Abstract
A nanoparticle preparation (PC@AgNPs), synthesized biogenically using Priva cordifolia leaf extract was found to possess quenching property of stable DPPH and ABTS in dose dependent manner. The PC@AgNPs showed well-established physicochemical stability with respect to temperature, PH and salinity. It showed a broad bactericidal effect against 10 pathogenic bacteria, notably Staphylococcus aureus, Escherichia coli, Shigella flexneri and Vibrio parahaemolyticus. Its non-toxicity up to 500 µg mL−1 concentration to peripheral blood mononuclear cells was recorded. However, its toxic effect against human lung carcinoma cells and human breast adenocarcinoma cell lines at a dose of 500 and 300 µg mL−1 respectively was clearly evident. The catalytic efficiency of PC@AgNPs was demonstrated in methylene blue and methyl orange dye degradation. The studies have suggested possible applications of PC@AgNPs in future biomedical, pharmaceutical, and environmental applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, green approach for nanoparticle synthesis becomes a safe and tuning protocol to increases or decreases the efficacy of the biological function which is applied through microbiology, biotechnology and Nanoscience using nanotechnology. Nanoparticle synthesizing using inorganic material hugely and also by microbes and plants. The nanotechnology defines the material of 1–100 nm in size which is having tremendous physicochemical and functional properties different from bulk material which is used for multi-domains of science including packaging, coating, cosmetics, electronics, medicine and in biotechnology also etc. [1, 2].
Many types of nanoparticles were reported by researches including Au, Ag, Pd and Pt synthesized through physical, chemical, and biological methods (supplementary Table 1) and also can be synthesizable using stabilizing agents, thermal decomposition in an organic solvents, photoreduction, and radiation methods. Mainly these methods are costly involves toxic and hazardous chemicals which create an environmental and biological risk [3] but, chemical methods popular due to its yield efficacy [4]. To synthesize silver nanoparticles (AgNPs) a required chemical reducing agent to convert Ag to AgNPs but, requires hazardous chemicals giving less biocompatible AgNPs for the biological function. In a physical method, requires high energy and pressure for the reaction [5] and have their own limitations like expensive, unsuitable for ecosystem [6].
Nanoparticles are synthesized employing bacteria [7, 8], enzyme [9], fungi [10] and plant extracts [11,12,13]. Synthesizing from biological protocol ginned tremendous momentum having nontoxic and environmentally safe. Among many metals, synthesizing silver nanoparticle proved its tremendous applications in medicine and industries due to its microbial effect such as antifungal, antibacterial, antiparasitic, and larvicidal properties. Due to its may unrevealed benefits to human, need to process and tune the synthesis and access its properties of silver nanoparticles [14,15,16] including in the field of degenerative disease and cancer biology [17]. Many unrevealed plant components and its characteristics induce focused research scientists to look into that using the antioxidant character as basic property linked to many other properties such as anti-mutagenic, anti-carcinogenic, and anti-allergic [18]. The property of the antioxidant due to the redox potential of the phytoconstituents [19], which involved in the quenching singlet and triplet oxygen, decompose peroxided or neutralizing the generated free radicals. Therefore, it is hypothesized that higher nanoparticles (NPs) activity depends on the antioxidant property which is come from adsorbing antioxidant molecule from the plant extract onto the surface of the nanoparticles to drive this property was exportable.
The silver nanoparticles are known to possess the inhibitory effect on various bacterial strains involved in causing minor to major health risk globally [20]. Therefore, in medicines, silver and silver nanoparticles will be advantageous especially in skin ointments and creams containing silver to prevent infection of burns and open wounds [21]. In the green synthesis of NPs using plant has advantageous such as easy availability, cost-effectiveness, safety, broad availability of metabolites, as well as the extract act both as reducing and capping agents. The phytochemicals adsorbed on NPs will have increased stability, agglomeration, enhanced activity and broad biological properties.
