Abstract
A pigeon robot is an ideal experimental animal for research in flying animal robots. The majority of current research publications have entailed electrical stimulation of the motor nuclei to regulate movement forcibly, and although a “virtual fear” behavior model has been proposed, the structure, location, and function of the nuclei that generate fear emotions remain obscure. Previous studies have shown that the Stratum Griseum Periventriculare (SGP) of pigeons is homologous to the mammalian periaqueductal gray (PAG), which plays an essential role in mammalian fear. To reveal the role of fear mediated by the SGP in behavioral regulation, we evaluated the structure and location of the SGP by histologic identification combined with magnetic resonance imaging, and analyzed the behavior of the SGP by electrical stimulation. Finally, the function of the SGP was verified with escape testing and homing experiments in an open field. Our results showed that the SGP is located in the pigeon midbrain and divided into two subregions, the dorsal part of the stratum griseum periventriculare (SGPd) and the ventral part of the stratum griseum periventriculare (SGPv) (the ranges were AP1.5–4.75 mm, ML1.75–6.75 mm, and DV2.2–7.1 mm), and that wired and wireless electrical stimulation freezing was the dominant behavior. In the escape test, SGP electrical stimulation caused the pigeons to flee to a safe place, while in the open-field homing test, electrical stimulation of the SGP induced evasive behavior in pigeons away from their original homing route. These results confirm that the SGP plays a crucial role in fear, and that electrical stimulation of this nucleus induces corresponding fear behaviors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
An animal robot, also known as a cyborg, is an electronic device used to control the nervous system of living animals [1, 2]. In the past few decades, cyborgs have been created for different animals, including insects (Periplaneta Americana) [3, 4], beetles (Mecynorhina polyphemus and Mecynorhina torquata) [5], fish (Carassius auratus) [6], reptiles (Gekko gecko), mammals (rat) [7], and birds (Columba livia) [8, 9]. Cyborgs can be roughly divided into four types based on the area stimulated to provide animal movement regulation: stimulation of muscles [10, 11], stimulation of the sensory system, stimulation of motor-related nuclei in the brain [6, 8, 9], and stimulation of emotion-related nuclei [12, 13]. The method of using emotion to regulate behavior is effective, and not only strengthens behavior but also eliminates the defects caused by artificially forced interference with animal consciousness.
Rat cyborgs have been extensively studied and can complete various tasks according to human commands [13,14,15]. Talwar et al. induced the movement of rats by stimulating the Somatosensory Cortex (SI) of the sensory area of rats and the Medial Forebrain Bundle (MFB) belonging to the reward center. By stimulating the S1, rats mistakenly thought that they had encountered obstacles and changed the direction of their movements accordingly; and with SI stimulation, the rats would exhibit a sense of pleasure, and with long-term training, the motor behaviors of rats were linked with reward stimulation. In this way, the rats could achieve movement navigation in the complex path [13]. Zheng et al. applied fear emotion to regulation. They demonstrated that freezing behavior could be induced by stimulating the dorsolateral periaqueductal gray (dlPAG), which acts as the fear nucleus in rats. In this system, the behavioral states of the rats were visually sensed by a camera, and analyzed by a computer in real time, thus accomplishing the automatic training of the animal cyborg [12].
In contrast, far less research has been applied to principles of pigeon regulation. Although Su et al. proposed a regulation theory based on feeling, motivation, and emotion; most of the regulation methods in pigeons have not been applied to emotions [16]. The first pigeon cyborg experienced stimulation of the posterior part of the archistriatum and nucleus Dorsalis Intermedius Ventralis Anterior (DIVA), and would ascend into flight and turn left and right, respectively. Cai et al. explored more neural sites for pigeon cyborg regulation [8] and ascertained that the nucleus Intercollicularis (ICo), Locus Ceruleus (Loc), and the dorsalis part of the nucleus Lemnisci Lateralis (LLd) allowed pigeons to ascend and fly; and that the Formatio Reticularis Medialis Mesencephali (FRM) and Tractus Vestibulomesencephalicus (TVM) played important roles in turning behavior. Jang et al. demonstrated that pigeons were able to induce right and left body turns upon stimulation of the nuclei of the Tractus Occipito-mesencephalicus (OM), Nucleus Taeniae (TN), or Nucleus rotundus (RT); pigeons induced flight aviation for flapping and take-off when the nuclei of the Tractus Septo-mesencephalicus (TSM) or Archistriatum Ventrale (AV) were stimulated [17]. Zhou et al. also showed behavior control of pigeon cyborgs on a ground path by electrically stimulating their Posterior Amygdala (PoA) and DIVA [9]. Yang presented a novel, more efficient method that did not require training (virtual fear). These studies are principally devoted to the maintenance and control of animal motor function, but lack the involvement of pigeon emotion. The primary reason for this is that there is so little research on the emotional nucleus in pigeons [18].
Neuroscientists have long used homologous comparisons to explore the emotional nucleus of pigeons. Dubbeldam proposed that Central Mesencephalic Gray (GCt) in collared dove may be homologous to the PAG in mammals using immunohistochemical methods, and they presented a similar association in the emotion-motor pathway [19]. Kingsbury suggested that much of the ICo was homologous to the dorsal PAG using immunohistochemical comparisons in the finch [20], while Melleu et al. further studied the mesencephalic GCt–ICo complex in pigeons and indicated that SGPd may be functionally comparable to the mammalian PAG in a tonic immobility experiment [21]. From the above point of view, the SGP of the pigeon is homologous to the mammalian PAG. However, the structure and function of the SGP have not been well analyzed, and whether it can be used for regulating emotion and motivation remains unelucidated.
