Abstract
Nitrate levels are frequently high in ground and surface waters mainly because of anthropogenic activities. Electrocoagulation (EC) is a viable alternative to conventional coagulation in drinking water treatment and has been successfully applied to remove nitrate from drinking water. The objective of this study was to determine optimum operating conditions for maximizing nitrate removal from drinking water using electrocoagulation followed by settling and filtration. Batch experiments were carried out using iron electrodes (mild steel) and four types of water were tested (groundwater, tap water, untreated water from IIT Water Works and double distilled water). Experiments were conducted with direct current (DC) power or solar power. Operating parameters such as initial nitrate concentration, voltage, electrocoagulation time and settling time were varied to determine optimum conditions for achieving maximum removal efficiency. Nitrate removals after electrocoagulation and after settling were determined for all experiments. In 22 DC power experiments with all waters tested, maximum removal efficiencies were 37% (after EC) and 38.21% (after EC and settling). The highest removal efficiency was obtained in double distilled (DD) water at 10 V, initial nitrate concentration of 452 ppm, EC time of 4 h, settling time of 4 h and an initial pH of 9.89. Multiple linear regression analysis of DD water experiments was done. Model 1 based on removal efficiency after EC only was found to be a good fit and was statistically significant. Five solar experiments were carried out and the maximum removal efficiency obtained was 53% with 3 h of electrolysis time. The initial nitrate concentration was 95.53 mg/L, voltage was 14 V and initial pH was 10.8. Electrocoagulation coupled with solar energy can be used in rural areas in decentralized mode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Everybody requires clean and safe drinking water. However, finding the same is becoming increasingly difficult due to population growth, excessive urbanization and industrialization. Even groundwater can no longer be considered ‘safe’ since overuse of agricultural pesticides and fertilizers result in the leaching of several harmful contaminants including nitrate. Nitrate is a highly soluble and stable ion which makes its presence in drinking water quite dangerous and a serious health concern since it can result in the blue baby syndrome [1, 2]. Rural areas where agriculture is the dominant activity are especially vulnerable to nitrate contamination of groundwater. Households in rural India constitute 68% of the total households in the country and at least 65% of these rural households rely on groundwater as a source of drinking water [3]. Thus the removal of nitrate from drinking water is absolutely essential for making it safe for consumption. Further, nitrate is one of the priority pollutants in India under the National Drinking Water Mission.
The most common contaminant identified in ground water is dissolved nitrogen in the form of NO3−. Nitrate can be of natural or anthropogenic origin. It is formed by the reaction of atmospheric nitrogen and oxygen due to lightning and large amounts of this atmospheric nitrate can enter soil and water due to precipitation. Anthropogenic sources of nitrate in surface and ground water are agricultural and urban practices like excessive use of fertilizers, presence of septic tanks and discharges of industrial wastewaters from food and metal industries [4]. Nitrate occurs naturally at low levels in most waters but it is particularly prevalent in groundwater that has been impacted by agricultural, commercial, or industrial activities. Of specific concern are crop fertilization activities and discharges from animal operations, wastewater treatment facilities, and septic systems [5]. In other cases, nitrate originates by conversion of organic nitrogen. Ammonification and nitrification are processes that normally occur above the water table generally in the soil zone, where organic matter and oxygen are abundant [6]. Groundwater is particularly susceptible to contamination to a large extent along its flow paths in shallow phreatic or unconfined to semi-confined aquifers which are well connected through a horizontal network of fissures, pores and weathered mantle. In India, in a CGWB survey of 15,640 shallow wells all over India, 2429 wells had nitrate concentrations greater than 45 mg/L which is the drinking water standard for nitrate in India [6]. The national average concentration of nitrate in these wells was 155.9 mg/L (standard deviation of 145.4 mg/L) while the maximum nitrate concentration observed was 1750 mg/L in Jalna, Maharashtra [6].
