood extractives can be quantified directly out of the extract without evaporating the solvent first with several chromatographic methods like gas chromatography (GC), high-performance liquid chromatography (HPLC), size-exclusion chromatography, supercritical fluid chromatography, and thin-layer chromatography [64]. GC is the most widely used technique for analyzing the accessory compounds of wood with high selectivity and sensitivity [65, 66], even though the derivatization prior to the actual measurement includes some time-consuming steps [67]. Nisula [21], for example, successfully quantified wood extractives in her extensive work with long- and short-column gas chromatography; resin acids, fatty acids, sterols, diterpenoids, juvabiones, lignans, stilbenes, and flavonoids with long-column GC, the bigger steryl esters, triacylglycerols, and oligolignans with short-column GC. Poljanšek et al. [68], on the other hand, has recommended HPLC as the method of choice for analyzing particularly phenolic wood compounds. For lipophilic extractives, the resolution of HPLC is unfortunately not high enough to separate some individual wood extractives, particularly sterols and fatty acids [21].
Although the content of lipophilic extractives in wood is in general significantly lower than the hydrophilic one [69, 70], methods for analyzing lipophilic extractives are more important in pulp and paper manufacture since they cause many problems like sticky deposits [55, 71].
2.3 Effects on pulp and paper quality and production
The accessory compounds are extracted from wood in the papermaking process to a certain amount. Particularly lipophilic extractives such as fatty acids and resin acids tend to agglomerate in the process water forming so-called pitch deposits [9, 72]. In Kraft pulping, the alkaline conditions enable saponification of the glycerol esters to soluble soaps. On the contrary, other lipophilic extractives like sterols and sterol esters cannot be saponified and still form hydrophobic deposits [73]. The ratio of unsaponifiable to saponifiable components is, therefore, an important indicator of pitch formation in Kraft pulping. A higher ratio (for example, silver birch, which has one of the highest ratios among pulpwood species) indicates pitch problems [74] because the deposits stick to process equipment, causing contamination and piping blockages [75]. Pitch also sticks to different parts of the papermaking machines and stains the felts and canvas, even leading to major failures like web breaks of the machine [76]. Hence, pitch deposition originating from wood extractives is responsible for reduced production levels, higher operating costs, and higher equipment maintenance costs of papermaking processes [77]. In Kraft pulp mills, economic losses due to pitch problems are assumed to account for 1–2% of sales [78].
Once pitch deposits become released from the equipment, they contaminate the pulp in the form of dark spots, specks, and streaks, lowering the quality of the final product [79, 80]. Additionally to dark spots, specks, and streaks, extractives are considered to have a negative influence on the color and bleachability of pulps [81, 82]. Also, the oxidation products of some accessory compounds, particularly polyphenols, lead to the darkening of wood and pulp when exposed to sunlight and oxygen [82]. Pereira et al. [20] highlighted stilbenoids as the phenolic compounds that darken wood in the presence of sunlight, bringing up some problems when producing paper. Wei et al. [83] isolated the phenolic extractives of locust wood and observed color changes also when exposed to heat. However, chromophores as the UV/VIS absorbing groups responsible for the color of wood cannot be attributed exclusively to phenolic wood extractives [84]. They also originate from lignin to a large extent and sometimes even from organometallic extractive complexes [84, 85]. Especially heat- and light-induced color changes of wood and pulp result from complex reactions of hemicelluloses, lignin, and extractives [85].
Besides the negative impact on color and bleachability, wood extractives are also responsible for the odor of the pulp [86]. Ghadiriasli et al. [87] identified various volatile organic compounds (VOC), including terpenes and degradation products from fatty acids and lignin, as odor-active compounds in oak wood, while Schreiner et al. [88] stated more precisely that fatty acid degradation products represent about two third of all the odor-active compounds in pine wood. Among all fatty acid degradation products, aldehydes play an essential role as odor-active compounds because their odor threshold is very low, and their smell is generally considered highly unpleasant [12, 89]. Aldehydes are not part of the original wood extractives but formed through an auto-oxidation of unsaturated fatty acids, called lipid oxidation, catalyzed by enzymes and heavy metal ions in wood [90, 91]. Although different aldehydes are formed through these lipid oxidation processes, hexanal as a linoleic acid oxidation product accounts for most of the aldehydes content [90]. Today’s solution to deal with the odor from lipid oxidation in pulp and paper is using chelating agents like ethylenediaminetetraacetic acid (EDTA) to inhibit the catalyzing effect of heavy metal ions [12, 90]. However, EDTA is considered a risk for the aquatic ecosystem because it influences the heavy metals bioavailability and remobilization processes in the environment [92, 93]. Munn et al. [93] even concluded in the European Union’s risk assessment report about EDTA that there is a need for limiting the environmental risks of EDTA. However, in conventional wastewater treatment plants, sufficient degradation of EDTA cannot be achieved [92]. EDTA containing process waters must therefore undergo special treatment before releasing them into the environment, leading to higher process costs [94, 95].
Environmental legislation has led to higher wastewater treatment requirements and, consequently, multiple reuses of process waters in the papermaking industry. This system closure decreases the wastewater treatment costs and creates new challenges due to the accumulation of wood extractives in process waters [67]. Naicker and Sithole [96] observed increasing extractives concentration and chemical oxygen demand (COD) of the process water with decreasing fresh water consumption in their experiments. Other issues with system closure are corrosivity and toxicity of process waters, mainly due to wood extractives, and more precisely because of phenolic compounds together with resin acids [12, 97]. Krilov and Gref [98] explained some corrosion effects with the ability of some phenolic wood extractives to form iron chelates. More specifically, Hazlewood et al. [99] stated that the phenolic catechols together with resin acids highly increase the corrosivity of black liquor from the Kraft process. On the contrary, other wood extractives like palmitic acid (a saturated fatty acid) were found to decrease corrosivity [99]. Similar findings were published by Singh and Anaya [100], who even found significant differences between some HW and SW species when pulping them with the Kraft process. Besides corrosivity, toxicity is another important effect of wood extractives. This must be taken into account for treating the contaminated waste streams of the papermaking process, especially because there is increased focus on biotechnological processes for generating by-products out of waste streams like sludge [101] or fines [102]. Also, for conventional treatment of pulp mill sludge, wood extractives shall be removed by hydrothermal treatment, for example, to enhance anaerobic digestibility [103]. The accessory compounds of wood should thus be removed already during or even prior to pulping to avoid any further treatment in the papermaking process.
3 Removal of wood extractives during wood yard operations
Regardless of any subsequent pulp pre-treatment or the pulping itself, there are several opportunities for removing wood extractives at the wood yard in advance. The main tasks of wood yards include debarking the logs, chipping them (except groundwood pulping), screening the chips, and storing the wood [104]. The following sections explain the possible influences of these process steps on the wood extractives content.
3.1 Removal of bark and knots
Log debarking is, in general, the first process step at wood yards. It removes the dirt on the logs' outside and ensures that the resulting pulp is free of any bark [10]. On the other hand, it is also an efficient method for reducing the accessory compounds of the used raw material since the content of both lipophilic and hydrophilic extractives is usually very high in the bark. Among different species, wood extractives account for 20–40% of the dry matter of bark [23]. The experiments of Arisandi et al. [105] showed that eucalyptus bark contains not only more extractives than sapwood or heartwood but also more of every analyzed group of lipophilic extractives. Salem et al. [106] confirmed the findings with SW, showing that particularly fir and spruce bark contains up to 3 times as much lipophilic extractives than the wood itself. Balaban and Uçar [107] compared the content of the accessory compounds of oak wood with the one of the corresponding bark using different polar and non-polar solvents. All the bark extracts contained more extractives than the wood ones did [107]. The high extractives content of bark would negatively influence pulp quality and pulping; including extended cooking times and increased bleaching chemical consumption. Wood debarking prior to pulping has therefore become a well-established process. Nowadays, relatively low-cost drum debarkers are among the most used technologies, reaching final bark contents below 1% [104].
Also, in debarked wood, the accessory compounds are not evenly distributed. For example, the inner heartwood is richer in both lipophilic and hydrophilic wood extractives than the outer sapwood [108, 109]. The starting points of branches inside the stem referred to as knots, contain even more extractives than all the surrounding stemwood [50]. Pohjamo et al. [110] reported significantly larger quantities of the phenolic flavonoids in knots than in the stem of the same trees. Other hydrophilic compounds like lignans can account for even 20% of the mass of the knots [111]. Willför et al. [112] analyzed knots for lipophilic wood extractives and found large amounts from 4.5 wt% up to 32 wt%. Besides the higher extractives content, knots also differ in the mechanical properties from wood since they are much denser and contain shorter and stiffer fibers. As so-called reaction wood, they have to withstand higher mechanical stress [21]. They are not fully cooked in chemical pulping and therefore leave the digester as large and dark impurities. Subsequent pressure screens remove the knots (called knotting) from the pulp stream [113]. However, the high extractives content of knots can negatively influence the entire pulp and paper manufacturing process, as already explained. Removing knots even before pulping should thus be considered. When chipping the logs, knots mainly result in over-thick chips because they are adamant and rigid [114]. Based on this finding and the fact that knots are denser than stemwood, Eckerman and Holmbom [115] developed a method for separating knots from the oversized chip fraction. This method, called “ChipSep”, relies on the phenomenon that dried knots are heavier than water, whereas dried stemwood, is lighter than water, provided that the dry matter content of the dried knots and stemwood is at least 85% (advantageously at least 87%), according to the patent of Eckerman and Holmbom [115]. Hence, knots containing wood sinks in water while knots-free wood rises towards the water surface [115].
3.2 Wood storage and biological treatment
Besides debarking and chipping, one primary purpose of a wood yard is wood storage. Delivered logs and cut wood chips are stored to ensure continuous supply of the subsequent pulping processes and compensate for any fluctuations in raw material supply [104]. Moreover, wood storage is even recommended to decrease the extractives content of the wood. During this wood seasoning, the accessory compounds of wood are reduced through volatilization, oxidative processes, and enzymatic hydrolysis in tree cells and by microorganisms [116]. In fact, the content of wood extractives starts to decrease immediately after felling the trees and continues during storage, in the shape of chips and even entire logs, as shown in the experiments of Gutiérrez et al. [117] and Silverio et al. [80]. The reaction rate of all the chemical and biochemical reactions occurring during storage is highly dependent on environmental conditions, i.e., temperature, UV irradiation, wind, rain, and storage conditions like ventilation, protection with covers and the duration of storage [23]. The main reactions include oxidation of wood resin, hydrolysis of glycerides and other esters, loss of volatile components, and microbial degradation [12]. Logs and particularly wood chips require well-suited storage conditions.
Otherwise, chip deterioration and wood decay due to sap stain and a fungal attack occur [104]. Ramnath et al. [118] even found variations in bacterial and fungal communities on frozen stored wood chips. They showed that storing the chips at − 20 °C had similar effects on the decrease of lipophilic wood extractives than seasoning does. During six months of storage, 25–44% reduction of lipophilic extractives was observed [118].
