Abstract
This work presents an innovative silica-layer coated capillary with comparison study of the silica-layer coated capillary and the fused-silica capillary for the separation of seven phenolic acids viz. p-hydroxyphenylacetic acid (PHPA), p-coumaric acid (PCA), p-hydroxybenzoic acid (PHBA), caffeic acid (CFA), (3,4-dihydroxyphenyl)acetic acid (DHPA), gallic acid (GLA), and 2,3,4-trihydroxybenzoic acid (THBA), together with caffeine (CF), by capillary electro-chromatography (CEC) and micellar electrokinetic chromatography (MEKC), respectively. The running buffer was 25.0 mM borate at pH 9.0, with addition of 50.0 mM sodium dodecyl sulfate for the MEKC mode. The non-coated capillary could not separate all seven phenolic acids by CEC or MEKC. This was achieved using the coated capillary for both CEC and MEKC. The innovative coated capillary with CEC had plate number N ≥ 2.0 × 104 m−1 and resolution Rs ≥ 1.6 for all adjacent pairs of peaks. The capillary was also able to separate GLA and THBA which are structural isomers. Although MEKC mode provided comparable efficiency and selectivity, the reduced EOF of the coated capillary led to longer separation time. The linear calibration range of the seven phenolic acids and caffeine were different but the coefficients of determinations (r2) were all > 0.9965. The precisions of the relative migration times and peak area ratios of analyte to internal standard were 0.1–1.8% and 1.8–6.8%, respectively. There were no statistical differences in the efficiency of separation of the phenolic acids and caffeine for three coated capillaries. It was applied to the analysis of caffeine and phenolic acids in brewed tea using tyramine as the internal standard. The tea samples were diluted prior to analysis by CEC. The separation was less than 15 min. Caffeine, gallic acid and p-coumaric acid were detected and quantified. Caffeine and gallic acid contents were 10.8–15.0 and 2.6–4.8 mg g−1 dry tea leaves, respectively. p-Coumaric acid was detected in only one of the samples with a content of 0.4 mg g−1. Percent recoveries of spiked diluted samples were 90 ± 9 to 106 ± 13%, respectively.
Article highlights
-
Silica-layer coated capillary is first reported for simultaneous separation of seven phenolic acids by non-MEKC analysis.
-
Performance between coated, and non-coated capillaries with analysis by CEC and MEKC were compared.
-
Plate number, resolution, capillary reproducibility, and electroosmotic flow mobility are investigated.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Tea is a health-promoting drink as shown by nutrition research [1]. The chemical composition of tea depends on its production process. One type of compounds found in all tea is the flavanols which are antioxidants [2]. A typical serving of tea (250–300 mg dry tea leaves) contains 30–40% w/w catechin and 3–6% w/w caffeine [3]. Caffeine is well known to help stimulate attention, and reduce fatigue [4]. Another class of compounds found in tea is the phenolic acids, such as gallic acid (GLA), p-coumaric acid (PCA), various derivatives of quinic acid, isomers of caffeoylquinic acid and caffeoyl glucose [5]. The main gallic ester component of tea, (−)-epigallocatechin gallate (EGCG) and its dimer, can decompose to gallic acid at high temperature during the manufacturing process and polymerize into pigments [2, 6]. The latter influences the pH and color of tea [7, 8]. Phenolic acids have health promoting properties, such as antioxidant, antimicrobial, anticancer, anti-inflammatory, and anti-mutagenic [9, 10].
Reports of HPLC–UV/DAD analysis of phenolic acids in tea are listed in Table S1 (Supplementary Information A). Sample extraction was either with hot water or alcohols [11,12,13,14,15]. Capillary electrophoresis with UV detection method (CE-UV) for analysis of phenolic acids and polyphenols are given in Table 1 [8, 16,17,18,19,20, 29]. Most studies employed micellar electrokinetic chromatography (MEKC) using borate buffer and sodium dodecyl sulfate (SDS) [16, 17, 19, 20]. MEKC analysis with conventional non-coated capillary is the most common method for quantitative analysis of phenolic acids and polyphenol compounds. MEKC analysis employs surfactants to form micelles as a “pseudo-stationary phase” [21]. The concentration of the surfactant must be higher than its critical micellar concentration, which is ca. 8.2 mM at 25 °C. However, too high surfactant concentration leads to large electrophoretic current that can cause Joule heating problem and increased viscosity of the running buffer [22,23,24]. The analytical features of the analysis of gallic acid and caffeine in fruit juices, wheat flour, and tomato samples including tea samples, viz. linear range, limit of detection (LOD), precision, recovery, and analysis time, are also listed in Table 1.