Guidelines for nitrate and nitrite in drinking water were revised recently by WHO [7]. High nitrate concentrations can lead to the blue baby syndrome [1, 6]. Nitrate should not be more than 50 mg/L as nitrate ion (11 mg/L as nitrate-nitrogen) and nitrite should not be more than 3 mg/L as nitrite ion (0.9 mg/L as nitrite-nitrogen) to prevent the occurrence of methemoglobinemia in bottle-fed infants [7]. This guideline value has been derived from epidemiological studies taking into account the nitrate and nitrite values reported in drinking water in the respective study areas.
Nitrate can be removed from drinking water by adsorption, ion-exchange, heterogeneous catalysis, biological treatment methods, membrane filtration, and electrochemical methods. However, most physicochemical processes either produce a lot of concentrated nitrate rejects whose treatment and disposal become an added problem or form secondary species like ammonia or nitrite which are toxic [8, 9]. Biological treatment can only be applied to waters which have low C:N ratios [8]. Electrocoagulation (EC) is a viable alternative to conventional coagulation (CC) and has several advantages as compared to CC [10, 11]. In EC, the coagulant species, mostly hydroxides of iron or aluminium, are produced in situ which makes it highly effective. The pollutant to be treated is co-precipitated or adsorbed on the coagulant-floc that is formed in solution and is eventually removed either after it settles as sludge or floats to the top [12,13]. EC has been applied widely to remove nitrate from different types of waters including drinking water [14,15,16]. The pH of the solution often increases in EC unlike CC where the pH decreases. Therefore, there is no need for post-neutralization in most cases with EC. The mechanism for EC is simple and no moving parts are required. However, the electrodes need to be replaced from time to time and electricity consumption is greater than in CC.
The general objective of this study was to understand the factors that affect nitrate removal using electrocoagulation, settling, and filtration. Specifically, optimum operating conditions for maximizing nitrate removal using electrocoagulation, settling and filtration were determined. In this study, 27 batch experiments were performed using four types of water to evaluate the efficiency of electrocoagulation for nitrate removal to meet drinking water standards. Operating parameters such as voltage, initial concentration, initial pH, electrocoagulation time and settling time were varied and their effect on nitrate removal efficiency was observed. Experimental results were analysed using MATLAB R2016a and modelled using multiple linear regression equations.
2 Materials and methods
2.1 Sample collection and characterization
2.1.1 Sampling locations
All water samples were collected from the academic cum residential campus of Indian Institute of Technology Kharagpur in Kharagpur, West Bengal, India. The campus lies at the southern extreme of Kharagpur city located in West Bengal, India. The city is famous for having one of the longest railway platforms in the world. It is surrounded by rural areas and agriculture is the dominant activity in this area.
Samples of four different types of drinking waters were collected from different locations as explained below and are shown in the map (Fig. 1):
-
a.
Groundwater samples were collected from the tubewell in Dandakaranya pump house, IIT Kharagpur, India. Fresh water samples were collected for each experiment and some were characterized for various water quality parameters. The depth of the tubewell is 250 feet.
-
b.
Water from IIT waterworks (water treatment plant) is from river Kansabati. River Kansabati is located around 10 km from IIT Kharagpur. Water was collected from the water treatment plant located inside the campus before treatment.
-
c.
Tapwater was taken from the Environmental Engineering and Management lab (EEM lab), Civil Engineering Department, IIT Kharagpur. The water is treated water from IIT waterworks.
-
d.
Double distilled water was produced in EEM lab and used for most of the experiments.
Each type of water was collected in clean plastic bottles and preserved till the experiment. Raw (untreated) water samples were collected afresh for each set of experiments.
2.1.2 Sample characterization
Each type of raw water was characterized for alkalinity, hardness, pH, turbidity, conductivity, TDS, Fe, Free and total chlorine as per Standard Methods [17].
