Abstract
Natural synthesis of metal nanoparticles is gaining more attention in recent years. This article demonstrates the phytochemical synthesis of silver nanoparticles (AgNPs) by using Indoneesiella echioides (L) leaf extract as a reducing and stabilizing agent. Biosynthesis of AgNPs was monitored by UV–visible spectroscopy which revealed intense surface plasmon resonance bands at 420 nm. Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction were employed to identify various functional groups and crystalline nature of AgNPs. High-resolution transmission electron microscopy studies demonstrated that synthesized particles were spherical with average size of ~29 nm. In vitro antioxidant effects were analyzed by 2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH), which exhibited 69 and 71 % of scavenging activity, respectively. The antimicrobial activity of green AgNPs displayed better zone of inhibition against selected human pathogens. The present study also investigated the toxicity effect of biogenic AgNPs against human lung adenocarcinoma cancer cells (A549) and normal human epithelial cells (HBL-100) in vitro, and the inhibitory concentrations (IC50) were found to be 30 and 60 µg/mL, respectively. Herein, we propose a previously unexplored medicinal plant for the biological synthesis of AgNPs with potent biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent times, stupendous efforts have been taken for the development of efficient methodology for the synthesis of metal nanoparticles with unique physicochemical and optoelectronic properties and their important applications in optics, electronics, biomedicine, magnetic, mechanics, catalysis, energy science, and so on (Dua and Jiang 2007). The synthesis of monodispersed nanoparticles with different sizes and shapes has been a great challenge in nanotechnology. Although various physical and chemical methods are extensively used to produce monodispersed nanoparticles, the stability and the use of toxic chemicals are the subjects of paramount concern (Rao and Cheetham 2001). The secrets discovered from nature have led to the development of biomimetic approaches to the growth of advanced nanomaterials (Singaravelu et al. 2007).
Green-assisted synthesis of nanoparticles using plant materials are effortless, capable and eco-friendly in comparison with chemical-mediated or microbe-mediated synthesis (Anamika et al. 2012). In combination with metallic nanoparticles such as copper, zinc and silver are most promising because of their biomedical properties. However, the mechanism and mode of action are still a matter of debate, due to their superior antimicrobial properties against bacteria, viruses and fungi comparing with standard antibiotics (Rai et al. 2009; Weir et al. 2008). Green-prepared silver nanoparticles (SNPs) have the physical properties of a larger specific surface area, smaller in size and high dispersion (Sharma et al. 2009). We have selected Indoneesiella echioides, an herbal plant widely scattered in the dry places of stifling India and Sri Lanka (Gamble 1956). In Indian traditional medicine, the leaf extract of I. echioides was used as a remedy for fever (Kirtikar and Basu 1975) and also the plant from genus Andrographis is used to treat goiter, liver diseases (Nadkarni and Nadkarni 1976), fertility disorders, bacterial (Qadrie et al. 2009), malarial and fungal diseases. These phytochemically synthesized SNPs have a potential effect such as good nanostructure (Huh and Kwon 2011) and antibacterial (Savithramma et al. 2011), antioxidant (Swamy et al. 2014) and anticancer activity (Vasanth et al. 2014).
This present investigation deals with the biosynthesis of silver nanoparticles using I. echioides leaf extract as a reducing and stabilizing agent; antioxidant activity of biosynthesized AgNPs was evaluated for ABT and DPPH assays and also antibacterial activity against gram-positive (Rhodococcus rhodochrous), gram-negative (Aeromonas hydrophila, Staphylococcus aureus, Pseudomonas aeruginosa) bacterial pathogens and fungal pathogen (Candida albicans). Furthermore, the anticancer activity of synthesized silver nanoparticles was examined in human lung adenocarcinoma cancer cell line (A549) and normal human epithelial cells (HBL-100).
Materials and methods
Preparation of plant extract
The healthy, matured and disease-free leaves of I. echioides were collected (Kolli Hills, Namakkal District, Tamil Nadu, India) and rinsed thoroughly in deionized water. Then, the cleaned leaves were sterilized using 0.02 % mercuric chloride. The sterilized leaves were then dried and finely powdered. The extract was prepared by mixing 2.5 g of the powdered leaf in 100 mL of deionized water, and this mixture was boiled at 80 °C for 5 min before decanting.