To address these problems, we first studied the structure and location of the SGP which provided assurance for the accuracy of electrode implantation in electrical stimulation experiments. The function of SGP was then explored by wired electrical stimulation. The narrow environment will limit the behavior of pigeons, so we designed a wireless electrical stimulation experiment to verify the role of SGP, on the other hand, the selection of the parameters of the wireless stimulator will also provide assistance for subsequent experiments. Next, we designed the escape test to demonstrate the role of SGP in fear, and finally we completed the navigation of the SGP to pigeons through the homing test. We used two wireless stimulators, a wireless current stimulator and a wearable behavior control system, as reported previously for pigeon cyborgs [22, 23].
2 Materials and Methods
2.1 Experimental Animals
Adult pigeons (Columba livia) were housed in single cages (cage size, 80 cm × 60 cm × 60 cm) under a normal day/night light cycle with food and water ad lib. This experiment was conducted in accordance with the Guide of Laboratory Animal Management Ordinance of China, and approved by the Animal Care and Use Committee of Zhengzhou University (Henan, China).
2.2 Structure of the SGP and Selection of Stimulation Sites
Adult pigeons were overdosed with 3% sodium pentobarbital (0.16 mL/0.1 kg) via pectoral intramuscular injection, and brains were removed and stored in 4% paraformaldehyde solution. Tissues were then transferred to 15% and 30% sucrose solutions in PBS and cut at 40 µm by frozen sectioning. Sections were stained with Nissl staining and compared with the pigeon brain atlas and images from magnetic resonance imaging (MRI).
For the Nissl staining, sections were fixed at 37 °C for 10–12 h; immersed in 100%, 95%, 80%, and 75% alcohol for de-fatting; and stained in 0.1% cresyl violet solution for 10–15 min at 37 °C. Following rinsing in distilled water, sections were sequentially dehydrated in 75%, 80%, 95%, and 100% ethyl alcohol for 2 min each. After clearing in xylene for 2 × 5 min, the sections were mounted with neutral resin and covered with glass coverslips. We referred to pigeon brain atlas by Karten and Hodos to calibrate the location and boundaries of nuclear clusters or structural regions [24]. A three-dimensional MRI-based atlas of the pigeon brain published by Güntürkün was used as coordinates to determine the range of nucleus [25].
The stimulation electrode was implanted using a stereotaxic apparatus. Six sites were randomly selected from the SGP, and three areas were implanted into each of the two subregions (SGPd and SGPv) (Table 1).
2.3 Electrode-implantation Surgery
Adult pigeons (380–450 g) were used in the electrical stimulation experiment. Pigeons were anesthetized with 3% sodium pentobarbital (0.14 mL/0.1 kg) via pectoral intramuscular injection, with the dose varying by body weight.
When the pain reflex disappeared after anesthesia, the pigeon’s head was affixed to a head adapter of the stereotaxic apparatus such that the orientation of the skull conformed to the pigeon atlas. Subcutaneous injection of local anesthetics was applied to the surgical field 15 min after anesthesia using a mixture of 2% lidocaine hydrochloride (0.1 mL). The skin above the surgical field was then removed carefully, followed by removal of the cranium with a cranial drill and removal of the dura and arachnoid, ultimately exposing the brain. The electrode was constructed of stainless steel wire (No. 304 Stainless Steel Wire, Φ0.1 mm, California Fine Wire Company, USA), which was implanted accurately into the target nucleus. After implantation, the electrodes were fixed with dental cement, and we disinfected the wounds around the head of the pigeon and applied an appropriate amount of erythromycin ointment to the damaged scalp. Seven days after the operation, the pigeon was fully recovered and moved about normally, and then executed the electrophysiologic stimulation experiment.
2.4 Electrical Stimulation Protocols
We implemented a cross-flow stimulus to reduce the impact of increased impedance, and applied the stimulus pattern in a two-way pulse. In this way, the damage caused by direct current stimulation to the target nucleus was alleviated, and in a two-way pulse, the total amount of charge output was zero and did not cause a charge difference between inside and outside the neuronal membrane. The wired and wireless experiments were performed with the same stimulus parameters: pulse number = 20, pulse width = 5 ms, wave interval = 50 ms, and stimulus intensity/pulse frequency = 16 Hz. However, a parametric difference was that the stimulus intensity of the former was 0.1–1.0 mA, while that of the latter was 0.3–1.0 mA.
2.5 Wired Electrical Stimulation Experiment
In order to study the function of SGP, we designed a wire stimulation experiment. The pigeon implanted with an electrode is connected to the YC-2 device (Chengdu, China) with a wire. We placed the pigeon in a behavior box cylinder (R = 80 cm, h = 80 cm), conducted an electrical stimulation experiment on the pigeon with the YC-2 device, recorded the changes in the pigeons’ movement behavior before and after the electrical stimulation, and analyzed the resulting videos.