2.2 EC experiments
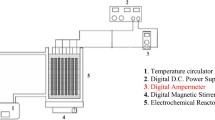
The setup for EC included two mild steel electrodes submerged in a glass beaker (2 L glass beaker) filled with raw water as shown in Fig. 2. The beaker was mounted on a magnetic stirrer (REMI 2ML magnetic stirrer) and conductivity and pH probes (LabQuest, Vernier International, USA) were immersed in the beaker for continuous data acquisition. The dimensions of the MS electrodes were 14.5 × 2.5 × 0.1 cm (Fig. 3) and submerged depth was 6 cm. The effective submerged area of the electrodes was about 97.8 cm2. Previous batch experiments in the same laboratory showed that an inter-electrode distance of 2 cm was optimum for turbidity removal [18]. Therefore, all experiments in this study were conducted with an inter-electrode distance of 2 cm.
The beaker was placed on a magnetic stirrer to facilitate continuous mixing throughout the EC period. The electrodes were connected to a DC power supply (0–30 V and 0–3 A). The raw water was initially spiked with NaNO3 (Merck India) so as to adjust the initial nitrate concentration to the desired value. The voltage was kept constant at a particular value and changes in current, pH, conductivity, turbidity and nitrate concentration were monitored throughout the experiment. Samples were collected every 15 min during the first hour of EC, and then every hour until the end of the experiment. Each sample was filtered through a 0.45 µm Whatman filter before analyzing it for anion concentrations using Ion Chromatography.
Solar experiments were carried out by connecting the two electrodes to the positive and negative ends of a 17 V, 5 W rated solar panel (Solar PV module, Model: SLP005-12). In solar powered experiments, there is no control over the voltage generated since voltage is dependent on sunlight intensity which varies during the day and from day-to-day. Photos of the experimental set-up are shown in Fig. 4a and b. The treated water of this experiment was exceptionally clear as seen in Fig. 4b. All the suspended solids had floated to the top as scum. This phenomenon, called ‘electroflotation’, occurs when the current density is high and a large number of bubbles are generated causing the floc to attach and float up. Sample collection and analysis were done in the same way as in the DC experiments.
A total of 27 experiments (22 of which were performed with DC power supply and 5 were performed with solar power) were conducted using the four types of raw waters. The voltage, initial concentration, electrocoagulation time, settling time and initial pH were varied within certain ranges. Voltage was varied from 10 to 30 V, initial concentration was varied between 45 and 570 mg/L, initial pH from 4.4 to 11, EC time and settling time, both, from 1 to 5 h. These ranges were selected based on experimental results obtained previously in our laboratory.
Electrocoagulation (EC) time is the time for which EC was performed during the experiment. After EC time, the voltage and stirring were switched off and the sludge was allowed to settle. The objective behind doing this was to determine if there was any improvement in nitrate and turbidity removals due to settling.
3 Results and discussion
Electrocoagulation experiments were conducted with four different types of waters: double distilled (DD) water, ground water (GW), tap water, and untreated water from the local water works plant (IIT Water Works).
3.1 Characterization of raw waters
Results of raw water characterization are shown in Table 1. Turbidity of river water was the highest while the pH of groundwater was the highest in these samples. River water samples were taken from the water treatment plant inlet.
3.2 Batch Nitrate experiments with DC power
A total of 22 batch experiments were done and the results are summarized in Table 2. The best removal efficiencies with each of the raw waters are highlighted in bold. Differences in removal efficiencies after EC only and after EC and settling were insignificant in almost all experiments. The final turbidity values ranged from 1 to 115.7 NTU. The average value was 51.9 NTU and standard deviation was 48.9 NTU. The best removal efficiency was observed in Experiment 20 and is described in the next section.