Priva cordifolia (L.f). Druce is an ethno medicinal plant indicated as useful for the treatment of wounds [22], diarrhea (https://www.giz.de/expertise/downloads/giz2014-en-aids.pdf) and migraine (http://opendata.keystone-foundation.org/priva-cordifolia-l-f-druce). P. cordifolia leaf extract have biological macromolecules viz, polyphenols, flavonoids, steroids, saponins and Phytosterol were responsible for reducing silver nitrate and acting as a capping agent to synthesize PC@AgNPs within 10 min of reaction time without any hazardous bi-product chemicals. Lawrence et al. [23] have reported the plant P. cordifolia assisted synthesis of silver nanoparticles employing water extract of the plants leaves without any detailed on the characteristics or data on application potential of the nanoparticles.
Further details on the physic-chemical and biological attributes of P. cordifolia-assisted silver nanoparticles were reported by the present authors [24]. It was considered necessary to obtain extra information to substantiate the potential of the nanoparticle preparation in biomedical, pharmaceutical and environmental and the data obtained are presented in this article.
2 Materials and methods
2.1 Chemicals and bacterial strains
All the chemicals used in this study were of analytical grade. 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), silver nitrate, Tris-buffer, and butylated hydroxytoluene (BHT) were purchased from Merck, Mumbai, India. Dimethyl sulfoxide (DMSO) was procured from Hi-media Mumbai, India. All lyophilized bacterial strains were procured from Microbial Type Culture Collection (MTCC), Chandigarh, India and American Type Culture Collection (ATCC). Enterobacter faecalis-439, Escherichia coli-1610, Salmonella typhimurium-98, Shigella flexneri-1457, Vibrio cholera-3904, Vibrio paraheamolyticus-451, Pseudomonas aeruginosa-1688, Staphylococcus aureus-96, Bacillus cereus-430, Enterobacter aerogenes-13048 and strains were cultured in recommended broth as per the revival procedure provided by MTCC and ATCC. Cell lines Hela (Human cervical cancer), peripheral blood mononuclear cell (PBMC) and A549 (Human lung carcinoma cells) were purchased from the National Centre for Cellular Sciences, Pune, India.
2.2 Preparation of Priva cordifolia leaf extract
25 g of dried leaves were ground to a fine powder and refluxed with 225 mL of methanol for 5 h. Then the extract was filtered with Whatman paper and evaporated to dry paste was used in the analysis.
2.3 Qualitative and quantification phytochemical analysis
The preliminary assessment of the extracted phytochemicals was performed by the methods of Harbor [25] to test for the presence of steroids, carbohydrates, alkaloids, flavonoids, and saponins.
2.4 Phytochemical characterization by using GCMS
The presence of phytochemicals in the plant extract was characterized by gas chromatography with mass spectra (Thermo scientific ITQ 900) system interfaced with a TG-95 MS column (5% diphenyl/95% dimethyl polysiloxane, 30 × 0.25 mm ID × 0.25). Helium gas (99.999%) was used as a carrier with pragmatic 70 eV ionizing energy in an electron ionization with a flow rate of 1 mL/min. 2 µL of the sample was injected to system with a split ratio of 10:1. The GC injector temperature was set to 250 °C, ion source temperature was kept at 200 °C. Oven temperature was preset to rise from 60 to 200 °C at 5 °C/min, and then to 280 °C at 10 °C/min and it stable for 9 min. The mass spectra of fragmented compounds were measured from 45 to 450 Da. The entire run time of programmer was 36 min. The peaks were compared with known spectra with a database of National Institute of Standards and Technology.
2.5 Synthesis and characterization of PC@AgNPs
The synthesized PC@AgNPs was confirmed by the absorption spectrum obtained using Shimadzu UV–visible spectrometer. The PC@AgNPs was detail characterized by FTIR, SEM, TEM, EDX, DLS and XRD in a previous report and same material was used for further studies [24].
2.6 Physicochemical interaction of PC@AgNPs
The physicochemical interaction of synthesized PC@AgNPs was amply studied with respect to ionic strength, pH and temperature [26]. The salinity interaction of PC@AgNPs was studied from 0.2 to 1 M at a constant pH of 7.0 and the colloidal solution was measured by UV–Vis spectroscopy. The consistency of PC@AgNPs with respect to temperature and PH were monitored in the range of 30–90 °C and 2–10 respectively.
2.7 Colloidal stability
100 µg mL−1 of synthesized PC@AgNPs was dissolved in Millipore water and the colloidal stability was monitored by using UV–Vis spectroscopy at 415 nm at different interval of time.
