Abstract
A simple, green, and cost-effective synthesis of ZnO nanoparticles particles (NPs) using an extract of Mucuna pruriens utilis is reported. The nanoparticles were characterized by X-ray diffraction, UV–vis spectroscopy, SEM, and TEM measurements. XRD results showed diffraction patterns that are consistent with the hexagonal phase of the wurtzite ZnO structure. Spherical morphology with irregular size and particle distribution was confirmed by the microscopic characterization. The antioxidant activity of the nanoparticles showed a concentration-dependent profile with an IC50 of 4.10 µg mL− 1, which was quite lower than that of the standard ascorbic acid (4.72 µg mL− 1), and indicated a significant free radical scavenging activity of the nanomaterials. The cytotoxicity properties of the nanoparticles were evaluated against human cancer cell lines HeLa and HEK 293 by the MTT assay, and the anticancer drug (5-Fluorouracil) was used as a control. The results showed selective toxicity of the nanoparticles towards cancerous cell lines and non-toxicity to normal cells. The study provides a simple and non-toxic protocol for biosynthesis of ZnO nanoparticles with potential biomedical applications as anticancer and antioxidant agents. However, further studies are necessary to ascertain the biochemical reactions and mechanisms responsible for the antioxidant and anticancer activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Zinc oxide (ZnO) nanoparticles have been explored in several studies [1] and one of the most versatile nanomaterials with their unique electrical and optical properties [2]. They are the most studied metal oxide nanomaterials [3]. Their notable optical, structural, and antimicrobial properties make them potential materials for application in agriculture [4]. Some of their great attributes in agriculture include their potency as bacteriostatic agents for the control of pathogenic infections as well as their enhancement of seed germination, seedling vigor, and radicle elongation [5]. They have been conventionally synthesized via several routes including hydrothermal, solvothermal and thermolysis of single source precursor compounds [6]. Compared to these previous methods, the green synthesis approach has recently provided a facile and environmentally benign route to their synthesis. This method involves the use of plant extracts as nanoparticle reducing and capping agents [7, 8]. Multitudes of plants and their anatomical components have been employed in green synthesis of ZnO NPs but very few studies have used extracts from the seeds of mucuna legume [9, 10].
Mucuna [Mucuna pruriens (L.) DC var. utilis (aka velvet bean)] is a tropical leguminous plant and a member of the Fabaceae family [11]. It is one of the significant crops widely grown in tropical and subtropical regions as forage and cover crop [12]. It tolerates diverse abiotic stresses such as acidic soils and droughts [13]. The legume possesses a plethora of phytochemical compounds some of which have antioxidant activities that help either in the treatment or reduction of the risk of many diseases [14,15,16]. In addition to alkaloids, isoflavanones, and lectins with analgesic, anti-inflammatory, antihemolytic, and many other bioactivities [17, 18], mucuna seeds contain L-3,4-dihydroxyphenylalanine (L-DOPA) as its main phytochemical compound that possesses antioxidant properties as demonstrated in their ability to inhibit lipid peroxidation [19]. Also, the plant’s seeds contain potent anticancer activities [20]. Hence, it is postulated that the use of the seeds to synthesize nanoparticles would yield nanomaterials with both anticancer and antioxidant attributes that would be of benefit to biological systems. The objective of this study was to biosynthesize ZnO nanoparticles using the aqueous extract of mucuna seeds, characterize them, and evaluate their anticancer and antioxidant activities.
2 Experimentals
2.1 Materials
Zinc acetate [Zn(CH3CO2)2]·2H2O, dimethyl sulfoxide (CH3)2SO, and sodium hydroxide (NaOH) were purchased from Merck (Pty) Ltd, South Africa. Dry mucuna seeds were collected from the Faculty of Agriculture, University of Eswatini (UNESWA), Eswatini.