3.2.1 Experiment 20
The best removal efficiency of 37.0% was observed in Experiment 20 with double distilled (DD) water after EC only and increased to 38.21% after settling, since there were no competing ions to interfere with the removal of nitrate from the raw water. The initial concentration for this experiment was 451.6 ppm and both, EC and settling time were 4 h each. Initial pH was adjusted to ≈10 which increased to 11 while EC was going on and decreased again to 10.7 after settling. Since the voltage applied was low (10 V), the energy consumption was 8.3 Wh/L. The average conductivity was around 1000 µS/cm during the experiment because of the high initial nitrate concentration. Change in anode weight was minimal and high nitrate removal was observed resulting in 53,919 mg of nitrate removed / gram of Fe consumed. The initial turbidity was 0.52 NTU to start with and increased up to 19.8 NTU till EC continued. After that it dropped to 2 NTU at the end of four hours of settling. The final turbidity was well within the limits for drinking water (5 NTU).
3.3 Solar experiments
All solar experiments were performed from 10 am to 4 pm since sunlight was intense during that period. The solar panel was set facing the sun and its position was manually changed twice or thrice per day. A voltage of 14–17 V was obtained during maximum sunlight hours. All solar experiments were conducted with untreated IIT Water Works water and tap water.
The average removal efficiency obtained after EC was 18.51% with a standard deviation of 20.19% while that after settling was 12.78% with a standard deviation of 11.12%. The energy consumed per litre was also substantially lower (5.49 Wh/L), though it should be kept in mind that the sample size is very small. The variability of these experiments is much higher than that of DC powered experiments, though the highest removal efficiency was also much higher than that obtained with DC power. Therefore, coupling the EC apparatus to solar panels seems to have great promise and further experimentation may lead to better results. In 5 solar experiments, the highest removal efficiency of 53% was observed in experiment no. 4 (Table 3) and is described in the following section.
3.3.1 Experiment 4—Solar
At a voltage of 14 V, initial concentration of 95.53 mg/L, an EC time of 3 h and a settling time of 5 h, the removal efficiency obtained after EC was 53% (Fig. 5). The removal efficiency decreased as soon as electrocoagulation stopped which is in accordance with the results of Hashim et al. who reported that removal efficiency increased with electrolysis time since a constant amount of coagulant ions are generated [19]. The voltage had dropped down to 10 V between 120 and 180 min. In this experiment, the initial pH was 10.80 and the final pH was 9.24. These results are similar to other studies where the optimal pH range for nitrate removal was between 9 and 11 in one study [20] and 10 and 11 in another study [21]. The energy consumed in this experiment was 6.6 Wh/L. The initial turbidity was 4.4 NTU which stabilized to 58.5 NTU at the end of EC. The final turbidity was 41 NTU. There was a sudden increase in the turbidity during settling which may be due to some disturbance.
Results of replicate nitrate experiments shown in Table 2 were analysed for repeatability and the analysis is shown in Table 4. Two sets of experiments with DD water had less variation in comparison to the experiments with IIT Water Works samples. The coefficient of variation was least for experiments 14–15 and very low for experiments 16–18. In all cases, the coefficient of variation for removal efficiency after EC was much less than that after settling indicating greater variability in results after settling.
Experiments 9–13 (Table 3) were performed with IIT Water Works’ samples and are exact replicates based on experimental conditions. As can be seen in Table 4, removal efficiency in experiment 13 is extremely low and therefore, based on the other results, it is an obvious outlier. Taking only experiments 9–12, the average after EC was 24.7% (standard deviation was 3.29%) and after settling was 25.1% (standard deviation was 5%). These results show that EC experiments for nitrate removal have high replicability.
3.4 Multiple linear regression (MLR) analysis
Experimental results for DD water and DC power were analysed by multiple linear regression using MATLAB R2016a. Several experiments were done with different water types, but regression analysis was done only for experiments with double distilled water since there is no interference due to other ions, compounds or particulate matter.
Five factors which affect the efficiency of defluoridation were chosen based on the literature and our own studies. Thus, five independent (x) and two dependent variables (y) were chosen for analysis. Apart from voltage (x1), initial concentration (x2) and initial pH (x3), two types of ‘time’ were considered – EC time (x4) and settling time (x5) as the independent variables. Two dependent variables, i.e., the removal efficiency after EC (y1) and the removal efficiency after EC and settling (y2) were considered.