Synthesis of Ag nanoparticles
For the synthesis of silver nanoparticles, 5 mL of I. echioides leaf extract was added dropwise into 45 mL of 1 mM silver nitrate (HiMedia) with constant stirring. As soon as I. echioides extract was mixed in an aqueous solution of silver ions, the reaction mixture turned from whitish to yellowish brown color. Finally, the samples were centrifuged at 6000 rpm for 20 min, and pellet was lyophilized, and nanoparticles were stored at 4 °C.
Characterization of silver nanoparticles
UV–Vis spectra and FTIR analysis
The bioreduction of Ag+ ions in solution was monitored using UV–visible spectroscopy (Synergy HT Multi-mode Microplate Reader, Bio-Tek Instruments, Inc., Winooski, VT, USA). Further, Fourier transform infrared spectroscopy (FTIR) measurements were taken using JASCO (FT/IR-6200) spectrophotometer to identify the functional groups in the dried form of SNPs and the plant leaf powder.
XRD measurement
After bioreduction, the residual solutions consisting of hydrosols and biomass were dried at 60 °C, and the dried mixture was collected for the determination of the formation of Ag by an X’Pert Pro X-ray diffractometer (PANalytical BV, The Netherlands) operated at a voltage of 40 kV and a current of 30 mA with Cu Kα radiation. The 2θ values were calculated by using the Debye–Scherrer’s formula, D = 0.9 λ/β cos θ, where D is the mean diameter of the nanoparticles, λ is the wavelength of X-ray radiation source, and β is the angular FWHM of the XRD peak at the diffraction angle θ.
High-resolution transmission electron microscopy (HR-TEM) analysis
Surface morphology and size of silver nanoparticles were determined by HR-TEM. A sample of the aqueous suspension of silver nanoparticles was prepared by placing a drop of the suspension on carbon-coated copper grids and allowing water to evaporate. TEM observations were performed on electron microscope (TechnaiG2 analyzer). Size distribution of the resulting nanoparticles was estimated on the basis of TEM micrographs. High-resolution TEM images were obtained, and crystalline structure was confirmed with the help of SAED pattern.
In vitro antioxidant assay
ABTS scavenging assay
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) scavenging activity was determined by modified method described by Rameshkumar and Sivasudha (2012). The working solution was prepared by mixing two stock solutions of 7 mM ABTS solution and 2.4 mM potassium persulfate solution in equal amounts and allowed to react for 12 h at room temperature in the dark condition. One milliliter of the resulting solutions was allowed to react with 1 mL of the aqueous nanoparticles in different concentration ranging from 10 to 50 μg/mL, and the reaction mixture was vortexed, and absorbance was measured at 734 nm after 6 min interval. Similar procedures were carried out for the butylated hydroxytoluene (BHT) standard at various concentrations. The percentage of inhibition capacity of ABTS by the silver nanoparticles was calculated from the following equation;
where A o control absorbance and A t sample absorbance.
DPPH scavenging assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was carried out using modified method described by Rameshkumar and Sivasudha (2012). One milliliter of 0.135 mM DPPH prepared in methanolic was mixed with 1 mL of aqueous nanoparticles with various concentrations ranging from 10 to 50 μg/mL. The reaction mixture was vortexed thoroughly and left in dark at room temperature for 30 min. The percentage of inhibition capacity of DPPH by the silver nanoparticles was calculated from Eq. 1.
Agar diffusion assay
Microbial cultures were grown overnight in nutrient broth medium and centrifuged at 5000 rpm for 2 min. Then, the pellet was washed with 1× PBS and resuspended in fresh nutrient broth medium and allowed to grow for 6 h. The 100 μL of the suspended cultures (gram-positive Rhodococcus rhodochrous (MTCC 265), gram-negative bacterial pathogens Aeromonas hydrophila (MTCC 1739), Staphylococcus aureus (MTCC 2940), Pseudomonas aeruginosa (MTCC 2453) and fungal pathogen Candida albicans (MTCC 227)) was spread uniformly on nutrient agar plates, and the plates were incubated at 37 °C for 30 min. The AgNPs at different concentrations (100, 150 and 200 μg/mL) were loaded into the wells. The zone of inhibition was determined by measuring the diameter (mm) of bacterial clearance after 24 h.
