Abstract
There is growing interest in the effect of dietary interventions in people living with memory impairment and delaying cognitive decline. Investigation of alterations in glucose metabolism and dietary-induced ketogenesis in older adults is a recent growing area of research. Ketone bodies are an important alternative energy source in the brain and may be beneficial to people developing or who already have memory impairment or those with Alzheimer’s disease. This scoping review aims to evaluate the available evidence on dietary-induced ketogenesis and its effect on cognition in older adults and the factors affecting feasibility of the dietary interventions to inform the design of future studies. The scoping review methodology explored the current knowledge about dietary interventions related to dietary-induced ketogenesis and cognition in older adults and identify gaps in the literature. Eleven dietary intervention studies included in the review demonstrated that both medium-chain triglyceride supplementation and ketogenic diets induce ketosis. Nine of these studies demonstrated that dietary-induced ketogenesis could lead to an improvement in cognitive functions, but the evidence remains inconclusive. Most of the included studies showed evidence to suggest that dietary-induced ketogenesis improves cognitive functions in older adults. However, the number of published papers is small and there were differences in the design and types of the dietary interventions (medium-chain triglyceride supplementation, ketogenic diet) along with high drop-out rates in some studies which limits the generalization of the findings. Although methodologies used in the studies vary, the findings warrant the need for further research with larger sample sizes in people at different stages of cognitive impairment, and to develop strategies to improve adherence to the intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dementia is a global problem that is increasing rapidly due to an increasing ageing population [1]. Worldwide, there are over 9.9 million new cases annually, implying a new diagnosis every 3.2 seconds [1]. With no effective pharmacological treatment for dementia, research has addressed the efficacy of dietary interventions on prevention and progression reduction of the disease [2]. Dietary interventions could slow or reverse cognitive decline in older adults, and improve their overall quality of life and cognitive performance. Recently, there has been growing attention on the importance of nutrition on cognitive functions through the life span. Studies have investigated the relation between whole diets (Mediterranean diet, dietary approaches to stop hypertension (DASH) diet) and dementia risk reduction [3,4,5]. Some research on dietary prevention of Alzheimer’s disease (AD) is focused on the consumption of the DASH and Mediterranean-DASH intervention for neurodegenerative delay (MIND) diets [6, 7]. These diets are recommended because of the relationship between high blood circulating cholesterol concentrations and increased risk of AD [8, 9].
However, the role of dietary modulation on cognitive functions is a growing area of research, especially to understand the alterations in glucose metabolism and dietary-induced ketogenesis in older adults. It has been shown that cerebral glucose hypo-metabolism is often related to neuronal loss and cognitive impairment [10, 11]. The cause of glucose hypo-metabolism although remains unclear, has been attributed to mitochondrial impairment, defects in brain glucose transport, disruption in glycolysis, or impairment in insulin functions [8, 12]. Carriers of the APO E4 gene have alterations in brain energy metabolism [13] as they demonstrate reductions in measures of glucose metabolism and mitochondrial functions in comparison to non-carriers of APO E4 [13]. Apo E4 has been recognized as a risk factor for sporadic and late-onset familial AD [14].Cerebral glucose hypo-metabolism could potentially be a key factor that contributes to cognitive decline in older adults [11]. However, the use of ketone production to improve energy uptake by the brain could reduce the progression of cognitive impairment [11].
Research has also shown that bypassing systematic glucose metabolism in the brain by inducing ketosis can increase ketone availability for neurons [11], thus providing an alternative energy source. Ketones such as beta-hydroxyl butyrate (ß-OHB) and acetoacetate are the by-products of the breakdown of fatty acids in the body and may be beneficial to people who are developing or already have memory impairment or AD diagnosis [3, 15, 16]. Therefore, increasing cerebral ketone uptake to combat the effect of glucose hypo-metabolism on brain functions has become a target for therapeutic interventions in AD [3, 15, 16].
Studies have shown that dietary-induced ketogenesis (DK) can increase ketone availability to the brain, which has beneficial cognitive effects in individuals with mild to moderate AD and MCI [17,18,19,20,21]. DKs can be achieved either by low carbohydrate ketogenic diets (with 20–50 g intake of carbohydrate a day) [22] or the supplementation of 20–70 g of medium chain triglycerides (MCT)/day (especially those containing the eight and ten carbon fatty acids or the usage of ketone esters) [20]. The effectiveness of ketogenesis in increasing ketone levels can be determined either by measuring beta-hydroxyl butyrate in blood or ketone bodies in urine [20]. However, little is known about the effect of different DK interventions on cognitive functions of older adults.
Therefore, this scoping review aimed to explore the current literature on the effectiveness of DK on ketone levels and its effect on the cognitive functions in older adults.