2.6 The Wireless Electrical Stimulation Experiment Performed Indoors
In order to get rid of the interference of the size of the field and the wire to the behavior of pigeons, we designed the wireless stimulation experiment indoors. We removed the behavior box, placed the pigeons indoors, and loaded them with a wireless stimulator backpack. The signal transmitter (Bluetooth module) was then inserted into the computer equipped with the stimulus software, and the pigeon was placed in an open space. After 30 min, the pigeons gradually acclimated to the surroundings and moved freely, then began to perform the experiment. The entire process was tracked and recorded by a DV video recorder (SONY) and we analyzed the video.
2.7 Escape Test
We designed an escape test to prove that the behavior of electric stimulation of pigeons is caused by fear. The test was divided into two phases. During the early stage of experiment (adaptation period) the four experimental areas were filled with food, and the pigeons were placed into the experimental area and allowed to explore and adapt to the entire experimental area. In this period, there was no electrical stimulation to the four experimental areas, while during the later stage of experiment (experimental period), three of the four experimental areas were electrically stimulated and the other remained unstimulated. As long as pigeons stayed in the electrically stimulated area, they would receive microcurrent stimulation to the SGP every 5 s. The escape behavior of pigeons from the electrically stimulated area to the non-electrically stimulated area after the electrical stimulation was recorded and observed.
2.8 Homing Test in an Open Field
To illustrate the role of fear in flight, we designed a free-flying experimental paradigm for the pigeon cyborg. In this homing test, we chose a starting point away from the nest to allow the pigeons to form a stable homing direction. The experiment was divided into experimental and control groups. The pigeons in the two groups were reared together while undergoing surgery for implantation of the SGP stimulation electrode using the same remote stimulation equipment. During the experiment, the experimental group was then stimulated with a microcurrent on its way home, while the control group flew normally without any stimulation. We ultimately observed the pigeons’ real-time flight trajectory and change in flight direction after the stimulation.
2.9 Statistical Analysis
Data are expressed as Mean ± SEM. The significance of differences was analyzed by Student’s t test, the test was considered significant with a P value < 0.05.
3 Results
3.1 Structure of the SGP and Selection of Stimulation Sites
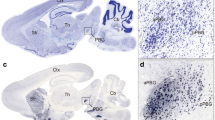
To describe the SGP in detail we employed different atlas levels to outline the SGP nucleus and its surrounding structures in Fig. 1a–n. These figures are shown in the Nissl coronal section of the pigeon brain at the anterior–posterior coordinates A1.50–A4.75.
SGP structure and schematic diagram of SGP subregion. a–n A series of coronal sections through the rostral to caudal regions that described the SGP nucleus started at approximately A1.50 and ended at A5.00 (the interval between photographs from A to N was 0.25 mm; scale bars = 2000 μm). o Schematic diagram of the six implant sites and SGPd/SGPv shown with MRIcroGL. The yellow area is SGPd and the green area is SGPv. The red diamond dots are the three implantation sites of SGPd, and the purple round dots are the three implantation sites of SGPv
The SGP nucleus is localized in the midbrain, encases a ventricle, begin to appears at A1.50, and vanishes by A4.75; structurally, the SGP is oblate, long, and hollow. Beginning at anterior positions and moving posteriorly, and due to the presence of the ventricles at A1.75, we divided the SGP into the following subregions: the dorsal part of the SGPd and the SGPv. The SGPd is located above the ventricle and extends into the GCt. Outside the SGPd are gray matter structures such as SAC, SGC, SGF and SOp. The SGPv is located below the ventricle and is closely adjacent to MLD and ICO. The nuclei under SGPv includes Imc, Ipc and SP.
In order to better evaluate the function of the SGP nucleus, we randomly selected six sites where the electrodes were implanted—three in the SGPd and three in the SGPv (Fig. 1o, Table 1). The coordinates were obtained with reference to the atlas of the pigeon brain and the three-dimensional digital atlas [25].
3.2 Effects of Electrical Stimulation on Pigeon Behavior
A series of differential motor behaviors were obtained in this study by electrical stimulation within the SGP, both in wired- and wireless experiments. The induced motor behaviors included freezing, taking off, running forward, and turning. We divided these behaviors into three categories for convenience in conducting behavioral analyses, and principally focused on freezing and moving behaviors (Table 2).
The pigeon (n = 18) was placed in the behavior box. After the pigeon became familiar with the environment, the freely moving pigeon was subjected to electric stimulation experiment (Fig. 2a). The intensity of the stimulus was 0.1–1.0 mA. Each intensity of electrical stimulation was given three times, and the interval of each stimulation was at least 1 min. The freely moving pigeons induced freezing behavior in all these six sites by wire electrical stimulation. In all electrical stimulation experiments, the freezing response rate was 45% and 46% in SGPd and SGPv, respectively (Fig. 2f, g). This result showed that both SGPd and SGPv could cause the pigeon to be frozen under electric stimulation, and the SGPv is slightly more effective than SGPd. To evaluate the function of the subregion, the two subregions’ regulation of freezing behavior was not significant (P = 0.6983) (Fig. 2h). Our result indicated that the optimal stimulus parameter is 0.5 mA, the response rates of freezing behaviors are generally higher than those of other behaviors. When the electric stimulation intensity was 0.5 mA, the freezing response rate reached 63.42% and 66.67% in SGPd and SGPv, respectively (Fig. 2d, e). While the current intensity was too high (more than or equal to 0.8 mA) or too low (less than or equal to 0.3 mA), the freezing behavior of pigeons was not obvious.
