Abstract
The current study was carried out to examine the spatial and temporal variations of physicochemical water quality parameters of Lake Bunyonyi. The observations were made on the surface water of Lake Bunyonyi for 1 year to determine the water quality. The basic 12 variables used to determine the quality of water were measured monthly at nine stations. Water temperature, dissolved oxygen (DO), turbidity, electric conductivity (EC), pH and Secchi depth (SD) were measured in the field, while parameters like total nitrogen (TN), total phosphorus (TP), nitrite-nitrogen (NO2-N), nitrate-nitrogen (NO3-N), soluble reactive phosphorus (SRP) were determined following APHA 2017 standard guidelines for physicochemical analysis. Taking into account standard guidelines for drinking water by the Uganda National Bureau of Standards (UNBS) and the World Health Organization (WHO), the water quality index (WQI) was used to determine the water quality. Temperature, DO, pH, turbidity and EC did not differ significantly among the study stations (p > 0.05) but showed significant temporal variations among the study months (p < 0.05). Likewise, TN, TP, NO2-N, NO3-N and SRP did not differ significantly among the study stations (p > 0.05) but showed significant temporal variations among the study months (p < 0.05). The WQI values ranged from 28.36 to 49 across and from 28.2 to 56.2 between study months with an overall mean value of 36.9. The measured water quality variables did not exceed the UNBS and WHO standards for drinking water in all months and at all stations. According to these values, the water quality of Lake Bunyonyi generally belongs to the ‘good’ class in terms of drinking water quality based on the WQI classification. The study findings are fundamentally important for policy makers in setting guidelines for effective lake management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Although water is a vital element in human life, it is most threatened worldwide [21, 48]. Globally, anthropogenic activities, namely rapid urbanization, industrialization, extensive use of agrochemicals such as chemical fertilizers, herbicides and pesticides and the unrelenting population growth, have resulted in the deterioration of water quality of freshwater bodies [37, 41]. High levels of pollutants such as persistent organic chemicals in the lake ecosystems may make the water unfit for recreation, swimming/ bathing, drinking and fishing, while excess nutrients (nitrogen and phosphorus) massive algal blooms that result in eutrophication and its associated effects on aquatic life [29, 41, 48, 55, 56]. Thus, aquatic environments require appropriate maintenance through interventions that require systematic knowledge of the ecosystem functions controlling the lake as a source of water and a habitat for both aquatic and benthic organisms. Since many factors are responsible for the deterioration of water quality in a given water body, the need for periodic monitoring of the lakes’ water chemistry is absolute [18].
The physicochemical water quality characteristics of any lake are influenced principally by natural and anthropogenic factors. The natural factors include relief, rainfall, weathering, geology and inputs from the catchment and atmosphere, mixing of riverine freshwater from rivers and saline water and climate variability. The disturbance from the catchment area due to pollution emanating from domestic sewage, agricultural activities and industrial settings are the anthropogenic factors. Agricultural run-off is an important source of freshwater pollution and has profound effects on aquatic ecosystems, and human health through the drinking water supply [15].
In Uganda and elsewhere in the world, it is historical that people have lived close to freshwater bodies because of health and well-being benefits. According to McDougall et al. [24], over 50% of the global population live within 3 km of freshwater body. Nevertheless, population increase, intensive farming practices and infrastructure developments have contributed to excessive water withdrawal while increasing the level of external nutrient loading in the remaining natural freshwater systems. As a result, major changes in the physical and chemical characteristics of freshwater bodies have occurred, often showing a significant shift from clear water to the turbid state.
In Kigezi, Lake Bunyonyi is the main source of drinking water, the harbour for faunal and floral biodiversity, and livelihood support [42]. Nevertheless, the Lake’s physicochemical characteristics are influenced by anthropogenic activities such as infrastructural developments on the lake’s 29 islands, campsites and business centres, agricultural activities and fish caging. All the above-mentioned anthropogenic activities are likely to cause spatio-temporal changes in the physicochemical characteristics which may cause eutrophication and subsequently water quality deterioration.
Although some studies have been undertaken to assess the status of Lake Bunyonyi [11, 42, 54] none have illustrated the spatio-temporal variability in physicochemical water quality characteristics of the Lake. Therefore, the need to conduct a comprehensive study to improve understanding of spatio-temporal variations of physicochemical water quality characteristics of Lake Bunyonyi was necessary. The results will contribute to a better understanding of the lake’s ecosystem responses and ultimately support its sustainable management.
2 Materials and methods
2.1 Study area
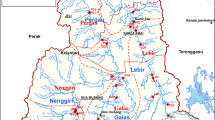
The study was conducted on Lake Bunyonyi which is shared by the districts of Kabale and Rubanda in Southwestern Uganda (Fig. 1). Geographically, Lake Bunyonyi is located between 1.2953° S and 29.9133° E and an average altitude of 1973 m above sea level. The lake is long and narrow with a total surface area of 56 km2 with an average depth of 40 m [19]. The climate of the study area is warm and temperate influenced by altitude and latitude. It is characterized by a bimodal rainfall distribution with the long rainy season occurring between March and May. The short rains occur between October and November while June to August is driest. The mean annual rainfall ranges from 800 to 1000 mm. The temperature in the lake catchment ranges from 23.7 °C in March to 24.8 °C in August [42]. Lake Bunyonyi catchment is a high potential and densely populated area with extensive subsistence farming where crops such as sweet potatoes, beans, sorghum, Irish potatoes and vegetables are grown. On a small scale, fishing and aquaculture are carried out in the lake water [19]. Thus, the livelihood activities of the people in the lake catchment largely depend on the health of the lake ecosystem.