2.8 Determination of antioxidant activity
2.8.1 DPPH radical scavenging activity
The free radical quenching property of PCE and PC@AgNPs was measured by using stable free radical chemical 2,2-diphenyl-1-picrylhydrazyl (DPPH) in accordance with Phull [27]. Briefly, different concentration of PCE and PC@AgNPs was mixed 2 mL of methanolic DPPH solution (40 mg−L) and 1 mL of 50 mM tris HCl. Ascorbic acid and BHT were used as a positive control and distilled water as a negative control. The reaction mixture was incubated at room temperature in dark for 30 min and absorbance was recorded at 517 nm. All the experiments were carried out in triplicates. The percentage of free radical quenching property was calculated as follows.
2.8.2 ABTS radical scavenging activity
The free radical quenching property of PCE and PC@AgNPs was measured by using stable chemical 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) in accordance with Arnao [28]. Briefly, ABTS stock solution was prepared by mixing 7 mM ABTS and 2.45 mM potassium per sulfite in methanol and incubated in a dark at room temperature. The working solution of ABTS was prepared by diluting the ABTS stock solution to 0.7 absorbance at 734 nm by using UV–vis spectrometer. Different concentration of 50 µL of PCE and PC@AgNPs was mixed with 2.95 mL of ABTS working solution. Absorbance was recorded after 30 min of incubation in a dark at 734 nm. Ascorbic and BHT were used as a positive control. All the experiments were carried out in triplicates. The percentage of free radical quenching property was calculated as follows.
2.9 Antibacterial activity of PC@AgNPs
The antibacterial activity was tested by using disc diffusion method according to Ghanwate [29] with minor changes to work out the PC@AgNPs susceptibility. The bacterial cultures were prepared from the overnight culture and 1 × 107 CFU mL−1 cells and inoculated on to nutrient agar, then sterile disc (6 mm) was loaded with 5 µl of different serially diluted PC@AgNPs (1, 2, 3, 4, 5 and 10 mg disc−1). The sterile saline water used as negative control and Streptomycin (10 µg disc−1) as a positive control. The plates were inverted incubated at 37 °C for 24 h to examine the zone of inhibition (ZOI).
2.9.1 Minimum inhibitory concentration by resazurin assay
The resazurin solution was prepared by 270 mg of powder dissolved in 40 mL of sterile water. 100 μL of sterile Baird Parker broth was dispensed in 96 well [30]. 50 μL of different concentration of plant extract (1 to 5 mg mL−1) and PC@AgNPs (10, 20, 40, 60, 80 & 100 µg mL−1) dissolved in DMSO was mixed with media. The tips were discarded and freshly used for each transfer. To each well had a 10 μL of resazurin indicator solution. Finally, 10 μL of bacterial suspension (5 × 106 cfu mL−1) was added to each well to achieve a concentration of 5 × 105 cfu mL−1. A broad-spectrum of antibiotic used as positive control Ciprofloxacin and Tetracycline (1 mg mL−1). A column with all solutions and exception of the test compound (positive control) and a column with all solutions without the addition of bacterial culture (negative control) instead of 10 μL of nutrient broth were added. Each plate was wrapped loosely with cling film to ensure that culture media did not become dehydrated and incubate at 37 °C incubator for 24 h.
2.10 Cell membrane damage study by SEM
The S. aureus and E. coli culture were used as model organisms and the cell membrane damage was studied by treating with minimum inhibitory concentration (MIC) of PC@AgNPs for 2 h. The treated culture was centrifuge at 1000 rpm for 5 min. Finally, cells were smeared on a glass slide with help of 2.5% glutaraldehyde in phosphate buffer solution. The deposited cells treated with 30–100% ethanol in stepwise to dry and SEM images were taken after 2 days [31].
2.11 Nano-toxicity of PC@AgNPs
The Peripheral blood mononuclear cells (PBMC), Human breast adenocarcinoma cells (MCF-7) and Human lung carcinoma cells (A549) cell lines were used to examine the toxical effect of PCE and PC@AgNPs. Prior to starting the experiment all the cell lines were cultured in Dulbecco’s Modified Eagle’s medium (DMEM) in media, supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 1 mM NaHCO3, 100 μg mL−1 streptomycin and 100 units mL−1 penicillin. All the cell cultures were incubated at 37 °C in 5% CO2 incubator.