All values were standardized prior to analysis by subtracting the mean of all values for a particular variable and then dividing it by the standard deviation, i.e., \({X}_{i}={(x}_{i}-\stackrel{-}{x})/\sigma\) where Xi = standardized value of the variable, xi = the observed value of the variable, \(\stackrel{-}{x} = \) mean of all observed values and σ = standard deviation of all observed values of the variable.
Two statistical models were developed for multiple regression analyses:
Model 1 The first model was used to analyse the parameters affecting removal efficiency after EC, consisting of four independent and one dependent variable. Removal efficiency after EC (y1) was measured immediately after EC was stopped, i.e., before settling started. Hence, in Model 1, settling time (x5) was not considered.
Model 2 The second model was used to analyse all the parameters previously considered in Model 1 as well as the influence of settling time on the total removal efficiency resulting in five independent variables and one dependent variable. In this case, removal efficiency after settling was measured after EC and settling both had taken place. Hence, in this part of analysis, EC time (x4) and settling time (x5) both were considered as independent variables and the dependent variable was (y2).
The general equations for multiple linear regression are as follows:
Removal efficiency after EC: y1 = c1 + a1x1 + a2x2 + a3x3 + a4x4.
Removal efficiency after EC and settling: y2 = c2 + a1x1 + a2x2 + a3x3 + a4x4 + a5x5.
The multiple linear regression equations obtained with standardized values of the variables for Models 1 and 2 are:
The other parameters are presented in a tabular form below (Table 5).
The R2 and p value of a model are two basic statistical parameters that determine how good a correlation is. R2 indicates how well the model ‘fits’ the data, i.e., the variation, while the p value indicates the significance of the model. In this case, the p value for each term tests the null hypothesis that the coefficients of the independent variables are equal to zero, i.e., that the parameters tested have no effect on the dependent variable. A low p value indicates that the null hypothesis can be rejected. The best scenario is when R2 is high and the p value is low, which means that the model explains a lot of variation within the data and the results are significant. In Model 1, for removal efficiency after EC (Eq. 1), the p value is much lower than 0.05 (at the 95% confidence level) and R2 is 0.9996, which indicates that the null hypothesis is rejected and this model is significant. In Model 2, the second model for total removal efficiency (Eq. 2), a high R2 of 0.9631 was obtained, while the p value is > 0.05. Thus, though the model is a good fit, it is not statistically significant.
The variance impact factor (VIF) gives us a measure of the multi-collinearity of the individual parameters. Severe multi-collinearity (a condition in which predictors are correlated to other predictors in the model) can prove to be a problem since it can increase the variance of coefficient estimates and make the estimates very sensitive to minor changes in the model. In the first model, all independent variables have low multi-collinearity, since the VIF values are close to 1 [22]. However, high multi-collinearity exists in the second model where VIF values are much greater than 1.
Further, since all independent variables were standardized, their coefficients can be compared directly to determine their relative contribution to removal efficiency. From Eq. 1, the most important factor affecting removal efficiency after EC is initial concentration > EC time > voltage > initial pH. These results are similar to those found by other researchers who reported that nitrate adsorption increased with increasing initial nitrate concentration [23,24,25]. They found that adsorption was rapid initially, but then saturated with time. However, some researchers have experimentally found that removal efficiency is indirectly proportional to initial concentration [26].
The model developed for EC removal efficiency for double distilled water (y1) was applied to the experiments performed with other types of water. Non-standardized values were taken so that their units remain the same as the observed values. The data for experiment 1 was not taken as it was an outlier. Good correlation was observed with an R2 value of 0.75 (R = 0.87) Any raw water other than double distilled water contains a certain amount of impurities in terms of dissolved solids, hardness, or co-existing anions. These can lower removal efficiencies which may explain why the R2 is not higher. These results show that Model 1 provides reasonably good predictions for removal efficiencies after EC for all types of raw waters.