2.2 Sample collection, preparation and storage
Sampling was done monthly for 1 year (October 2019 to September 2020) from nine stations (Table 1) giving a total of 108 samples. Water samples were collected (between 6: 00 and 12 noon) by dipping 1 L polyethene plastic bottles below the surface of the water (0.3 m). The sampling stations were selected in the three study sites that varied in extent and type of human activities in the lake and the lake peripheral. Nyombe (U1), Ndarura (U2) and Kariko (U3) stations were located in the upper Bunyonyi site; Harutinda (M1), Akampene (M2) and Mugyera (M3) stations were located the middle Bunyonyi site, whereas Heissesero (L1), Rugarambiro (L2) and Hamukaka (L3) stations were located in the lower Bunyonyi site. Before the actual collection of samples, the sampling bottles were labelled according to their station codes and rinsed with the Lake water four times to remove any possible form of contamination. All the samples were stored in the icebox with ice and then transported to the National Water and Sewerage Corporation (NWSC) Central Laboratories in Kampala for analysis within 24 h. While in the laboratory, samples were stored in the refrigerator at 4 °C before analysis.
2.3 Analytical methods
The physicochemical water quality parameters such as water temperature, DO, turbidity and electrical conductivity (EC), Secchi depth (SD) and pH were measured on-site. DO was measured using the DO meter (DO 5510 M.R.C model) while water temperature was measured using a temperature sensor in the DO meter. The pH and EC were measured using a water-resistant hand-held pH meter (HI8314 HANNA instruments) and using a conductivity meter (HI 9033 HANNA instruments), respectively, and the turbidity measured was using a turbidity meter (2100P, HACH). Likewise, SD was measured using Secchi disc (20 cm diameter and painted black and white alternately in a radial fashion). All measurements were taken in triplicate and the average values are reported herein.
In the laboratory, nutrient parameters such as TN and TP were determined following the standard producers described in the HACH Method 10,072 and HACH Method 8190, respectively. To determine NH3-N concentration in the water samples, 1 ml of phenol solution, 1 ml sodium nitroprusside solution and 2.5 ml of the oxidizing solution were added to 25 ml of filtered water sample in a 50-ml Erlenmeyer flask, with thorough mixing after each addition. The mixture was covered with a plastic wrap and kept at room temperature (22 to 27 °C) in subdued light for at least 1 h for colour development. The absorbance was measured at 640 nm and NH3-N concentrations contained in the samples were recorded directly from the DR 6000 Spectrophotometer.
To measure NO2-N concentration in water samples, the contents of one pillow Nitraver 3 were added to 25 ml of water sample in a graduated mixing cylinder. The mixture was then covered with palm, held firmly and inverted several times to dissolve the powder. In not more than 20 min after the addition of Nitraver 3 reagent powder pillow, pink colour was observed in samples with nitrites. Between 10 min and 2 h afterwards, absorbance was measured at 543 nm at UV spectrophotometer (DR6000, HACH Laboratory Instruments). The NO2-N concentration was directly recorded from the reading from the spectrophotometer [6]. For NO3-N determination, the content of one pillow Nitraver 6 was added to 25 ml of water sample in a graduated mixing cylinder. The mixture was covered with palm, held firmly and inverted several times to dissolve the powder. Thereafter, the contents of one Nitraver 3 reagent powder pillow were added and a similar procedure for analysis of NO2-N was followed. For SRP determination in the water sample, 50 mL of filtered water sample was mixed with ammonium molybdate to form molybdo-phosphoric acid in a dry 125-mL Erlenmeyer flask. The acid was reduced by ascorbic acid to a blue complex (molybdenum blue). Thereafter, the colour intensity proportional to the concentration of phosphate in the sample was measured by the spectrophotometer at a wavelength of 880 nm [6]. The SRP concentration was recorded directly from DR 6000 Spectrophotometer.
2.4 Water quality index estimation
The WQI was computed using the nine physicochemical parameters based on their importance in water quality analysis. These parameters are temperature, turbidity, pH, DO, EC, NO2-N, NO3-N, SRP and NH3-N. The values used for each parameter were the mean value of each station under investigation. The standards for drinking water used in this study are recommended by WHO [51, 53] and the Uganda National Bureau of Standards [47]. The selected parameters were assigned weight (AW) which varied from 1 to 4.4 based on the collective expert opinions taken from previous studies (Table 25). The average values of the AW values of the selected variables along with the reference are listed (Table 2). Thereafter, the relative weights (RW) were calculated by dividing the weight for each variable by the sum of the weights for all variables (Eqs. 1–4). The weighted arithmetic index method [9] was used for the computation of WQI.
where Qn = quality rating of nth water quality parameter. Wn = the unit weight of the nth water quality parameter.
Qn was computed using Eq. (2).
where Vn = the concentration value of nth variable;
Vi = the ideal value (Vi = 0, except for DO (Vi = 14.6 mg/L) and pH (Vi = 7). Sn = the standard permissible value for the nth variable.
The unit weight (Wn) was computed using Eq. (3)
where K = the constant of proportionality computed using Equation 4.