2.2 Preparation of Mucuna Extract
Mucuna seeds were collected and rinsed with distilled water before air-drying in the laboratory for 3–5 days [21]. Thereafter, they were pulverized into fine powders and sieved using a 40 mm mesh. About 8 g of the powdered seed was mixed with distilled water (400 mL) and heated at 80 ℃ for 1 h. This was followed with the cooling of the product to room temperature and filtering (Whatman No. 1). The filtrate was then kept in a reduced temperature.
2.3 Synthesis of ZnO Nanoparticles
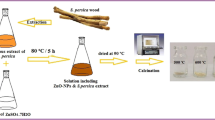
About 20 mL solution of the seed extract in a 100 mL flask, sodium hydroxide solution was added drop wise to adjust the pH to approximately 10. Another 20 mL solution of 0.05 M [Zn(CH3CO2)2]·H2O was prepared and heated up to 80 ℃ while stirring steadily on a magnetic stirrer to ensure a homogeneous solution. Thereafter, the 20 mL solution of mucuna extract was introduced drop-wise to the [Zn(CH3CO2)2]·H2O and the resulting solution heated for 2 h at 85 ºC [22]. During heating the process, a change in solution color from black to brown occurred, signifying the end of the reaction. The solution was cooled to room temperature and centrifuged at 5000 rpm for 30 min, followed by washing 3 times with a solution of ethanol and water to isolate the product. The product was dried in the oven at 50 ℃ for 3 h, and thereafter calcinated at 350 ℃ for 2 h to afford ZnO NPs (Fig. 1).
2.4 Characterization of ZnO Nanoparticles
Crystallinity and phase identification of the nanoparticles was ascertained using a Bruker D8 Advance X-ray diffractometer over 2θ range of 20–80º. A Cary 30 UV–visible spectrophotometer by Agilent was used in the wavelength range of 200 to 900 nm to evaluate the UV–Vis spectrum. The morphological characterization was by TECNAI G2 (ACI) electronic microscope and a Quanta FEG 250 (FEI Inc., USA) scanning electron microscope (SEM) for the measurement of the internal and external morphologies respectively. The particle size measurement as well as their distribution was obtained from the TEM micrographs using Image J software. Energy-dispersive X-ray spectroscopy (EDS) attached to the SEM machine was used to examine the atomic-level compositions of the nanoparticles.
2.5 Cytotoxicity Analysis of ZnO Nanoparticles
The cytotoxicity assay was carried out following the methods previously reported with slight modifications [23]. The assay utilized the human embryonic kidney (HEK293) and cervical carcinoma (HeLa) cells lines purchased from ATCC, Manassas, USA. The cell culturing was done using Dulbecco’s Modified Eagle’s Medium (DMEM) in a 25 cm2 tissue culture flasks and 10% fetal bovine serum. Other reagents include penicillin (100 U/mL) and streptomycin (100 µg/mL). The MTT assay was conducted using a 96-well plate that contained 2.5 × 103 cells/well in DMEM (100 µL). The cells were incubated overnight at 37 ℃ before treatment with the samples, and ZnO nanoparticle solutions of 10, 25, 50, and 100 µg/mL were added to the cells, which were then incubated further for 48 h at 37 ℃, and then followed by the MTT assay. 5-fluorouracil (5-FU) was used as standard to compare the efficiency of the nanoparticles, and the fresh medium containing 10% MTT reagent was used to replace media in the wells for 4 h incubation at 37 ºC. After incubation, crystals of formazan were dissolved in 100 µL of dimethyl sulfoxide (DMSO), and absorbance recorded using a Mindray MR-96 A microplate reader (Vacutec, Hamburg, Germany) at 570 nm in DMSO solution used as a blank. The analysis was done in triplicate and absorbance values obtained by calculating the average of the measurements. The calculation of cell viability was done using the Eq. (1) as reported by Mthana et al. [22].