Methods
This review was guided by the methodological framework developed by Levac et al. [23] which is the updated version to the initial scoping review framework developed by Arksey and O’Malley [24]. The framework consists of five major steps: (1) identifying the research question, (2) searching for relevant studies, (3) selecting studies, (4) charting the data, collating, summarizing, and (5) reporting the results. This methodology has been recommended for areas of research that have yet to be thoroughly reviewed, as it allows the exploration of the existing literature for the identification of research gaps when the research conducted to date in a specific area is diverse [23].
Identifying the Research Question: Inclusion and Exclusion Criteria
Studies that used a dietary intervention with any study design (ketogenic diet or medium-chain triglyceride supplementation) to induce ketogenesis in older adults (aged 60 years and above) were included in this review. Participants aged 60 years and above was used to define older adults as it is the standard cut off point used by the UN (United Nations) and WHO (World Health Organization) [25]. Interventional studies, such as randomized control trials (RCTs), case studies, pilot, and feasibility studies that documented the effect of ketogenesis on cognitive functions in older adults were included. Studies that were conducted on individuals living with different types of cognitive impairment (MCI, AD, or dementia) were included in the review. Studies that were not published in English were excluded.
Searching for Relevant Studies: Search Terms
A search on the relevant material was undertaken to provide an overview of the current available knowledge to help identify the research questions. The literature search was carried out using search terms that represented the population, intervention, and outcome. Only peer reviewed interventional studies were included.
Population
Older-adults or “Older adults” or Geriatrics or Seniors or Dementia.
Intervention
Ketosis or “ketogenic diet” or “ketogenic agents” or “ketone body metabolism” or “ketone synthesis” or ketones or “ketonic acids” or hyperketonaemia or keto* or ketone* or MCT or “medium chain triglycerides”.
Outcome
Cognition or Memory or Mnemosyne or “Memory testing” or “cognitive functions” or “cognitive impairment”.
A search was run through the databases of Medline (1971–2019), Psych Info (1998–2019), Cochrane (CENTRAL) (2014–2019), PubMed, Scopus (1970–2019), Web of Science, CINAHL (1971–2019), and Elsevier (2003–2019). The search included published peer reviewed literature from the date of inception of each database.
Selecting Studies
The electronic search strategy was developed based on key terms from other studies and the usage of MESH terms in the afore-mentioned databases. The reference lists of key papers were checked and key word searches in Google Scholar were performed to identify studies. The database search was conducted between July 2018 and March 2019. All studies were exported into Endnote Bibliographic software for screening. Duplicates were deleted and abstracts were screened for eligibility. Studies were independently selected by the authors based on the afore-mentioned inclusion/exclusion criteria. In case of discrepancies in selection, the entire research team was consulted, and a discussion was held regarding inclusion/exclusion of the study.
Charting the Data
Data from the chosen studies were extracted using a checklist that was adapted from the Cochrane data extraction and assessment form [26]. Data extracted included type of study, methods, randomization, sample size, location, duration, outcome measures, intervention, administration method, medical condition of target population, age of participants, APO E4 status of participants, exclusion and inclusion criteria, adherence, drop-out rates, and funding sources. The quality of the included studies was assessed using the Critical Appraisal Skills Programme (CASP) checklist for RCT’s and case control studies [27].
Results
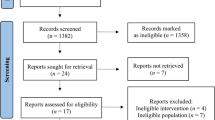
There were 115 studies identified across all databases. Of these, 79 studies were excluded after the initial screening, which included the title and abstract (refer to Fig. 1). The remaining 36 studies were fully screened for eligibility and only 11 studies of these met the eligibility criteria for inclusion in the review (Table 1). Narrative and descriptive numerical analysis were used to report all the study results. Studies were grouped together according to the type of intervention, MCT supplementation, or ketogenic diet.
PRISMA 2009 flow diagram of screened and included studies. (Source: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. https://doi.org/10.1371/journal.pmed1000097)
Study Setting
Across the 11 included studies, participants were recruited from different settings, either through databases of universities or community research centres, care homes, hospitals, and memory clinics. Most of the studies were conducted in the USA (n 8) [17, 20, 21, 28,29,30,31] and Japan (n 3) [32,33,34]. Six of the eleven studies were RCTs [17, 20, 21, 30, 32, 34], three were controlled pilot studies [31, 33, 35], and only two were case studies [28, 29] (refer to Table 2).
Intervention Characteristics
Type of Dietary Intervention
All the 11 studies reported interventions that involved MCT supplementation or low carbohydrate (CHO) diets. Of these, most of the studies used MCT supplementation (n 8) [17, 21, 28,29,30, 32,33,34], low CHO ketogenic diet (n 2) [20, 35], or both (n 1) [31] to induce ketosis in participants.