2.11.1 Cytotoxicity by MTT assay
The cytotoxicity effect of PCE and PC@AgNPs was measured by MTT assay in accordance with Mossaman [32]. Briefly, 100 µL of culture medium was dispensed in 96 well plates and all cell lines were inoculated to the well at 1 × 105 cells mL−1 density. The different concentration of PCE and PC@AgNPs (0–1000 µg mL−1) and 10 μL of MTT (5 mg mL−1) per well were mixed and incubated at 37 °C for additional 3 h at 37 °C in 5% CO2 incubator for 24 h. The intensity of culture solution was measured at 540 nm with the ELISA micro plate reader (Spectra MAX Plus; Molecular Devices; supported by SOFT max PRO-5.4). The percent of cell viability was determined with reference to the control (without test compound). Assay performed in triplicates and repeated thrice.
2.12 Catalytic degradation of methyl orange and methylene blue
The catalytic activity of green syntheses PC@AgNPs was assessed to measure the degradation potency of methyl orange and methylene blue dies by using sodium borohydride [33]. A stock solution (100 ppm) was prepared by dissolve the dye in Millipore water. 10 mL of dye was dispensed in a beaker, and then treated with different concentration of synthesized PC@AgNPs. 1 mL (1 g in 10 mL) of NaBH4 was mixed with dye at room temperature. The dye degradation was measured at λmax 460 and 664 for MO and MB respectively at every 5 min intervals. The percentage of dye degradation was calculated by using the formula.
where A0 = Absorbance of untreated dye, At = Absorbance of treated dye with PC@AgNPs at different interval is time and C = percentage of dye degradation.
3 Result and discussion
3.1 Phytochemical data
The preliminary study of leaves methanolic extract of Priva cordifolia showed the presence of phenolics, flavonoids, steroids and saponins (Supplementary Table 2). Therefore, we have chosen the methanolic extract for the synthesis of PC@AgNPs.
3.2 GC–MS data
The data on GC–MS analysis of the methanolic extract of Priva cordifolia leaves was given in supplementary figures 1 and 2. The 3 predominant peaks were found in the plant extract viz, Decanedioic acid bis (10-ethoxycarbonyldecyl) ester (RT-22.85), Docosahexaenoic acid, 1,2,3-propanetriyl ester (RT-28.83) and beta-Sitosterol (RT-45.85). It clearly indicated the presence of a variety of bioactive compounds with different retention times (RT). The compounds identified by their RT, molecular weight, molecular formulas, structure are illustrated in Supplementary Tables 3 and 4, along with their, summarizes the nature of the identified compounds and their biological activities. Therefore, we opted this extract to increase its therapeutic activity by synthesizing the PC@AgNPs.
3.3 Synthesis and characterization of PC@AgNPs
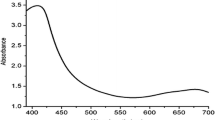
The PC@AgNPs was synthesized by adding plant methanolic extract as reducing agent to 1 mM AgNO3. The development of the nanoparticles was evident by altering the color of the solution from the yellowish color to brown color. This clearly confirmed that the plant extract contains polyphenols, flavonoids, saponins, carotenoids and Phytosterol were responsible for reduced and acting as a capping agent (Supplementary Table 2). The development of colloidal PC@AgNPs was confirmed by UV–Vis spectral pattern. The dispersed PC@AgNPs showed maximum absorption at 415 nm after 10 min of reaction and also retained the stability, shape, point of absorption band even after a month (Fig. 1).
UV–Vis spectra of P. cordifolia leaf silver nanoparticles (PC@AgNPs). Bio-synthesized PC@AgNPs was dissolved in the Millipore water and absorbance readings measured from 300 to 1000 nm wave length ranges. The observed λ max band at 415 nm for PC@AgNPs and symmetry of relative stability after 1 month
3.4 Physicochemical interaction of PC@AgNPs
The biological applications of synthesized nanoparticles depend on size, surface chemistry and silver ions release. Decreases in the stability of nanoparticles are a result of self-aggregation of the materials and this reduction finally affects overall function. Due to the reduction in the stability, biological properties such as cellular uptake, bioavailability. Therefore, it is very essential to characterize the influence of factors such as pH, ionic strength and temperature on nanoparticles.