2.6 Evaluation of Antioxidants Activity
The antioxidant analysis followed a similar procedure with previously reported studies [22, 24, 25] with modifications. The standard in vitro protocol for investigating the radical scavenging potential of ZnO nanoparticles was adopted. The deep violet coloured 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), which is considered one of the most utilized compounds for free radicals assay, was used to measure the antioxidant activity molecules [26]. The positive control used was ascorbic acid. In a volumetric flack, 12.8 mg of DPPH was mixed with 50 mL DMSO and the mixture properly shaken, wrapped in aluminum foil paper and kept in the dark for 30 min. About 10 mg of the ZnO nanoparticles and ascorbic acid were mixed with 10 mL DMSO and thoroughly sonicated. The scavenging activity was studied using different concentrations of 3.13, 6.25, 12.5, 25, and 50 µg/mL to assess the scavenging trend. The control sample was established by not adding the test samples (i.e., ZnO NPs). All the samples were prepared in triplicate. The absorbance was recorded at 540 nm in an AMR-Microplate. The IC50 values that represent effective concentration (for scavenging at 50%) for both standard and ZnO NPs were measured from the sigmoidal curve in the graph of percentage inhibition versus concentration, using linear regression analysis [27]. The inhibition percentage was calculated by using the Eq. (2).
where Ac is the absorbance of the control and As is the absorbance of the test sample (ZnO NPs and standard) [28, 29].
3 Results and Discussion
3.1 Synthesis and Characterization
The properties of nanoparticles are dependent on their shape, size, structure, and surface area dispersity. The observed colour change when the mucuna extract was added to the zinc acetate solution indicated the initial stage in the formation of ZnO NPs [30]. This reaction was observed because of the phytochemicals from the seed extract and their interaction with the Zn(II) ions in solution, which was responsible for the reduction of the precursor compound into the ZnO NPs [31].
3.2 X-ray Diffraction Patterns of ZnO Nanoparticles
The XRD patterns of the biosynthesized ZnO NPs (Fig. 2) showed eight diffraction peaks corresponding to (100), (002), (101), (102), (110), (103), (200) and (112) lattice planes [30]. The sharp peaks clearly indicated the crystalline nature of the biosynthesized ZnO NPs and corresponded to the reference pattern (JCPDS Card No: 00-036-1451) confirming the successful preparation of ZnO NPs [2]. The nanoparticle crystallite size was evaluated using the Scherrer’s Eq. (0.9λ)/(β cosθ) [32] and found to be 46.5 nm.
3.3 3 SEM, TEM Images and EDS Analysis Of ZnO Nanoparticles
The morphology and size of ZnO nanoparticles were studied using SEM and TEM techniques. The SEM image (Fig. 3a) showed spherically shaped nanoparticles with some level of agglomeration [33]. The agglomeration may be attributed to the polarity and electrostatic attraction between the ZnO NPs [8]. The TEM images also confirmed the spherical shape of the ZnO NPs (Fig. 3b), and the particle size distribution histogram (Fig. 3c) showed a size range from 21.60 to 47.16 nm, with an average of 30.50 nm, which is within the estimated range of the crystalline size from the XRD pattern. The EDS spectra (Fig. 3d) showed that the nanoparticle was composed of Zn and O, and confirmed the high purity for the synthesized ZnO NPs, with the carbon arising from the presence of the mucuna plant or the carbon tape on the surface of the sample holder [34].