Wired electrical stimulation modulates pigeon freezing behavior. a Schematic diagram of wired electrical stimulation experiment. b Schematic diagram of electrode implantation in the brain of a pigeon robot. c The freezing behavior was induced by wired electrical stimulation (C1–C8); when the blue light went on, the electrical stimulation began (C5) and proceeded to the end of electrical stimulation (C8). d The frequency of behavior that stimulated the SGPd. e The frequency of behavior that stimulated SGPv. f The proportion of wire stimulation behavior in the SGPd. g The proportion of wire stimulation behavior in the SGPv. h Comparison of freezing behavior in the SGPd and SGPv (the two subregions were not significantly different with respect to freezing (t test, P = 0.6983)
On the contrary, the movement behavior reached 58.85% and 66.67% when the electric stimulation intensity was 0.9 mA and 0.1 mA in SGPd and SGPv, respectively. The other behavior is generally lower than those of freezing and movement behaviors, which the highest response rates being only 27.45% and 11.11% in SGPd and SGPv, respectively.
3.3 Optimization of Pigeon Cyborg Indoor
Compared with the experiment of wire electric stimulation, pigeons have more space to move in the wireless stimulation experiment, which plays a decisive role in verifying specific nuclear groups of pigeons.
In this experiment, pigeons implanted with the electrode were loaded with an 18 g wireless receiver; this will not affect the free movement of pigeons (Fig. 3a). The electrical stimulation experiment began after the pigeon adapted to its surroundings. The parameters of electric stimulation are consistent with wire electrical stimulation, the difference was that only 0.3–1.0 mA as the current selected for electrical stimulation. Pigeons behave in various ways when stimulated wirelessly, but freezing behavior is the primary behavior. Our results showed that both the SGPd/SGPv could induce freezing behavior in pigeons and that the optimal electric stimulation intensities were 0.5 mA and 0.6 mA, respectively. At this intensity, the SGPd’s freezing response rate was 50%, and the SGPv’s response rate was 66.67% (Fig. 3d, e).
Indoor testing of pigeon robots. a Schematic diagram of wireless electrical stimulation experiment. b Magnification of the brain of the pigeon robot shows the implantation site. c The freezing behavior was induced by wireless electrical stimulation (C1–C8). The blue light continued to indicate that the device was communicating well; and when the red light was activated, the electrical stimulation began (C6) and proceeded to the end of the electrical stimulation (C8). d The freezing frequency of the SGPd under different electrical stimulation intensities. e The freezing frequency of the SGPv under different electrical stimulation intensities
These results were similar to the influence of wired stimulation, indicating that the subregional function of the SGP was consistent, and that the SGP was closely related to the freezing behavior of pigeons. Under intensities of 0.5 mA and 0.6 mA, the most suitable nerve transmission signals of pigeon were simulated, achieving the optimal electric-stimulation effect.
3.4 Escape Behavior of the Pigeon Cyborg
In this experiment, we measured the behavioral response rate of pigeons after electric stimulation and the success rate of escape to the non-stimulation area. Our data revealed no significant difference between the response rate of pigeons in the early- and later-stage experiment, indicating that different pigeons could receive electrical stimuli and produce behavior (Fig. 4e). The success rate of pigeons escaping to the no-stimulus area after electric stimulation increased gradually with the development of the experiment, and we noted a significant difference in the success rates between the early and late stages of the experiment (Fig. 4d). These results indicated that the pigeons were able to find a safe area after a period of learning, and also confirmed that SGP stimulation produced fear in pigeons and induced flight behavior.
The escape behavior of the pigeon robot. a Schematic diagram of the escape test. b The trajectory of the pigeons in the early experiment. c The trajectory of the pigeons in the late stage of the experiment. The red dots in the figure represent the actual movement of the pigeons, and the density of the red dots indicates the duration of the pigeons’ stay at the location. d The success rate of pigeons escaping to safety after electrical stumulation (P = 0.0026). e Behavioral response rate of pigeons to electrical stimulation (P = 0.8770)
3.5 Homing Test of the Pigeon Cyborg in Open Space
We released pigeons that had undergone surgery and that were equipped with remote stimulation devices into a three-dimensional, obstacle-free-flying environment at the starting point. Moreover, we observed that after stimulating a pigeon’s SGP on their way home, the pigeons deviated from the stable direction of home and swerved toward the left, indicating that the pigeons felt virtual fear generated by the electrical stimulation and redirected their flying activity to the left so as to avoid any perceived threat. As the stimulus increased, experimental pigeons deviated farther away from home (Fig. 5, solid red line), while pigeons in the control group, which were not stimulated, continued to fly in a steady homing direction (Fig. 5, solid blue line).