The amount of MCT administered ranged widely between studies from 6 g/day [32] to 165 g/day [29]. It is suggested that DK can be achieved by the supplementation of 20–70 g of MCT/day [20] to the normal diet. Abe and colleagues (2017) supplemented participants with less than 20 g/day of MCT and participants were provided with 6 g/day MCT along with 1.2 g of L-Leucine amino acid and 20 µg of cholecalciferol [32]. It is unknown if ketosis was achieved in this study as circulating blood ketone concentrations were not tested. L-Leucine amino acids and cholecalciferol were supplemented to increase muscle strength and functions [32]. L-leucine and cholecalciferol play a role in improving muscle function but had no impact on cognition in this study. Individuals taking the MCT (with L-leucine and cholecalciferol) supplements showed a 30.6% improvement in MMSE (Mini-Mental State Examination) score in comparison to participants supplemented with long-chain fatty acids (with L-leucine and cholecalciferol) or the control group where no supplementation was provided [32].
In another study, a multivitamin (containing vitamin D, calcium, and phosphorus) was provided in addition to very high fat ketogenic diet with MCT supplementation [31]. The multivitamin was provided to prevent micronutrient deficiencies due to the strict diet that might be lacking in some micronutrients [31]. In a case study conducted by Newport et al., the participants were provided with 165 ml of MCT per day along with 35 ml of coconut oil [29]. Coconut oil was supplemented with MCT as it is a rich source of medium-chain fatty acids that aids in inducing ketosis [29].
Administration of Dietary Intervention
MCT
The approach to administer MCT differed between the studies included. The MCT were supplemented either through mixing powdered MCT sachets with liquid or meal replacement drinks [19], ketogenic meal (Meihi817-B 50) made of mixing MCT in hot water [34], or mixing MCT with food [32]. Table 3 outlines the different methods that MCT were used to induce ketosis. In a feasibility study, Ohnuma and colleagues used “Axona Graduating Dosing Plan” which is a four-step titration method recommended by Accera Inc. [33]. Initially, supplementation of Axona was 10 g for 2 days, then the volume of MCT was increased gradually every 2 days to reach 20 g/day, then 30 g/day and finally 40 g/day remaining at this intake for 3 months [33]. This approach was used to limit gastro-intestinal symptoms associated with MCTs such as nausea, abdominal pain, and flatulence in participants with mild to moderate sporadic AD [33]. The participants in the study reported a reduction in the number of adverse events compared to a previous study using Axona as a source of MCT [19] which helped improve their adherence to the intervention (90% had more than 80% compliance to the intervention). However, 2 out of 24 participants (8%) reported flatulence and abdominal pain and dropped out of the study due to their inability to tolerate the MCT [33].
Ketogenic Diet
There were 3 studies that examined the effect of a ketogenic diet by reducing the amount of dietary carbohydrate (CHO) to induce ketosis in older adults [20, 31, 35] (refer to Table 4). All of these studies limited CHO intake of participants to no more than 20 g/day [20, 31, 35].
Krikorian and colleagues [20] restricted CHO intake to 20 g/day but did not alter total dietary energy, fat, and protein intake for 6 weeks in older adults with MCI in two of the studies. Participants were randomized to either a high CHO diet (50% of total energy intake) or a low CHO ketogenic diet (5–10% CHO intake). They were provided with educational and counselling sessions at the baseline visit to help them follow the dietary requirements [20]. Participants were provided with oral and written instructions for food and beverage portion estimation using a portion poster (Nutrition Consulting Enterprises, Framingham, MA, USA) along with instructions for recording quantities of food and beverages consumed [20] to help them fill in the 7-day food diaries that were used to assess their adherence to the intervention. They were also contacted weekly to ensure protocol compliance and to allow them to ask any questions regarding the diet [20]. The results of the dietary assessment showed that all the participants adhered to the diet. However, at the end of the study, only one participant out of 12 expressed the willingness to continue the low CHO diet [20]. Ketone bodies measured in urine increased in participants who followed the low CHO diet [20]. A follow-up study conducted by the same research team followed the same dietary adjustments on 7 participants; but in this study, blood ketone levels were measured to assess ketosis [35] as blood and capillary measurement of beta-hydroxyl butyrate (ß-OHB) are more sensitive than urinary ketone measurements to reflect metabolic status [36]. Of the seven participants, two were removed from analysis due to the lack of available data to permit robust quantification. Five participants with MCI followed the low CHO diet for 6 weeks. A significant increase in blood ketone levels was detected in the study (p = 0.03).
Taylor and colleagues used a combination of MCT supplementation and a very high fat (VHF) ketogenic diet to achieve optimum ketosis [31]. In this study, participants were also provided with a multivitamin to prevent micronutrient deficiencies [31].The diet restricted CHO intake to 20 g/day (2–10% of total energy intake), with protein and fat accounting for 20% and 70% respectively of the total energy intake to achieve 1:1 ratio of food between fat and non-fat sources. Participants gradually added MCT to the diet through mixing of MCT oil with food and beverages after attending cooking demonstrations. In the first week, MCT supplied 10% of the total fat intake, which was increased to 40% of energy intake by the end of 3 months duration of the study. The dietary intervention achieved ketosis which was evidenced by the significant increase in plasma ß-OHB (p < 0.001) in plasma. There was a high drop-out rate in the study (n 5; 33%) which was attributed to increased carer burden due to the restrictiveness of the diet, especially in individuals with advanced dementia [31]. Overall, the diet was tolerated as evidenced by the three day food records, but adverse events such as diarrhoea due to MCT oil were reported by all the study participants [31].