3.4 4 UV–Vis Characterization
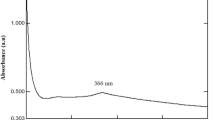
UV–vis spectra were measured using the ethanol solution of ZnO. The UV absorption spectrum presented in Fig. 4 shows a maximum absorption peak at 350 nm (3.54 eV), which indicates a blue shift with respect to the absorption maximum of bulk ZnO (~ 376 nm; 3.3 eV) and this could be ascribed to quantum confinement effects [35, 36]. This is confirmation of the successful synthesis of ZnO NPs. The direct band gap of the ZnO NPs was estimated from the Tauc graph of hv versus (αhν)2. Here, α is the absorption coefficient, and it is related to the band gap Eg as (αhν)2 = k(hν − Eg), where hν denotes the incident light energy and k is a constant. From this graph, the extrapolation of a straight line to the point where (αhν)2 = 0 is the estimated value of the band gap energy Eg. In the current study, the optical band gap (Eg) was estimated to be 3.75 eV, which denotes an increase in the band gap of the ZnO nanoparticles and a confirmation of reduction in particle size with respect to bulk ZnO. Similar studies have reported band gap energies of ZnO NPs of 4.52 and 3.40 eV using extracts of Ocimum tenuiflorum and Camellia sinensis [37, 38], and the variation in the band gap energy may be attributed to the difference in temperature of synthesis, volume of plant extract used, the concentration of precursors as well as type of extract used. Jayachandran et al. [39]. reported that different conditions of synthesis have various influences on the properties of ZnO NPs.
3.5 Anticancer Evaluation
Nanoparticles possess enormous potential as anticancer agents. Many studies have reported the substantial cytotoxicity property of ZnO NPs against various types of cancer cells compared to their macro-sized counterpart materials [40]. In the current study, ZnO NPs showed selective toxicity towards the cancerous cell lines and proved non-toxic to normal cells. Both cells treated with ZnO NPs showed > 50% decrease (Fig. 5) in cell viability at 100 µg mL− 1 concentration. These results showed the ability of ZnO NPs to impede the proliferation of cancer cells, and this effect was concentration-dependent. Similar results showing anticancer effects of bio-synthesized ZnO nanoparticles have previously been reported [41, 42]. The IC50 value of 50.44 µg mL− 1 (Table 1), for the obtained ZnO NPs exhibited a lower toxic effect than the 5-FU drug (17.48 µg mL− 1). Notwithstanding, other studies have demonstrated higher cytotoxic potential of green ZnO NPs in HeLa cells than that reported in our study. For example, ZnO NPs synthesized using the extract of Tagetes erecta flower displayed 50% cell viability at 26.53 µg mL− 1 [43]. In contrast to our results, the percentage cell viability was recorded at 88.6% at the highest conc. (100 µg mL− 1) when ZnO NPs were biosynthesized using A. arabica leaf extract [44]. In another study, the IC50 concentration required for Tradescantia pallida mediated ZnO NPs was 62.5 µg mL− 1 [45]. The discrepancies could be ascribed to differences in the concentrations of nanoparticles used. It could also be due to the variations in cellular density and the differences in the morphology and particle size of the NPs [39]. Further, it was noted in the HEK 293 cell lines that the cytotoxic impact of ZnO NPs increased with the increase in their concentration. The ZnO NPs used in this study exhibited higher cytotoxicity (Table 1) than those synthesized using Acantholimon serotinum extract that had an IC50 value of 60 µg mL− 1 [46]. Mkhize et al. [47]. reported 56 µg mL− 1 for Pleurotus ostreatus mushroom-mediated ZnO NPs. Also, ZnO NPs prepared from extract of Hertia intermedia had IC50 of 240 µg mL− 1 [48]. However, Holigarna grahamii mediated ZnO NPs displayed a higher cytotoxicity with an IC50 value of 9.060 µg mL− 1, which showed higher activity than the NPs reported in this study [49]. The variations in these reports for the HEK 293 cell lines may be attributed to properties including size of particles, surface area, and surface reactivity. Smaller-sized particles are reported to be more toxic than the larger particles [47], since they are able to penetrate the cellular walls.
3.6 Antioxidant Activity Analysis
The antioxidant activity of the green-synthesized ZnO NPs was assessed by evaluating their ability to scavenge free radicals generated by DPPH by measuring the reduction in the DPPH absorbance at 504 nm wavelength. The deep violet colour of the DPPH solution gradually changed to pale orange in the presence of ZnO nanoparticles and also with ascorbic acid (used as reference), which confirmed the antioxidant capacity of the nanoparticles, as previously observed [50]. Notably, the ZnO nanoparticles displayed better antioxidant potential (Table 2) compared to ascorbic acid, which may be attributed to the size of the nanoparticles and the phytochemicals in the mucuna plant extract used for nanoparticle synthesis [51]. These results agree with similar studies that reported higher antioxidant properties of ZnO NPs synthesized from the leaf extract of Tecoma castanifolia [52].