Homing test in open space. A redrawn road map using Amap (Auto Navi Map). The red raindrop-shaped dots indicate the position of the pigeons. The red solid line shows the flight trajectory generated by electrical stimulation during homing in real time, and the red circles indicate the start of the stimulus. The red dotted line indicates the way home without stimulation, the blue solid line shows the flight trajectory without electrical stimulation, and the arrow represents the direction of a steady direction home (scale bar = 50 m)
4 Discussion
The pigeon robot constitutes a model of intense interest in the area of animal robotics. Although theories based on emotion and motivation have been proposed for the pigeon, emotion-related nuclei have still not been accurately identified. Our results indicated that the SGP is a crucial nucleus for fear processing in pigeons, and that its stimulation generates fear behavior, which can then be applied to the behavioral regulation of pigeon robots.
Pigeons comprise a model species for animal robot research. If it is necessary to precisely control the motion of pigeon robot, a clear understanding of brain structure is the basis of research. The research on pigeon brain focuses on the afferent and efferent of nucleus and the distribution of neurotransmitters [26,27,28]. Pigeon brain structure studies include the hippocampus, PoA, central caudal nidopallium (NCC), basal ganglia, cerebellum and midbrain, which are more finely divided into subregions [29,30,31,32,33,34]. In addition, there are methods based on MRI and Functional Ultrasound to study brain structure [25, 35]. However, there is a lack of research on emotional nuclei. Through an extensive review of papers, we found that SGP and PAG are homologous and may be involved in the regulatory pathway(s) of fear in pigeons. We herein studied the structure of the SGP, and combined histologic and magnetic MRI to characterize and localize its structures, facilitating and corroborating our follow-up and subsequent electrical stimulation experiments.
Papini suggested that we cannot directly study animal emotions yet, because we do not have access to the subjective experience of organisms. Therefore, we must rely on an animal’s behavior and physiology to judge its emotional expression. Therefore, the formulated mammalian emotional criteria will be applied to pigeons [36]. We explored the function of the SGP in wired electrical stimulation experiments. When we electrically stimulated the pigeons, we found that they exhibited various behaviors such as continuous movement, wing spreading, and freezing. These behaviors can be interpreted as strategies that animals use in the face of fear—including fight, flight, and freezing [37,38,39]. Given that these behaviors occur, it is possible that the behavior box may have limited the pigeons’ movements. In addition, the control of the pigeon robot also needs the help of wireless devices; we, therefore, removed the behavior box and let the pigeons walk freely in the room. Via a wireless electrical stimulation experiment, we uncovered that the motor patterns of both the SGPd and SGPv were similar to those of wired stimulation, and that there was no difference in the freezing behavior between the two subregions (P = 0.6983). This result, on the one hand, proved that SGP can regulate pigeons’ behavior, and on the other hand, the parameters of wireless electrical stimulation experiment also provide help for subsequent experiments.
Animals in a state of fear will produce escape and avoidance behavior [40, 41]. In order to confirm that the behavior induced by SGP is caused by fear, we designed an escape experiment. During the early stage of the experiment when pigeons received SGP stimulation in the electrical stimulation area, the birds stayed away from their current position and avoided direction without purpose; thus, they did not directly flee to the non-stimulation location. However, later in the experiment when the pigeons received SGP stimulation in the electrical stimulation area, they escaped to the non-stimulated area immediately. The pigeons’ flight to safety further supported the function of the SGP in the processing of fear. These results were consistent with the defensive responses observed by other researchers in the cat and mouse with PAG stimulation [42, 43]. The pigeon SGP is thus an emotion-related brain region that can be used to regulate the movement of a pigeon robot.
To better explain the behavior of the SGP in regulating pigeons in three-dimensional space, we conducted a homing test. Homing is a pigeon’s natural ability to find its way wherever it is [44, 45]. But when we electrically stimulated the SGP, pigeons stayed away from the homing route. This action was likely due to the electrical stimulation inducing fear in the pigeon, leading it to believe that the way home was dangerous. These results revealed that pigeons primarily showed avoidance behavior when they felt danger. These behaviors demonstrated that pigeons would show the avoidance strategy when they felt danger during flight, although this behavior did not directly regulate movement, it provided a cognitive impact.
Investigative research on pigeon cyborgs still requires further exploration, and developments in avian neuroscience are crucial to the study of the pigeon cyborg. In robotic control, brain structure is the basis of brain function; and although the telencephalon of birds has been renamed, other brain regions remain less studied, and other nuclei with functions similar to the SGP cannot be discounted [18, 46]. In addition, novel regulatory modalities will also occupy a key role in the future. For example, optogenetics is used to regulate the movement of mice, and the pigeon robot would constitute an excellent control scheme if it were to be applied in a similar fashion.
5 Conclusion
In this study, the structure and location of SGP were studied by histologic methods and MRI software, which provided a guarantee for electrical stimulation implantation in all subsequent experiments; then, the function of SGP was explored by wired electrical stimulation; then, wireless electrical stimulation not only confirmed the function of SGP, but also provided parametric support for subsequent pigeon navigation; next, through escape experiment, it was confirmed that the behavior after electrical stimulation was caused by fear; finally, the homing experiment confirmed that SGP is an important nucleus to deal with fear emotions, and suggested that it is possible to apply emotional brain regions to pigeon cyborg. Compared with the previous studies on forced control of pigeons’ behavior, the control method in this study eliminates the interference of human compulsion on the pigeon’s consciousness, but uses the mood of the pigeon to make its own judgment and generate corresponding behaviors. This provides a new brain nucleus and a new idea for closed-loop regulation of pigeon cyborg. Our series of experiments suggest that SGP is a nucleus of fear, but we need to use more mammalian methods to study fear in pigeons from innate fear and learned fear [47]. We suspect that there will be more other nuclei with similar functions to SGP, and the structure of these nuclei can be studied by other methods, such as neural circuit and neurotransmitter distribution. In addition, with the development of methods for regulating movement, optogenetics technology and chemical genetics can also be used to precisely control the activity of neurons, which will become the goal of joint efforts of researchers in the field of animal robots.