Outcome Measures of Cognition and Ketogenesis
The outcome measure in 8 studies was altered ketone concentrations in blood and urine [19,20,21, 30, 31, 33,34,35]. Circulating blood ß-OHB concentrations were measured in 6 studies [19, 21, 30, 33,34,35]. All of the studies that measured blood ketone concentrations demonstrated increased ketones in individuals taking MCT [19, 21, 30, 33,34,35]. Krikorian and colleagues measured urinary ketones using urine strips [20] while Taylor used both blood and urine tests to measure ketone concentrations [31].
All the eleven studies included measured the changes in cognitive functions as primary outcomes that included scores of MMSE, ADAS-Cog, Trail Making test, VPAL, and measures of verbal fluency (refer to Table 5). Of these, seven showed statistically significant improvement in either overall cognitive functions [19, 30,31,32] or improvement in specific subsets of memory functions [20, 34, 35]. The most commonly used measure to assess overall cognitive functions was MMSE [19, 21] and ADAS-Cog [28, 32] or both [1, 29,30,31, 33]. Other domain-specific cognitive measures were used such as Trail Making test, verbal fluency, and VPAL [20, 21, 30, 34, 35]. Most of the studies showed significant improvement in cognitive and memory functions in relation to ketogenesis in comparison to pre-intervention scores and the scores of the control group. In some studies, improvement in cognitive functions was detected through changes in cognitive test results; however, the improvement was not statistically analyzed in three studies [21, 28, 29]. In the case studies conducted by Maynard and Geblum [28], participants who had mild AD demonstrated improvement in cognitive functions by the end of the intervention after supplementing 30 g/day of caprylic triglycerides to their dietary intake. However, 2 of the 8 participants recruited used only 10 g/day of the caprylic triglycerides [28] and in both cases the patients were declared stable. There was also an annual decrease in their MMSE scores (annual decrease of 1.8 and 2.4) in comparison to the other participants who took 20 g/day in which MMSE scores increased in 4 out of 6 participant who took 20 g/day caprylic triglycerides. The duration of the caprylic triglycerides intake ranged from 6 months to 4 years [28]. Greater improvement in MMSE scores was detected in patients who were diagnosed with Mild AD prior to the intervention in comparison to their counterparts who had moderate AD. APO lipo protein E4 status was screened in 6 out the 11 studies, and the results show an increased effect of ketogenesis on cognitive functions in APO E4-negative participants [19, 21, 28,29,30, 33].
Discussion
Cerebral glucose hypo-metabolism could lead to chronic brain energy deprivation which causes a deterioration in neuronal functions leading to a reduction in synaptic functionality and further decline in glucose metabolism [11]. Thus, this creates a viscous cycle of neuronal damage leading to the exacerbation of cognitive impairment [11]. Glucose supplies about 95% of the brain’s energy needs; however, it utilizes ketones instead in case of an increase in plasma ketone concentration [11, 37]. The cerebral metabolic rate of ketones (CMRk) which measures brain ketone utilization is dependent on plasma ketone concentration. Studies using positron emission tomography imaging and a ketone tracer (11C-acetoacetate) demonstrated that brain ketone uptake remains normal in ageing, MCI, and AD [11, 37]. Thus, increasing cerebral ketone uptake to combat the effect of glucose hypo-metabolism on brain functions has become a target for therapeutic interventions in AD [3, 15,16,17]. This method is often referred to as “brain energy rescue” [11]. Recent clinical studies have shown the association between brain energy rescue using ketones and improvement in cognitive functions in MCI and AD [38].
This scoping review shows that for most studies identified, there was a positive association between DK and cognitive functions in older adults. Studies that were conducted on individuals with no to mild cognitive impairment showed increased improvements in cognition [21, 28, 30] in comparison to studies conducted on individuals with more advanced cognitive impairments [28, 30, 31, 33]. This would suggest that interventions to induce DK are likely to be more effective in earlier stages of dementia (MCI or mild AD) in comparison to interventions in later stages of the disease. The reason for this observation could be attributed to the ability of participants to adhere to the intervention or to other factors such as extent of neuronal damage though further research is warranted.