This work describes the first application of CEC with highly efficient innovative silica-layer coated capillary for the separation of seven phenolic acids viz. p-hydroxyphenylacetic acid (PHPA), p-coumaric acid (PCA), p-hydroxybenzoic acid (PHBA), caffeic acid (CFA), (3,4-dihydroxyphenyl)acetic acid (DHPA), gallic acid (GLA), and 2,3,4-trihydroxybenzoic acid (THBA), and caffeine (CF). The method, with tyramine as internal standard, is applied to the analysis of caffeine and the seven phenolic acids in brewed tea samples using direct injection into the capillary electrophoresis system. The developed method is validated in terms of linear calibration range, limit of detection, precision and recovery of spiked samples.
2 Experimental
2.1 Chemicals
Gallic acid (GLA, 3,4,5-trihydroxybenzoic acid), p-coumaric acid (PCA, (2E)-3-(4-hydroxyphenyl)acrylic acid), p-hydroxyphenylacetic acid (PHPA, (4-hydroxyphenyl)acetic acid), p-hydroxybenzoic acid (PHBA), 4-hydroxybenzoic acid), 2,3,4-trihydroxybenzoic acid (THBA), caffeine (CF, 1,3,7-trimethylpurine-2,6-dione) and tyramine, 4-(2-aminoethyl)phenol) were purchased from Sigma-Aldrich (St. Louis, MO, USA). (3,4-Dihydroxyphenyl)acetic acid (DHPA) and caffeic acid (CFA, (2E)-3-(3,4-dihydroxyphenyl)acrylic acid) were purchased from TCI (Portland, OR, USA). Sodium dodecyl sulfate (SDS) was from Alfa Aesar (Lancashire, UK). Methanol was purchased from Scharlau (Sentmenat, Spain). Boric acid was purchased from Merck (Darmstadt, Germany). Ultrapure water (18.0 MΩ·cm) was produced from Milli-Q Advantage A10 water purifying system (Merck, Darmstadt, Germany).
2.2 Preparation of standard solutions and samples
Stock 100.0 mM standard solutions of individual phenolic acid, viz. PHPA, PHBA, GLA, DHPA, and THBA, were prepared in 1.00 mL of MeOH–H2O (20:80 v/v), while PCA and CFA were prepared in 1.00 mL of MeOH–H2O (40:60 v/v). Stock 100.0 mM caffeine standard was prepared in 10.00 mL ultrapure water. The internal standard (IS) was 6.0 mM tyramine in methanol. All stock solutions were stored at 4 °C. For constructing the calibration plots working standard solutions were freshly prepared from the stock standards by diluting with the running buffer. The running buffer (25.0 mM borate buffer, pH 9.0) was prepared from boric acid and adjusting the pH to 9.0 with 0.1 M NaOH. For MEKC, the borate buffer contained 50.0 mM SDS.
Tea samples were purchased from local supermarkets in Bangkok, Thailand. An accurate weight of dry tea leaves is infused with 15.0 mL of water at 80 °C for 10.0 min. After cooling to room temperature, the solution is first filtered with Whatman no. 1 filter paper to remove the tea leaves, and then with a 0.45 µm cellulose acetate syringe filter (Filtrex Technologies, Bangkok, Thailand). An aliquot of the clear filtrate is diluted with ultrapure water. A second aliquot of the diluted tea solution is further diluted with the running buffer and the internal standard added. The final dilution depends on the tea sample (T1, T2 or T3) and the analyte to be quantified (caffeine, GLA or PCA). In all cases, the final solution for injection into the CEC-UV system contains the same concentration of the IS (i.e. 0.15 mM tyramine), including the standard solutions of caffeine and phenolic acids employed for calibration.
2.3 Capillary electrophoresis with UV detection
The capillary electrophoresis instrument with a UV detector is an in-house assembled instrument (see picture of the system in Fig. S1 of Supplementary Information B). It consists of a UV detector (Applied Biosystem, 785A UV detector, CA, USA), a high voltage (HV) power supply (Spellman CZE1000R, Hauppauge, USA). The analog output from the detectors are recorded via a data acquisition system from eDAQ (Denistone East, NSW, Australia). The fused-silica capillaries (50 µm i.d., 360 µm o.d.) are from Polymicro Technologies (Phoenix, AZ, USA). The capillary used for the electrophoresis is 60.0 cm long, with 38.0 cm effective length. The capillary is conditioned prior to use by rinsing with 0.1 M NaOH, ultrapure water, and running buffer for 5, 10 and 5 min, respectively, using a syringe pump (model CEC-MSP-001, Unimicro Technologies, CA, USA) connected to a 0.25-mm i.d. Tygon® tubing (Tygon S3™, Cole-Parmer®, IL, USA). The running buffer for CEC is 25.0 mM borate buffer (pH 9.0) and with addition of 50.0 mM SDS for MEKC. Electrokinetic injection is at 354 V cm−1 (21,000 V) for 3 s. The separation field strength is 354 V cm−1 (21,000 V) and UV-absorbance detection is at 280 nm.