Data Availability
The data that support the finding of this study are available from the corresponding author upon reasonable request.
Abbreviations
- SGPd:
-
The dorsal part of the stratum griseum periventriculare
- SGPv:
-
The ventral part of the stratum griseum periventriculare
- SGP:
-
The stratum griseum periventriculare
- SAC:
-
Stratum album central
- SGC:
-
Stratum griseum central
- SGF:
-
Stratum griseum et fibrosum superficiale
- SOp:
-
Stratum opticum
- Ipc:
-
Nucleus isthmi, pars parvocellularis
- Imc:
-
Nucleus isthmi, pars magnocellularis
- V:
-
Ventriculus
- ICo:
-
Nucleus intercollicularis
- MLd:
-
Nucleus mesencephalicus lateralis, pars dorsalis
- MLv:
-
Nucleus mesencephalicus lateralis, pars ventralis
- FRL:
-
Formatio reticularis lateralis mesencephali
- GCt:
-
Substantia grisea centralis
- ToS:
-
Torus semicircularis
- SP:
-
Nucleus subpretectalis
- EM:
-
Nucleus ectomamillaris
- PT:
-
Nucleus retundus
- PST:
-
Tractus pretecto-subpretectalis
- IPs:
-
Nucleus interstitio-pretecto-subpretectalis
- NCC:
-
Central caudal nidopallium
References
Service, R. F. (2017). Bioelectronics herald the rise of the cyborg. Science, 358(6368), 1233–1234. https://doi.org/10.1126/science.358.6368.1233
Wu, Z. H., Zhou, Y. D., Shi, Z. Z., Zhang, C. S., Li, G. L., Zheng, X. X., Zheng, N. G., & Pan, G. (2016). Cyborg intelligence: Recent progress and future directions. IEEE Intelligent Systems, 31(6), 44–50. https://doi.org/10.1109/MIS.2016.105
Holzer, R., & Shimoyama, I. (1997). Locomotion control of a bio-robotic system via electric stimulation. IEEE/RSJ International Conference on Intelligent Robots & Systems. https://doi.org/10.1109/IROS.1997.656559
Sato, H. (2009). Remote radio control of insect flight. Frontiers in Integrative Neuroscience, 3, 24. https://doi.org/10.3389/neuro.07.024.2009
Sato, H., Peeri, Y., Baghoomian, E., Berry, C. W., & Maharbiz, M. M. (2009). Radio-controlled cyborg beetles: A radio-frequency system for insect neural flight control. IEEE. https://doi.org/10.1109/MEMSYS.2009.4805357
Kobayashi, N., Yoshida, M., Matsumoto, N., & Uematsu, K. (2009). Artificial control of swimming in goldfish by brain stimulation: Confirmation of the midbrain nuclei as the swimming center. Neuroscience Letters, 452(1), 42–46. https://doi.org/10.1016/j.neulet.2009.01.035
Zhang, J. C., Xu, K. D., Zhang, S. M., Wang, Y. M., Zheng, N. G., Pan, G., Chen, W. D., Wu, Z. H., & Zheng, X. X. (2019). Brain-machine interface-based rat-robot behavior control. Advances in Experimental Medicine and Biology, 1101, 123–147. https://doi.org/10.1007/978-981-13-2050-7_5
Cai, L., Dai, Z. D., Wang, W. B., Wang, H., & Tang, Y. Z. (2015). Modulating motor behaviors by electrical stimulation of specific nuclei in pigeons. Journal of Bionic Engineering, 12(4), 555–564. https://doi.org/10.1016/S1672-6529(14)60145-1
Zhou, Z. Y., Liu, D. H., Sun, H., Xu, W. B., Tian, X. M., Li, X. Y., Cheng, H., & Wang, Z. L. (2021). Pigeon robot for navigation guided by remote control: System construction and functional verification. Journal of Bionic Engineering, 18(1), 184–196. https://doi.org/10.1007/s42235-021-0013-3
Cao, F., Zhang, C., Choo, H. Y., & Sato, H. (2016). Insect–computer hybrid legged robot with user-adjustable speed, step length and walking gait. Journal of the Royal Society Interface, 13(116), 20160060. https://doi.org/10.1098/rsif.2016.0060
Sanchez, C. J., Chiu, C. W., Zhou, Y., Gonzalez, J. M., Vinson, S. B., & Liang, H. (2015). Locomotion control of hybrid cockroach robots. Journal of the Royal Society Interface, 12(105), 20141363. https://doi.org/10.1098/rsif.2014.1363
Lin, J., Yu, C., Jia, J., Zhang, S., Wang, Y., Chen, W., & Zheng, X. (2010). Using dlpag-evoked immobile behavior in animal-robotics navigation. International Conference on Computer Science & Education. https://doi.org/10.1109/ICCSE.2010.5593729
Talwar, S. K., Xu, S., Hawley, E. S., Weiss, S. A., Moxon, K. A., & Chapin, J. K. (2002). Rat navigation guided by remote control. Nature, 417(6884), 37–38. https://doi.org/10.1038/417037a