2.5 Statistical analysis of data
Statistica software (version 10) was used for statistical analysis of data. The mean values of the measured parameters for the study stations were compared to UNBS [47] guidelines for natural drinking water sources, the WHO standards for drinking water quality and the USEPA guidelines for recreational waters. One-way ANOVA was used to establish whether there were significant spatial and temporal differences in physicochemical quality characteristics. For data not normally distributed, the Kruskal–Wallis test was conducted to establish whether there existed significant differences in the physicochemical quality parameters measured. Paired samples t-test was used to establish whether there were significant seasonal variations among the measured parameters. For non-normally distributed data, the Mann–Whitney U test was used to determine whether there were significant differences in measured parameters between the dry season and wet seasons. Pearson’s rank correlation analysis was performed to establish whether there existed a relationship among the measured physicochemical water quality parameters.
3 Results
3.1 Spatial variations in physicochemical water quality parameters
The values of water temperature varied from 20.9 ± 1.2 to 21.8 ± 1.2 °C at Ndarura (U2) and Heissesero (L1) stations, respectively (Table 3). DO concentration values varied between 6.5 ± 1.2 mg/L at Mugyera station (M3) and 7.2 ± 1.7 mg/L at Harutinda (M1). The pH values ranged from 7.4 ± 0.5 to 7.9 ± 0.6 recorded at Mugyera (M3) and Akampene (M2) stations, respectively (Table 3). Turbidity values ranged from 2.8 ± 0.6 to 4.3 ± 1.6 NTU at Akampene (M2) and Mugyera (M3) station, respectively. Likewise, electric conductivity values varied between 241.1 ± 11.1µS/ cm and 266.8 ± 61.9 µS/cm obtained at Mugyera (M3) and Harutinda (U2), respectively (Table 3). Secchi depth values ranged from 0.8 ± 0.2 m to 1.2 ± 0.4 m recorded at Nyombe (U1) and Heissesero (U3) stations, respectively. Statistically, water temperature, DO, turbidity, pH and EC and SD values showed no significant differences between study stations (p > 0.05).
For the case of nutrients, NH3-N values ranged between 0.07 ± 0.01 mg/L and 0.13 ± 0.07 mg/L obtained at Akampene (M2) and Heissesero (L1) station, respectively. TN concentration varied between 1.0 ± 0.7 mg/L and 2.9 ± 2.1 mg/L obtained at Nyombe (U1) and Rugarambiro (L2) stations, respectively (Table 4). NO2-N ranged from 0.003 ± 0.002 to 0.006 ± 0.003 mg/L obtained at Nyombe and Rugarambiro stations, respectively (Table 3). NO3-N concentration values ranged between 0.02 ± 0.02 to 0.03 ± 0.03 mg/L obtained at Harunda (U2) and Rugarambiro (L2) stations, respectively (Table 4). In addition, SRP concentration ranged from 0.05 ± 0.02 mg/L at Hamukaka (L3) station to 0.18 ± 0.3 mg/L at Nyombe station (U1). TP concentration ranged between 0.08 ± 0.04 mg/L and 0.21 ± 0.17 mg/L obtained at Kariko station (U3) and Nyombe (U1) stations, respectively (Table 4). Although the Kruskal–Wallis test revealed significant differences in NH3-N concentration values (H (8, N = 108) = 29.265, p = 0.003), no significant differences were recorded in TN, NO2-N, NO3-N, SRP and TP concentration values between the study stations (p > 0.05).
3.2 Temporal variations in physicochemical water quality variables
The values of water temperature varied between 19.8 ± 0.5 to 23.0 ± 0.6 °C obtained in December 2019 and August 2020, respectively (Table 5). DO values varied between 4.4 ± 0.5 mg/L and 8.4 ± 0.7 mg/L obtained in September and April 2020, respectively (Table 5). Likewise, pH values varied between 7.0 and 8.5 ± 0.1 recorded in September and December 2020, respectively. Turbidity values ranged from 2.2 ± 0.3 NTU in June to 4.8 ± 1.1NTU in April 2020. EC values ranged from 234.7 ± 5 µS/cm in July 2020 to 266.2 ± 58.5µS/cm in January 2020 (Table 5). SD values ranged from 0.6 ± 0.2 to 1.3 ± 0.4 m obtained in May and June 2020, respectively (Table 4). Statistically, the mean values for the measured physical parameters were significantly different between sampling months (p < 0.05).
Nutrients varied distinctly between sampling months. NH3-N values ranged from 0.07 ± 0.01 to 0.14 ± 0.1 mg/L recorded in June and March 2020, respectively (Table 5). TN concentration ranged from 0.6 ± 0.4 to 3.8 ± 2.1 mg/L recoded in August and March 2020, respectively (Table 5). The NO2-N values ranged from 0.002 ± 0.001 to 0.008 ± 0.004 mg/L recorded in March and August 2020, respectively. NO3-N concentration ranged from 0.01 ± 0.00 mg/L in January and September 2020 to 0.06 ± 0.03 mg/L in February 2020. Likewise, SRP values in water samples varied between 0.019 ± 0.01 mg/L in October 2019 to 0.23 ± 0.3 mg/L in March 2020. TP values varied from 0.05 ± 0.01 mg/L in June 2020 to 0.27 ± 0.2 mg/L in March 2020 (Table 5). Statistically, the mean values for all the nutrient parameters were significantly different between sampling months (p < 0.05).