2.4 Preparation of silica-layer coated capillary by hydrothermal sol–gel process
The process for coating the inner wall of the fused-silica capillary using a hydrothermal sol–gel method was previously developed by our group [25], based on previous reported methods [26,27,28]. The sol–gel mixture consists of cyclohexane (3.00 mL), TEOS (500 µL), ultrapure water (3.00 mL), CTAB (100 mg), urea (60.0 mg), 0.10 mM acetic acid (440 µL), and n-pentanol (92 µL). The mixture is homogenized using an ultrasonic probe (2 kHz, 20 watts) for 30 s. The sol–gel mixture is then immediately pumped into the capillary using a syringe pump at flow rate of 3.0 µL min−1 for 5 min. The capillary had been conditioned previously by flushing with 50% MeOH, 1.0 M NaOH and ultrapure water, respectively. The ends of the capillary are then sealed with GC septa and the capillary heated in a gas chromatograph oven (HP 6890A, Agilent, Santa Clara, CA, USA) for 4 h at 70 °C. After cooling to room temperature, the capillary is rinsed with ethanol and ultrapure water and kept in an oven at 50 °C until required. Morphology of the inner wall of the coated capillary was observed by scanning electron microscopy (SEM). Figure S2(a)(iii) of Supplementary Information C shows that a layer of uniform silica beads of ca. 100 nm thickness (see Fig. S2(a)(ii)) is deposited on the inner wall of the capillary [25]. The composition of deposited silica layer was characterized by FT-IR spectra. IR bands are observed at 786 cm−1, 963 cm−1 and 1052 cm−1, which are characteristics of the Si‒O, Si‒OH and Si‒O‒Si bonds, respectively [25].
3 Results and discussion
3.1 Comparison of separation performance of non-coated and innovative silica-layer coated capillaries using MEKC and CEC with UV detection
3.1.1 Separation mode
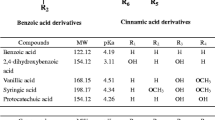
Table 2 shows the chemical structures of the seven phenolic acids, caffeine and the tyramine (the internal standard) and their abbreviations. The compounds were employed in the study of their separation using CEC and MEKC employing conventional fused-silica capillary and the innovative silica-layer coated capillary, respectively. Running buffers were 25.0 mM borate buffer (pH 9.0) for CEC with addition of 50.0 mM SDS for MEKC.
The optimized concentration of 50.0 mM SDS was employed for MEKC mode (see Fig. S3, Supplementary Information D). Figure 1 shows the electropherograms from this study. The CEC with non-coated capillary is able to separate only four compounds as shown in Fig. 1a (i), viz. PHPA (Peak 1), PCA (Peak 2), PHBA (Peak 3), and caffeine (Peak CF). The MEKC mode is more effective (see Fig. 1a (ii)) with separation of the phenolic acids, PHPA (Peak 1), PCA (Peak 2), and THBA (Peak 7). However, it was not able to separate CFA (Peak 4), DHPA (Peak 5), and GLA (Peak 6). Figure 1b shows that the coated capillary gave baseline separation of all 8 compounds for both CEC and MEKC modes of separation. The separation of CFA, DHPA, GLA and THBA have not been previously reported using MEKC with conventional capillaries [19, 20, 29]. This is also the first report of the separation of the two isomers, GLA and THBA, which are structural isomers (see Table 2 for structures).