To summarize, wood seasoning can be applied as a strategy for reducing wood extractives without a significant decrease in the quality of the wood, but wood might be attacked by microorganisms, resulting in chip deterioration and mass losses of cellulose and hemicelluloses [16]. Seasoning offers no control over the microbial species growing on wood chips, leading to unpredictable wood effects, like loss of brightness or strength. Nevertheless, methods for controlled seasoning have been developed using microorganisms that effectively remove extractives without causing discoloration or strength loss [119]. This biological treatment method includes different bacteria strains and fungi, such as white-rot fungi, and is referred to as biopulping. The employed microorganisms can also digest lignin to a certain extent [120, 121]. Dorado et al. [122] proved white-rot fungi to decrease the amount of accessory wood compounds successfully. Their experiments removed up to 51% of resin acids and up to 87% of free fatty acids from pine wood within two weeks, while total loss of wood mass was below 12% [122]. Similar findings were published by Thao et al. [123], who removed up to 89% of fatty acids and fatty alcohols and 79% of free sterols from acacia wood within 30 days by means of a white-rot fungus. Košíková et al. [124] investigated four yeast strains concerning their wood extractives removal behavior. They could decrease the extractives content up to 63% and the fatty and resin acids up to 78% within four weeks. Experiments on bacterial strains done by Burnes et al. [125] and Kallioinen et al. [126] showed that the total wood extractives content can be bacterially decreased up to 41% (the lipophilic extractives even up to 67%) within two weeks without any visible discoloration of the wood. However, the experiments about biopulping mentioned above showed that microbiological treatment has to be carried out in a bioreactor under controlled conditions and takes quite long (several weeks) compared to mechanical or chemical pulping (minutes to hours).
4 Extractives’ removal through conventional pulping
The two conventional technological principles to produce paper pulp out of wood are mechanical and chemical processes, differing in the yields (80–95% at mechanical pulping comparing to 45–55% at chemical pulping), properties, and economic potential [8].
The defibration during pulping leads to exposure of the resin channels, making oleoresin accessible for dissolution and chemical reactions. On the one hand, this can lead to pitch problems at mechanical pulping, but on the other hand, it enables deresination in chemical pulping [12]. The parenchyma resin, on the contrary, is more difficult to remove because the pulping liquors must diffuse into the parenchyma cells, dissolve the resin or form soap micelles with the sterols and esters and diffuse out again afterwards. This process is enhanced by breaking the cell walls through chemical or mechanical pulping [21].
4.1 Mechanical and semi-chemical pulping
Mechanical pulping can be divided into two main categories, depending on the defibering method; groundwood pulp (GWP) obtained by grinding processes and refined mechanical pulp (RMP) produced with refining processes [8]. In semi-chemical pulping processes, the wood undergoes mild chemical treatment preceded by a mechanical refining step [2]. The most important refining process is thermomechanical pulping (TMP). Before refining, the wood chips are heated with steam (to 100–130 °C) to soften the lignin [10, 127]. The thermal treatment can also be combined with a chemical treatment to produce chemi-thermomechanical pulp (CTMP), representing the transition from mechanical to chemical pulps [2, 128].
Except for CTMP, the mechanical pulp contains most of the original lignin and still many accessory compounds [82]. However, the high temperatures of the defibering processes lead to partial degradation of cellulose and hemicelluloses. The resulting saccharides are then dissolved in the process water. Increased temperatures (above approximately 160 °C) at the TMP process even enhance these mechanisms, as shown by the work of Schneider et al. [129]. Besides saccharides, also extractives are liberated to a certain amount and released into the process water afterwards. These extracted accessory compounds do not only include hydrophilic extractives but also lipophilic extractives like fatty and resin acids [130]. Resin acids and phenolic compounds contribute the most to the toxicity of mechanical pulping process waters [12].
4.2 Chemical pulping
Compared to mechanical pulping, in chemical pulping, lignin is rather removed by degradation and dissolution than just softened. Depending on the chemicals used for cooking the wood chips, there are mainly two types of processes: the sulfite process and the sulfate or Kraft process as the dominant pulping process [131]. Johansson et al. [132] stated as early as 1987 that most of the world’s chemical pulp is produced by the Kraft process because of several advantages, including versatility in dealing with different raw materials and efficient recovery of cooking chemicals. Sixta et al. [9] defined Kraft pulping 2006 still the dominating chemical pulping process with almost 90% of the produced pulp worldwide, although sulfite pulping has accounted for approximately 70% of the world’s dissolving pulp production [9]. Especially for dissolving pulp, wood extractives are important disruptive factors since they decrease the reactivity of cellulose with carbon disulfide when producing viscose out of dissolving pulp [133]. The accessory compounds of wood are usually mainly removed through chemical pulping but lead to increased consumption of cooking chemicals since they compete with lignin and hinder its removal [81].
The pH-flexible sulfite process uses aqueous sulfur dioxide and a base for cooking the wood chips. The dominating sulfite process in Europe is the acid magnesium bisulfite process [10]. While hemicelluloses are removed directly through hydrolysis during a cooking process called digestion, lignin must be sulfonated by the bisulfite ions to make it soluble in the cooking liquor [15]. In addition to hemicelluloses and lignin, most of the accessory compounds of wood are also removed through sulfite pulping. Unfortunately, some extractives participate in the sulfonation reactions, competing with lignin and increasing cooking liquor consumption [81]. Nevertheless, Rodrigues et al. [133] removed with the acid sulfite process nearly 85 wt% (based on initial dry wood) of acetone-soluble wood extractives, leaving mainly just extractives consisting of fatty acids and sterols in the unbleached pulp [133]. Nevertheless, Sixta et al. [9] listed the extractives content of sulfite pulp and Kraft pulp for both spruce and beech wood, showing that the sulfate process even removed about twice as much extractives as the sulfite one, independent of the wood class (SW/HW). Duan et al. [134] published similar findings and attributed them to the alkaline conditions of the Kraft process, which promote saponification and subsequent extractives removal.
In Kraft pulping, lignin is depolymerized by cooking wood chips with an alkaline cooking liquor consisting of caustic soda and sodium sulfide. The resulting phenolic fragments are then dissolved into the cooking liquor [135]. For producing dissolving pulp, hemicelluloses have to be removed from wood by prehydrolysis prior to pulping. The prehydrolysis can be done with diluted acid (acid-prehydrolysis) or just hot water, called autohydrolysis because wood typically releases acetic acid through autohydrolysis [136]. Although autohydrolysis is technically a hot-water extraction, Da Silva Morais et al. [137] and Li et al. [138] found an increased extractive content, especially at more severe prehydrolysis conditions. Da Silva Morais et al. [137] explained that phenomenon with the partial degradation of some macromolecules (mainly hemicelluloses) at high prehydrolysis temperatures. Therefore, the macromolecules are mobilized, meaning that the degradation products can easily be extracted in the case of subsequent extractions. When determining the extractives content gravimetrically by evaporating the solvent from the extract, the degradation products are then distorting the extractives content. As already mentioned, experiments showed, that the sulfate process removes about twice as many extractives as the sulfite one due to saponification. The alkaline black liquor converts resin and fatty acids into soluble sodium soaps [12]. However, Dunlop-Jones et al. [139] published that in typical Kraft pulping, only half of the potentially saponifiable wood resin was saponified in the digester. Moreover, Shin et al. [140] found sterols, steryl esters, and fatty acids as the main compounds in residual Kraft pulp extractives. Therefore, there is a need to further eliminate the remaining accessory wood compounds through pulp bleaching, for example, removing the unsaturated fatty acids by oxygen delignification [140].
4.3 Pulp bleaching
Extended cooking for removing color in pulp originating from lignin, extractives and other colored impurities would significantly decrease pulp quality due to enhanced cellulose degradation [141]. Thus, pulp bleaching has been developed, which is defined by Sixta et al. [141] as a chemical process for removing the color sources in pulps, has been introduced. To quantify the results of these bleaching processes, brightness has been established as an indication of the pulp’s whiteness [10]. Starting with chlorine as the main bleaching agent, in the 1980s, so-called elemental chlorine-free (ECF) bleaching processes have been developed to substitute chlorine with chlorine dioxide (D). The recent development is the totally chlorine-free (TCF) bleaching process, applying oxygen (O), hydrogen peroxide (P) and/or ozone (Z) as bleaching agents, and an alkaline extraction stage (E) [141]. Since mechanical pulp aims to minimize mass loss, lignin of mechanical pulps is rather just brightened than removed compared to chemical pulps. This so-called lignin-preserving bleaching is accomplished commercially, primarily with hydrogen peroxide and/or sodium dithionate [142].
Alkaline hydrogen peroxide bleaching as lignin-preserving bleaching method decreases the lipophilic extractives content of TMP just slightly [143]. Ekman and Holmbom [144] examined alkaline peroxide bleaching of mechanical pulps with spruce groundwood. Hydrophilic extractives were oxidized almost wholly, whereas resin acids were just partly oxidized and fatty acids even unaffected [144]. As already mentioned, chemical pulping typically eliminates far more wood extractives than mechanical pulping, showed by Rodrigues et al. [133], with a removal rate of 85 wt% (based on initial dry wood) acetone-soluble wood extractives through the acid sulfite process. With subsequent E–O–P bleaching, they removed further 11 wt%, leaving just four wt% of the initial extractives in the bleached pulp, mainly consisting of fatty acids and sterols [133]. España Orozco et al. [145] achieved comparable results with oxygen-reinforced alkaline extraction of mixed HW sulfite pulp, followed by Z and P bleaching. They decreased the gravimetric acetone extract of pulp by 68% through the TCF bleaching sequence, with oxygen-reinforced alkaline extraction as the most effective stage, leaving mainly just sterols and sterol esters in the pulp. For lipophilic extractives (determined by GC–FID), the highest drop was observed after Z bleaching (− 38%). Freire et al. [146] and Gutiérrez et al. [147] compared different bleaching sequences with eucalyptus Kraft pulp. They observed that unsaturated sterols and fatty acids were extensively degraded during D and Z bleaching but only partially degraded during O and P bleaching. The wood extractives removed during bleaching are not only degraded and oxidized but also dissolved to a certain extent into bleaching and washing liquids, as shown through analysis of pitch deposits in bleaching stages by Del Rio et al. [75] and evaluation of precipitated extractives on bleached Kraft pulp by Koljonen et al. [148]. For avoiding such problems during bleaching, the extractives content can be reduced by extrations before bleaching or even before pulping which is explained in the following chapter.
5 Alternative methods for pulping and removing extractives
Currently, high-purity cellulose fibers are commercially produced from wood with the sulfite or the Kraft process. These pulping methods are considered cellulose-first strategies since fractionation of biomass usually targets high-quality cellulose. The resulting lignin has different properties than the native one (i.e., it is less reactive) and is mainly burned for energy recovery instead of using as a by-product [149]. The approach for using all the components of the biomass feedstock as products are so-called lignocellulose biorefineries. Besides pulping, lignocellulose biorefineries also include separation and purification processes, which contrasts the increased revenue streams with the high capital and operating costs of up to 20–50% of the total capital and operating costs of biorefineries [150]. However, there is growing interest in organosolv pulping processes using organic solvents instead of inorganic pulping liquors. The recovery of satisfying by-products is easier than in the Kraft process [151].
5.1 Organosolv pulping and extraction with organic solvents
Kleinert and Tayenthal [152] successfully carried out experiments as early as 1931 for fractionating different woods with aqueous ethanol. This so-called organosolv pulping uses—as already mentioned—organic solvents. It is a two-stage process where hydrolysis degrades hemicelluloses, and lignin is dissolved into the organic solvent to a certain extent depending on the pulping conditions [153]. Johansson et al. [132] showed that the organosolv process has considerable potential in terms of delignification selectivity. Compared to pulps produced through conventional pulping, organosolv SW pulps can have higher yields at equal kappa numbers, which indicates the lignin content of the pulp [132]. Good delignification of the organosolv process was also proved by the experiments of Botello et al. [28] and Kirci and Akgül [154]. They concluded that organosolv pulping could be a suitable alternative for producing dissolving pulps with very high cellulose contents. Moreover, organosolv pulps can have better optical and strength properties than conventional Kraft pulp [155].