Assessment of in vitro cytotoxicity
The cancer cell line A549 and normal human epithelial cells (HBL-100) were purchased from the National Center for Cell Sciences (NCCS, Pune, India). The cells were maintained in Dulbecco’s modified eagles medium (DMEM) and McCoy’s 5a medium, respectively, in supplement with non-essential amino acids. The cells were maintained at 37 °C with 5 % CO2 in a humidified CO2 incubator. The cells were seeded (1 × 104 cells/well) in a 96-well plate and incubated for 48 h into 75 % confluence. The medium was replaced with fresh medium containing serially diluted AgNPs, and the cells were further incubated for 48 h. The culture medium was removed, and 100 µL of the MTT [3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl tetrazolium bromide] (HiMedia) solution was added to each well and incubated at 37 °C for 4 h. Purple color formazan crystals were observed, and these crystals were dissolved with 100 µL of dimethyl sulfoxide (DMSO) and read at 620 nm in a multi-well ELISA plate reader (Thermo, Multiskan, USA). Optical density (OD) value was subjected to sort out percentage of viability by using the following formula
Observation of cytomorphological alterations
The A549 and HBL-100 cells that were grown on cover slips (1 × 105 cells/cover slip) were incubated with biogenic AgNPs at the IC50 concentration and then fixed in methanol:acetic acid solution (3:1, v/v). The cover slips were gently mounted on glass slides for the morphometric analysis. The morphological changes in AgNPs-treated A549 and HBL-100 cells were analyzed using Nikon (Japan) bright field inverted light microscopy at 400 × magnification.
Result and discussion
Characterization of silver nanoparticles
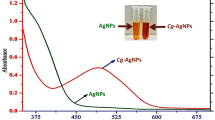
Synthesized silver nanoparticles were initially identified by the color change in the reaction from yellow to brown color. Further confirmation was done by UV–visible spectroscopy (Fig. 1). UV–visible spectroscopy has shown the absorption peak at 420 nm. An UV–visible spectrum is one of the important techniques to verify the formation of metal nanoparticles provided surface plasmon resonance exists for that metal. It is known from the spectra that the silver surface plasmon resonance band occurs between 410 and 420 nm (Maliszewska et al. 2009; Ashok et al. 2010; Kaviya et al. 2011; Kanipandian and Thirumurugan 2014).
The powdered form of prepared silver nanoparticles was used for FTIR analysis to identify the presence of functional groups which are responsible for reduction of AgNPs from plant leaf. The intensity peak values ranging from ~3420.94, 2270.25, 1709.83, 1160.21, 1369, 1227.19, 1071.53 and 540.82 cm−1 were identified. Figure 2 and Table 1 represent the FTIR spectra and corresponding functional groups of synthesized AgNPs from the I. echioides plant leaf extract. The following functional groups were identified such as aromatic primary amine (C–N stretch), secondary amine N–H bend, organic nitrates, carbonate ions and aliphatic fluoro compounds C–F stretch. The IR bands at ~1709.83 and 1369 cm−1 are characteristic of C–O and C–O stretching modes, respectively, of the carboxylic group (Kathiraven et al. 2015). The strong band at ~1037 cm−1 arises from C–O–C and C–OH vibrations (Solomun et al. 2004; Ankamwar et al. 2005; Huang et al. 2007; Li et al. 2007; Kannan and Abraham John 2008). Earlier studies revealed that the absorbance bands at ~1109 and 1726 cm−1 in curve were associated with the stretch vibration of –C–O and –C=C respectively (Zhu 2000). Hence, it is possible that proteins/enzymes play a vital role in reduction of metal ions by the oxidation of aldehydes to carboxylic acid. Amide II band is observed at ~1538 cm−1, and amide I band got merged in the broad envelope around ~1743 cm−1 (Solomun et al. 2004).
XRD analysis
The XRD analysis (Fig. 3) shows that the diffraction peaks at 2θ are 38.10°, 44.22°, 64.52° and 77.36° and can be assigned to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of a face-centered cubic (fcc) structure of silver nanoparticles compared with JCPDS card no: 65-2871 (Yugandhar and Savithramma 2015). XRD patterns were analyzed to determine peak intensity, position and full width at half maximum (FWHM) data were used with the Debye–Scherrer’s formula to determine the crystallite size, and the estimated crystallite size was 19 nm. Further, the silver nanoparticles were crystalline nature, and the current XRD data were well correlated with the existing literature (Ashok et al. 2010; Shashi Prabha et al. 2010; Dwivedi and Gopal 2010).