3.3 The relationship between physicochemical water quality parameters
The Pearson’s correlation was computed to establish whether there existed significant relationship among physicochemical parameters measured in Lake Bunyonyi (Table 7). Water temperature had a positive significant correlation with SD (r = 0.367; p < 0.01) but with a negative significant correlation with DO (r = -0.046, p < 0.01), turbidity (r = − 0.416, p < 0.01), NO2-N (r = − 0.290; p = 0.02); and NO3-N (r = − 0.323, p = 0.001) and TN (r = − 0.329, p = 0.001) (Table 6). TP had a positive significant correlation with NO3-N (r = 0. 335, p < 0.01), NH3-N(r = 0.279, p = 0.003), SRP (r = 0.5502, p < 0.01, TN (r = 0.198, p = 0.040) but significantly negatively correlated with SD (r = − 0.217; p = 0.024) (Table 6). Likewise, DO levels were significantly positively correlation with turbidity (r = 0.508; p < 0.05) and pH level (r = 0.342, p < 0.01), turbidity (r = 0.483, p < 0.01), NO2-N (r = 0.329, p < 0.01) but significantly negatively correlated with SD (r = -0.268, p < 0.01). Besides, the TN values were significantly positively correlated with turbidity (r = 0.245, p = 0.010), NH3-N (r = 0.255, p = 0.008), NO3-N (r = 0.571, p < 0.01) but significantly negatively correlated with temperature (r = − 0.329, p < 0.01), pH (r = − 0.210, p = 0.029) and SD (r = − 0.255, p = 0.004).
3.4 Water quality index
The WQI used to assess the water quality was prepared using values of the nine physicochemical variables selected based on their importance in water quality. They include; temperature, turbidity, pH, DO, EC, NO2-N, NO3-N, SRP and NH3-N. The values used for each variable were the mean value of the stations under investigation. The threshold limit values by WHO [51], [53] except for SRP and NH3-N values derived from Uganda National Bureau of Standards [47] were used for computation of the WQI values (Table 8). The WQI values (Table 7) in the Lake varied from 28.36 to 49.4 obtained at Harutinda and Heissesero stations, respectively. Based on the WQI classification, the recorded WQI range values fall under the ‘good’ class, which reflects that water from Lake Bunyonyi is good for drinking, recreation and domestic uses on account of physicochemical variables (Table 8).
The water quality index signifies as scale of water quality (Table 9). As indicated in Table 10, the WQI between 0 to 25 means ‘Excellent’ quality of water; 25 to 50 means ‘Good’ quality of water; 51 to 75 means ‘Poor’ quality of water; 76 to 100 means ‘Very poor’ quality of water; and > 100 means the water is ‘Unsuitable’ for drinking [29].
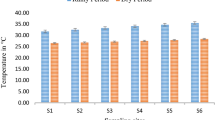
On a temporal scale, it was observed that March 2020 recorded the highest WQI value (56.2) while in July 2020, the lowest index value (28.2) was obtained (Fig. 2). Based on the WQI classification, it was observed that WQI values obtained during the sampling period fall under the ‘good’ category unlike the values for March and April 2020 which fell under the ‘poor’ category (51–75). When examined changes in the WQI values between seasons (Fig. 2), it was observed that the mean WQI value for the wet season (41.7) was higher than the value recorded in the dry season (33.5) and both fell under the ‘good’ category.
4 Discussion
4.1 Variability in physical water quality parameters
The observed surface temperature values were found to be within the WHO permissible limit (25 °C) for aquatic living [50]. Similar to our findings, Tibihika et al. [42] recorded an overall mean water temperature value of 21.3 ± 1.4 °C at Lake Bunyonyi. The observed somewhat low temperature was attributed to two main factors. Firstly, the water mass that enters Lake Bunyonyi through River Kagoma and other minor streams take longer to warm up. Secondly, Lake Bunyonyi is situated between steep hills with several barriers that limit the amount of sunlight heating the surface waters of the lake.
The observed DO concentrations in the current study were > 2 mg/L. These values according to Keister et al. [17] do not cause stress to aquatic organisms and ecosystems and can rarely result in changes in the communities through direct organisms mortality and reduction in the population growth of sensitive species. In the aquatic ecosystem, DO provides a broad indication of water quality and depends on many factors such as microbial activity, temperature, level of organic matter, pressure and the sampling time [10, 27]. Similar to study findings, Tiémoko et al. [43] reported a DO concentration range of 4.6 to 7.7 mg/L at Lake Taabo and Kossou. With regard to EC, the recorded values in the current study did not exceed the WHO maximum permissible limits of 2500 μS/cm as stated in the global national regulations and standards for drinking water. Therefore, our study results rightly indicate that water in the Lake Bunyonyi was not considerably ionized and has a relatively low ionic concentration level. The observed high EC values at Harutinda station are possibly attributed to the possible pollution from Crater Bay Cottages and Lake Bunyonyi Overland camp whereas low EC values at Nyombe station indicates an untainted aquatic environment. The turbidity range across all study stations did not exceed the maximum value (20 NTU) for the global national regulations and standards for drinking water [53]. Similar to our study results, Anjusha et al. [5] reported a mean turbidity range of 2.5–3.9 NTU, slightly lower than the values reported in the present study. On the other hand, Umer et al. [46] reported a turbidity range of 28.5 to 63.0 NTU in Lake Beseka significantly higher than recorded values in the current study attributed to the rapid expansion of the Lake water level.