The efficacy of DK on cognition was investigated by measuring changes in cognitive functions. MMSE and ADAS-Cog were the most commonly used outcome measures in the majority of studies identified. These tests measure the overall cognitive functions [39, 40] of individuals and might not reflect the small changes that might occur in different areas of memory (e.g. executive memory, orientation which might improve quality of life). The effect of the intervention on subsets of cognitive functions was further confirmed by studies that demonstrated an improvement in long-term or executive memory despite the lack of change in overall cognitive results tested by MMSE or ADAS-Cog [20, 31, 35].
The four studies that looked at the benefits of DK showed statistically significant improvement on cognitive functions [17, 30,31,32], while three studies showed improvement in subsets of cognitive functions such as executive functions and verbal memory [20, 34, 35]. Whilst the studies showed a significant correlation between ketogenic diet and cognitive functions, the small sample size and the high drop-out rate limit the generalizability of the results. Moreover, the inconsistencies within and between studies in terms of study designs, types of interventions, and outcome measures might limit the application of such interventions in a clinical setting. Thus, the examination of the 11 studies demonstrated that the benefits of DK on cognitive functions of older adults remains unclear.
APO E4
Apo-lipoprotein E (Apo E) is a polymorphic protein with three common alleles, APO epsilon 2, APO epsilon 3, and APO epsilon 4 [14]. Carriers of the APO E4 gene have alterations in brain energy metabolism [13] as they demonstrate reductions in measures of glucose metabolism and mitochondrial functions in comparison to non-carriers of APO E4 [13]. Apo E4 has been recognized as a risk factor for sporadic and late-onset familial Alzheimer disease (AD) [14]. APOE4 homozygotes have up to 15 times increased risk of AD while APOE4 heterozygotes have up to 4 times the risk for AD in comparison to risk neutral APOE3 homozygotes [13]
Previous studies have shown that APO E4 status affects metabolism in relation to ketone absorption and utilization; however, the mechanism remains unclear [18]. It is suggested that APO E4 brain might potentially possess an upper limit for ketone metabolism and utilization in compensation for metabolic dysfunction [41]. Thus, APO E4 brain would be incapable of complete metabolism of exogenous ketone bodies to facilitate energy production and maintain neuronal functions [41]. Henderson and colleagues [19] showed an increase in blood ß-OHB concentrations in APO E4 positive compared with APO E4-negative participants after 120 min of administration of MCT. This association is also reflected by the effect of DK on cognitive functions as individuals who were APO E4 negative demonstrated an increased improvement in their cognitive functions in comparison to their APO E4-positive counterparts [19, 21, 28, 30]. On the other hand, in the study conducted by Maynard and Geblum, APO E4 had no effect on overall cognition in AD patients as the APO E4-positive participants who were supplemented with caprylic triglycerides showed an improvement in MMSE scores after the intervention [28]. However, APO E4 status of participants was measured in 6 out of the 11 studies. Thus, more studies are needed to understand the effect of APO E4 gene on DK and cognition.
Limitations
Limitations of the review are the relatively few numbers of studies and the small sample sizes and the inclusion of only English language studies. Some of the findings of the current review are consistent with those of a previous review conducted by Lilamand and colleagues [42]. However, this review looked into the effect of ketogenic diets only in Alzheimer’s disease. We go beyond the findings of this review as we have identified the limitations of current studies and offer recommendations for the design of future studies to better understand the effect of DK on cognition in older adults (including MCI and Alzheimer’s disease) as follows.
Generalizability and Credibility
Most of the studies reviewed were pilot studies, which explains their short duration and small sample size. Seven studies did not report planned sample size or power calculations [21, 30,31,32,33,34,35] and three studies did not report funding sources [21, 29, 31]. Five studies were funded by industry [17, 28, 30, 32, 33] which could impact the credibility of the results due to the conflict of interest [43]. Furthermore, the combination of healthy older adults with cognitive impairment patients (MCI, AD) makes it difficult to evaluate the effect of the intervention in specific stages of life/dementia.
Inconsistencies Between Studies
A major limitation of these studies was the lack of consistency and replicability between studies. The majority of the studies used MCTs to induce DK in older adults (n 8) [17, 21, 28,29,30, 32,33,34]. There was a lack in consistency in interventions as the method of administration, volume, and duration of the intervention differed between studies that used MCT supplementation to induce ketosis. The review showed that despite the different administration methods, volume, and duration of MCT supplementation, such dietary interventions induce ketosis which affects cognitive functions in older adults. The most common cognitive outcome measures used were the ADAS-Cog [19, 21], MMSE [28, 32], or both [29, 30, 33]. However, some studies used specific cognitive measures such as Trail Making and VPAL [20, 31]. The method for measuring ketones in the body also varied between studies as some studies measured blood ß-OHB concentrations [17, 21, 30, 31, 33,34,35] while others measured urinary ketones [20, 31]. A study comparing measures of ketones in urine and blood has demonstrated that blood and capillary measurement of ß-OHB are more sensitive than urinary ketone measurements to reflect the individual’s metabolic status [36]. The duration of the dietary intervention in studies ranged from 3 weeks to 6 months. There were also differences in the way in which MCT were administered with MCT provided intravenously, added to food in the form of powder or oil, or mixed with drinks. The amount of MCT differed between studies, ranging from 6 g/day to 150 g/day with different kinds of MCT used (AC-1202, caprylic triglycerides).