Electropherograms of caffeine and seven standard phenolic acids using a fused-silica capillary and b silica-layer coated capillary. (i) CEC mode. (ii) MEKC mode. Sample: 100 µM caffeine (Peak CF), 200 µM PHPA (Peak 1), 50 µM PCA (Peak 2), 100 µM PHBA (Peak 3), 200 µM CFA (Peak 4), 200 µM DHPA (Peak 5), 200 µM GLA (Peak 6), and 200 µM THBA (Peak 7) (abbreviations as given in Table 2). The separation parameters are: running buffer: 25.0 mM borate buffer (pH 9.0) for CEC mode, addition of 50.0 mM SDS for MEKC mode; electrokinetic injection: 21,000 V for 3 s; field strength: 21,000 V; UV detection: 280 nm
The coated capillary with MEKC has a much longer separation time due to partitioning of the compounds into the anionic micelles [8, 19]. The CEC analysis has a total separation time of 15 min, which is about 4 times faster than the MEKC separation. In addition, MEKC mode has a higher electrophoretic current than the CEC. The electrophoretic current of the MEKC is 18 µA for both coated or non-coated capillaries, compared with 6 µA for CEC. Thus the CEC systems produce less Joule heating and lower band broadening.
3.1.2 Separation efficiency
Separation efficiency, expressed as plate number per effective capillary length (m−1), was calculated by the equation N = 5.54 × (tm /W1/2)2 × (1/leff), where tm is migration time (s), leff is the effective length of the capillary (m) and W1/2 is the peak width at half maximum height using the PowerChrom software (eDAQ, Denistone East, NSW, Australia). For the silica-layer coated capillary the efficiencies are in the range of 0.3–13 × 104 m−1 for both modes. Figure 2a shows the bar graphs of the efficiencies for the 7 phenolic acids. Measurements of the resolution Rs for all pairs of adjacent peaks in the four electropherograms in Fig. 1 are listed in Table S2 (Supplementary Information E). The Rs values for the silica-layer coated capillary are in the range of 1.6–22.0, respectively.
Bar graphs of a plate number N per one meter of effective capillary length of the silica-layer coated capillary for CEC (dark blue) and MEKC (light blue) calculated using the peak widths of caffeine (CF) and 7 phenolic acids (PHPA, PCA, PHBA, CFA, DHPA, GLA, and THBA). b Bar graphs of plate number N per meter of the 7 phenolic acids and caffeine (CF) for three different coated capillaries. Concentrations of the compounds are: 100 µM caffeine (CF), 200 µM PHPA, 50 µM PCA, 100 µM PHBA, 200 µM CFA, 200 µM DHPA, 200 µM GLA, 200 µM THBA. The separation parameters are: running buffer: 25.0 mM borate buffer (pH 9.0); electrokinetic injection: 21,000 V for 3 s; field strength: 21,000 V; UV detection: 280 nm. Data are mean ± SD (n = 3). See Table 2 for abbreviations of the phenolic acids
3.1.3 Electroosmotic flow mobility
The effect of the layer of silica coating on the electroosmotic flow was also studied. The EOF mobility was determined from conductivity measurements as described in a previous report [30]. The measured values of the EOF mobilities (cm2 V−1 s−1) are given in Table 3. As expected, coating of the silica capillary decreased the EOF mobilities for both the CEC and MEKC modes. The EOF mobilities of coated capillary for different buffers, viz. 25.0 mM phosphate buffer pH 2.70, 25.0 mM acetate buffer pH 4.00 and 25.0 mM borate buffer pH 9.00 are 0.78 ± 0.08, 2.57 ± 0.07 and 4.02 ± 0.04 × 10−4 cm2 V−1 s−1, respectively. The increase of mobility at the higher pH is as expected as more silanol groups on the surface of the silica wall are ionized.
The EOF mobilities for MEKC are always lower than for the CEC. The low EOF of the coated capillary for MEKC mode leads to longer migration times and band broadening of analytes with low net mobilities. More efficient dissipation of Joule heating is observed with a coated capillary, especially with MEKC, as it is more sensitive to temperature variation than the CEC mode [22]. The use of coated capillary is of benefit when using in-house CE system that has no temperature control.
3.2 Analytical features of CEC-UV separation of phenolic acids and caffeine using the silica-layer coated capillary
3.2.1 Calibration
Table 4 lists the linear ranges, calibration equations, coefficients of determination, instrumental LOD, precisions of relative migration time and peak area ratio (ratio of peak areas of analyte and internal standard in the same electropherogram) for the CEC-UV separation with the innovative silica-layer coated capillary [31]. Tyramine is used as the internal standard. The linear calibration range of the seven phenolic acids and caffeine have different ranges but the coefficients of determinations (r2) are > 0.9965.
3.2.2 Precision
The precisions of relative migration times and peak area ratios are calculated from three replicate injections of a standard mixture containing 50 µM caffeine, 100 µM PHPA, 16 µM PCA, 60 µM PHBA, 50 µM CFA, 40 µM DHPA, 20 µM GLA, 20 µM THBA, and 0.15 mM tyramine (IS). The precisions are 0.1–1.8%RSD and 1.8–6.8%RSD, respectively.