The results also indicated that as the concentration of DPPH increased, the maximum DPPH radical scavenging activities increased to 55.25% (Fig. 6). This implies that the free radical scavenging ability of the ZnO nanoparticles is dose-dependent. The good antioxidant potential of ZnO NPs relates to their lower IC50 value (Table 2) of 4.10 µg mL− 1 IC 50, which indicated quite a lower value than that of standard 4.72 µg mL− 1. Hence, the results of our study showed that the ZnO nanoparticles displayed higher antioxidant potency than the standard ascorbic acid which is in contrast to other reports by Chandra, Patel [27]. This could be attributed to the effect of size and shape of ZnO nanoparticles [53], as well as the effect of phytochemicals in mucuna seeds [54,55,56]. These results suggest that ZnO NPs could be used as a source of antioxidants in antioxidant-based therapies.
4 Conclusion
Zinc oxide nanoparticles, with average of size of 46.5 nm, were successfully synthesized by an environmentally friendly route using M. pruriens utilis seed extract. The crystallinity, morphological and optical properties, and composition of the ZnO NPs were determined by the different analytical techniques. The synthesized ZnO NPs showed good cytotoxic and antioxidant activities as they displayed significant percentage inhibition with IC50 values better than the reference compound. The results demonstrate the potential utility of the green synthesized ZnO NPs in different biological applications especially in tumor cells. Further research is needed to explore their applications at the in vivo level, and to ascertain the biochemical reactions and mechanisms responsible for the antioxidant and anticancer activities.
Change history
06 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42250-023-00764-7
References
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. The Scientific World Journal, 2014
Nguyen DTC, Le HT, Nguyen TT, Nguyen TTT, Bach LG, Nguyen TD, Van Tran T (2021) Multifunctional ZnO nanoparticles bio-fabricated from Canna indica L. flowers for seed germination, adsorption, and photocatalytic degradation of organic dyes. J Hazard Mater 420:126586
Chaudhuri SK, Malodia L (2017) Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: characterization and its evaluation on tree seedling growth in nursery stage. Appl Nanosci 7(8):501–512
Ukidave VV, Ingale LT (2022) Green Synthesis of Zinc Oxide Nanoparticles from Coriandrum sativum and Their Use as Fertilizer on Bengal Gram, Turkish Gram, and Green Gram Plant Growth International. Journal of Agronomy, 2022
García-López JI, Zavala-García F, Olivares-Sáenz E, Lira-Saldívar RH, Díaz E, Barriga-Castro NA, Ruiz-Torres (2018) Zinc oxide nanoparticles boosts phenolic compounds and antioxidant activity of Capsicum annuum L. during germination. Agronomy 8(10):215
Faisal AD, Ismail RA, Khalef WK, Salim ET (2020) Synthesis of ZnO nanorods on a silicon substrate via hydrothermal route for optoelectronic applications. Opt Quant Electron 52(4):1–12
Mbenga Y, Mthiyane M, Botha TL, Horn S, Pieters R, Wepener V, Onwudiwe DC (2022) Nanoarchitectonics of ZnO nanoparticles mediated by Extract of Tulbaghia Violacea and their cytotoxicity evaluation. J Inorg Organomet Polym Mater, p. 1–11
Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Rajesh K, Kyzas GZ (2020) Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct 1211:128107
Agarwal H, Menon S, Shanmugam VK (2020) Functionalization of zinc oxide nanoparticles using Mucuna pruriens and its antibacterial activity. Surf Interfaces 19:100521
Sardjono RE, Khoerunnisa F, Musthopa I, Khairunisa D, Suganda PA, Rachmawati R (2018) Synthesize of zinc nanoparticles using Indonesian velvet bean (Mucuna pruriens) extract and evaluate its potency in lowering catalepsy in mice. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing
Saranya G, Sruthi D, Jayakumar K, Jiby M, Nair RA, Pillai PP, Jayabaskaran C (2021) Polyphenol oxidase (PPO) arm of catecholamine pathway catalyzes the conversion of L-tyrosine to L-DOPA in Mucuna pruriens (L.) DC var. Pruriens: an integrated pathway analysis using field grown and in vitro callus cultures. Plant Physiol Biochem 166:1032–1043
Travlos I, Roussis I, Roditis C, Semini C, Rouvali L, Stasinopoulou P (2018) D. Bilalis, allelopathic potential of velvet bean against rigid ryegrass, Notulae Botanicae Horti Agrobotanici Cluj-Napoca, p 173–176.