HR-TEM analysis and SAED pattern
HR-TEM was employed to analyze the morphology and size of biosynthesized AgNPs. TEM studies demonstrated that synthesized particles were spherical with average size of ~29 nm (Fig. 4). Figure 5 shows that the selected-area electron diffraction (SAED) pattern of green-synthesized AgNPs confirms the crystalline (fcc) nature of the silver nanoparticles (Rajkumar et al. 2015).
In vitro antioxidant assay
Figure 6 displays the antioxidant image of the green-synthesized AgNPs. The ABTS assay was used to detect the antioxidant property of AgNPs depicted in Fig. 6a. The highest percentage of inhibition was found to be 71 % at 1000 µg/mL concentration of AgNPs, and BHT (standard) exhibited 81 % of scavenging ability at the same concentration.
DPPH assay was used to determine the high antioxidant potential of AgNPs and is shown in Fig. 6 (b). The highest percentage of inhibition was found to be 69 %, and BHT (control) displayed 81 % at 1000 µg/mL.
The involvement of active oxygen and free radicals in the pathogenesis of certain human diseases, including cancer, aging and atherosclerosis, is increasingly being recognized (Moskovitz et al. 2002). Active oxygen and free radicals, such as superoxide anion (O2), hydrogen peroxide (H2O2) and hydroxyl radical (OH), are constantly formed in the human body by normal metabolic action. Their action is opposed by a balanced system of antioxidant defenses, including antioxidant compounds and enzymes. Upsetting the radical balance causes oxidative stress, which can lead to cell injury and death (Halliwell and Gutteridge 1999). Therefore, much attention has been focused on the use of antioxidants, especially natural antioxidants, to inhibit or to protect against the damage of free radicals (Vendemiale et al. 1999). This investigation provides evidence for the uses of biosynthesized silver nanoparticles as natural antioxidant or potential radical scavenger against deleterious damages caused by the free radicals.
Antimicrobial activity of silver nanoparticles
The well diffusion method was used to evaluate the antimicrobial activity of silver nanoparticles against the pathogens such as gram-positive bacteria Rhodococcus rhodochrous (MTCC 265), gram-negative bacterial pathogens Aeromonas hydrophila (MTCC 1739), Staphylococcus aureus (MTCC 2940), Pseudomonas aeruginosa (MTCC 2453) and fungal pathogen Candida albicans (MTCC 227). The zone of inhibition tests were conducted for three different concentrations (100, 150 and 200 µg/mL) of Ag nanoparticles. The potent antimicrobial nature of the silver nanoparticles was confirmed by the zone of inhibition around the wells. Figure 7 and Table 2 indicate the diameter of zone of inhibitions formed around the colonies of microorganisms.
Previous studies have revealed that ionic silver has the property to strongly interact with thiol groups of vital enzymes and inactivate the bacterial cells. Experimental evidence has shown that DNA loses its replication ability once the bacteria have been treated with silver ions (Jose Ruben et al. 2005). Most importantly, silver attacks a broad range of targets in the microbes, so it is difficult for them to develop resistance against silver, because this would require developing a host of mutations to protect themselves (Sharvil et al. 2009). Our findings proved the capability of silver nanoparticles to inhibit the pathogens, and it suggests that AgNPs can be used for antimicrobial applications.
Anticancer efficacy of biogenic AgNPs
Silver nanoparticles are gaining more attention from research community and also from the biomedical industry for their potential biomedical applications. At the same time, only limited studies were conducted to evaluate the anticancer effects of biologically synthesized AgNPs, against cancer cell lines. In this study, an in vitro cytotoxicity assay for AgNPs on A549 and HBL-100 cells was conducted using an MTT reduction assay (Fig. 8). After the treatment of cells with different concentrations (10–100 µg/mL) of AgNPs, the inhibitory percentage against proliferation of cells was determined. The cytotoxicity effect on A549 cell lines was increased with increased concentration of AgNPs. In the present study, dose-dependent cytotoxicity was observed in AgNPs-treated A549 cells, and fifty percentage of cancer cell death (IC50) was found to be at the silver nanoparticle concentration of 30 µg/mL. The HBL-100 cells failed to show cytotoxic effect at lower concentrations, and cytotoxicity increases with increasing concentration of AgNPs by 60 µg/mL. It is evident from these results that when compared to the cancer cells IC50 concentration (30 µg/mL), control/normal cells used for this study required double the volume (60 µg/mL), i.e., silver nanoparticles at a concentration of 30 µg/mL were non-toxic to control/normal cells. Our present findings provide the evidence for cytotoxic effect of biogenic AgNPs from extract of I. echioides against lung cancer A549 cell line compared with HBL-100 normal cell lines.