3.2.3 Limit of detection
The instrumental limit of detection (LOD) is calculated from 3 × (SD of regression)/(slope of regression) [32, 33]. The values are 1.8–19.4 µM for the acids and 3.4 µM for caffeine.
3.2.4 Reproducibility of capillary efficiency
The values of plate number N per one meter of effective capillary length of 3 different coated capillaries for separation of seven phenolic acids (see examples of electropherograms in Fig. S4 of Supplementary Information F), viz. PHPA, PCA, PHBA, CFA, DHPA, GLA, and THBA and caffeine are: 0.9–1.1 × 105 m−1, 1.3–1.6 × 105 m−1, 1.3–1.4 × 105 m−1, 5.8–6.9 × 104 m−1, 5.0–6.9 × 104 m−1, 3.5–5.9 × 104 m−1, 0.4–3.1 × 104 m−1, and 1.9–2.6 × 104 m−1, respectively (see Fig. 2b). The mean N (n = 3) of all seven phenolic acids and caffeine are 1.0 × 105 m−1, 1.5 × 105 m−1, 1.3 × 105 m−1, 6.5 × 104 m−1, 6.2 × 104 m−1, 4.6 × 104 m−1, 1.5 × 104, and 2.1 × 104 m−1, respectively. The values of N of all seven phenolic acids and caffeine of the 3 capillaries tested lie within the range of mean ± 3SD, indicating that there is no statistical difference between the capillaries. Thus, the coating is highly uniform and reproducible for all the 3 capillaries.
3.3 Analysis of tea samples by CEC-UV using innovative silica-layer coated capillary
Samples of tea leaves were infused in hot water, cooled, filtered and diluted. The dilution factors of each tea sample are given in Table 5. These factors were obtained from prior experimental tests. Figure 3a shows the electropherograms of diluted sample T1 for dilution factors of 10-, 20-, and 60-fold, respectively, and for the 60-fold dilution with spiked standard mixture (Fig. 3a (i), (ii), (iii) and (iv), respectively). Although salicylic acid is not a phenolic acid, it was added to assist the identification of peaks when there are multiple unknown peaks. The electropherogram of standard mixture is also given in Fig. 3a (v) for comparison. High dilution factors lead to reduction of matrix peaks and increased resolution of peaks. Dilution factors of 20- to 60-fold gave baseline separatuion of PCA and GLA, respectively, and factors of 60- to 100-fold for CF. Figure 3b shows representative electropherograms of sample T1. Figure 3b (i) is the electropherogram of the 40-fold diluted tea sample and Fig. 3b (ii) is of the same sample but spiked with PCA (at 5 µM) and GLA (at 10 µM), respectively. The quantitation of caffeine and phenolic acids in the three diluted tea samples are given in Table 5. Caffeine (CF) and gallic acid (GLA) were detected in all samples, but p-coumaric acid (PCA) was found in only sample T1. The other 5 phenolic acids were not detected in all samples.
a Electropherograms for different dilution factors of sample T1. (i) Tenfold dilution, (ii) 20-fold dilution, (iii) 60-fold dilution, (iv) 60-fold dilution spiked with 50 µM PHPA, 3.5 µM PCA, 15 µM PHBA, 50 µM salicylic acid (SA), 12.5 µM CFA, 50 µM DHPA, 45 µM GLA, and 60 µM THBA and (v) mixture of standard phenolic acids and caffeine at 25 µM caffeine (CF), 100 µM PHPA, 7 µM PCA, 30 µM PHBA, 170 µM SA, 25 µM CFA, 100 µM DHPA, 90 µM GLA, and 120 µM THBA. See peak identifications are as shown in Fig. 1, *: unidentified peak. b Electropherograms of 40-fold diluted tea sample T1: non-spiked (red) and spiked (blue) with 10 µM caffeine (CF), 5 µM PCA (peak 2) and 10 µM GLA (peak 6), respectively. Inset is the expanded views of PCA and GLA peaks. Peak identification: IS: tyramine; CF: caffeine; peak 2: PCA; peak 6: GLA; \(*\): unidentified peak
Percent recoveries of CF, PCA and GLA in diluted sample T1, and of CF and GLA in diluted samples T2 and T3, were carried out. The percent recovery was calculated from % Recovery = [(S1 − S2)/S0] × 100, where S0 is the peak area ratio of the standard solution, S1 is the peak area ratio of spiked diluted sample, and S2 is the peak area ratio of the non-spiked diluted sample. The percent recoveries are in the range of 90 (± 9) % to 106 (± 13) % (n = 3). The small shift in migration times between non-spiked and spiked samples in Fig. 3a (iii) and (a) (iv) and also in Fig. 3b is due to the siphoning effect resulting from the imprecision in the placement of the buffer container inherent with the in-house capillary system.