Makhaye N, Aremu AO, Gruz J, Magadlela A (2021) Effects of soil nutrients and microbe symbiosis on the nutrient assimilation rates, growth carbon cost and phytochemicals in Mucuna pruriens (L.) DC. Acta Physiol Plant 43(12):1–11
Agbafor K, Nwachukwu N (2011) Phytochemical analysis and antioxidant property of leaf extracts of Vitex doniana and Mucuna pruriens. Biochemistry Research International, 2011
Pathania R, Chawla P, Khan H, Kaushik R, Khan MA (2020) An assessment of potential nutritive and medicinal properties of Mucuna pruriens: a natural food legume. Biotech 3(6):1–15
Theansungnoen T, Nitthikan N, Wilai M, Chaiwut P, Kiattisin K, Intharuksa A (2022) Phytochemical analysis and antioxidant, antimicrobial, and antiaging activities of ethanolic seed extracts of four Mucuna Species. Cosmetics 9(1):14
Dendup T, Prachyawarakorn V, Pansanit A, Mahidol C, Ruchirawat S, Kittakoop P (2014) α-Glucosidase inhibitory activities of isoflavanones, isoflavones, and pterocarpans from Mucuna pruriens. Planta medica 80(7):604–608
Lacerda RR, Moreira IC, Nascimento JSJd, Lacerda ACSd, Cabral NL, Lucetti DL (2015) C.A.d.A. Gadelha, Lectin isolated from Brazilian seeds of velvet bean (Mucuna pruriens (L) DC.) Presents analgesic, anti-inflammatory and antihemolytic action.
Spencer JP, Jenner A, Butler J, Aruoma OI, Dexter DT, Jenner P, Halliwell B (1996) Evaluation of the pro-oxidant and antioxidant actions of L-DOPA and dopamine in vitro: implications for Parkinson’s disease. Free Radic Res 24(2):95–105
Rajeshwar Y, Gupta M, Mazumder UK (2005) Antitumor activity and in vivo antioxidant status of Mucuna pruriens (Fabaceae) seeds against Ehrlich ascites carcinoma in swiss albino mice. Iran J Pharmacol Ther 4(1):46–40
Osuntokun J, Onwudiwe DC, Ebenso EE (2019) Green synthesis of ZnO nanoparticles using aqueous Brassica oleracea L. var. Italica and the photocatalytic activity. Green Chem Lett Rev 12(4):444–457
Mthana MS, Mthiyane DM, Onwudiwe DC, Singh M (2022) Biosynthesis of ZnO Nanoparticles using Capsicum chinense Fruit Extract and their in vitro cytotoxicity and antioxidant assay. Appl Sci 12(9):4451
Adeyemi JO, Onwudiwe DC, Nundkumar N, Singh M (2020) Diorganotin (IV) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study. Open Chem 18(1):453–462
Ajiboye TO, Imade EE, Oyewo OA, Onwudiwe DC (2022) Silver functionalized gC3N4: photocatalytic potency for chromium (VI) reduction, and evaluation of the antioxidant and antimicrobial properties. J Photochem Photobiol A 432:114107
Sharma A, Nagraik R, Sharma S, Sharma G, Pandey S, Azizov S, Kumar D (2022) Green synthesis of ZnO nanoparticles using Ficus palmata: antioxidant, antibacterial and antidiabetic studies. Results Chem 4:100509
Chinnathambi A, Ali Alharbi S, Joshi D, Lenin H (2022) Anticancer and Free Radical Scavenging Competence of Zinc Oxide Nanoparticles Synthesized by Aqueous Leaf Extract of Phyllanthus acidus Bioinorganic Chemistry and Applications, 2022
Chandra H, Patel D, Kumari P, Jangwan J, Yadav S (2019) Phyto-mediated synthesis of zinc oxide nanoparticles of Berberis aristata: characterization, antioxidant activity and antibacterial activity with special reference to urinary tract pathogens. Mater Sci Engineering: C 102:212–220