The pH effect on PC@AgNPs stability was investigated with different pH ranges from 2 to 10. The data indicated that PC@AgNPs was most stable with high pH at 10 an evidenced by the absorbance at 415 nm (Fig. 2a). The ionic strength of PC@AgNPs was evaluated by dissolved in PBS with 0.2–1 M NaCl and absorbance was measured in UV–Vis spectra. PC@AgNPs has a characteristic peak at 415 nm. The data indicated PC@AgNPs was stable up to 0.2 M and beyond this concentration broaden the peak (Fig. 2b). Finally, effect of temperature was investigated in the temperature range of 30–90 °C. PC@AgNPs was stable in the temperature range of 35–75 °C (Fig. 2c) [26].
3.5 DPPH radical scavenging activity of analysis of PC@AgNPs
The potent antioxidant property of PCE and PC@AgNPs were estimated by DPPH radical scavenging activity. The dose dependent DPPH activity of PC@AgNPs were observed and showed DPPH radical (purple) into neutralized form (yellow) which is statistically significant (P ≤ 0.05) represented in Fig. 3a. The IC50 values of PCE and PC@AgNPs are 49.02 ± 0.24 and 43.63 ± 0.18 μg mL−1 respectively compared to standard ascorbic acid and BHT (IC50 value is 5.02 ± 0.08 and 27.90 ± 0.10 μg mL−1). With increasing concentration of PC@AgNPs change in color was observed due to scavenging of DPPH radical by donating a hydrogen atom to neutralize the reactive DPPH molecule. This shows a responsible decrease in absorbance at 517 nm and it is attributed due to the functional groups of PC adhered to NPs present in the PC@AgNPs. The data indicated the scavenging activity was increased to 5.39 ± 0.08 μg mL−1 after the silver was reduced by the plant extract.
DPPH and ABTS radical scavenging activity of PCE and PC@AgNPs. a The data represented dose dependent DPPH radical scavenging activity with IC50 value of Vit-C, BHT, PCE and PC@AgNPs at the concentration of 5.02 ± 0.08, 27.9 ± 0.10, 49.02 ± 0.24 and 43.63 ± 0.18 μg mL−1 respectively. b Indicated ABTS radical scavenging activity with IC50 value of Vit-C, BHT, PCE and PC@AgNPs at the concentration of 5.3 ± 0.08, 7.37 ± 0.06, 114.40 ± 0.60 and 77.04 ± 0.32 μg mL−1 respectively
The free radicle quenching of PCE and PC@AgNPs were estimated by another stable chemical ABTS assay. The dose dependent ABTS activity of PC@AgNPs were witnessed and showed ABTS radical (blue) into neutralized form (colorless) which is statistically significant (P ≤ 0.05) represented in Fig. 3b. The IC50 values of PCE and PC@AgNPs are 114.40 ± 0.80 and 74.29 ± 0.30 μg mL−1 respectively compared to standard ascorbic acid and BHT (IC50 value is 5.3 ± 0.04 and 7.37 ± 0.08 μg mL−1).
3.6 Zone of inhibition (ZOI) by PC@AgNPs against pathogenic bacteria
Currently, emergence of drug resistant microbes is a serious worldwide health issue. The PC@AgNPs shown significant inhibition zone against bacteria compared to the standard antibiotic (Fig. 4, Table 1; Supplementary Table 5 and Fig. 3).