Morphological analysis of AgNPs-treated cells
The cancer cells and normal cells grown on glass cover slips were incubated with biosynthesized AgNPs. After the treatment with AgNPs, some morphological alterations were observed during the microscopic examination. The changes in treated cancer cells were compared with treated normal cells. The morphological changes such as cell shrinkage, retardation of cell growth, cytoplasmic condensation and membrane blebbing were detected in the cancer cells (Fig. 9). The similar results were observed in the earlier report (Kanipandian et al. 2014). However, no such effects were seen in HBL-100 cells, and it was shown with normal morphology (image not given).
Conclusion
The biological route of the synthesizing nanoparticles was known to be the eco-friendly, simple and cost-effective. In this study, the silver nanoparticles were successfully synthesized using the plant leaf extract of Indoneesiella echioides and were characterized by UV–visible spectroscopy, FTIR, XRD, HR-TEM and SAED pattern. The presence of the spherical silver nanoparticles with average size of ~29 nm was synthesized. The present study also confirms the antioxidant activity of biosynthesized silver nanoparticles, which can be effectively used as the drug to eradicate the free radicals to prevent the cellular injury. Due to their high antimicrobial activity, the silver nanoparticles can also be used in the antimicrobial applications. The present study also dealt with the anticancer potential of AgNPs against human lung adenocarcinoma cancer cell line (A549) and normal human epithelial cells (HBL-100), and further study on silver nanoparticles will be carried out for drug delivery, food and pharmaceutical applications.
References
Anamika M, Sanjukta C, Prashant MR, Geeta W (2012) Evidence based green synthesis of nanoparticles. Adv Mater Lett 3:519–525
Ankamwar B, Chaudhary M, Sastry M (2005) Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorg Met 35:19–26
Ashok B, Bhagyashree J, Ameeta R, Kumara B, Smita Z (2010) Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf A Physicochem Eng Asp 368:58–63
Dua B, Jiang H (2007) Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5a and its application on direct electrochemistry of hemoglobin. Electrochem Commun 9:1165–1170
Dwivedi AD, Gopal K (2010) Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A Physicochem Eng Asp 369:27–33
Gamble JS (1956) Flora of the presidency of Madras. Botanical survey of India, Calcutta
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, Wang H, Wang Y, Shao W, He N, Hong J, Chen C (2007) Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 18:105104–105111
Huh AJ, Kwon YJ (2011) Nanoantibiotics: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156(2):128–145
Jose Ruben M, Jose Luis E, Alejandra C, Katherine H, Juan BK, Jose Tapia R, Miguel Jose Y (2005) The bactericidal effect of silver nanoparticles. J Nanotechnol 16:2346–2353
Kanipandian N, Thirumurugan R (2014) A feasible approach to phyto-mediated synthesis of silver nanoparticles using industrial crop Gossypium hirsutum (cotton) extract as stabilizing agent and assessment of its in vitro biomedical potential. Ind Crops Prod 55:1–10
Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R (2014) Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull 49:494–502
Kannan P, Abraham John S (2008) Synthesis of mercaptothiadiazole functionalized gold nanoparticles and their self-assembly on Au substrates. Nanotechnology 19(8):085602
Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T (2015) Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci 5:499–504
Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K (2011) Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A 79:594–598
Kirtikar KR, Basu BD (1975) In Indian medicinal plants-3. Periodical Experts, New Delhi
Li S, Shen Y, Xie A, Yu X, Zhang X, Yang L, Li C (2007) Rapid, room-temperature synthesis of amorphous selenium/protein composites using Capsicum annuum L. extract. Nanotechnology 18:405101