Another advantage of the organosolv process is the effective removal of extractives from wood. As already described, the accessory compounds of wood can be extracted with (aqueous solutions of) organic solvents. This is even considered as the method of choice for the exclusive extraction of accessory wood compounds. The two most commonly used standards, T204 [59] and NREL/TP-510–42,619 [60], for quantitatively determining the wood extractives content, are based on a mild extraction with organic solvents through a Soxhlet apparatus. According to the standards, the wood shall be extracted with organic solvents at ambient pressure and low temperatures (below the solvent's boiling point) for a long period (up to 24 h). The extraction works automatically in a closed loop with a boiler on the bottom, a condenser on the top, and the extraction thimble with a siphon as well as a vapor bypass in the middle. There are many siphon cycles within the entire extraction period, with fresh solvent for each cycle [59, 60]. The long period for complete extraction can be compensated with high pressures. National Renewable Energy Laboratory [60] describes a method to use a speed extractor with high pressures, which allows quantitatively extracting the wood in a few minutes. This method is called accelerated solvent extraction (ASE). Table 3 compares the ASE with the traditional Soxhlet extraction, as well as different solvents.
Vek et al. [156] showed that with the same solvent(s), ASE yields comparable amounts of extractives but takes just 10–20% of the time of Soxhlet extraction. The reason for the difference in extraction time is that Soxhlet extraction is carried out under atmospheric pressure and temperatures between 7 °C and 10 °C below the boiling point of the solvent compared to temperatures above the boiling point and pressures higher than 100 bar at the speed extraction [158, 160]. The solvent itself, on the contrary, can have a significant influence on the determined extractives content. It can be seen in Table 3 that solely extracting with non-polar solvents like Cyclohexane is not appropriate for quantitatively removing wood extractives. Non-polar solvents mainly extract lipophilic accessory compounds of wood, which are less in amount than the hydrophilic ones, as already mentioned. To enhance the quantitative removal of wood extractives, non-polar solvents should be mixed with miscible polar solvents, or a subsequent extraction with a polar solvent should be added [69, 156, 159]. Moodley [157] and Schwanninger and Hinterstoisser [160] could increase the amount of extracted material up to 150% with a hot-water extraction in addition to the organic solvent extraction step. On the other hand, one must be aware of autohydrolysis occurring during hot-water extraction, dissolving considerable amounts of wood compounds that are not wood extractives by definition, i.e., hemicellulose degradation products [161]. Krogell et al. [162] and Song et al. [163] removed considerable amounts of hemicelluloses from the wood meal by pressurized hot-water extraction (PHWE) within 20 min. Increasing the wood particle size significantly decreased the amount of total dissolved solids (TDS) [162, 163]. The influencing factor particle size was also investigated by Bertaud et al. [158] at extractions of coarse-crushed wood and groundwood with acetone and cyclohexane and significantly influenced the extraction behavior. A lower particle size led to sharply higher amounts of extracted wood extractives [158]. In terms of process design for an industrial application of organic solvent extraction for removing wood extractives, different extraction methods and conditions should thus be compared using the actual wood particle size of the process later on. Thurbide and Hughes [164] carried out experiments comparing Soxhlet extraction and ASE with TMP instead of wood meal as the standards T204 [59] and NREL/TP-510–42,619 [60] require. The obtained extractives content was quite similar, meaning that even at industrial wood particle sizes like TMP, long extraction times can be compensated by high temperatures and pressures and vice versa [164].
Soxhlet extraction is just a lab-scale method because of the long extraction period and the high energy demand for recycling the vast amount of organic solvent needed. Nevertheless, organic solvent extraction, in general, would technically be an interesting technology for removing wood extractives as pulp (pre-)treatment as well as for pulping itself. Shin et al. [165], for example, could significantly decrease the chromophore groups of aspen Kraft pulp just by extracting the pulp with organic solvents to remove residual extractives. Baptista et al. [166] extracted wood with organic solvents even prior to Kraft cooking. The results showed better bleachability and less chlorine dioxide consumption to reach the same brightness levels when bleaching the pulp [166].
Despite all the advantages of extracting wood with organic solvents and aqueous solutions of organic solvents, like good pulp properties and superior capabilities of removing lignin as well as extractives (depending on the process conditions), it is unfortunately not an economic pulp (pre-)treatment method [167]. Notably, organic solvent recycling has a significant impact on the energy consumption of the process, as it remains one of the main challenges towards an economically viable design [168, 169].
5.2 Novel extraction and fractionation methods
In the organosolv process, as already mentioned, not only the accessory compounds of wood are extracted, but also lignin and hemicelluloses by breaking down the internal bonds. The lignin obtained as a by-product of this process is characterized by high purity and quality [170]. On the other hand, organic solvents are associated with flammability, volatility, toxicity, environmental hazards, and increased costs [171, 172]. Thus, extensive research has been done to develop various (pre-)treatment processes with different methods and solvents, particularly environmentally friendly or green solvents [172, 173].
One alternative method with green solvents is supercritical extraction (SCE), more precisely, the extraction with supercritical fluids. The advantages of using supercritical fluids are the low viscosity, high diffusivity, high dissolving power, and low surface tension, leading to higher solvent mass transfer when penetrating cell wall layers. Typical SCE solvents are water, carbon dioxide, and mixtures with organic co-solvents [171]. Supercritical extraction with carbon dioxide (SC-CO2) has become an established technology since CO2 is non-toxic, non-flammable, and recyclable. Furthermore, carbon dioxide is considered non-polar and dissolves lipophilic compounds, i.e., resin and fatty acids [158]. Another alternative process is steam explosion, which is technically the treatment of wood through hot steam under pressure with a subsequent pressure drop to atmospheric pressure. This leads to the destruction of cell wall layers and easier solubilization of wood compounds [174]. Table 4 compares different supercritical extractions and steam explosion treatments with Soxhlet as the reference method for quantitatively extracting the accessory wood compounds.
As it can be seen in Table 4, supercritical extraction and steam explosion can yield significantly more extractives than a Soxhlet extraction. Still, one should be aware that SCE and especially steam explosion degrades hemicelluloses to a certain extent [170]. This means that the extractives content gravimetrically determined by evaporating the solvent from the extract can incorrectly be too high because the evaporation residue contains extractives and hemicellulose degradation products. Nevertheless, Demirbaş [175], for example, extracted four times more extractives and ten times more amount of fatty acids with SCE than the Soxhlet extractions. The big advantage of steam explosion is the lower capital costs and the higher energy efficiency, which makes it suitable for lab-scale and commercialized applications from an economical point of view [176]. Despite the benefits of SC–CO2 as low solvent costs and low temperatures, the exceptionally high pressure of up to several hundred bars is a barrier for upscaling this process to an industrial scale [177].
One relatively new approach to overcome the disadvantages of using volatile organic solvents, or applying high pressures for SCE or steam explosion, for example, are ionic liquids (ILs) which are also considered to have lower environmental impacts [178]. ILs are salts and therefore composed of anions and cations. The properties resulting from this composition are thermal stability, remarkable solvating ability, nonvolatility, and near-zero vapor pressure [179]. Especially in biorefineries, ILs were proven to be an effective solvent for fractionating wood and even dissolving entire wood particulates after an autohydrolysis step, as shown by Deb et al. [180]. On the other hand, Papa et al. [181] could extract significant amounts of the terpene α-pinene from pine wood applying IL treatment, and Kilulya et al. [182] demonstrated the removal of lipophilic wood extractives from dissolving pulp with ILs in amounts comparable to other extraction technologies. Additionally, ILs exhibit favorable behavior when producing food supplements or drugs out of wood, i.e., antioxidants. Pinkert et al. [183] successfully applied food-additive-derived ILs for extracting wood lignin without dissolving or degrading cellulose. However, the drawback was the requirement of recycling the ILs more than 100 times for an economically feasible process [183]. The reason are high costs for ILs due to synthesis, purification, and downstream processing procedures, which are currently the limiting factors for using ILs at a large scale [179]. Other disadvantages of ILs are poor biodegradability and biocompatibility, and usually, fossil resources are needed to produce ILs [184]. In 2007, Fukaya et al. [185] developed so-called “Bio-ILs” completely derived from biomaterials to overcome these problems. However, Yiin et al. [184] concludes that further research has to be done on Bio-ILs, because studies are still limited in 2021.
One of the drawbacks of all the treatment methods mentioned above is the heat transfer resistance limiting the process efficiency. Microwave (MW) technology, which has received increased attention in recent years, can overcome this limitation because MWs penetrate materials and deliver energy throughout the material volume. Therefore, this so-called in-core volumetric heating generates heat directly inside the volume instead of heat transfer through conduction and convection when conventionally heating from outside [186]. The advantages are enhancement of reaction rate, reduction of the reaction time, increased product yield, and less formation of by-products, provided that the solvent and/or the biomass are high-microwave-absorption materials [187]. For example, Xu et al. [188] proved MW technology as an efficient method by liquefying about 75% of the initial wood in methanol at 180 °C in just 15 min. However, applying microwave extraction can lead to local overheating since composition, geometry, and size affect microwave power distribution, making it challenging to up-scaling the process [171].
Another novel method applying high-energy radiation is ultrasonic-assisted extraction (UEA) [189]. The principle behind UAE is disrupting the solid structure with ultrasonic waves [190]. In detail, the ultrasonic waves degrade cell walls and rupture bonds between lignin, hemicelluloses, and cellulose [171]. He et al. [191] proved the ultrasound-induced mechanical damage of wood structure in his work and observed a significant reduction of wood extractives. Wang et al. [192] could increase the extraction efficiency even by factor 2.6 when extracting hemicellulosic and phenolic compounds from bamboo wood with water. Similar findings were published by Sillero et al. [193], who successfully increased the extraction of phenolic wood extractives from different HW species with intensified UEA. Although UEA has been successfully proved as an effective extraction method, much more research has to be carried out for using UEA in large industrial-scale processes, i.e., optimization in terms of ultrasound power input and frequency [189].
6 Utilization of wood extractives
When applying extractive methods as pulp (pre-)treatment, the accessory compounds of wood cannot only be removed but also isolated as side products since they have many interesting properties.
Wood extractives play an essential role in protecting living trees against fungal attacks and other pests like insects [19]. Especially phenolic extractives and resin acids act as natural fungicides of trees [12, 194]. The extract of Delonix regia (also known as flame tree or flamboyant) in the study of Salem et al. [195] showed inhibition of fungal growth and antioxidant activity. Additionally, the extract exhibited antibacterial behavior against different bacterial strains, i.e., Escherichia coli, suggesting the usage to control plant and human pathogens [195]. Hosseini Hashemi and Jahan Latibari [196] found that walnut-heartwood-extractives-coated poplar wood had significantly less mass loss after exposing it to white-rot fungus than untreated poplar wood. Belt [197] observed pine knot extract to have a significant antifungal character, but suggested more study before approving pine extractives as preservative chemicals. However, wood extractives have enormous potential in wood protection because they have many different antimicrobial properties, are renewable, and are less eco-toxic than chemical biocides [198].