The relative migration times (RMT) are shown in Table 5. The values are 1.29–1.38 for caffeine, 4.62–8.80 for GLA and 2.97 for PCA. The intra-day precisions are in the range of 0.36–4.12%RSD. Using the dilution factor, the initial volume of the brew (15.0 mL) and the accurate weight of the samples of tea leaves (ca. 1.0 g), the contents of caffeine (CF), gallic acid (GLA) and p-coumaric acid (PCA) can be calculated in unit of mg g−1 tea leaves. The results are given in Table 5. Caffeine contents are 10.8 ± 0.5, 15.0 ± 1.1 and 15.0 ± 1.3 mg g−1, and the GLA contents are 2.6 ± 0.1, 3.9 ± 0.1, and 4.8 ± 0.4 mg g−1, for samples T1, T2 and T3, respectively. Sample T1 also has PCA at 0.35 ± 0.04 mg g−1. The LOD of analysis are 1.0–1.8 mg g−1 for caffeine, 0.7–1.2 mg g−1 for GLA and 0.2 mg g−1 for PCA, respectively, depending on the dilution factor. The contents of caffeine and the two phenolic acids are similar to previous reports [11, 12, 14, 15, 17].
This method, with tyramine as internal standard, is the first application of coated capillary with CEC for the analysis of caffeine and phenolic acids in tea samples. The separation time is 15 min with only dilution prior to direct injection into the capillary electrophoresis system. Table 1 lists the application of MEKC-UV for analysis of phenolic acids and polyphenols. Most of the reports employ conventional non-coated capillary. MEKC has limitation when using SDS concentrations very much above the CMC due to the Joule heating effect. This is of particular problem for in-house assembled capillary electrophoresis with no efficient temperature control.
4 Conclusions
An innovative silica coated capillary was successful in separating seven phenolic acids and caffeine using CEC. Previous methods employed the MEKC mode with conventional non-coated capillary (see Table 1). The running electrolyte in this work was boric acid at pH 9.0 and detection was UV absorbance at 280 nm. The coated capillary has a lower EOF mobility than the non-coated capillary but still has a total separation time of 15 min. The modified capillary has a plate number N ≥ 2.0 × 104 m−1 and peak resolution Rs ≥ 1.6 for all adjacent pairs of peaks of the 8 compounds. The plate numbers of all analytes measured using three silica coated capillaries lay within mean ± 3SD. The linear calibration range of the seven phenolic acids are different but span 2–250 µM. The coefficients of determination (r2) for the acids are > 0.9965, with instrumental LOD in the range of 1.8–19.4 µM. Caffeine has a linear calibration range of 5–100 µM (r2 > 0.9994), with instrumental LOD of 3.4 µM.
The system was applied to the analysis of caffeine and phenolic acids in infused tea samples prepared with hot water. After cooling, filtering and serial dilution with ultrapure water and running buffer, the samples were analyzed using electrokinetic injection. Caffeine and gallic acid were detected and quantified in all three samples, using tyramine as the internal standard. p-Coumaric acid was also detected in one sample. The method is convenient, rapid with separation time of only 15 min and has potential for convenient determination of phenolic acids and caffeine content for assessment of tea quality.