Concerning pH levels, the recorded range of values reflected a neutral to a slightly alkaline aquatic environment and was within the acceptable range (6.5–8.5) for a healthy aquatic life [53]. The observed variations in pH are possibly due to the high influx of freshwater from the upper Bunyonyi site and River Kagoma that flows into the lake. Similar to the present study findings, Ongom et al. [30], Song et al. [39] and Muduli and Pattnaik [26] obtained pH ranges reflecting neutral to slightly alkaline lake conditions. According to Niyoyitungiye et al. [28], the optimal pH range below pH 6.5 influences the slow growth of some aquatic species while pH value > 6.5 affects the capacity of some organisms to preserve their salt equilibrium and can cause a stoppage in reproduction unlike the reported pH range is conducive. Concerning SD, the observed temporal variability is possibly attributed to the seasonal variations. For instance, Lake Bunyonyi experiences high run-off inflow from the catchment and increased as a result of high discharge experience in the rain season. Similar to study results, Sitoki et al. [38], Tran et al. [45], reported the SD range value of 0.8–1.7 m (Lake Kyoga) and 0.17–2.3 m (Edward), respectively. On the other hand, Stoyneva-Gärtner et al. [40], Vundo et al. [49] and Ozguven & Demir Yetis [32] reported an SD range significantly higher than the recorded values in the present study.
4.2 Variability in the nutrient concentrations
TN concentration is the measure of both organic and inorganic nitrogen concentration. The role of nitrogen in freshwater bodies varies according to the relative amounts of the forms of nitrogen present. Generally, higher TN concentration values were found in the lower Bunyonyi site where run-off from agricultural land enters the lake through the Rugarambiro sampling station. This confirms the frequently experienced opinion that farm activities in the lake watershed are an important source of nitrogen in freshwater lakes [14]. Similar tour study findings, Tibihika et al. [42] and Ozguven and Demir Yetis [32] reported TN concentration values of 103.6 ± 8.4 μg/L in Lake Bunyonyi and 4.9 ± 16.0 mg/L in Big Soda Lake Van, respectively. However, the observed study findings were higher than 0.88 ± 0.05 mg/L for Lake Qiandaohu as reported by Zhou et al. [58].
In all study stations, the observed NH3-N concentrations were greater than 0.025 mg/L, the recommended limit for freshwater environments beyond which, it acts as toxic to freshwater organisms [13]. Similar to our study results, Tilahun and Ahlgren [44] obtained 0.09 ± 0.08 mg/L in Lake Chamo of Ethiopia. The observed high NH3-N concentration at Heissesero is possibly due to the demineralization of submerged macrophytes. The assumption is built based on the fact that rivers that flow into the lake understudy showed low NH3-N concentration and the NH3-N concentration in the lake did not change significantly during the rainy season.
The observed NO2-N concentration range values were within the WHO concentration threshold of 0.9 mg/L for drinking water [51]. Thus, water from Lake Bunyonyi is less likely to cause health concerns and thus Lake Bunyonyi still meets the minimal standards to serve as a source for drinking water. The observed NO2-N values were lower than 32.9 ± 0.7 μg/L and 0.82 ± 0.25 mg/L recorded at Lake Bunyonyi, and Lake Tonga by Tibihika et al. [42] and Loucif et al. [22], respectively. On the other hand, the recorded NO2-N values were higher than 1.3 ± 0.7 μg/L obtained at Lake Oubeira reported by Keyombe and Waithaka [18]. The presence of nitrites in freshwater lakes can give rise to the presence of nitrosamines by reaction with organic compounds and thus cause carcinogenic effects [52].
The observed NO3-N concentration values did not exceed 11 mg/L; the WHO recommended NO3-N limits for drinking water [51]. The recorded highest NO3-N concentration value (0.01 ± 0.004) at Ndarura station perhaps emanates from farmlands around the study station. In comparison with other previous studies, Tibihika et al. [42], Maryam et al. [23] and Tibebe et al. [41] obtained the average NO3-N values of 33.8 ± 2.1 μg/L, 0.46 mg/L and 0.21 mg/L slightly higher the values obtained in the current study. In aquatic environments, NO3-N are important nutrients necessary for the growth of aquatic plants; nevertheless, the concentration > 90 mg/L is toxic to aquatic organisms [4]. The major pathways that naturally increase NO3-N content in lake ecosystems are rain, fog, snow, decomposition of organic matter and fertilizer application in agricultural fields.
TP is the measure of both inorganic and organic forms of phosphorus. Due to the longer residence time in lakes, TP is considered the most critical nutrient [33]. Similar to the results of the current study, Opiyo et al. [31] reported high TP values (3.09 ± 0.09 mg/L) in the wet season than in the dry season. The observed spatial variations in TP concentrations were attributed to proximity to agricultural farmlands which are the major sources of nutrients in the lake. Likewise, the variations in TP concentration during the study period are possibly attributed to seasonal changes in rainfall amounts which influence nutrient deposition intensity. The current study findings, however, disagrees with that of Opiyo et al. [31] who reported the mean average of 2.9 ± 0.08 mg/L in Lake, respectively, higher than reported TP values in the current study. This attributed to several years of nutrients inflows from the Lake sub-catchment that experiences intense agricultural activities [31].
4.3 Water quality index of Lake Bunyonyi
The WQI of Lake Bunyonyi fell within the ‘good’ category of WQI classification across study stations. Nevertheless, the WQI values for March and April 2020 fell under the ‘poor’ category (51–75). Similar to our results, Shah and Joshi [36] revealed the quality of water at Station 1 of River Sabarmati of India was of good quality with a WQI range of 19.84 to 44.58 obtained from 2005 to 2008. Similarly, Nihalani and Meeruty (2020) reported a WQI range of 30 to 50 for River Mahi, and 28 to 52 for River Narmada, all of which are substantially related to the observed WQI range of the current study. In other related studies, Kükrer and Mutlu [20] reported that WQI values for Saraydüzü Dam Lake ranged between 17.62 and 29.88, implying that Saraydüzü Dam Lake water belonged to the ‘very good’ class of WQI classification in terms of drinking water quality.