Pine extractives have been used by humans for hundreds of years for paintings and coatings, particularly for caulking seams of wooden ships [199]. Since distilling wood extractives, the two resulting products, turpentine and rosin, have served as important platform chemicals. Today, the overwhelming predominance of petroleum-based chemicals has led to much less usage of wood extractives than their potential would be. Nevertheless, the rising demand for renewable materials and chemicals increases interest in wood extractives [200].
Currently, wood extractives resulting from thermomechanical pulping are wasted because today’s approaches for managing them with additives during pulping do not allow efficient recovery [201]. In Kraft pulping of SW, on the other hand, the volatile accessory compounds of wood can be isolated, yielding approximately 10 kg/ton of pulp. These volatile extractives mainly consist of monoterpenes and are distilled for removing impurities. The resulting fraction is called turpentine, with the major constituent α-pinene. Turpentine is mainly used for producing chemicals, fragrances, and flavors. The non-volatile accessory compounds of wood, mainly soaps of resin acids and fatty acids, are separated from the cooking liquor after the Kraft cooking process. The addition of sulfuric acid liberates the free fatty acids, giving crude tall oil (CTO) with a yield of up to 50 kg/ton of pulp [202]. The CTO production in 2018 was about 2.5 million metric tons worldwide. From that amount, globally, two million metric tons were refined, while the rest was used for heat and power generation [203]. The distillation products of CTO are 30–50% pitch, 30–50% fatty acids, and 15–35% resin acids [204]. In the rosin fraction (colophony), Holmbom [205] found 62–80% of the resin acids from the crude tall oil feed.
From a product point of view, CTO products can be divided into chemical intermediates, biodiesel, and tall oil pitch. Pitch is currently just used for heat and power generation, whereas the chemical intermediates, rich in resin acids, are used as adhesives and in paintings and printing inks [203]. The fatty acids of tall oil can be converted into biodiesel by esterification with the challenge of removing the remaining sulfur [206]. However, tall oil biodiesel is one way to add more value to CTO. In Finland, the first industrial-scale CTO biodiesel plant worldwide produced more than 100,000 tons of biodiesel per year, already in 2015 [207, 208].
7 Concluding remarks and outlook
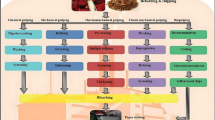
Wood extractives are still a big issue for the pulp and paper industry. They lower pulp quality and cause many problems for the pulping process itself due to the formation of sticky deposits. Some established process steps already decrease the extractives content to a certain extent, i.e., debarking, chemical pulping, and bleaching. Additionally, many alternative technologies for reducing accessory wood compounds have been developed to replace or supplement the conventional process steps, as shown in Fig. 1.
Overview of established and alternative methods for removing wood extractives at pulp production. Description: Blue solid-line frames are established conventional processes, green double-line frames are novel alternative methods. Solid lines inside the frames separate different options at the respective process stage; dashed lines indicate possible combinations of different methods
Wood debarking as a well-established technology is a very effective method to reduce the wood extractives content. In bark, wood extractives account for 20–40% [23] of the dry matter compared to less than in wood. Research about the extractives content of different wood parts showed that besides bark, also knots contain large amounts of extractives from 4.5 wt% up to 32 wt% [112]. Knots are also denser and stronger than heart- and sapwood, which allows rejecting knot-containing chips. Wood seasoning after chipping can also reduce the accessory compounds even prior to pulping. Still, it requires well-suited storage conditions and long storage times for significantly lowering the extractives content. Otherwise, wood decay and/or fungal attack might lead to quality losses. On the other hand, several studies proved different fungi and bacterial strains to effectively decrease the extractives content by more than 60% [124] without decreasing wood quality. However, the main drawback for an industrial-scale use is the requirement of controlled conditions in a bioreactor for several weeks required for such a biological treatment.
Conventional pulping also removes wood extractives to a certain extent. At mechanical pulping, just a small part of hydrophilic and lipophilic wood extractives are released into the process water, but Rodrigues et al. [133], for example, could remove in their work nearly 85 wt% (based on initial dry wood) of acetone-soluble wood extractives by the acid sulfite process. Kraft pulping was found to remove even more extractives than the sulfite process does. Furthermore, pulp bleaching plays an important role in decreasing the amount of accessory wood compounds. With the right choice of pulping stages, the residual extractives content can be lowered significantly, leaving mainly just sterols and sterol esters in the bleached pulp.
Additionally, a number of organic-solvent-based pulping and extraction methods have been developed to remove the accessory compounds from wood and pulps. The extraction with organic solvents, as one of the most used extraction techniques for woody biomass, even allows quantitatively removing wood extractives and is therefore also the method of choice for determining the extractives content. However, the typical organic solvents like ethanol, acetone, or cyclohexane are quite volatile and highly flammable. This has led—together with economic challenges for solvent recycling—to the development of novel extraction methods, ranging from alternative solvents like supercritical fluids or ILs through MW technology as an alternative heating method to UAE for enhancing the extraction by mechanically damaging cell wall structures. All these extraction-based methods are not only technically suitable for removing wood extractives but can also be used as an alternative pulping method for wood fractionation and delignification, as proved by many studies. Moreover, the extraction-based removal of wood extractives allows using the extracted accessory compounds of wood, i.e., as natural biocides or platform chemicals. However, further research is required to overcome the problems of novel extraction methods which are currently hindering the application for removing wood extractives on an industrial scale. On the one hand, there is a need for better synthesis and recycling processes to reduce operating costs (ILs). On the other hand, there are several technical issues, like high pressures (SCE) and irradiation power input and distribution for large geometries (MW technology and UAE).
This need for research has also been intensified by enhancing environmental legislation. On the one hand, state-of-the-art processes for avoiding negative impacts of wood extractives on pulp and paper quality by adding chemicals will most likely be restricted by law. Most additives, i.e., complexing agents against lipid oxidation of wood, are already considered as risks for the aquatic ecosystems. On the other hand, higher wastewater treatment requirements and fresh water usage regulations have led to increased process water system closure and, consequently, to an accumulation of wood extractives in process waters. The resulting lower product quality and higher maintenance costs (due to blockages and web breaks, for example) can only be avoided by expensive process water treatment. An alternative solution is removing wood extractives as pulp (pre-)treatment, which is still in the research and development stage. Nevertheless, some novel methods, like extracting wood with supercritical fluids or ILs and using MW and UAE technologies, are still performed on a lab scale but already delivering promising results for solving this issue in the near future.
Abbreviations
- ASE:
-
Accelerated solvent extraction
- COD:
-
Chemical oxygen demand
- CTMP:
-
Chemi-thermomechanical pulp
- CTO:
-
Crude tall oil
- D (bleaching):
-
Chlorine dioxide (bleaching)
- E (bleaching):
-
Alkaline extraction (bleaching)
- ECF (bleaching):
-
Elemental chlorine-free (bleaching)
- EDTA:
-
Ethylenediaminetetraacetic acid
- FT-IR:
-
Fourier-transform infrared spectroscopy
- FT-NIR:
-
Fourier-transform near-infrared spectroscopy
- FT-RAMAN:
-
Fourier-transform RAMAN spectroscopy
- GC:
-
Gas chromatography
- GWP:
-
Groundwood pulp
- HPLC:
-
High-performance liquid chromatography
- HW:
-
Hardwood
- ILs:
-
Ionic liquids
- MW:
-
Microwave
- O (bleaching):
-
Oxygen (bleaching)
- P (bleaching):
-
Hydrogen peroxide (bleaching)
- PHWE:
-
Pressurized hot-water extraction
- RMP:
-
Refined mechanical pulp
- SC-CO2:
-
Supercritical extraction with carbon dioxide
- SCE:
-
Supercritical extraction
- SD:
-
Standard deviation
- SW:
-
Softwood
- TCF (bleaching):
-
Totally chlorine-free (bleaching)
- TDS:
-
Total dissolved solids
- TMP:
-
Thermomechanical pulp
- UEA:
-
Ultrasonic-assisted extraction
- VOC:
-
Volatile organic compounds
- Z (bleaching):
-
Ozone (bleaching
References
Gellerstedt G (2009) The worldwide wood resource. In: Ek M et al (eds) Wood chemistry and wood biotechnology. Walter de Gruyter GmbH & Co KG, Berlin, pp 1–12
Sixta H (2006) Introduction. In: Sixta H (ed) Handbook of pulp, vol 1. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 3–20
FAO Forestry Department (2017) Global forest products facts and figures 2016. Food and agriculture organization of the United Nations. http://www.fao.org/3/I7034EN/i7034en.pdf. Accessed 14 Nov 2019.
Aadil KR, Barapatre A, Jha H (2016) Synthesis and characterization of Acacia lignin-gelatin film for its possible application in food packaging. Bioresour Bioprocess 3:27. https://doi.org/10.1186/s40643-016-0103-y
Hazarika A, Baishya P, Maji TK (2015) Bio-based wood polymer nanocomposites: a sustainable high-performance material for future. In: Thakur VK, Thakur MK (eds) Eco-friendly polymer nanocomposites: advanced structured materials, vol 75. Springer, India, New Delhi, pp 233–257
Rafiqul ISM, Sakinah AMM (2012) Design of process parameters for the production of xylose from wood sawdust. Chem Eng Res Des 90(9):1307–1312. https://doi.org/10.1016/j.cherd.2011.12.009
Food and Agriculture Organization of the United Nations (2019) Global production and trade of forest products in 2017. Food and Agriculture Organization of the United Nations. http://www.fao.org/forestry/statistics/80938/en/. Accessed 14 Nov 2019.