Abbreviations
- CEC:
-
Capillary electro-chromatography
- CF:
-
Caffeine
- CFA:
-
Caffeic acid
- CMC:
-
Critical micelle concentration
- CTAB:
-
Cetyltrimethyl ammonium bromide
- DHPA:
-
(3,4-Dihydroxyphenyl)acetic acid
- EOF:
-
Electroosmotic flow
- GLA:
-
Gallic acid
- HPLC:
-
High Performance Liquid Chromatography
- LOD:
-
Limit of detection
- MEKC:
-
Micellar electrokinetic chromatography
- PCA:
-
p-Coumaric acid
- PHBA:
-
p-Hydroxybenzoic acid
- PHPA:
-
p-Hydroxyphenylacetic acid
- RMT:
-
Relative migration time
- RSD:
-
Relative standard deviation
- SDS:
-
Sodium dodecyl sulfate
- SEM:
-
Scanning electron microscopy
- THBA:
-
2,3,4-Trihydroxybenzoic acid
References
Khan N, Mukhtar H (2013) Tea and health: studies in humans. Curr Pharm Des 19:6141–6147. https://doi.org/10.2174/1381612811319340008
Du G-J, Zhang Z, Wen X-D, Yu C, Calway T, Yuan C-S et al (2012) Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 4:1679–1691. https://doi.org/10.3390/nu4111679
Mukhtar H, Ahmad N (1999) Cancer chemoprevention: future holds in multiple agents. Toxicol Appl Pharmacol 158:207–210. https://doi.org/10.1006/taap.1999.8721
Camandola S, Plick N, Mattson MP (2019) Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem Res 44:214–227. https://doi.org/10.1007/s11064-018-2492-0
Lorenzo JM, Munekata PES (2016) Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac J Trop Biomed 6:709–719. https://doi.org/10.1016/j.apjtb.2016.06.010
Zhou J, Wu Y, Long P, Ho C-T, Wang Y, Kan Z et al (2019) LC-MS-based metabolomics reveals the chemical changes of polyphenols during high-temperature roasting of large-leaf yellow tea. J Agric Food Chem 67:5405–5412. https://doi.org/10.1021/acs.jafc.8b05062
Obanda M, Owuor PO, Taylor SJ (1997) Flavanol composition and caffeine content of green leaf as quality potential indicators of kenyan black teas. J Sci Food Agric 74:209–215. https://doi.org/10.1002/(SICI)1097-0010(199706)74:2%3C209::AID-JSFA789%3E3.0.CO;2-4
Aucamp JP, Hara Y, Apostolides Z (2000) Simultaneous analysis of tea catechins, caffeine, gallic acid, theanine and ascorbic acid by micellar electrokinetic capillary chromatography. J Chromatogr A 876:235–242. https://doi.org/10.1016/s0021-9673(00)00145-x
Kumar N, Goel N (2019) Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol Rep 24:e00370. https://doi.org/10.1016/j.btre.2019.e00370
Hayat K, Iqbal H, Malik U, Bilal U, Mushtaq S (2015) Tea and its consumption: benefits and risks. Crit Rev Food Sci Nutr 55:939–954. https://doi.org/10.1080/10408398.2012.678949
Zuo Y, Chen H, Deng Y (2002) Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 57:307–316. https://doi.org/10.1016/S0039-9140(02)00030-9
Fernández PL, López A, Pablos F, González AG, Martín MJ (2003) The use of catechins and purine alkaloids as descriptors for the differentiation of tea beverages. Microchim Acta 142:79–84. https://doi.org/10.1007/s00604-003-0002-8
Peng L, Song X, Shi X, Li J, Ye C (2008) An improved HPLC method for simultaneous determination of phenolic compounds, purine alkaloids and theanine in Camellia species. J Food Compos Anal 21:559–563. https://doi.org/10.1016/j.jfca.2008.05.002
Yin X-L, Wu H-L, Gu H-W, Zhang X-H, Sun Y-M, Hu Y et al (2014) Chemometrics-enhanced high performance liquid chromatography-diode array detection strategy for simultaneous determination of eight co-eluted compounds in ten kinds of Chinese teas using second-order calibration method based on alternating trilinear decomposition algorithm. J Chromatogr A 1364:151–162. https://doi.org/10.1016/j.chroma.2014.08.068
Azevedo RSA, Teixeira BS, Sauthier MCdS, Santana MVA, dos Santos WNL, Santana DdA (2019) Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chem 273:39–44. https://doi.org/10.1016/j.foodchem.2018.04.030
Bonoli M, Colabufalo P, Pelillo M, Gallina Toschi T, Lercker G (2003) Fast determination of catechins and xanthines in tea beverages by micellar electrokinetic chromatography. J Agric Food Chem 51:1141–1147. https://doi.org/10.1021/jf020907b
Hsiao H-Y, Chen RLC, Cheng T-J (2010) Determination of tea fermentation degree by a rapid micellar electrokinetic chromatography. Food Chem 120:632–636. https://doi.org/10.1016/j.foodchem.2009.10.048
Vochyanova B, Opekar F, Tuma P (2014) Simultaneous and rapid determination of caffeine and taurine in energy drinks by MEKC in a short capillary with dual contactless conductivity/photometry detection. Electrophoresis 35:1660–1665. https://doi.org/10.1002/elps.201300480