Koh, C. S., Park, H., Shin, J., Kong, C., Park, M., Seo, I., Koo, B., Jung, H. H., Chang, J. W., & Shin, H. (2020). A novel rat robot controlled by electrical stimulation of the nigrostriatal pathway. Neurosurgical Focus, 49(1), E11. https://doi.org/10.3171/2020.4.FOCUS20150
Yu, Y. P., Pan, G., Gong, Y. Y., Xu, K. D., Zheng, N. G., Hua, W. D., Zheng, X. X., & Wu, Z. H. (2016). Intelligence-augmented rat cyborgs in maze solving. PLoS ONE, 11(2), e147754. https://doi.org/10.1371/journal.pone.0147754
Su, X. C., Huai, R. T., Yang, J. Q., Wang, H., & Lv, C. Z. (2012). Brain mechanism and methods for robo-animal motor behavior control. Scientia Sinica (Informationis), 42(9), 1130–1146. https://doi.org/10.1360/112012-522
Jang, J., Baek, C., Kim, S., Lee, T., Choi, G. J., Shim, S., Yun, S., Jung, Y., Lee, C. E., Ko, S., Seo, K., Seo, J. M., Won, M. H., Kim, S. J., & Song, Y. K. (2021). Current stimulation of the midbrain nucleus in pigeons for avian flight control. Micromachines, 12(7), 788. https://doi.org/10.3390/mi12070788
Yang, J. Q., Huai, R. T., Wang, H., Lv, C. Z., & Su, X. C. (2015). A robo-pigeon based on an innovative multi-mode telestimulation system. Bio-Medical Materials and Engineering, 26(Suppl 1), S357–S363. https://doi.org/10.3233/BME-151323
Dubbeldam, J. L., & den Boer-Visser, A. M. (2002). The central mesencephalic grey in birds: Nucleus intercollicularis and substantia grisea centralis. Brain Research Bulletin, 57(3–4), 349–352. https://doi.org/10.1016/s0361-9230(01)00689-x
Kingsbury, M. A., Kelly, A. M., Schrock, S. E., & Goodson, J. L. (2011). Mammal-like organization of the avian midbrain central gray and a reappraisal of the intercollicular nucleus. PLoS ONE, 6(6), e20720. https://doi.org/10.1371/journal.pone.0020720
Melleu, F. F., Lino-de-Oliveira, C., & Marino-Neto, J. (2016). The mesencephalic GCt–ICo complex and tonic immobility in pigeons (Columba livia): A c-fos study. Brain Structure and Function, 222, 1253–1265. https://doi.org/10.1007/s00429-016-1275-0
Yang, L., Li, M. M., Wan, H., & Shang, Z. G. (2019). The wearable behavior control system for robo-animal. Chinese Automation Congress (CAC), 2019, 5777–5780. https://doi.org/10.1109/CAC48633.2019.8996922
Zhao, K., Wan, H., Shang, Z. G., Liu, X. Y., & Liu, L. (2019). Intracortical microstimulation parameters modulate flight behavior in pigeon. Journal of Integrative Neuroscience, 18(1), 23–32. https://doi.org/10.31083/j.jin.2019.01.14
Karten, H. J., & Hodos, W. (1968). A stereotaxic atlas of the pigeon brain. The American Journal of Psychology, 81(2), 289–290. https://doi.org/10.2307/1421283
Güntürkün, O., Verhoye, M., De Groof, G., & Van der Linden, A. (2013). A 3-dimensional digital atlas of the ascending sensory and the descending motor systems in the pigeon brain. Brain Structure and Function, 218(1), 269–281. https://doi.org/10.1007/s00429-012-0400-y
Atoji, Y. (2016). Gene expression of ionotropic glutamate receptor subunits in the tectofugal pathway of the pigeon. Neuroscience, 316, 367–377. https://doi.org/10.1016/j.neuroscience.2015.12.032
Atoji, Y., & Sarkar, S. (2019). Localization of ampa, kainate, and nmda receptor mrnas in the pigeon cerebellum. Journal of Chemical Neuroanatomy, 98, 71–79. https://doi.org/10.1016/j.jchemneu.2019.04.004
Atoji, Y., & Wild, J. M. (2004). Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. The Journal of Comparative Neurology, 475(3), 426–461. https://doi.org/10.1002/cne.20186
Atoji, Y., Saito, S., & Wild, J. M. (2006). Fiber connections of the compact division of the posterior pallial amygdala and lateral part of the bed nucleus of the stria terminalis in the pigeon (Columba livia). The Journal of Comparative Neurology, 499(2), 161–182. https://doi.org/10.1002/cne.21042
Atoji, Y., Sarkar, S., & Wild, J. M. (2018). Differential projections of the densocellular and intermediate parts of the hyperpallium in the pigeon (Columba livia). Journal of Comparative Neurology, 526(1), 146–165. https://doi.org/10.1002/cne.24328
Atoji, Y., & Wild, J. M. (2006). Anatomy of the avian hippocampal formation. Reviews in the Neurosciences, 17(1–2), 3. https://doi.org/10.1515/revneuro.2006.17.1-2.3