Blechschmidt J, Heinemann S (2006) Introduction. In: Sixta H (ed) Handbook of pulp, vol 2. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1071–1072
Sixta H, Potthast A, Krotschek AW (2006) Chemical pulping processes. In: Sixta H (ed) Handbook of pulp, vol 1. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 109–510
Bajpai P (2010) Environmentally friendly production of pulp and paper. Wiley, Hoboken
Koch G (2006) Raw material for pulp. In: Sixta H (ed) Handbook of pulp, vol 1. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 21–68
Björklund Jansson M, Nilvebrant N-O (2009) Wood extractives. In: Ek M (ed) Wood chemistry and wood biotechnology, vol 1. Walter de Gruyter GmbH & Co. KG, Berlin, pp 147–171
Cibik T (2003) Untersuchungen am System NMMO/H2O/Cellulose. Dissertation, Technische Universität Berlin
Hanemann O (1996) Untersuchungen zum Lösungsverhalten von Cellulose in verschiedenen Lösungsmitteln. Dissertation, Universität Karlsruhe
Johnson T, Stockman L (1967) Die Technologie der Zellstoff-Fabrikation. In: Götze K (ed) Chemiefasern nach dem Viskoseverfahren, 3rd edn. Springer-Verlag, Berlin, Heidelberg, New York, pp 143–194
Silverio FO, Barbosa LCA, Fidencio PH, Cruz MP, Maltha CRA, Pilo-Veloso D (2011) Evaluation of chemical composition of eucalyptus wood extracts after different storage times using principal component analysis. J Wood Chem Technol 31(1):26–41. https://doi.org/10.1080/02773811003650463
Umezawa T (2000) Chemistry of extractives. In: Hon DN-S, Shiraishi N (eds) Wood and cellulosic chemistry, 2nd edn. Marcel Dekker Inc, New York, Basel, pp 213–241
Hillis WE (1971) Distribution, properties and formation of some wood extractives. Wood Sci Technol 5(4):272–289. https://doi.org/10.1007/BF00365060
Syofuna A, Banana A, Nakabonge G (2012) Efficiency of natural wood extractives as wood preservatives against termite attack. Maderas Cienc tecnol 14(2):155–163. https://doi.org/10.4067/S0718-221X2012000200003
Pereira H, Graça J, Rodrigues JC (2003) Wood chemistry in relation to quality. In: Barnett J, Jeronimidis G (eds) Wood quality and its biological basis. Biological Sciences Series. Blackwell Publishing Ltd., Oxford, Carlton, pp 53–86
Nisula L (2018) Wood extractives in conifers: a study of stemwood and knots of industrially important species. Åbo Akademi University Press, Åbo
Ekeberg D, Flæte P-O, Eikenes M, Fongen M, Naess-Andresen CF (2006) Qualitative and quantitative determination of extractives in heartwood of Scots pine (Pinus sylvestris L.) by gas chromatography. J Chromatogr A 1109(2):267–272. https://doi.org/10.1016/j.chroma.2006.01.027
Routa J, Brännström H, Anttila P, Mäkinen M, Jänis J, Asikainen A (2017) Wood extractives of Finnish pine, spruce and birch – availability and optimal sources of compounds: a literature review. Natural resources and bioeconomy studies 73/2017
Fengel D, Wegener G (1989) Extractives. In: Fengel D, Wegener G (eds) Wood: chemistry, ultrastructure, reactions. Walter de Gruyter & Co., Berlin, New York, pp 182–226
Collard F-X, Blin J, Bensakhria A, Valette J (2012) Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J Anal Appl Pyrol 95:213–226. https://doi.org/10.1016/j.jaap.2012.02.009
Demirbas A (2004) Combustion characteristics of different biomass fuels. Prog Energ Combust 30(2):219–230. https://doi.org/10.1016/j.pecs.2003.10.004
Nik-Azar M, Hajaligol MR, Sohrabi M, Dabir B (1997) Mineral matter effects in rapid pyrolysis of beech wood. Fuel Process Technol 51(1–2):7–17. https://doi.org/10.1016/S0378-3820(96)01074-0
Botello JI, Gilarranz MA, Rodríguez F, Oliet M (1999) Preliminary study on products distribution in alcohol pulping of Eucalyptus globulus. J Chem Technol Biot 74(2):141–148
Oliet M, García J, Rodríguez F, Gilarrranz MA (2002) Solvent effects in autocatalyzed alcohol–water pulping comparative study between ethanol and methanol as delignifying agents. Chem Eng J 87(2):157–162. https://doi.org/10.1016/S1385-8947(01)00213-3
Penín L, Santos V, Río JCd, Parajó JC (2019) Assesment on the chemical fractionation of Eucalyptus nitens wood: characterization of the products derived from the structural components. Bioresour Technol 281:269–276. https://doi.org/10.1016/j.biortech.2019.02.098
Teramoto Y, Lee SH, Endo T (2009) Cost reduction and feedstock diversity for sulfuric acid-free ethanol cooking of lignocellulosic biomass as a pretreatment to enzymatic saccharification. Bioresour Technol 100(20):4783–4789. https://doi.org/10.1016/j.biortech.2009.04.054
Clark IL, Hicks JR, Harris EE (1947) Extractives of douglas fir and douglas fir lignin residue. J Am Chem Soc 96(12):3142–3143. https://doi.org/10.1021/ja01204a502
Graham HM, Kurth EF (1949) Constituents of extractives from douglas fir. Ind Eng Chem 41(2):409–414. https://doi.org/10.1021/ie50470a035
Ewanick SM, Bura R, Saddler JN (2007) Acid-catalyzed steam pretreatment of lodgepole pine and subsequent enzymatic hydrolysis and fermentation to ethanol. Biotechnol Bioeng 98(4):737–746. https://doi.org/10.1002/bit.21436
Kilpeläinen A, Peltola H, Ryyppö AM, Sauvala K (2003) Wood properties of Scots pines (Pinus sylvestris) grown at elevated temperature and carbon dioxide concentration. Tree Physiol 23(18):889–897. https://doi.org/10.1093/treephys/23.13.889
Sable I, Grinfelds U, Jansons A, Vikele L, Irbe I, Verovkins A, Treimanis A (2012) Properties of wood and pulp fibers from Lodgepole Pine (Pinus Contorta) as compared to scots pine (Pinus Sylvestris). BioRes 7(2):1771–1783. https://doi.org/10.15376/biores.7.2.1771-1783
Von Schenck A, Berglin N, Uusitalo J (2013) Ethanol from Nordic wood raw material by simplified alkaline soda cooking pre-treatment. Appl Energ 102:229–240. https://doi.org/10.1016/j.apenergy.2012.10.003
Butler E, Devlin G, Meier D, McDonnell K (2013) Characterisation of spruce, salix, miscanthus and wheat straw for pyrolysis applications. Bioresour Technol 131:202–209. https://doi.org/10.1016/j.biortech.2012.12.013
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N-O (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Tech 24(3–4):151–159. https://doi.org/10.1016/S0141-0229(98)00101-X
Pielhop T, Larrazábal GO, Rohr PRV (2016) Autohydrolysis pretreatment of softwood – enhancement by phenolic additives and the effects of other compounds. Green Chem 18(19):5239–5247. https://doi.org/10.1039/c6gc01447j
Ket Soon L, Chiang K (2012) Influence of different extraction solvents on lipophilic extractives of acacia hybrid in different wood portions. Asian J Appl Sci 5(2):107–116. https://doi.org/10.3923/ajaps.2012.107.116
Hakkila P, Verkasalo E (2009) Structure and properties of wood and woody biomass. In: Kellomäki S (ed) Forest resources and sustainable management: papermaking science and technology, vol 2, 2nd edn. Paperi ja Puu Oy, Helsinki, pp 133–215
Uprichard JM, Llyod JA (1980) Influence of tree age on the chemical composition of radiata pine. New Zeal J For Sci 10(3):551–557
Kasmani JE, Nemati M, Samariha A, Chitsazi H, Mohammadi NS, Nosrati H (2011) Studying the effect of the age in Eucalyptus camaldulensis species on wood chemical compounds used in pulping process. AEJAES 11(6):854–856
Miranda I, Sousa V, Ferreira J, Pereira H (2017) Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 12(6):e0179268. https://doi.org/10.1371/journal.pone.0179268
Morais MC, Pereira H (2011) Variation of extractives content in heartwood and sapwood of Eucalyptus globulus trees. Wood Sci Technol 46(4):709–719. https://doi.org/10.1007/s00226-011-0438-7
Tasooji M, Wan G, Lewis G, Wise H, Frazier CE (2017) Biogenic formaldehyde: content and heat generation in the wood of three tree species. ACS Sustain Chem Eng 5(5):4243–4248. https://doi.org/10.1021/acssuschemeng.7b00240
Dunlop-Jones N, Jialing H, Allen LH (1991) Analyse der Aceton-Extraktstoffe aus Holz und Rinde von Zitterpappel Auswirkungen auf Entharzung und Harzkontrolle. J Pulp Pap Sci 17(2):J60–J66
Ferreira JPA, Miranda I, Gominho J, Pereira H (2016) Chemical characterization of cork and phloem from Douglas fir outer bark. Holzforschung 70(5):475–483. https://doi.org/10.1515/hf-2015-0119
Kebbi-Benkeder Z, Colin F, Dumarçay S, Gérardin P (2014) Quantification and characterization of knotwood extractives of 12 European softwood and hardwood species. Ann For Sci 72(2):277–284. https://doi.org/10.1007/s13595-014-0428-7
Mutton DB (1962) Wood resins. In: Hillis WE (ed) Wood extractives and their significance to the pulp and paper industries. Academic Press, New York, London, pp 331–363
Sjöström E (1993) Extractives. In: Sjöström E (ed) Wood chemistry, fundamentals and applications, 2nd edn. Academic Press Inc, San Diego, London, pp 90–108
Back EL (2002) Pattern of parenchyma and canal resin composition in softwoods and hardwoods. J Wood Sci 48:167–170. https://doi.org/10.1007/BF00771362
Hillis WE (1962) The distribution and formation of polyphenols within the tree. In: Hillis WE (ed) Wood extractives and their significance to the pulp and paper industries. Academic Press, New York, London, pp 59–131
Vercoe D, Stack K, Blackman A, Yates B, Richardson D (2005) An innovative approach characterising the interactions leading to pitch deposition. J Wood Chem Technol 24(2):115–137. https://doi.org/10.1081/wct-200026562
Caron G, Ermondi G, Scherrer RA (2007) Lipophilicity, polarity, and hydrophobicity. In: Taylor JB, Triggle DJ (eds) Comprehensive medicinal chemistry II, vol 5, 2nd edn. Elsevier Ltd., Amsterdam, pp 425–452
Jaynes WF (2008) Hydrophilicity, hydrophobicity. In: Chesworth W (ed) Encyclopedia of soil science. Springer, Dortrecht, pp 328–330
Reichardt C, Welton T (2011) Solvents and solvent effects inorganic chemistry, 4th edn. Wiley-VCH Verlag GmbH & Co, KGa, Weinheim
Technical Association of the Pulp and Paper Industry (2007) Solvent extractives of wood and pulp. T 204 cm-97
National Renewable Energy Laboratory (2008) Determination of extractives in biomass: laboratory analytical procedure (LAP). NREL/TP-510–42619
Wagner K, Musso M, Kain S, Willfor S, Petutschnigg A, Schnabel T (2020) Larch wood residues valorization through extraction and utilization of high value-added products. Polymers 12(2):359. https://doi.org/10.3390/polym12020359
Schnabel T, Barbu MC, Tudor EM, Petutschnigg A (2021) Changing in larch sapwood extractives due to distinct ionizing radiation sources. Materials 14(7):1613. https://doi.org/10.3390/ma14071613