There were also differences in the range of people recruited within studies as they had different levels of cognitive impairment. Some studies included people with MCI and AD [30, 31], frail elderly [32, 34], and only individuals with MCI [20, 21, 35] or AD [17, 28, 29, 33], other kinds of cognitive impairment such as sporadic AD [29, 33]. It is likely that the dietary interventions may affect people differently depending on the level cognitive impairment or dementia. Thus, more studies with larger sample sizes are needed to investigate this effect among people with different levels of cognitive impairment.
Factors Affecting Feasibility
Adherence
Most of the studies associated the high drop-out rates to the inability of participants to adhere to the intervention either due to the restrictiveness of the ketogenic diet [20, 31, 35] or the gastro-intestinal side effects of MCT intake such as diarrhoea and bloating [19, 21, 28, 33]. The application of a 4-day titration method to supplement the body with MCT showed a reduction in the gastro-intestinal implications that usually accompany MCT consumption in comparison to a previous study that applied a 2-day titration method [19]. Thus, in future studies, this approach using a 4-day titration of MCT could help to reduce drop-out rates by reducing risks of MCT-associated adverse events.
Dietary modifications using reduced dietary CHO have been studied to achieve ketosis. However, the high drop-out rate revealed the impracticality of utilizing a highly restrictive diet on individuals with advanced cognitive impairments [31]. The high drop-out rate could be related to the sugar cravings of some individuals with AD, which has been established in previous studies [44, 45]. Furthermore, the long-term application of this diet in relation to its restrictiveness could be a burden on participants and their caregivers.
Risks
Ketogenic diets in older adults could play a role in exacerbating other diseases such as cardiovascular or renal diseases due to the substitution of carbohydrates with fat or protein [20]. High fat intake has been associated with cardiovascular diseases due to an increase in blood cholesterol and triglycerides levels [46],while a high protein diet could exacerbate kidney disease and increase risk of proteinuria, diuresis, and nephrolithiasis [47]. Furthermore, evidence demonstrates a relationship between high blood cholesterol concentrations and increased dementia risk [48,49,50]. Thus, a high fat low CHO ketogenic diet might lead to an increase in blood cholesterol level and consequently increase dementia risk in older adults. Krikorian and colleagues had some concerns regarding the reduced dietary fibre intake of participants associated with the low CHO diet and its impact on their gastro-intestinal functions especially constipation, which is a common issue with older adults [20]. Gastro-intestinal side effects of consumption of MCT such as nausea, bloating, diarrhoea, and abdominal pain were reported in the studies [19, 21, 28, 33]. The side effects of MCT consumption on the gastro-intestinal tract are well known from previous studies [51, 52]. Thus, the long-term consumption of MCT or ketogenic diet might pose a risk on the health and physical well-being of older adults. This was also demonstrated in a review conducted by Lilamand and colleagues [42], which discussed the effect of KD on dementia risk.
Conclusion
There is evidence to suggest that DK can lead to an improvement in cognitive functions in older adults. This review has shown that despite the differences between the included studies (intervention methods and outcome measures) taken together these studies suggest a promising relationship between DK and cognitive functions. However, robust studies with larger sample sizes are needed. Future research should focus on the feasibility of dietary interventions using DK to improve adherence and reduce the potential for high drop-out observed in some studies. APO E4 genetic screening would also provide more knowledge about the effect of the gene on ketone metabolism and cognitive functions.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- DK:
-
Dietary-induced ketogenesis
- MCI:
-
Mild cognitive impairment
- AD:
-
Alzheimer’s disease
- MCT:
-
Medium chain triglycerides
- ADAS-cog:
-
Alzheimer’s Diseases Assessment Scale-Cognitive
- MMSE:
-
Mini-Mental State Examination
- Apo E4:
-
Apo-lipoprotein E4
- RCT:
-
Randomized controlled trial
- CHO:
-
Carbohydrate
- VHF-KD:
-
Very high fat ketogenic diet
- ß-OHB:
-
Beta-hydroxyl butyrate
- M:
-
Males
- F:
-
Females
References
Alzheimer’s Disease International. The global impact of dementia: an analysis of prevalence, incidence, cost and trends. London; 2015. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
Canevelli M, Lucchini F, Quarata F, Bruno G, Cesari M. Nutrition and dementia: evidence for preventive approaches? Nutrients. 2016;8(3):144. https://doi.org/10.3390/nu8030144.
Allès B, Samieri C, Féart C, Jutand M-A, Laurin D, Barberger-Gateau P. Dietary patterns: a novel approach to examine the link between nutrition and cognitive function in older individuals. Nutr Res Rev. 2012;25:207–22.
Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, et al. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11(5):677–708. https://doi.org/10.1586/ern.11.56.
Tang EYH, Harrison SL, Albanese E, Gorman TJ, Rutjes AWS, Siervo M, et al. Dietary interventions for prevention of dementia in people with mild cognitive impairment. Cochrane Database Syst Rev. 2015(10). https://doi.org/10.1002/14651858.cd011909.
Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–22.
Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT, et al. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–14.
Hoyer S. Oxidative energy metabolism in Alzheimer brain. Studies in early-onset and late-onset cases. Mol Chem Neuropathol. 1992;16. https://doi.org/10.1007/bf03159971.
Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014;71(2):195–200.
Ciavardelli D, Piras F, Consalvo A, Rossi C, Zucchelli M, Di Ilio C, et al. Medium-chain plasma acylcarnitines, ketone levels, cognition, and gray matter volumes in healthy elderly, mildly cognitively impaired, or Alzheimer’s disease subjects. Neurobiol Aging. 2016;43:1–12. https://doi.org/10.1016/j.neurobiolaging.2016.03.005.
Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, et al. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27(1):3–20. https://doi.org/10.1016/j.nut.2010.07.021.
Hertz L, Chen Y, Waagepetersen HS. Effects of ketone bodies in Alzheimer’s disease in relation to neural hypometabolism, β-amyloid toxicity, and astrocyte function. J Neurochem. 2015;134(1):7–20. https://doi.org/10.1111/jnc.13107.
Perkins M, Wolf AB, Chavira B, Shonebarger D, Meckel JP, Leung L, et al. Altered energy metabolism pathways in the posterior cingulate in young adult apolipoprotein E ɛ4 carriers. J Alzheimers Dis. 2016;53(1):95–106. https://doi.org/10.3233/JAD-151205.
Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103(15):5644–51.
Costantini LC, Barr LJ, Vogel JL, Henderson ST. Hypometabolism as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008;9:1–9. https://doi.org/10.1186/1471-2202-9-s2-s16.
Freemantle E, Vandal M, Tremblay-Mercier J, Tremblay S, Blachère J-C, Bégin ME, et al. Omega-3 fatty acids, energy substrates, and brain function during aging. Prostaglandins Leukot Essent Fatty Acids. 2006;75(3):213–20. https://doi.org/10.1016/j.plefa.2006.05.011.
Henderson ST. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics. 2008;5. https://doi.org/10.1016/j.nurt.2008.05.004.
Henderson ST, Poirier J. Pharmacogenetic analysis of the effects of polymorphisms in APOE, IDE and IL1B on a ketone body based therapeutic on cognition in mild to moderate Alzheimer’s disease; a randomized, double-blind, placebo-controlled study. BMC Med Genet. 2011;12:137. https://doi.org/10.1186/1471-2350-12-137.
Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial. Nutr Metab. 2009;6(1):31. https://doi.org/10.1186/1743-7075-6-31.
Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging. 2012;33(2):425. https://doi.org/10.1016/j.neurobiolaging.2010.10.006.
Rebello CJ, Keller JN, Liu AG, Johnson WD, Greenway FL. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: a randomized controlled trial. BBA Clin. 2015;3(Supplement C):123–5. https://doi.org/10.1016/j.bbacli.2015.01.001.
Westman EC, Feinman RD, Mavropoulos JC, Vernon MC, Volek JS, Wortman JA, et al. Low-carbohydrate nutrition and metabolism. Am J Clin Nutr. 2007;86. https://academic.oup.com/ajcn/article/86/2/276/4633078
Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. https://doi.org/10.1186/1748-5908-5-69.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. https://doi.org/10.1080/1364557032000119616.
United Nations. Provisional guidelines on standard international age classifications. statistical papers: United Nations; 1982. https://digitallibrary.un.org/record/32113?ln=en
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions version 6.0. 2019. www.training.cochrane.org/handbook. Accessed 2020.
CASP: Critical Appraisal Skills Programme- Quantitative Checklist. 2019. https://casp-uk.net/casp-tools-checklists/. Accessed 2019.
Maynard SD, Gelblum J. Retrospective case studies of the efficacy of caprylic triglyceride in mild-to-moderate Alzheimer’s disease. Neuropsychiatr Dis Treat. 2013;9:1629–35. https://doi.org/10.2147/ndt.s49895.
Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 2015;11(1):99–103. https://doi.org/10.1016/j.jalz.2014.01.006.
Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson GS, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–4.
Taylor MK. Influence of diet in Alzheimer’s disease: the role of carbohydrate intake and ketogenic therapy. ProQuest Information & Learning; 2018. https://hdl.handle.net/1808/25883
Abe S, Ezaki O, Suzuki M. Medium-Chain triglycerides in combination with leucine and vitamin D Benefit cognition in frail elderly adults: a randomized controlled trial. J Nutr Sci Vitaminol. 2017;63(2):133–40. https://doi.org/10.3177/jnsv.63.133.