Maliszewska I, Sadowski Z, Waszak K (2009) Biological synthesis of silver nanoparticles. J Phys Conf Ser 146:012–024
Moskovitz J, Yim MB, Chock PB (2002) Free radicals and disease. Arch Biochem Biophys 397:354–359
Nadkarni AK, Nadkarni KM (1976) Indian materia medica, vol 1. Popular Prakashan, Bombay
Qadrie ZL, Jacob B, Anandan R, Raj Kapoor B, Rahamathulla M (2009) Antibacterial activity of ethanol extract of Indoneesiella echioides. Pak J Pharm Sci 22:123–125
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antibacterials. Biotechnol Adv 27:76–83
Rajkumar KS, Kanipandian N, Thirumurugan R (2015) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci. doi:10.1007/s13204-015-0417-7
Rameshkumar A, Sivasudha T (2012) In vitro antioxidant and antimicrobial activity of aqueous and methanolic extract of Mollugo nudicaulis Lam. Leaves. Asian Pac J Trop Biomed 2(2):S895–S900
Rao CNR, Cheetham AK (2001) Science and technology of nanomaterials: current status and future prospects. J Mater Chem 11:2887–2894
Savithramma N, Rao ML, Devi PS (2011) Evaluation of antibacterial efficacy of biologically synthesized silver nanoparticles using stem barks of Boswellia ovalifoliolata Bal. and Henry and Shorea tumbuggaia Roxb. J Biol Sci 11:39–45
Sharma VK, Yngard RA, Lin Y (2009) Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloids Interf Sci 145:83–96
Sharvil SP, Ravindra SD, Mithun VV, Paradkar AR, Khanna PK (2009) Synthesis and antibacterial studies of chloramphenicol loaded nano-silver against Salmonella typhi. Metal Org Nanometal Chem 39:65–72
Shashi Prabha D, Manu L, Mika S (2010) Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A Physicochem Eng Asp 364:34–41
Singaravelu G, Arockiamary JS, Ganesh Kumar V, Govindaraju K (2007) A novel extracellular synthesis of monodisperse gold nanoparticles using marine algae, Sargassum wightii Greville. Colloids Surf B Biointer 57:97–101
Solomun T, Schimanski A, Sturm H, Illenberger E (2004) Reactions of amide group with fluorine as revealed with surface analytics. Chem Phys Lett 387:312–316
Swamy MK, Sudipta KM, Jayanta K, Balasubramanya S (2014) The green synthesis, characterization, and evaluation of the biological activities of silver nanoparticles synthesized from Leptadenia reticulata leaf extract. Appl Nanosci. doi:10.1007/s13204-014-0293-6
Vasanth K, Ilango K, Mohankumar R, Agrawal A, Dubey GP (2014) Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B 1:354–359
Vendemiale G, Grattagliano I, Altomare E (1999) An update on the role of free radicals and antioxidant defense in human disease. Int J Clin Lab Res 29:49–55
Weir E, Lawlor A, Whelan A, Regan F (2008) The use of nanoparticles in anti-microbial materials and their characterization. Analyst 133:835–845
Yugandhar P, Savithramma N (2015) Biosynthesis, characterization and antimicrobial studies of green synthesized silver nanoparticles from fruit extract of Syzygium alternifolium (Wt.) Walp. an endemic, endangered medicinal tree taxon. Appl Nanosci. doi:10.1007/s13204-015-0428-4
Zhu M (2000) Apparatus analyses. Higher Education Press, Beijing
Acknowledgments
The authors are thankful to the UGC-DAE Consortium for Scientific Research, Indore, for providing financial support through collaborative research project (Ref. No: CSR–I/CRS–71/2015-2016/2101). We are also grateful to UGC-SAP DRS-II for the Instrumentation facilities in the Department of Animal Science, Bharathidasan University, Tiruchirappalli, Tamil Nadu, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kuppurangan, G., Karuppasamy, B., Nagarajan, K. et al. Biogenic synthesis and spectroscopic characterization of silver nanoparticles using leaf extract of Indoneesiella echioides: in vitro assessment on antioxidant, antimicrobial and cytotoxicity potential. Appl Nanosci 6, 973–982 (2016). https://doi.org/10.1007/s13204-015-0514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-015-0514-7