Moosavinejad SM, Madhoushi M, Vakili M, Rasouli D (2019) Evaluation of degradation in chemical compounds of wood in historical buildings using FT-IR and FT-Raman vibrational spectroscopy. Maderas Cienc tecnol 21(3):381–392. https://doi.org/10.4067/s0718-221x2019005000310
Holmbom B (1999) Extractives. In: Sjöström E, Alén R (eds) Analytical methods in wood chemistry, pulping, and papermaking. Springer series in wood science. Springer, Berlin Heidelberg, pp 125–148
Gellerstedt G (2009) Analytical methods. In: Ek M (ed) Wood Chemistry and wood biotechnology. Walter de Gruyter GmbH & Co KG, Berlin, pp 195–217
Rigol A, Lacorte S, Barceló D (2003) Sample handling and analytical protocols for analysis of resin acids in process waters and effluents from pulp and paper mills. Trend Anal Chem 22(10):738–749. https://doi.org/10.1016/S0165-9936(03)01011-2
Valto P, Knuutinen J, Alén R (2012) Overview of analytical procedures for fatty and resin acids in the papermaking process. BioRes 7(4):6041–6076. https://doi.org/10.15376/biores.7.4.6041-6076
Poljanšek I, Vek V, Oven P (2015) Razvoj metode za HPLC-analizo izbranih fenolnih spojin lesa. Acta Silvae et Ligni 108:11–17. https://doi.org/10.20315/ASetL.108.2
Vek V, Oven P, Poljanšek I, Ters T (2015) Contribution to understanding the occurrence of extractives in red heart of beech. BioRes 10(1):970–985. https://doi.org/10.15376/biores.10.1.970-985
Benouadah N, Pranovich A, Aliouche D, Hemming J, Smeds A, Willför S (2018) Analysis of extractives from Pinus halepensis and Eucalyptus camaldulensis as predominant trees in Algeria. Holzforschung 72(2):97–104. https://doi.org/10.1515/hf-2017-0098
Gutierrez A, del Rıo JC, Gonzalez-Vila FJ, Martın F (1998) Analysis of lipophilic extractives from wood and pitch deposits by solid-phase extraction and gas chromatography. J Chromatogr 823(1–2):449–455
Lee R, Stack K, Richardson DE (2011) Aggregation studies of pinus radiata wood extractives under increased system closure. In: Conference proceedings of the 65th Appita annual conference and exhibition, Appita Inc., Rotorua, pp. 10–13 April 2011
Gutiérrez A, Río JCd, González-Vila FJ, Martín F (1999) Chemical composition of lipophilic extractives from eucalyptus globulus labill. Wood Holzforschung 53(5):481–486. https://doi.org/10.1515/hf.1999.079
Smeds AI, Vähäsalo L, Rahkila J, Eklund PC, Willför SM (2019) Chemical characterisation of polymerised extractives in bleached birch kraft pulp. Holzforschung 73(11):1017–1033. https://doi.org/10.1515/hf-2019-0067
Del Rio JC, Romero J, Gutierrez A (2000) Analysis of pitch deposits produced in Kraft pulp mills using a totally chlorine free bleaching sequence. J Chromatogr A 874(2):235–245
Farrell RL, Hata K, Wall MB (1997) Solving pitch problems in pulp and paper processes by the use of enzymes or fungi. In: Eriksson K-EL (ed) Biotechnology in the pulp and paper industry advances in biochemical engineering/biotechnology, vol 57. Springer, Berlin, Heidelberg, pp 197–211
Gutiérrez A, Del Rıo JC, Martinez AT (2010) Fungi and their enzymes for pitch control in the pulp and paper industry. In: Hofrichter M (ed) Industrial applications. The mycota, vol 10, 2nd edn. Springer, Berlin, Heidelberg, pp 357–377
Allen LH (2000) Pitch control in paper mills. In: Black EL, Allen LH (eds) Pitch control, wood resin and deresination. TAPPI Press, Atlanta, pp 265–287
Christopher LP (2013) Integrated forest biorefineries: current state and develpoment potential. In: Christopher LP (ed) Integrated forest biorefineries: challenges and oppurtunities. The Royal Society of Chemistry, Cambridge, pp 1–66
Silverio FO, Barbosa LCA, Maltha CRA, Fidencio PH, Cruz MP, Veloso DP, Milanez AF (2008) Effect of storage time on the composition and content of wood extractives in Eucalyptus cultivated in Brazil. Bioresour Technol 99(11):4878–4886. https://doi.org/10.1016/j.biortech.2007.09.066
Gardner JAF, Hillis WE (1962) The influence of extractives on the pulping of wood. In: Hillis WE (ed) Wood extractives and their significance to the pulp and paper industries. Academic Press, New York, London, pp 367–403
Hillis WE, Swain T (1962) The influence of extractives on the color of groundwood and newsprint. In: Hillis WE (ed) Wood extractives and their significance to the pulp and paper industries. Academic Press, New York, London, pp 405–418
Wei Y, Wang M, Zhang P, Chen Y, Gao J, Fan Y (2017) The role of phenolic extractives in color changes of locust wood (Robinia pseudoacacia) during heat treatment. BioRes 12(4):7041–7055. https://doi.org/10.15376/biores.12.4.7041-7055
Paulsson M, Parkås J (2012) Review: light-induced yellowing of lignocellulosic pulps – mechanisms and preventive methods. BioRes. https://doi.org/10.15376/biores.7.4.5995-6040
Tribulová T, Kačík F, Evtuguin D, Čabalová I (2016) Assessment of chromophores in chemically treated and aged wood by Uv-Vis diffuse reflectance spectroscopy. Cellulose Chem Technol 50(5–6):659–667
Ezhumalai R (2013) Wood science and technology. In: Raj AJ, Lal SB (eds) Forestry: principles and applications. Scientific Publishers, Jodhpur, pp 332–349
Ghadiriasli R, Wagenstaller M, Buettner A (2018) Identification of odorous compounds in oak wood using odor extract dilution analysis and two-dimensional gas chromatography-mass spectrometry/olfactometry. Anal Bioanal Chem 410(25):6595–6607. https://doi.org/10.1007/s00216-018-1264-7
Schreiner L, Bauer P, Buettner A (2018) Resolving the smell of wood - identification of odour-active compounds in Scots pine (Pinus sylvestris L.). Sci Rep 8:8294. https://doi.org/10.1038/s41598-018-26626-8
Wilken R, Kleebauer M (1999) Ermittlung und Bewertung von flüchtigen Inhaltsstoffen in Verpackungskartons. PTS München. https://www.ptspaper.de/fileadmin/PTS/PTSPAPER/06_Forschung/Dokumente/Forschungsprojekte/AiF_11367.pdf. Accessed 05 Sep 2019.
Wilken R, Kleebauer M (2001) Ursachen und Vermeidung der Aldehydbildung in Lebensmittelverpackungspapieren. PTS München. https://www.ptspaper.de/fileadmin/PTS/PTSPAPER/06_Forschung/Dokumente/Forschungsprojekte/AiF_12149.pdf. Accessed 05 Sep 2019.
Ramis-Ramos G (2003) Synthetic antioxidants. In: Caballero B (ed) Encyclopedia of food sciences and nutrition, 2nd edn. Academic Press, San Diego, London, pp 275–282
Oviedo C, Rodríguez J (2003) EDTA: the chelating agent under environmental scrutiny. Quím Nova 26(6):901–905. https://doi.org/10.1590/S0100-40422003000600020
Munn SJ, Allanou R, Aschberger K, Berthault F, Cosgrove O, de Bruijn J, Musset C, O’Connor S, Pakalin S, Paya-Perez A, Pellegrini G, Scheer S, Schwarz-Schulz B (2004) European Union Risk Assessment Report about Edetic acid (EDTA). European Chemicals Agency. https://echa.europa.eu/documents/10162/65615721-ab6d-4f28-b48f-73cf9d8cc529. Accessed 04 Feb 2020.
Pirkanniemi K, Metsarinne S, Sillanpaa M (2007) Degradation of EDTA and novel complexing agents in pulp and paper mill process and waste waters by Fenton’s reagent. J Hazard Mater 147(1–2):556–561. https://doi.org/10.1016/j.jhazmat.2007.01.050
Van Ginkel CG, Virtapohja J, Steyaert JAG, Alén R (1999) Treatment of EDTA-containing pulp and paper mill wastewaters in activated sludge plants. TAPPI J 82(2):138–142
Naicker D, Sithole B (2016) Using a Rapid-Kothen paper machine to simulate the effect of system closure on the contamination load of whitewater. Nord Pulp Pap Res J 31(4):600–607. https://doi.org/10.3183/npprj-2016-31-04-p600-607
Lacorte S, Latorre A, Barceloó D, Rigol A, Malmqvist A, Welander T (2003) Organic compounds in paper-mill process waters and effluents. Trend Anal Chem 22(10):725–737. https://doi.org/10.1016/S0165-9936(03)01009-4
Krilov A, Gref R (1986) Mechanism of sawblade corrosion by polyphenolic compounds. Wood Sci Technol 20:369–375. https://doi.org/10.1007/BF00351589
Hazlewood PE, Singh PM, Hsieh JS (2006) Role of wood extractives in black liquor corrosiveness. Corrosion 62(10):911–917. https://doi.org/10.5006/1.3279901
Singh PM, Anaya A (2007) Effect of wood species on corrosion behavior of carbon steel and stainless steels in black liquors. Corros Sci 49(2):497–509. https://doi.org/10.1016/j.corsci.2006.04.020
Takano M, Hoshino K (2016) Lactic acid production from paper sludge by SSF with thermotolerant Rhizopus sp. Bioresour Bioprocess 3:29. https://doi.org/10.1186/s40643-016-0106-8
Min BC, Bhayani BV, Jampana VS, Ramarao BV (2015) Enhancement of the enzymatic hydrolysis of fines from recycled paper mill waste rejects. Bioresour Bioprocess 2:40. https://doi.org/10.1186/s40643-015-0068-2
Baroutian S, Robinson M, Smit A-M, Wijeyekoon S, Gapes D (2013) Transformation and removal of wood extractives from pulp mill sludge using wet oxidation and thermal hydrolysis. Bioresour Technol 146:294–300. https://doi.org/10.1016/j.biortech.2013.07.098
Ressel JB (2006) Wood yard operations. In: Sixta H (ed) Handbook of pulp, vol 1. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 69–108
Arisandi R, Ashitani T, Takahashi K, Marsoem SN, Lukmandaru G (2019) Lipophilic extractives of the wood and bark from Eucalyptus pellita F Muell grown in Merauke, Indonesia. J Wood Chem Technol 40(2):146–154. https://doi.org/10.1080/02773813.2019.1697295
Salem MZM, Zeidler A, Böhm M, Mohamed MEA, Ali HM (2015) GC/MS analysis of oil extractives from wood and bark of Pinus sylvestris, Abies alba, Picea abies and Larix decidua. BioRes 10(4):7725–7737. https://doi.org/10.15376/biores.10.4.7725-7737
Balaban M, Uçar G (2001) Extractives and structural components in wood and bark of endemic oak Quercus vulcanica boiss. Holzforschung 55(5):478–486. https://doi.org/10.1515/HF.2001.079
Benouadah N, Aliouche D, Pranovich A, Willför S (2018) Chemical characterization of Pinus halepensis sapwood and heartwood. Wood Mater Sci Eng 14(3):157–164. https://doi.org/10.1080/17480272.2018.1448436
Li Y, Deng X, Zhang Y, Huang Y, Wang C, Xiang W, Xiao F, Wei X (2019) Chemical characteristics of heartwood and sapwood of red-heart Chinese Fir (Cunninghamia lanceolata). For Prod J 69(2):103–109. https://doi.org/10.13073/FPJ-D-18-00042
Pohjamo SP, Hemming JE, Willför SM, Reunanen MHT, Holmbom BR (2003) Phenolic extractives in Salix caprea wood and knots. Phytochemistry 63(2):165–169. https://doi.org/10.1016/s0031-9422(03)00050-5