3.5 Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was performed on the sludge samples and tiny elongated needle-like crystals were seen upon magnification. These crystal structures were present in clusters and are shown in Fig. 6. Further study of the sludge can help us in understanding the chemistry involved in the reactions taking place as EC proceeds.
3.6 Energy dispersive X-ray spectroscopy (EDX) results
EDX spectra for sludge samples were obtained on the basis of areas or points considered. Some nitrogen and oxygen was found in the sludge samples where an area was considered, and relatively less nitrogen was found where point samples were taken. Large quantities of iron and oxygen were present in the sludge samples along with nitrogen and sodium. EDX analysis of the anode scrapings (Table 6) showed oxygen (43%), nitrogen (19.6%), oxygen (43%) and calcium (17.4%) in high amounts along with carbon (10%) and phosphorous (7.3%). This phenomenon suggests that nitrate was being removed primarily by deposition on the electrodes as well as by adsorption on floc.
3.7 Cost analysis
For any experiment or method to be useful to the common man or lay person, it has to be economically feasible. The average energy consumption for all the DC powered experiments was 10.86 Wh/L. However, if the 3 experiments which were performed with 30 V are excluded since their removal efficiencies were lower than experiments conducted at 10 V (maximum removal efficiency), the average energy consumption of the remaining experiments was only 7.48 Wh/L. The average energy consumption of all the solar experiments was 5.05 Wh/L, which is lower than that obtained with DC powered experiments. Solar experiment no. 4 in which the highest removal efficiency was obtained had an energy consumption of just 4.4 Wh/L. Thus, parameter optimization can also lead to greater efficiency in energy consumption.
4 Conclusions
After conducting several batch experiments for nitrate removal using different types of raw water and DC or solar power, it is clear that electrocoagulation using iron electrodes can be used for removing nitrate from drinking water and making it safe for consumption. Major findings of this study are:
-
1.
The best nitrate removal efficiencies were obtained at high initial concentrations of nitrate, high EC and settling times and high pH.
-
2.
Optimization of working parameters like initial concentration, voltage, electrocoagulation time and settling time is necessary for further improvement in removal efficiency as well as reduction in energy consumption.
-
3.
The statistical model applied shows that EC time contributes much more to the removal efficiency rather than settling time, and initial concentration is the most important parameter. The statistical model provided reasonably good estimates of the measured values with an R2 of 0.75.
-
4.
The use of solar power coupled with electrocoagulation can substantially improve removal efficiency, decrease working costs and further reduce energy consumption.
References
Adimalla N, Qian H (2019) Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, South India. Ecotoxicol Environ Saf 176:153–161
Kapoor A, Viraraghavan T (1997) Nitrate removal from drinking water—review. J Environ Eng 123:371–380
Office of the Registrar General of India and Census Commissioner (2011) Provisional population totals census of India 2011. https://censusindia.gov.in/2011-Common/CensusData2011.html. Accessed 11 Jan 2017
Adimalla N (2019) Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Human Ecol Risk Assess Int J. https://doi.org/10.1080/10807039.2018.1508329
UC Davis (2012) Drinking water treatment for nitrate with a focus on Tulare Lake Basin and Salinas Valley groundwater; Davis, CA, USA. https://groundwaternitrate.ucdavis.edu/files/139107.pdf. Accessed 2 Apr 2018
CGWB (2010) Ground water quality in shallow Aquifers of India. https://cgwb.gov.in/documents/Waterquality/GW_Quality_in_shallow_aquifers.pdf. Accessed 27 Sept 2016