Antibacterial activity of PC@AgNPs against pathogenic bacteria. The disc diffusion assay was done to measure the MIC values of PC@AgNPs against disease causing pathogens. a Typical plate displaying concentration of PC@AgNPs correspondingly added in tested plates (N: Negative control; Positive control: PC@AgNPs at 1 (1); 2 (2); 3 (3); 4 (4); 5 (5) and 10 (6) mg disc−1). b S. aureus; c B. cereus; d E. faecalis, e E. aerogens; f E. coli; g P. aeruginosa; h S. typhimurium; i S. flexneri; j V. cholera and k V. parahaemolyticus
The high antibacterial activity showed at a concentration of 10 mg mL−1 against Gram-positive S. aureus, B. cereus, E. faecalis and Gram-negative E. coli, E. aerogenes, S. typhimurium, V. cholera, P. aeruginosa, S. flexneri, and V. parahaemolyticus. The PC@AgNPs having strong ZOI for S. aureus (9.38 ± 0.06), E. coli (9.20 ± 0.09), S. flexneri (9.60 ± 0.11) and V. parahaemolyticus (9.10 ± 0.13) mm clearance zone observed after overnight incubation. While no ZOI was observed for the negative control (sterile distilled water) in any case. In the resazurin assay, indicated the increase of MIC concentration values after synthesis of PC@AgNPs against commonly encounter food pathogens compared to crude extract (Table 2). The antibacterial activity was dependent on particle size; shape and importantly capping agent loaded verify the important role in the bactericidal activity. The bacterial cell wall composed of thick peptidoglycan consisting of a similar type of linear polysaccharide chains cross-linked by short peptides formed into the rigid structure [35]. The plants are rich source of antioxidants containing different phytochemical involved in antioxidant activity by neutralizing reactive oxygen species or free radicals, as anti-microbials, as anti-helminthic, as anti-inflammatory, anti-diabetic, and as anti-thrombus etc. [36,37,38]. The high bactericidal property of cationic Ag+ ions leading to damage the bacteria intern stops the growth of the microorganisms [36]. The possible antibacterial mechanism of metal toxicity is due to protein dysfunction, production of reactive oxygen species (ROS), depletion of antioxidants, impaired membrane function, interfere with nutrient assimilation and also be gene-toxicity. The coating of Ag+ ions widely used in food industries medical and pharmaceutical industries [34]. The increasing of synthetic antibiotics and its improper use without knowledge, bacteria resistant and cause minor to major health issues. This may lead to cause severe life threatening diseases worldwide. There is a need to anticipate and develop potent antimicrobial agent against multidrug resistant microbes. The present study highlights the loading of active bactericidal PCE to synthesize PC@AgNPs and play a potent role in inhibiting the growth of both Gram-positive and Gram-negative microorganisms.
3.7 Bacterial cell damage
The bacterial cell damage was studied by SEM of E. coli and S. aureus cells treated with PC@AgNPs are presented in the Fig. 5.
The SEM images showing cell membrane damage. The after treatment of nanoparticles with S. aureus and E. coli overnight of incubation exhibited the alterations in the membranes of bacteria (a, b) respectively, compared to the control (without treatment). The alteration in the membrane was showed by arrow indications
The electron microscopy determined the distribution and location of the silver nanoparticles, as well as the morphology of the bacteria after treatment with silver nanoparticles. In the controlled untreated sample, the surface of bacterial cells was smooth and showed typical characters of surface of native cells, such as smooth and intact, while cells treated with NPs appeared damaged severely. Some cells showed large leakage, others misshapen many pits and gaps appeared in their membrane [25].
3.8 Cytotoxity analysis
The synthesized PC@AgNPs demonstrates no toxic effect to the non-cancerous peripheral blood mononuclear (PBMC) cells up to 500 µg mL−1 (Fig. 6a). When PCE (Fig. 6a) treatment to PBMC cells in a dose dependent manner (20–1000 µg mL−1) induced to kill PBMC cells at 300 µg mL−1 concentration itself and started to prevent growth of lung cancer (A549) and breast cancer (MCF-7) cells at concentrations of 300 and 500 µg mL−1 respectively. Thus, a free active component of PCE was toxic to both cancerous and non-cancerous cells at higher doses. On the other hand, PC@AgNPs showed toxicity at 500, 500 and 300 µg mL−1 against PBMC, A549, and MCF-7 cells respectively (Fig. 6b), indicating silver NPs with PCE active synergistic components increased the bioavailability at lower doses apart from increasing its therapeutic efficacy.