Holmbom B, Eckerman C, Eklund P, Hemming J, Nisula L, Reunanen M, Sjöholm R, Sundberg A, Sundberg K, Willför S (2003) Knots in trees – a new rich source of lignans. Phytochem Rev 2(3):331–340. https://doi.org/10.1023/B:PHYT.0000045493.95074.a8
Willför S, Hemming J, Reunanen M, Holmbom B (2003) Phenolic and lipophilic extractives in scots pine knots and stemwood. Holzforschung 57(4):359–372. https://doi.org/10.1515/HF.2003.054
Krotschek AW (2006) Pulp screening, cleaning, and fractionation. In: Sixta H (ed) Handbook of pulp, vol 1. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 561–608
Van der Merwe J-P, Ackerman P, Pulkki R, Längin D (2016) The impact of log moisture content on chip size distribution when processing Eucalyptus pulpwood. Croat J For Eng 37(2):297–307
Eckerman C, Holmbom B (2001) Method for recovery of compression wood and/or normal wood from oversize chips. International Patent WO 02/09893, 2 Aug 2001
Breuil C, Iverson S, Gao Y (1998) Fungal treatment of wood chips to remove extractives. In: Young RA, Akhtar M (eds) Environmentally friendly technologies for the pulp and paper industry. Wiley, New York, pp 541–566
Gutiérrez A, del Ŕio JC, González-Vila FJ, Romero J (1998) Variation in the composition of wood extractives from Eucalyptus globulus during seasoning. J Wood Chem Technol 18(4):439–446. https://doi.org/10.1080/02773819809349591
Ramnath L, Sithole B, Govinden R (2018) The effects of wood storage on the chemical composition and indigenous microflora of Eucalyptus species used in the pulping industry. BioRes 13(1):86–103. https://doi.org/10.15376/biores.13.1.86-103
Henriksson G, Teeri T (2009) Biotechnology in the forest industry. In: Ek M (ed) Wood chemistry and wood biotechnology, vol 1. Walter de Gruyter GmbH & Co. KG, Berlin, pp 273–300
Kirk TK, Burgess RR, Koning JW (1992) Use of fungi in pulping wood: an overview of biopulping research. In: Leatham GF (ed) Frontiers in industrial mycology. Springer, Boston, pp 99–111
Abdel-Hamid AM, Solbiati JO, Cann IK (2013) Insights into lignin degradation and its potential industrial applications. Adv Appl Microbiol 82:1–28. https://doi.org/10.1016/B978-0-12-407679-2.00001-6
Dorado J, van Beek TA, Claassen FW, Sierra-Alvarez R (2001) Degradation of lipophilic wood extractive constituents in Pinus sylvestris by the white-rot fungi Bjerkandera sp. and Trametes versicolor. Wood Sci Technol 35(1–2):117–125. https://doi.org/10.1007/s002260000077
Thao PTH, Lien NTH, Van Hieu N, Linh NVM, Nhung DT, Huong TT, Van Son C (2021) Characteristics of Pleurotus sp. TD36 and its ability to reduce wood extractives in pretreatment for pulping. Eur J Wood Prod 79(5):1315–1324. https://doi.org/10.1007/s00107-021-01687-1
Košíková B, Sláviková E, Sasinkova V, Kačík F (2006) The use of various yeast strains for removal of pine wood extractive constituents. Wood Res Slovakia 51(4):47–53
Burnes TA, Blanchette RA, Farrell RL (2000) Bacterial biodegradation of extractives and patterns of bordered pit membrane attack in pine wood. Appl Environ Microb 66(12):5201–5205. https://doi.org/10.1128/aem.66.12.5201-5205.2000
Kallioinen A, Vaari A, Rättö M, Konn J, Siika-aho M, Viikari L (2003) Effects of bacterial treatments on wood extractives. J Biotechnol 103(1):67–76. https://doi.org/10.1016/s0168-1656(03)00051-8
Blechschmidt J, Heinemann S (2006) Mechanical pulping processes. In: Sixta H (ed) Handbook of pulp, vol 2. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1079–1112
Sixta H (2006) Pulp properties and applications. In: Sixta H (ed) Handbook of pulp, vol 2. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 1009–1068
Schneider T, Roffael E, Windeisen E, Wegener G (2004) Einfluss der Aufschlusstemperatur auf lösliche Kohlenhydrate bei der TMP-Herstellung. Holz Roh Werkst 62(4):321–322. https://doi.org/10.1007/s00107-004-0491-0
Yang J, Zasadowski D, Edlund H, Norgren M (2019) Biorefining of spruce tmp process water: selective fractionation of lipophilic extractives with induced air flotation and surface active additive. BioRes 14(2):4124–4135. https://doi.org/10.15376/biores.14.2.4124-4135
Wingerson RC (2002) Method of treating lignocellulose biomass to produce cellulose. US Patent 6,419,788, 16 Jul 2002
Johansson A, Aaltonen O, Ylinen P (1987) Organosolv pulping – methods and pulp properties. Biomass 13(1):45–65. https://doi.org/10.1016/0144-4565(87)90071-0
Rodrigues PF, Evtyugin DD, Evtuguin DV, Prates A (2018) Extractive profiles in the production of sulfite dissolving pulp from Eucalyptus globulus wood. J Wood Chem Technol 38(5):397–408. https://doi.org/10.1080/02773813.2018.1513037
Duan C, Li J, Ma X, Chen C, Liu Y, Stavik J, Ni Y (2015) Comparison of acid sulfite (AS)- and prehydrolysis kraft (PHK)-based dissolving pulps. Cellulose 22(6):4017–4026. https://doi.org/10.1007/s10570-015-0781-1
Lachenal D (2016) Kraft Pulping. In: Belgacem N, Pizzi A (eds) Lignocellulosic fibers and wood handbook. Scrivener Publishing LLC, Salem, pp 207–223
Saukkonen E, Kautto J, Rauvanto I, Backfolk K (2012) Characteristics of prehydrolysis-kraft pulp fibers from Scots pine. Holzforschung 66(7):801–808. https://doi.org/10.1515/hf-2011-0158
Da Silva Morais AP, Sansigolo CA, de Oliveira NM (2016) Effects of autohydrolysis of Eucalyptus urograndis and Eucalyptus grandis on influence of chemical components and crystallinity index. Bioresour Technol 214:623–628. https://doi.org/10.1016/j.biortech.2016.04.124
Li P-P, Lei Y-C, Chen C, Meng Q-J (2012) Effect of hot water prehydrolysis on the major components of the pine and the hydrolysate. China Pulp Pap 31(11):1–6
Dunlop-Jones N, Douek M, Jialing H, Allen LH, Dorris GM (1989) The effects of certain chemical additives on the deresination of trembling aspen in kraft pulping. J Wood Chem Technol 9(3):365–386. https://doi.org/10.1080/02773818908050305
Shin S-J, Cho N-S, Lai Y-Z (2007) Residual extractives in aspen kraft pulps and their impact on kappa number and Klason lignin determination. J Wood Sci 53(6):494–497. https://doi.org/10.1007/s10086-007-0894-8
Sixta H, Süss H-U, Potthast A, Schwanninger M, Krotschek AW (2006) Pulp bleaching. In: Sixta H (ed) Handbook of pulp, vol 2. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 609–932
Hintz HL, Lawal SA (2018) Paper: pulping and bleaching. In: Hashmi S (ed) Reference module in materials science and materials engineering. Elsevier Inc., London, pp 1–7
Holmbom B (1999) Analysis of papermaking process waters and effluents. In: Sjöström E, Alén R (eds) Analytical methods in wood chemistry pulping and papermaking. Springer series in wood science. Springer, Berlin Heidelberg, pp 269–285
Ekman R, Holmbom B (1989) The wood extractives in alkaline peroxide bleaching of groundwood from Norway spruce. Nord Pulp Pap Res J 4(3):188–191. https://doi.org/10.3183/npprj-1989-04-03-p188-191
España Orozco S, Bischof RH, Barbini S, Sriranganadane D, Fackler K, Potthast A (2021) Fate of lipophilic wood extractives in oxygen-based cellulose bleaching. ACS Sustain Chem Eng 9(13):4840–4849. https://doi.org/10.1021/acssuschemeng.1c00109
Freire CSR, Silvestre AJD, Pascoal Neto C (2005) Oxidized derivatives of lipophilic extractives formed during hardwood Kraft pulp bleaching. Holzforschung 57(5):503–512. https://doi.org/10.1515/HF.2003.075
Gutiérrez A, Romero J, Río JCd (2001) Lipophilic extractives from eucalyptus Globulus pulp during Kraft cooking followed by TCF and ECF bleaching. Holzforschung 55(3):260–264. https://doi.org/10.1515/hf.2001.043
Koljonen K, Österberg M, Kleen M, Fuhrmann A, Stenius P (2004) Precipitation of lignin and extractives on kraft pulp: effect on surface chemistry, surface morphology and paper strength. Cellulose 11(2):209–224. https://doi.org/10.1023/B:CELL.0000025424.90845.c3
Si X, Lu F, Chen J, Lu R, Huang Q, Jiang H, Taarning E, Xu J (2017) A strategy for generating high-quality cellulose and lignin simultaneously from woody biomass. Green Chem 19(20):4849–4857. https://doi.org/10.1039/c7gc02492d
Huang H-J, Ramaswamy S (2013) Overview of biomass conversion processes and separation and purification technologies in biorefineries. In: Ramaswamy S et al (eds) Separation and purification technologies in biorefineries. Wiley, Chichester, pp 3–36
Garrote G, Eugenio ME, Díaz MJ, Ariza J, López F (2003) Hydrothermal and pulp processing of Eucalyptus. Bioresour Technol 88(1):61–68. https://doi.org/10.1016/S0960-8524(02)00256-0
Kleinert T, Kv T (1931) Über neuere versuche zur trennung von cellulose und inkrusten verschiedener hölzer. Z Angew Chem 44(39):788–791. https://doi.org/10.1002/ange.19310443903
Rodríguez A, Espinosa E, Domínguez-Robles J, Sánchez R, Bascón I, Rosal A (2018) Different solvents for organosolv pulping. In: Kazi SN (ed) Pulp and paper processing. IntechOpen, London, pp 33–54
Kirci H, Akgül MZ (2002) Production of dissolving grade pulp from poplar wood by ethanol-water process. Turk J Agric For 26:239–245
Shatalov AA, Pereira H (2008) Arundo donax L. reed: new perspectives for pulping and bleaching. 5. Ozone-based TCF bleaching of organosolv pulps. Bioresour Technol 99(3):472–478. https://doi.org/10.1016/j.biortech.2007.01.014
Vek V, Oven P, Poljanšek I (2018) Comparison of two extraction and two chromatographic methods in analysis of beech wood extractives. Eur J Wood Prod 76(1):389–392. https://doi.org/10.1007/s00107-017-1216-5
Moodley P (2011) Characterisation and Quantification of Wood Extractives and their Impact on Pitch. Master thesis, University of KwaZulu-Natal
Bertaud F, Crampon C, Badens E (2017) Volatile terpene extraction of spruce, fir and maritime pine wood: supercritical CO2 extraction compared to classical solvent extractions and steam distillation. Holzforschung 71(7–8):667–673. https://doi.org/10.1515/hf-2016-0197
Uçar G, Fengel D (1995) Variation in composition of extractives from wood of Pinus nigra varieties. Phytochemistry 38(4):877–880. https://doi.org/10.1016/0031-9422(94)00734-B
Schwanninger M, Hinterstoisser B (2002) Comparison of the classical wood extraction method using a Soxhlet apparatus with an advanced extraction method. Holz Roh Werkst 60(5):343–346. https://doi.org/10.1007/s00107-002-0312-2
Testova L, Vilonen K, Pynnönen H, Tenkanen M, Sixta H (2009) Isolation of hemicelluloses from birch wood: distribution of wood components and preliminary trials in dehydration of hemicelluloses. Lenzinger Berichte 87:60–67