World Health Organization (WHO) (2017) Guidelines for drinking water quality. https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/. Accessed 15 Dec 2019
Yehya T, Balla W, Chafi M, Audonnet F, Vial C, Essadki A, Gourich B (2015) Assessment of denitrification using electrocoagulation process. Can J Chem Eng 93:241–248
Govindan K, Noel M, Mohan R (2015) Removal of nitrate ion from water by electrochemical approaches. J Water Process Eng 6:58–63
Apshankar KR, Goel S (2018) Review and analysis of defluoridation of drinking water by electrocoagulation. J Water Supply Res Technol AQUA 67:297–316
Apshankar KR (2018) Electrocoagulation-filtration for the removal of fluoride or nitrate from drinking water, Ph.D. thesis, IIT Kharagpur, India
Sivakumar M, Emamjomeh MM (2005) Electrochemical method for fluoride removal: Measurement, speciation, and mechanisms. in: proceedings of the environ postgrad conference on environmental change: making it happen; School of Civil & Chemical Engineering, RMIT, Australia: Wollongong, Australia, pp 1–7. https://ro.uow.edu.au/engpapers/1661/. Accessed 21 Sept 2015
Zhao H, Yang W, Zhu J, Ni J (2009) Defluoridation of drinking water by combined electrocoagulation: Effects of the molar ratio of alkalinity and fluoride to Al(III). Chemosphere 74:1391–1395
Kumar NS, Goel S (2010) Factors influencing arsenic and nitrate removal from drinking water in a continuous flow electrocoagulation ( EC ) process. J Hazard Mater 173(5):28–533
Lacasa E, Cañizares P, Sáez C, Fernández FJ, Rodrigo M (2011) Removal of nitrates from groundwater by electrocoagulation. Chem Eng J 171:1012–1017
Mook WT, Chakrabarti MH, Aroua MK, Khan GMA, Ali BS, Islam MS, Abu Hassan MA (2012) Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: a review. Desalination 285:1–13
APHA, AWWA, WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA, Washington
Adapureddy SM, Goel S (2012) Optimizing electrocoagulation of drinking water for turbidity removal in a batch reactor. In: International proceedings of chemical, biologival and environment engineering, vol 30. https://www.ipcbee.com/vol30/020--ICEST2012_N30001.pdf. Accessed 10 Apr 2012
Hashim KS, Shaw A, Al Khaddar R, Ortoneda Pedrola M, Phipps D (2017) Defluoridation of drinking water using a new flow column-electrocoagulation reactor (FCER)—experimental, statistical, and economic approach. J Environ Manag 197:80–88
Koparal AS, Ögütveren ÜB (2002) Removal of nitrate from water by electroreduction and electrocoagulation. J Hazard Mater 89:83–94
Emamjomeh MM, Sivakumar M (2009) Denitrification using a monopolar electrocoagulation/flotation (ECF) process. J Environ Manag 91:516–522
Heckman E (2015) What in the world is a VIF? https://blog.minitab.com/blog/starting-out-with-statistical-software/what-in-the-world-is-a-vif. Accessed 16 Nov 2017
Escobar C, Soto-Salazar C, Ines Toral M (2006) Optimization of the electrocoagulation process for the removal of copper, lead and cadmium in natural waters and simulated wastewater. J Environ Manag 81:384–391
Lakshmi J, Sozhan G, Vasudevan S (2013) Recovery of hydrogen and removal of nitrate from water by electrocoagulation process. Environ Sci Pollut Res Int 20:2184–2192
Sengil IA, Ozacar M (2006) Treatment of dairy wastewaters by electrocoagulation using mild steel electrodes. J Hazard Mater 137:1197–1205
Malakootian M, Yousefi N, Fatehizadeh A (2011) Survey efficiency of electrocoagulation on nitrate removal from aqueous solution. Int J Environ Sci Technol 8:107–114
Acknowledgements
The authors are grateful to K. Rajesh, Saikat Bhowmik and Tushar Satpati for their help with sample collection and analysis. Funding for this research work was obtained from Department of Science and Technology (Grant No. DST/WTI/2k9/144, dated: 07/06/2010), Government of India and is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Apshankar, K.R., Goel, S. Nitrate removal from drinking water using direct current or solar powered electrocoagulation. SN Appl. Sci. 2, 304 (2020). https://doi.org/10.1007/s42452-020-2069-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2069-9