Dulta K, Koşarsoy Ağçeli G, Chauhan P, Jasrotia R, Chauhan P (2021) A novel approach of synthesis zinc oxide nanoparticles by bergenia ciliata rhizome extract: antibacterial and anticancer potential. J Inorg Organomet Polym Mater 31(1):180–190
Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM (2018) Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme and microbial technology 117:91–95
Dulta K, Koşarsoy Ağçeli G, Chauhan P, Jasrotia R, Chauhan P (2022) Ecofriendly Synthesis of Zinc Oxide Nanoparticles by Carica papaya leaf extract and their applications. J Cluster Sci 33(2):603–617
Islam F, Shohag S, Uddin MJ, Islam MR, Nafady MH, Akter A, Cavalu S (2022) Exploring the Journey of Zinc Oxide Nanoparticles (ZnO-NPs) toward Biomedical Applications. Materials 15(6):2160
Hargreaves J (2016) Some considerations related to the use of the Scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Struct Reactivity 2(1–4):33–37Catalysis
Ansari MA, Murali M, Prasad D, Alzohairy MA, Almatroudi A, Alomary MN, Ashwini andBS (2020) Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules 10(2):336
Shashanka R, Esgin H, Yilmaz VM, Caglar Y (2020) Fabrication and characterization of green synthesized ZnO nanoparticle based dye-sensitized solar cells. J Science: Adv Mater Devices 5(2):185–191
Gu Y, Kuskovsky IL, Yin M, O’Brien S, Neumark G (2004) Quantum confinement in ZnO nanorods. Appl Phys Lett 85(17):3833–3835
Debanath M, Karmakar S (2013) Study of blueshift of optical band gap in zinc oxide (ZnO) nanoparticles prepared by low-temperature wet chemical method. Mater Lett 111:116–119
Upadhyay P, Jain VK, Sharma S, Shrivastav A, Sharma R (2020) Green and chemically synthesized ZnO nanoparticles: A comparative study. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing
Dhanemozhi AC, Rajeswari V, Sathyajothi S (2017) Green synthesis of zinc oxide nanoparticle using green tea leaf extract for supercapacitor application Materials Today: Proceedings, 4(2): p. 660–667
Baek M, Kim M, Cho H, Lee J, Yu J, Chung H, Choi S (2011) Factors influencing the cytotoxicity of zinc oxide nanoparticles: particle size and surface charge. In: Journal of Physics: Conference Series. IOP Publishing
Hussein BY, Mohammed AM (2021) Green synthesis of ZnO nanoparticles in grape extract: Their application as anti-cancer and anti-bacterial Materials Today: Proceedings, 42: p. A18-A26
Gomathi R, Suhana H, Paradesi D (2021) Characterization study of cytotoxicity of Green synthesized ZnO nanoparticles loaded with Anti-Cancer Doxorubicin Drug. ChemistrySelect 6(18):4533–4538
Vijayakumar S, Chen J, Kalaiselvi V, Tungare K, Bhori M, González-Sánchez ZI (2022) Durán-Lara, Marine polysaccharide laminarin embedded ZnO nanoparticles and their based chitosan capped ZnO nanocomposites: synthesis, characterization and in vitro and in vivo toxicity assessment. Environ Res 213:113655
Ilangovan A, Venkatramanan A, Thangarajan P, Saravanan A, Rajendran S, Kaveri K (2021) Green Synthesis of Zinc Oxide Nanoparticles (ZnO NPs) using Aqueous Extract of Tagetes Erecta flower and evaluation of its antioxidant, Antimicrobial, and cytotoxic activities on HeLa cell line. Curr Biotechnol 10(1):61–76