Krogell J, Korotkova E, Eränen K, Pranovich A, Salmi T, Murzin D, Willför S (2013) Intensification of hemicellulose hot-water extraction from spruce wood in a batch extractor – effects of wood particle size. Bioresour Technol 143:212–220. https://doi.org/10.1016/j.biortech.2013.05.110
Song T, Pranovich A, Holmbom B (2012) Hot-water extraction of ground spruce wood of different particle size. BioRes 7(3):4214–4225. https://doi.org/10.15376/biores.7.3.4214-4225
Thurbide KB, Hughes DM (2000) A rapid method for determining the extractives content of wood pulp. Ind Eng Chem Res 39(8):3112–3115. https://doi.org/10.1021/ie0003178
Shin S-J, Sung Y-J, Park J-M, Choe N-S (2009) Impact of residual extractives in Kraft pulps on brightness and color. J of Korea TAPPI 41(5):20–25
Baptista C, Belgacem N, Duarte AP (2006) The effect of wood extractives on pulp properties of maritime pine kraft pulp. Appita J 59(4):311–316
Lindemann M (2016) Celluloseabbau bei der Organosolvbehandlung. Master thesis, Technische Universität Wien
Schulze P, Leschinsky M, Seidel-Morgenstern A, Lorenz H (2019) Continuous separation of lignin from organosolv pulping liquors - combined lignin particle formation and solvent recovery. Ind Eng Chem Res 58(9):3797–3810. https://doi.org/10.1021/acs.iecr.8b04736
Viell J, Harwardt A, Seiler J, Marquardt W (2013) Is biomass fractionation by Organosolv-like processes economically viable? a conceptual design study. Bioresour Technol 150:89–97. https://doi.org/10.1016/j.biortech.2013.09.078
Haghighi Mood S, Hossein Golfeshan A, Tabatabaei M, Salehi Jouzani G, Najafi GH, Gholami M, Ardjmand M (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renwe Sust Energ Rev 27:77–93. https://doi.org/10.1016/j.rser.2013.06.033
Liu Y, Nie Y, Lu X, Zhang X, He H, Pan F, Zhou L, Liu X, Ji X, Zhang S (2019) Cascade utilization of lignocellulosic biomass to high-value products. Green Chem 21(13):3499–3535. https://doi.org/10.1039/c9gc00473d
Nanda B, Sailaja M, Mohapatra P, Pradhan RK, Nanda BB (2021) Green solvents: a suitable alternative for sustainable chemistry. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2021.06.458
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4:7. https://doi.org/10.1186/s40643-017-0137-9
Bogolitsyn K, Krasikova A, Gusakova M, Ivakhnov A, Gravitis J (2019) Selective extraction of terpenoid compounds of Juniperus communis L. wood in the medium of a binary solvent (supercritical CO2 with modifier). Phytochem Anal 30(6):609–616. https://doi.org/10.1002/pca.2833
Demirbaş A (1991) Analysis of beech wood fatty acids by supercritical acetone extraction. Wood Sci Technol 25(5):365–370. https://doi.org/10.1007/BF00226176
Tomás-Pejó E, Oliva JM, Ballesteros M (2008) Realistic approach for full-scale bioethanol production from lignocellulose: a review. J Sci Ind Res India 67(11):874–884
Agbor VB, Cicek N, Sparling R, Berlin A, Levin DB (2011) Biomass pretreatment: fundamentals toward application. Biotechnol Adv 29(6):675–685. https://doi.org/10.1016/j.biotechadv.2011.05.005
Ferreira AM, Morais ES, Leite AC, Mohamadou A, Holmbom B, Holmbom T, Neves BM, Coutinho JAP, Freire MG, Silvestre AJD (2017) Enhanced extraction and biological activity of 7-hydroxymatairesinol obtained from Norway spruce knots using aqueous solutions of ionic liquids. Green Chem 19(11):2626–2635. https://doi.org/10.1039/c7gc01091e
Sadaf A, Grewal J, Khare SK (2018) Ionic liquid stable cellulases and hemicellulases: application in biobased production of biofuels. In: Bhaskar T (ed) Waste biorefinery. Elsevier B.V., Amsterdam, Oxford, Cambridge, pp 505–532
Deb S, Labafzadeh SR, Liimatainen U, Parviainen A, Hauru LKJ, Azhar S, Lawoko M, Kulomaa T, Kakko T, Fiskari J, Borrega M, Sixta H, Kilpeläinen I, King AWT (2016) Application of mild autohydrolysis to facilitate the dissolution of wood chips in direct-dissolution solvents. Green Chem 18(11):3286–3294. https://doi.org/10.1039/c6gc00183a
Papa G, Kirby J, Murthy Konda NVSN, Tran K, Singh S, Keasling JD, Peter GF, Simmons BA (2017) Development of an integrated approach for α-pinene recovery and sugar production from loblolly pine using ionic liquids. Green Chem 19(4):1117–1127. https://doi.org/10.1039/c6gc02637k
Kilulya KF, Msagati TAM, Mamba BB, Ngila JC, Bush T (2012) Determination of lipophilic extractives in ionic liquid extracts of eucalyptus pulp by gas chromatography - mass spectrometry. Tanzan J Sci 38(3):14–26
Pinkert A, Goeke DF, Marsh KN, Pang S (2011) Extracting wood lignin without dissolving or degrading cellulose: investigations on the use of food additive-derived ionic liquids. Green Chem 13(11):3124–3136. https://doi.org/10.1039/c1gc15671c
Yiin CL, Yap KL, Ku AZE, Chin BLF, Lock SSM, Cheah KW, Loy ACM, Chan YH (2021) Recent advances in green solvents for lignocellulosic biomass pretreatment: potential of choline chloride (ChCl) based solvents. Bioresour Technol 333:1–12. https://doi.org/10.1016/j.biortech.2021.125195
Fukaya Y, Iizuka Y, Sekikawa K, Ohno H (2007) Bio ionic liquids: room temperature ionic liquids composed wholly of biomaterials. Green Chem. https://doi.org/10.1039/b706571j
Calcio Gaudino E, Cravotto G, Manzoli M, Tabasso S (2019) From waste biomass to chemicals and energy via microwave-assisted processes. Green Chem 21(6):1202–1235. https://doi.org/10.1039/c8gc03908a
Tsubaki S, Azuma J-i, Fujii S, Singh R, Thallada B, Wada Y (2018) Microwave-driven biorefinery for utilization of food and agricultural waste biomass. In: Bhaskar T (ed) Waste biorefinery. Elsevier B.V., Amsterdam, Oxford, Cambridge, pp 393–408
Xu J, Jiang J, Hse C, Shupe TF (2012) Renewable chemical feedstocks from integrated liquefaction processing of lignocellulosic materials using microwave energy. Green Chem 14(10):2821–2830. https://doi.org/10.1039/c2gc35805k
Singh S, Goyal A, Moholkar VS (2018) Synthesis of bioethanol from invasiveweeds: process design, optimization, and intensification with ultrasound. In: Bhaskar T (ed) Waste biorefinery. Elsevier B.V., Amsterdam, Oxford, Cambridge, pp 445–485
Abidin ZZ, Biak DRA, Yusoff HM, Harun MY (2013) Solid-liquid extraction in biorefinery. In: Ramaswamy S (ed) Separation and purification technologies in biorefineries. Wiley, Chichester, pp 351–374
He Z, Zhang Y, Wang Z, Zhao Z, Yi S (2015) Reducing wood drying time by application of ultrasound pretreatment. Drying Technol 34(10):1141–1146. https://doi.org/10.1080/07373937.2015.1099107
Wang C, Tallian C, Su J, Vielnascher R, Silva C, Cavaco-Paulo A, Guebitz GM, Fu J (2018) Ultrasound-assisted extraction of hemicellulose and phenolic compounds from bamboo bast fiber powder. PLoS ONE 13(6):e0197537. https://doi.org/10.1371/journal.pone.0197537
Sillero L, Morales A, Fernandez-Marin R, Hernandez-Ramos F, Davila I, Erdocia X, Labidi J (2021) Study of different extraction methods of bioactive molecules from different tree species. Chem Eng Trans 86:31–36. https://doi.org/10.3303/CET2186006
Fuchtner S, Brock-Nannestad T, Smeds A, Fredriksson M, Pilgard A, Thygesen LG (2020) Hydrophobic and hydrophilic extractives in norway spruce and kurile larch and their role in brown-rot degradation. Front Plant Sci 11:855. https://doi.org/10.3389/fpls.2020.00855
Salem MZM, Abdel-Megeed A, Ali HM (2014) Stem wood and bark extracts of Delonix regia (Boj. Ex. Hook): chemical analysis and antibacterial, antifungal and antioxidant properties. BioRes 9(2):2382–2395. https://doi.org/10.15376/biores.9.2.2382-2395
Hosseini Hashemi SK, Jahan Latibari A (2011) Evaluation and identification of walnut heartwood extractives for protection of poplar wood. Bio Res 6(1):59–69. https://doi.org/10.15376/biores.6.1.59-69
Belt T (2013) Wood preservative potential of Scots pine bark and knot extractives. Master thesis, Aalto University
Broda M (2020) Natural compounds for wood protection against fungi-a review. Molecules 25(15):1–24. https://doi.org/10.3390/molecules25153538
Zinkel DF (1989) Naval Stores. In: Rowe JW (ed) Natural products of woody plants: chemical extraneous to the lignocellulosic cell wall. Springer Series in Wood Science. Springer, Berlin, Heidelberg, pp 953–978
Bajpai P (2018) Wood-based products and chemicals. In: Bajpai P (ed) Biermann’s handbook of pulp and paper, 3rd edn. Elsevier Inc, Amsterdam, Oxford, Cambridge, pp 233–247
Singh S, Stack KR, Richardson DE, Lewis TW (2019) Review: the value of wood extractives from Pinus radiata. Appita J 72(2):70–81
Gellerstedt G (2009) Cellulose products and chemicals from wood. In: Ek M et al (eds) Wood chemistry and wood biotechnology. Walter de Gruyter GmbH & Co KG, Berlin, pp 173–193
Molinder R, Almqvist J (2018) Extractives in the Scandinavian pulp and paper industry: Current and possible future applications. RISE Processum AB, Örnsköldsvik
Konwar LJ, Mikkola J-P, Bordoloi N, Saikia R, Chutia RS, Kataki R (2018) Sidestreams from bioenergy and biorefinery complexes as a resource for circular bioeconomy. In: Bhaskar T (ed) Waste biorefinery. Elsevier B.V., Amsterdam, Oxford, Cambridge, pp 85–125
Holmbom B (1978) The behavior of resin acids during tall oil distillation. J Am Oil Chem Soc 55(12):876–880. https://doi.org/10.1007/BF02671411
Mikulášik R, Šurina I, Katuščák S, Cvengroš J, Polovka M (2008) Preparation of biodiesel from tall oil. Chem Listy 102(15):s552–s555
Hamaguchi M, Cardoso M, Vakkilainen E (2012) Alternative technologies for biofuels production in Kraft pulp mills—potential and prospects. Energies 5(7):2288–2309. https://doi.org/10.3390/en5072288
UPM Biofuels (2020) UPM Lappeenranta Biorefinery. https://www.upmbiofuels.com/about-upm-biofuels/production/upm-lappeenranta-biorefinery/. Accessed 05.10.2020
Acknowledgements
The authors acknowledge TU Wien Bibliothek for financial support through its Open Access Funding Program.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
ML carried out the literature research and drafted the work, MM and AF provided literature, and substantively revised the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
All the references used for the tables in this work are provided in or below the tables. Figure 1, as well as the graphical abstract, were created by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lehr, M., Miltner, M. & Friedl, A. Removal of wood extractives as pulp (pre-)treatment: a technological review. SN Appl. Sci. 3, 886 (2021). https://doi.org/10.1007/s42452-021-04873-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04873-1