Ohnuma T, Toda A, Kimoto A, Takebayashi Y, Higashiyama R, Tagata Y, et al. Benefits of use, and tolerance of, medium-chain triglyceride medical food in the management of Japanese patients with Alzheimer’s disease: a prospective, open-label pilot study. Clin Interv Aging. 2016;11:29–36. https://doi.org/10.2147/cia.s95362.
Ota M, Matsuo J, Ishida I, Hattori K, Teraishi T, Tonouchi H, et al. Effect of a ketogenic meal on cognitive function in elderly adults: potential for cognitive enhancement. Psychopharmacology. 2016;233(21–22):3797–802.
Krikorian R, Boespflug EL, Dudley JA, Norris MM, Chu W-J, Summer SS, et al. Enhanced cerebral bioenergetics with dietary ketosis in mild cognitive impairment. Nutrition & Aging. 2014;2(4):223.
Turan S, Omar A, Bereket A. Comparison of capillary blood ketone measurement by electrochemical method and urinary ketone in treatment of diabetic ketosis and ketoacidosis in children. Acta Diabetol. 2008;45(2):83–5. https://doi.org/10.1007/s00592-008-0026-y.
Croteau E, Castellano CA, Fortier M, Bocti C, Fulop T, Paquet N, et al. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp Gerontol. 2017. https://doi.org/10.1016/j.exger.2017.07.004.
Jennings A, Cunnane SC, Minihane AM. Can nutrition support healthy cognitive ageing and reduce dementia risk? BMJ. 2020;369. https://doi.org/10.1136/bmj.m2269
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. 1984. https://doi.org/10.1176/ajp.141.11.1356
Wu L, Zhang X, Zhao L. Human ApoE isoforms differentially modulate brain glucose and ketone body metabolism: implications for Alzheimer’s disease risk reduction and early intervention. J Neurosci. 2018;38(30):6665–81.
Lilamand M, Porte B, Cognat E, Hugon J, Mouton-Liger F, Paquet C. Are ketogenic diets promising for Alzheimer’s disease? A translational review. Alzheimers Res Ther. 2020;12(1):42. https://doi.org/10.1186/s13195-020-00615-4.
Nestle M. Corporate Funding of Food and Nutrition Research: Science or Marketing? JAMA Intern Med. 2016;176(1):13–4. https://doi.org/10.1001/jamainternmed.2015.6667.
Ikeda M, Brown J, Holland AJ, Fukuhara R, Hodges JR. Changes in appetite, food preference, and eating habits in frontotemporal dementia and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73(4):371–6. https://doi.org/10.1136/jnnp.73.4.371.
Kai K, Hashimoto M, Amano K, Tanaka H, Fukuhara R, Ikeda M. Relationship between eating disturbance and dementia severity in patients with Alzheimer’s disease. PLoS One. 2015;10(8):e0133666. https://doi.org/10.1371/journal.pone.0133666.
Ascherio A. Epidemiologic studies on dietary fats and coronary heart disease. Am J Med. 2002;113(9):9–12.
Friedman AN. High-protein diets: potential effects on the kidney in renal health and disease. Am J Kidney Dis. 2004;44(6):950–62.
Dufouil C, Seshadri S, Chene G. Cardiovascular risk profile in women and dementia. J Alzheimers Dis. 2014;42(s4):S353–63.
Iwagami M, Qizilbash N, Gregson J, Douglas I, Johnson M, Pearce N, et al. Blood cholesterol and risk of dementia in more than 1· 8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2(8):e498–506.
Peters R, Peters J, Booth A, Anstey KJ. Trajectory of blood pressure, body mass index, cholesterol and incident dementia: systematic review. Br J Psychiatry. 2020;216(1):16–28.
Jeukendrup AE, Aldred S. Fat supplementation, health, and endurance performance. Nutrition. 2004;20(7):678–88. https://doi.org/10.1016/j.nut.2004.04.018.
Marten B, Pfeuffer M, Schrezenmeir J. Medium-chain triglycerides. Int Dairy J. 2006;16(11):1374–82. https://doi.org/10.1016/j.idairyj.2006.06.015.
Author information
Authors and Affiliations
Contributions
The paper represents the original work of the authors. R. El Zein was responsible for researching, writing, and preparing the manuscript. J. L. Murphy, S. Shanker, and P. W. Thomas are the supervisors for R. El Zein’s PhD studentship and they provided their input regarding discussion of content and reviewed the manuscript before submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Zein, R., Murphy, J.L., Shanker, S. et al. The Effectiveness of Dietary-Induced Ketogenesis on Cognition in Older Adults: A Scoping Review of the Literature. SN Compr. Clin. Med. 4, 134 (2022). https://doi.org/10.1007/s42399-022-01211-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01211-3