Cytotoxicity analysis of PC@AgNPs. The normal cell PBMC, cancerous cells (A549 and MCF-7) were treated different concentrations (0–1000 µg mL−1) of PE (a) and PC@AgNPs (b) for 24 h and tested for cell viability using MTT assay. The PE indicated less toxic to PBMC up to 300 µg mL−1 and showed anti-cancer activity to A549 (300 µg mL−1) and MCF-7 (500 µg mL−1) concentration onwards. PC@AgNPs indicated toxic above 500 µg mL−1 concentrations onwards to PBMC and significantly responds to cancerous A549 (500 µg mL−1) and MCF-7 (400 µg mL−1)
3.9 Catalytic effect of PC@AgNPs
3.9.1 Degradation of methyl orange and methylene blue
We investigated the catalytic activity of PC@AgNPs against cationic (MB) and anionic (MO) dyes routinely used in textile, paper, printing, pharmaceutical, food industries and research laboratories. The release of residual of untreated dye through effluent discharge and it cause potent pollution to the environment and toxic to aquatic animals. Methylene blue and methyl orange dye degradation were studied by using synthesized PC@AgNPs and monitoring the degradation capacity at every 5 min intervals. The absorbance peak of methylene blue dye in water was recorded at 664 nm and 460 nm for methylene orange. In the presence of PC@AgNPs as a catalyst, absorbance spectra of MO and MB were decreased with a varying interval of time and also the color of the dye was disappeared at higher concentration (Figs. 7, 8). 250 µg of PC@AgNPs amount is adequate to reduce the 97.50% of MO dye in 15 min time intervals and 2 mg for MB (83.55%) (Table 3). The result has shown the synthesized PC@AgNPs work more potent in the degradation of anionic dye compare to the cationic dye and excellent catalyst on the reduction of hazardous dyes [33, 39].
4 Conclusion
Of the various known methods to generate nano-particles, the ‘green’ approaches using P. cordifolia leaf extract proved simple, but adapt to produce a functionally competent end product. The leaf extract apparently possessed appropriate reducing components to change and link silver to the right herbal factors leading to improved antibacterial properties and also possess other favorable attributes indicative of its potential for therapeutic applications, and also use in the process of degradation of hazardous dyes in an economically and ecofriendly. The present observations have provided an impetus for further detailed studies to realize, in practical terms, the said potential. However, understanding the toxicity and fate of AgNPs in ecosystems requires further investigation.
References
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32(1):79
Ananda AP, Manukumar HM, Umesha S et al (2017) A relook at food packaging for cost effective by incorporation of novel technologies. J Package Technol Res 1:67. https://doi.org/10.1007/s41783-017-0011-4
Ponarulselvam S, Panneerselvam C, Murugan K, Aarthi N, Kalimuthu K, Thangamani S (2012) Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed 2(7):574–580
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32(1):79
Rajasekharreddy P, Rani PU, Sreedhar B (2010) Qualitative assessment of silver and gold nanoparticle synthesis in various plants: a photobiological approach. J Nanopart Res 12(5):1711–1721
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84(2):151–157
Nair B, Pradeep T (2002) Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst Growth Des 2:293–298
Konishi Y, Uruga T (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653
Willner I, Baron R, Willner B (2006) Growing metal nanoparticles by enzymes. J Adv Mater 18:1109–1120
Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paraliker KM, Balasubramanya RH (2007) Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett 61:1413–1418
Shankar SS, Ahmed A, Akkamwar B, Sastry M, Rai A, Singh A (2004) Biological synthesis of triangular gold nanoprism. Nature 3:482
Jae YS, Beom SK (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84
Ahamed M, Khan MM, Siddiqui MKJ, Al Salhi MS, Alrokayan SA (2011) Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Physica E 43(6):1266–1271
Manukumar HM, Umesha S, Kumar HN (2017) Promising biocidal activity of thymol loaded chitosan silver nanoparticles (T-C@AgNPs) as anti-infective agents against perilous pathogens. Int J Biol Macromol 102:1257–1265
Manukumar HM, Chandrasekhar B, Rakesh KP, Ananda AP, Nandhini M, Lalitha P, Sumathi S, Qin H-L, Umesha S (2017) Novel TC@ AgNPs mediated biocidal mechanism against biofilm associated methicillin-resistant Staphylococcus aureus (Bap-MRSA) 090, cytotoxicity and its molecular docking studies. MedChemComm 8(12):2181–2194