Although WQI values for stations in the lower Bunyonyi site place them into the ‘good category, the declining water quality was noticed. The recorded somewhat high WQI values are perhaps a result of containments inflow from the upper and middle Bunyonyi sites to the lower site, thereby increasing pollutants concentration. Besides, the Heissesero station is situated adjacent to Muko Trading Centre where wastewater and surface run-off are directed into the Lake via run-off ways. The high WQI value for Hamukaka station is attributed to wastewater discharge from Bright Island and the agricultural run-off from crop fields. At Rugarambiro station, the increased agricultural practices (such as Irish potato growing) around the Lake increase pollution as a result of excessive use of fertilizers, and chemicals including pesticides.
4.4 Correlation of among physicochemical variables
The correlation analysis revealed that temperature was positively correlated with DO, EC, SRP, NH3-N, NO2-N and NO3-N. The reported significant negative correlation between temperature and NO3-N concentration values has been reported by Kangabam et al. [16] in Loktak Lake, India. Likewise, Bhattrai et al. [7] reported that DO was significantly negatively correlated with water temperature while positively correlated with turbidity, pH level, TP and TN of Lake Hwajinpo of South Korea. The results of significant correlation between surface water temperature and DO differed slightly from that of Ali and Khairy [3] who reported an increase in DO levels the period when surface water temperatures were lower due to gas solubility, which increases when temperatures and metabolic activity of organisms are low and the decreased, respectively. TP values were positively correlated with temperature, DO, turbidity, NH3-N, TP and SRP. Similar to the study findings, Yu et al. [57] reported a positive correlation that TN positively correlated with TP and NH3-N but significantly negatively with NO2-N.
5 Conclusion
Based on the results, we conclude that the quality of water in Lake Bunyonyi is generally good for drinking as per WQI classification. This is further supported by the fact that the mean values for all the measured physicochemical variables were within WHO, UNBS and USEPA permissible limits for drinking water and recreational waters, respectively. It was evident that the obtained values for temperature, DO, turbidity, EC, NH3-N, NO2-N and NO3-N of the samples fell below the permissible levels and thus may not put both human and aquatic life at health risks. Although remarkable temporal variations were recorded among the measured physicochemical parameters, no extreme variations were recorded in all the study stations. The current study findings are fundamentally important for policymakers in setting guidelines for effective lake management. We, therefore, recommend that further studies investigate in detail the point sources of contamination and the possible causes of high concentration of nutrients in Lake Bunyonyi be conducted.
Availability of data and material
All data generated and analysed during this study are included in this published article.
Abbreviations
- WHO:
-
World Health Organization
- APHA:
-
American Public Health Association
- Turb:
-
Turbidity
- NTU:
-
Nephelometric turbidity unit
- WQI:
-
Water quality Index
- USEPA:
-
The United States Environment Protection Agency
- SD:
-
Secchi depth transparency
- DO:
-
Dissolved oxygen
- NWSC:
-
National Water and Sewage Cooperation
References
Abdul MJ, Haider SA, & Bahram KM (2010) Application of water quality index for assessment of Dokan lake ecosystem, Kurdistan region, Iraq. J Water Resour Prot 2(9):792–798
Abrahão R, Carvalho M, Da Silva Jr WR, Machado T, Gadelha C, & Hernandez M (2007) Use of index analysis to evaluate the water quality of a stream receiving industrial effluents. Water Sa 33(4)
Ali EM, Khairy HM (2016) Environmental assessment of drainage water impacts on water quality and eutrophication level of Lake Idku, Egypt. Environ Pollut 216:437–449
Amić A, Tadić L (2018) Analysis of basic physical-chemical parameters, nutrients and heavy metals content in surface water of small catchment Area of Karašica and Vučica Rivers in Croatia. Environments 5(2):20
Anjusha KV, James AM, Thankachan FA, Benny J, Hezakiel VB (2020) Assessment of water pollution using GIS: a case study in Periyar River at Eloor Region. In: Green buildings and sustainable engineering, Springer, pp 413–420
APHA (2017) Standard methods for the examination of water and wastewater (23rd ed.). American Public Health Association. www.standardmethods.org
Bhattrai BD, Kwak S, Choi K, Heo W (2017) Assessment of long-term physicochemical water quality variations by PCA Technique in Lake Hwajinpo, South Korea. J Environ Prot 8(13):1636
Boyacioglu H (2007) Development of a water quality index based on a European classification scheme. Water Sa 33(1)
Brown RM, McClelland NI, Deininger RA, O’Connor MF (1972) A water quality index—crashing the psychological barrier. In: Thomas WA (ed) Indicators of environmental quality. Springer, US, pp 173–182. https://doi.org/10.1007/978-1-4684-2856-8_15
Das Kangabam R, Govindaraju M (2019) Anthropogenic activity-induced water quality degradation in the Loktak Lake, a Ramsar site in the Indo-Burma biodiversity hotspot. Environ Technol 40(17):2232–2241
Denny P (1972) Lakes of south-western Uganda. Freshw Biol 2(2):143–158
Dwivedi SL, Pathak V (2007) A preliminary assignment of water quality index to Mandakini River, Chitrakoot. Indian J Environ Prot 27(11):1036
EPA (2001) Parameters of water quality–interpretation and standards. Wexford. EPA. ISBN, 133.