Hayat S, Ashraf A, Zubair M, Aslam B, Siddique MH, Khurshid M, Naeem Z (2022) Biofabrication of ZnO nanoparticles using Acacia arabica leaf extract and their antibiofilm and antioxidant potential against foodborne pathogens. PLoS ONE 17(1):e0259190
Li C-y, Zhang Z-c, Mao J-y, Shi L-f, Zheng Y, Quan J-l (2017) Preparation of Tradescantia pallida-mediated zinc oxide nanoparticles and their activity against cervical cancer cell lines. Trop J Pharm Res 16(3):494–500
Karimzadeh MR, Soltanian S, Sheikhbahaei M, Mohamadi N (2020) Characterization and biological activities of synthesized zinc oxide nanoparticles using the extract of Acantholimon serotinum. Green Process Synthesis 9(1):722–733
Mkhize SS, Pooe OJ, Khoza S, Mongalo IN, Khan R, Simelane MBC (2022) Characterization and biological evaluation of Zinc Oxide Nanoparticles synthesized from Pleurotus ostreatus mushroom. Appl Sci 12(17):8563
Soltanian S, Sheikhbahaei M, Mohamadi N, Pabarja A, Abadi MFS, Tahroudi MHM (2021) Biosynthesis of zinc oxide nanoparticles using Hertia intermedia and evaluation of its cytotoxic and antimicrobial activities. BioNanoScience 11(2):245–255
Lokapur V, Jayakar V, Divakar M, Chalannavar RK, Lasrado L, Shantaram M (2022) ZnO nanoparticles with spectroscopically controlled morphology, bioinspired from Holigarna grahamii (Wight) Kurz and delving its antioxidant and anticancer potential on A498 cell line. Mater Today Commun 31:103338
Safawo T, Sandeep B, Pola S, Tadesse A (2018) Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) For antimicrobial and antioxidant activity assessment. OpenNano 3:56–63
Stan M, Popa A, Toloman D, Silipas T-D, Vodnar DC (2016) Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall Sinica (English Letters) 29:228–236
Sharmila G, Thirumarimurugan M, Muthukumaran C (2019) Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem J 145:578–587
Singh TA, Sharma A, Tejwan N, Ghosh N, Das J, Sil PC (2021) A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv Colloid Interface Sci 295:102495
Ravichandran V, Vasanthi S, Shalini S, Shah SAA, Harish R (2016) Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater Lett 180:264–267
Abdelbaky AS, Abd El-Mageed TA, Babalghith AO, Selim S, Mohamed AM (2022) Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 11(8):1444
More K GC-MS analysis and antioxidant potential of wild underutilized medicinally important legume, velvet bean (Mucuna pruriens L. DC.) Kamlakar C. MORE1*, Deepak B. SHELKE2*, Sunil TAYADE1, Prashant GAWANDE1, Hiralal B. SONAWANE3
Funding
Open access funding provided by North-West University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
On behalf of all authors, the corresponding author declares that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The first name “Doctor” of the author Doctor M. N. Mthiyane was omitted from the article and has now been added.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gamedze, N.P., Mthiyane, D.M.N., Mavengahama, S. et al. Biosynthesis of ZnO Nanoparticles Using the Aqueous Extract of Mucuna pruriens (utilis): Structural Characterization, and the Anticancer and Antioxidant Activities. Chemistry Africa 7, 219–228 (2024). https://doi.org/10.1007/s42250-023-00750-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00750-z