Marti R, Valcarcel M, Herrero-Martinez JM, Cebolla-Cornejo J, Rosello S (2017) Simultaneous determination of main phenolic acids and flavonoids in tomato by micellar electrokinetic capillary electrophoresis. Food Chem 221:439–446. https://doi.org/10.1016/j.foodchem.2016.10.105
Parveen S, Siyal AN, Memon N, Memon SQ, Khuhawar MY (2015) Extraction and determination of phenolic acids and vitamin B of sieved and unsieved wheat grain by MEKC. J Liq Chromatogr Relat Technol 38:143–152. https://doi.org/10.1080/10826076.2014.896812
Hancu G, Simon B, Rusu A, Mircia E, Gyéresi A (2013) Principles of micellar electrokinetic capillary chromatography applied in pharmaceutical analysis. Adv Pharm Bull 3:1–8. https://doi.org/10.5681/apb.2013.001
Deeb SE, Iriban MA, Gust R (2011) MEKC as a powerful growing analytical technique. Electrophoresis 32:166–183. https://doi.org/10.1002/elps.201000398
Billiot E, Billiot F, Warner IM (2008) Optimization of 12 chiral analytes with 8 polymeric surfactants. J Chromatogr Sci 46:757–763. https://doi.org/10.1093/chromsci/46.9.757
Schmitt-Kopplin P (2008) Capillary electrophoresis method and protocols. Humana Press, NJ
Obma A, Hemwech P, Phoolpho S, Bumrungpuech R, Wirasate S, Kaophong S et al (revised work, 2021) Silica nanolayer coated capillary by hydrothermal sol-gel process for amines separation and detection of tyramine in food products
Liu X, Sun S, Nie R, Ma J, Qu Q, Yang L (2018) Highly uniform porous silica layer open-tubular capillary columns produced via in-situ biphasic sol–gel processing for open-tubular capillary electrochromatography. J Chromatogr A 1538:86–93. https://doi.org/10.1016/j.chroma.2018.01.024
Liu X, Zhu X, Camara MA, Qu Q, Shan Y, Yang L (2019) Surface modification with highly-homogeneous porous silica layer for enzyme immobilization in capillary enzyme microreactors. Talanta 197:539–547. https://doi.org/10.1016/j.talanta.2019.01.080
Liu X, Azhar I, Khan H, Qu Q, Tian M, Yang L (2020) Capillary electrophoresis-immobilized enzyme microreactors for acetylcholinesterase assay with surface modification by highly-homogeneous microporous layer. J Chromatogr A 1609:460454. https://doi.org/10.1016/j.chroma.2019.460454
Lin Y-H, Huang H-C, Hsu W-L (2015) Application of capillary electrophoresis with electrokinetic supercharging and sweeping for the on-line preconcentration of phenolic acids. Anal Bioanal Chem 407:7093–7100. https://doi.org/10.1007/s00216-015-8869-x
Wang W, Zhou F, Zhao L, Zhang J-R, Zhu J-J (2007) Measurement of electroosmotic flow in capillary and microchip electrophoresis. J Chromatogr A 1170:1–8. https://doi.org/10.1016/j.chroma.2007.08.083
U.S. Food and Drug Administration Foods Program (2019). Guidelines for the validation of chemical methods in food, feed, cosmetics, and veterinary products, 3rd edition [online]. https://www.fda.gov/media/121751/download. Accessed 13 September 2020.
Miller JN, Miller JC (2010) Calibration methods: regression and correlation. In: Statistics and chemometrics for analytical chemistry, 6th edn. Pearson, Prentice Hall, pp 124–126
Christian GD, Dasgupta PK, Schug KA (2014) Chapter 3 statistics and data handling in analytical chemistry. In: Gayle A, Bull K (eds) Analytical chemistry, 7th edn. Wiley, Hoboken, pp 105–106
Acknowledgements
This work is funded by National Research Council of Thailand (NRCT): NRCT5-RSA63015-18. Thanks to Faculty of Science, Mahidol University. The Science Achievement Scholarship of Thailand (SAST) is gratefully acknowledged for scholarships to PH, and AO. The Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation, and International Research Network (IRN/502/2563) are gratefully acknowledged for support.
Author information
Authors and Affiliations
Contributions
PH: Investigation, Methodology, Validation, Visualization, Writing-original draft. AO: Methodology, Writing of sections. SD: Investigation. SW: Advise. PC: Funding acquisition, Resource. PW: Supervision, Review and Editing. RC: Conceptualization, Data curation, Funding acquisition, Project administration, Writing-Review, Editing, Supervision.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hemwech, P., Obma, A., Detsangiamsak, S. et al. Capillary electrophoresis-UV analysis using silica-layer coated capillary for separation of seven phenolic acids and caffeine and its application to tea analysis. SN Appl. Sci. 3, 872 (2021). https://doi.org/10.1007/s42452-021-04849-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04849-1