Huang J, Xu C, Ridoutt BG, Wang X, Ren P (2017) Nitrogen and phosphorus losses and eutrophication potential associated with fertilizer application to cropland in China. J Clean Prod 159:171–179
Ioannidou V, Stefanakis AI (2020) The use of constructed wetlands to mitigate pollution from agricultural runoff. In: Contaminants in Agriculture, Springer, pp 233–246
Kangabam RD, Bhoominathan SD, Kanagaraj S, Govindaraju M (2017) Development of a water quality index (WQI) for the Loktak Lake in India. Appl Water Sci 7(6):2907–2918
Keister JE, Winans AK, Herrmann B (2020) Zooplankton community response to seasonal hypoxia: a test of three hypotheses. Diversity 12(1):21
Keyombe J, Waithaka E (2019) Analysis of some aspects of water quality of Lake Naivasha. Int J Chemical Lifesci 6(1):2001–2005
Kizza CL, Tenywa MM, Majaliwa JGM, Kansiime F, Magunda M, Nakileza B, Barasa B, Gabiri G, Sebuliba E, Nampijja J (2017) Land use/cover change patterns in highland ecosystems of Lake Bunyonyi Catchment in western Uganda. Afr Crop Sci J 25(1):43–58
Kükrer S, Mutlu E (2019) Assessment of surface water quality using water quality index and multivariate statistical analyses in Saraydüzü Dam Lake, Turkey. Environ Monit Assess 191(2):71
Li C, Feng W, Chen H, Li X, Song F, Guo W, Giesy JP, Sun F (2019) Temporal variation in zooplankton and phytoplankton community species composition and the affecting factors in Lake Taihu—A large freshwater lake in China. Environ Pollut 245:1050–1057
Loucif K, Neffar S, Menasria T, Maazi MC, Houhamdi M, Chenchouni H (2020) Physico-chemical and bacteriological quality assessment of surface water at Lake Tonga in Algeria. Environ Nanotechnol Monit Manag 13:100284. https://doi.org/10.1016/j.enmm.2020.100284
Maryam AB, Ovie EA, Gambo J (2020) Monitoring and Exploring the Spatio-temporal Variation of Physico-chemical Variables of River Hadejia, Nigeria; Using Statistical Approach. Asian J Geogr Res 3(1):49–61
McDougall CW, Quilliam RS, Hanley N, Oliver DM (2020) Freshwater blue space and population health: An emerging research agenda. Sci Total Environ 737:140196
Meher PK, Sharma P, Gautam YP, Kumar A, Mishra KP (2015) Evaluation of water quality of ganges river using water quality index tool. EnvironmentAsia 8(1):124–132
Muduli PR, Pattnaik AK (2020) Spatio-temporal variation in physicochemical parameters of water in the Chilika Lagoon. In: Ecology, Conservation, and Restoration of Chilika Lagoon, India, Springer, pp. 203–229
Mutlu E (2019) Evaluation of spatio-temporal variations in water quality of Zerveli stream (northern Turkey) based on water quality index and multivariate statistical analyses. Environ Monit Assess 191(6):335. https://doi.org/10.1007/s10661-019-7473-5
Niyoyitungiye L, Giri A, Mishra B (2019) Assessment of physico-chemical characteristics of water at selected stations of Lake Tanganyika, Africa with special emphasis on pisciculture purposes. Int J Basic Appl Biol 6(3):211–217
Noori R, Berndtsson R, Hosseinzadeh M, Adamowski JF, Abyaneh MR (2019) A critical review of the application of the National Sanitation Foundation Water Quality Index. Environ Pollut 244:575–587
Ongom R, Andama M, Lukubye B (2017) Physico-chemical quality of lake kyoga at selected landing sites and anthropogenic activities. J Water Resour Prot 9(11):1225–1243
Opiyo S, Getabu AM, Sitoki LM, Shitandi A, Ogendi GM (2019) Application of the Carlson’s trophic state index for the assessment of trophic status of lake Simbi ecosystem, a deep alkaline-saline lake in Kenya. Int J Fish Aquat Stud 7(4):327–333
Ozguven A, Demir Yetis A (2020) Assessment of spatiotemporal water quality variations, impact analysis and trophic status of Big Soda Lake Van, Turkey. Water Air Soil Pollut 231(6):260. https://doi.org/10.1007/s11270-020-04622-x
Radbourne AD, Elliott JA, Maberly SC, Ryves DB, Anderson NJ (2019) The impacts of changing nutrient load and climate on a deep, eutrophic, monomictic lake. Freshw Biol 64(6):1169–1182
Ravikumar P, Mehmood MA, Somashekar RK (2013) Water quality index to determine the surface water quality of Sankey Tank and Mallathahalli Lake, Bangalore Urban District, Karnataka, India. Appl Water Sci 3(1):247–261
Rubio-Arias H, Contreras-Caraveo M, Quintana RM, Saucedo-Teran RA, Pinales-Munguia A (2012) An overall water quality index (WQI) for a man-made aquatic reservoir in Mexico. Int J Environ Res Public Health 9(5):1687–1698
Shah KA, Joshi GS (2017) Evaluation of water quality index for River Sabarmati, Gujarat, India. Appl Water Sci 7(3):1349–1358
Singh D, Singh BB, Kumar Y (2018) Spatio-temporal variability of physico-chemical and biological water quality parameters of river ganges in six cities situated on Indo-Gangetic plain transect. Am J Water Resour 6(6):235–245
Sitoki L, Gichuki J, Ezekiel C, Wanda F, Mkumbo OC, Marshall BE (2010) The environment of Lake Victoria (East Africa): Current status and historical changes. Int Rev Hydrobiol 95(3):209–223