Karthik CS, Manukumar HM, Ananda AP, Nagashree S, Rakesh KP, Mallesha L, Qin H-L, Umesha S, Mallu P, Krishnamurthy NB (2018) “Synthesis of novel benzodioxane midst piperazine moiety decorated chitosan silver nanoparticle against biohazard pathogens and as potential anti-inflammatory candidate: a molecular docking studies. Int J Biol Macromol 108:489–502
Nazem A, Mansoori GA (2008) Nanotechnology solutions for Alzheimer’s disease: advances in research tools, diagnostic methods and therapeutic agents. J Alzheimers Dis 13(2):199–223
Moure A, Cruz JM, Franco D, Domı́nguez JM, Sineiro J, Domı́nguez H et al (2001) Natural antioxidants from residual sources. Food Chem 72(2):145–171
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49(11):5165–5170
Jiang H, Manolache S, Wong ACL, Denes FS (2004) Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J Appl Polym Sci 93(3):1411–1422
Durán N, Marcato PD, Alves OL, De Souza GI, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3(1):8
Ayyanar M, Ignacimuthu S (2009) Herbal medicines for wound healing among tribal people in Southern India: ethnobotanical and scientific evidences. Int J Appl Res Nat Prod 2(3):29–42
Lawrence RAR, Raj TLS, Selvi AA (2017) Green synthesis and characterization of silver nanoparticles of leaf extracts of Priva cordifolia (LF) druce. Int J Ecotoxicol Ecobiol 2(1):52–55
Ananda AP, Manukumar HM, Krishnamurthy NB, Nagendra BS, Savitha KR (2019) Assessment of antibacterial efficacy of a biocompatible nanoparticle PC@ AgNPs against Staphylococcus aureus. Microb Pathog 126:27–39
Harborne JB (1998) Photochemical methods: a guide to modern techniques of plant analysis, 2nd edn. Chapman A. & Hall, London, pp 4–84
Goswami SR, Sahareen T, Singh M, Kumar S (2015) Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J Ind Eng Chem 26:73–80
Phull AR, Abbas Q, Ali A, Raza H, Zia M, Haq IU (2016) Antioxidant, cytotoxic and antimicrobial activities of green synthesized silver nanoparticles from crude extract of Bergenia ciliata. Future J Pharm Sci 2(1):31–36
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73:239–244
Ghanwate N, Thakare P, Bhise PR, Gawande S (2016) Colorimetric method for rapid detection of Oxacillin resistance in Staphylococcus aureus and its comparison with PCR for mec A gene. Sci Rep 6:23013
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42(4):321–324
Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS (2010) Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicrob Agents Chemother 54(8):3132–3142
Mossman BT, Marsh JP (1989) Evidence supporting a role for active oxygen species in asbestos-induced toxicity and lung disease. Environ Health Perspect 81:91
Jyoti K, Singh A (2016) Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J Genet Eng Biotechnol 14(2):311–317
Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M (2016) Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci 6(5):755–766
Chaloupka K, Malam Y, Seifalian AM (2010) Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol 28(11):580–588
Ananda AP, Nagendra BS, Krishnakantha TP, Joseph R (2012) Enhancement of antioxidant profile of Japanese cherry (Muntingia calabura Linn.) by alcoholic fermentation. Int J Pharm Life Sci 3(6):1743–1751
Manukumar HM, Prathima VR, Sowmya, Siddhagangaia, Thribhuvan KR (2013) Study of nutritional quality, phytochemical constituents and antioxidant activities by different solvents of nettle (Urtica urens) from Madikeri-Karnataka state. Int Res J Pharm Appl Sci 3(5):112–119
Manukumar HM, Ananda AP, Deepa V, Siddhagangaia BS (2013) Study of physicochemical parameters and antioxidant in honey collected from different locations of India. Int J Pharm Life Sci 4(12):3159–3165
Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M, Kumar ST, Rao MV (2015) Catalytic degradation of organic dyes using synthesized silver nanoparticles: a green approach. J Bioremediat Biodegrad 6(5):1
Acknowledgements
The author A. P. Ananda, thank to Mr. A. R. Subramanya, CEO of Ganesh Consultancy and Analytical Services, Mysore for providing laboratory facility.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ananda, A.P., Krishnamurthy, N.B., Savitha, K.R. et al. Biogenic synthesis of silver nanoparticles using Priva cordifolia leaf extract (PC@AgNPs) a potent antioxidant, antibacterial and catalytic activity. SN Appl. Sci. 1, 800 (2019). https://doi.org/10.1007/s42452-019-0818-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0818-4