Song J, Hou C, Liu Q, Wu X, Wang Y, Yi Y (2020) Spatial and temporal variations in the plankton community because of water and sediment regulation in the lower reaches of the Yellow River. J Clean Prod 261:120972. https://doi.org/10.1016/j.jclepro.2020.120972
Stoyneva-Gärtner MP, Morana C, Borges AV, Okello W, Bouillon S, Deirmendjian L, Lambert T, Roland F, Nankabirwa A, Nabafu E (2020) Diversity and ecology of phytoplankton in Lake Edward (East Africa): present status and long-term changes. J Great Lakes Res 46:741–751
Tibebe D, Kassa Y, Melaku A, Lakew S (2019) Investigation of spatio-temporal variations of selected water quality parameters and trophic status of Lake Tana for sustainable management, Ethiopia. Microchem J 148:374–384
Tibihika PDM, Okello W, Barekye A, Mbabazi D, Omony J, Kiggundu V (2016) Status of Kigezi minor Lakes: A limnological survey in the Lakes of Kisoro, Kabale and Rukungiri Districts. Int J Water Resour Environ Eng 8(5):60–73
Tiémoko GJL, Ouattara NK, Kouamé CKY, Ouattara A, Gourène G (2020) Spatial and temporal variation of faecal indicator bacteria in three reservoirs of Ivory Coast (Taabo, Kossou and Fae). J Environ Sci 6(1):408–411
Tilahun G, Ahlgren G (2010) Seasonal variations in phytoplankton biomass and primary production in the Ethiopian Rift Valley lakes Ziway, Awassa and Chamo-The basis for fish production. Limnologica 40(4):330–342
Tran PQ, McIntyre PB, Kraemer BM, Vadeboncoeur Y, Kimirei IA, Tamatamah R, McMahon KD, Anantharaman K (2019) Depth-discrete eco-genomics of Lake Tanganyika reveals roles of diverse microbes, including candidate phyla, in tropical freshwater nutrient cycling. BioRxiv. https://doi.org/10.1101/834861
Umer A, Assefa B, Fito J (2020) Spatial and seasonal variation of lake water quality: Beseka in the Rift Valley of Oromia region, Ethiopia. Int J Energy Water Resour 4(1):47–54
UNBS (2014) Uganda standards Template—World Trade Organization, 1st edn. Government of Uganda, Kampala
Varol M (2020) Spatio-temporal changes in surface water quality and sediment phosphorus content of a large reservoir in Turkey. Environ Pollut 259:113860
Vundo A, Matsushita B, Jiang D, Gondwe M, Hamzah R, Setiawan F, Fukushima T (2019) An overall evaluation of water transparency in Lake Malawi from MERIS data. Remote Sens 11(3):279
WHO (2008) Guidelines for drinking-water quality: Incorporating first and second addenda to the third edition, Vol. 1, Recommendations. Geneva: WHO Press, Geneve.
WHO (2011) Guidelines for drinking water quality. WHO Chron 4(38):104–108
WHO (2017) Guidelines for drinking-water quality: First addendum to the fourth edition.
WHO (2018) A global overview of national regulations and standards for drinking-water quality. World Health Organization.
Worthington EB (1932) A Report on the Fisheries of Uganda Investigated by the Cambridge Expedition to the East African Lakes, 1930–31 with 3 Appendices, 5 Maps and 21 Other Illustrations.
Wu T, Qin B, Brookes JD, Yan W, Ji X, Feng J (2019) Spatial distribution of sediment nitrogen and phosphorus in Lake Taihu from a hydrodynamics-induced transport perspective. Sci Total Environ 650:1554–1565
Wu Z, Wang X, Chen Y, Cai Y, Deng J (2018) Assessing river water quality using a water quality index in Lake Taihu Basin, China. Sci Total Environ 612:914–922
Yu Y, Song X, Zhang Y, Zheng F (2020) Assessment of water quality using chemometrics and multivariate statistics: a case study in chaobai river replenished by reclaimed water, North China. Water 12(9):2551
Zhou L, Wang X, Zhang X, Zhao Y, Zhu P, Zhao X, Li X (2020) Spatiotemporal variations in nitrogen and phosphorus in a large man-made lake and their relationships with human activities. Water 12(4):1106
Acknowledgements
We would like to thank NWSC, Kampala—Uganda, for accepting us to use their laboratory facilities. We also wish to thank the communities living around Lake Bunyonyi for their support during the study.
Author information
Authors and Affiliations
Contributions
AS conceived and designed the study, collected and analysed data and drafted the manuscript: TJL contributed to the conception and design of the study, assisted in data interpretation and revision of the manuscript for intellectual content. JM contributed to the conception and design of the study, assisted in data interpretation and revision of the manuscript for intellectual content. SP contributed to the conception and design of the study, assisted in data interpretation and revision of the manuscript for intellectual content. All the authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of competing interests.
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saturday, A., Lyimo, T.J., Machiwa, J. et al. Spatio-temporal variations in physicochemical water quality parameters of Lake Bunyonyi, Southwestern Uganda. SN Appl. Sci. 3, 684 (2021). https://doi.org/10.1007/s42452-021-04672-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-021-04672-8