Atoji, Y., & Wild, J. M. (2009). Afferent and efferent projections of the central caudal nidopallium in the pigeon (Columba livia). The Journal of Comparative Neurology, 517(3), 350–370. https://doi.org/10.1002/cne.22146
Bruce, L. L., Erichsen, J. T., & Reiner, A. (2016). Neurochemical compartmentalization within the pigeon basal ganglia. Journal of Chemical Neuroanatomy, 78, 65–86. https://doi.org/10.1016/j.jchemneu.2016.08.005
Pakan, J. M., & Wylie, D. R. (2006). Two optic flow pathways from the pretectal nucleus lentiformis mesencephali to the cerebellum in pigeons (Columba livia). The Journal of Comparative Neurology, 499(5), 732–744. https://doi.org/10.1002/cne.21108
Rau, R., Kruizinga, P., Mastik, F., Belau, M., de Jong, N., Bosch, J. G., Scheffer, W., & Maret, G. (2018). 3D functional ultrasound imaging of pigeons. NeuroImage, 183, 469–477. https://doi.org/10.1016/j.neuroimage.2018.08.014
Papini, M. R., Penagos-Corzo, J. C., & Pérez-Acosta, A. M. (2019). Avian emotions: Comparative perspectives on fear and frustration. Frontiers in Psychology. https://doi.org/10.3389/fpsyg.2018.02707
Bracha, H. S. (2004). Freeze, flight, fight, fright, faint: Adaptationist perspectives on the acute stress response spectrum. CNS Spectrums, 9(9), 679. https://doi.org/10.1017/S1092852900001954
Darwin, C., & Maudsley, H. (1978). Review of the expression of emotions in man and animals; The pathology of mind, by C. Darwin & H. Maudsley. The American Journal of Psychology, 94(1), 181–182. https://doi.org/10.2307/1422356
Floyd, N. S., Price, J. L., Ferry, A. T., Keay, K. A., & Bandler, R. (2000). Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. Journal of Comparative Neurology, 422(4), 556–578. https://doi.org/10.1002/1096-9861(20000710)422:4%3c556::aid-cne6%3e3.0.co;2-u
Rozeske, R. R., Jercog, D., Karalis, N., Chaudun, F., Khoder, S., Girard, D., Winke, N., & Herry, C. (2018). Prefrontal-periaqueductal gray-projecting neurons mediate context fear discrimination. Neuron, 97(4), 898–910. https://doi.org/10.1016/j.neuron.2017.12.044
Wang, W., Schuette, P. J., Nagai, J., Tobias, B. C., Cuccovia, V., Reis, F. M., Ji, S., de Lima, M. A. X., La-Vu, M. Q., Maesta-Pereira, S., Chakerian, M., Leonard, S. J., Lin, L., Severino, A. L., Cahill, C. M., Canteras, N. S., Khakh, B. S., Kao, J. C., & Adhikari, A. (2021). Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats. Neuron, 109(11), 1848–1860. https://doi.org/10.1016/j.neuron.2021.03.033
Bandler, R., & Shipley, M. T. (1994). Columnar organization in the midbrain periaqueductal gray: Modules for emotional expression? Trends in Neurosciences, 17(9), 379–389. https://doi.org/10.1016/0166-2236(94)90047-7
Behbehani, M. M. (1995). Functional characteristics of the midbrain periaqueductal gray. Progress in Neurobiology, 46(6), 575–605. https://doi.org/10.1016/0301-0082(95)00009-K
Hough, G. E. (2022). Neural substrates of homing pigeon spatial navigation: Results from electrophysiology studies. Frontiers in Psychology, 13, 867939. https://doi.org/10.3389/fpsyg.2022.867939
Shao, Y., Tian, H., Zhang, J. J., Kharrati-Koopaee, H., Guo, X., Zhuang, X. L., Li, M. L., Nanaie, H. A., Dehghani Tafti, E., Shojaei, B., Reza Namavar, M., Sotoudeh, N., Oluwakemi Ayoola, A., Li, J. L., Liang, B., Esmailizadeh, A., Wang, S., & Wu, D. D. (2020). Genomic and phenotypic analyses reveal mechanisms underlying homing ability in pigeon. Molecular Biology and Evolution, 37(1), 134–148. https://doi.org/10.1093/molbev/msz208
Reiner, A., Perkel, D. J., Bruce, L. L., Butler, A. B., Csillag, A., Kuenzel, W., Medina, L., Paxinos, G., Shimizu, T., Striedter, G., Wild, M., Ball, G. F., Durand, S., Gütürkün, O., Lee, D. W., Mello, C. V., Powers, A., White, S. A., Hough, G., … Jarvis, E. D. (2004). Revised nomenclature for avian telencephalon and some related brainstem nuclei. The Journal of Comparative Neurology, 473(3), 377–414. https://doi.org/10.1002/cne.20118
Gross, C. T., & Canteras, N. S. (2012). The many paths to fear. Nature Reviews Neuroscience, 13(9), 651–658. https://doi.org/10.1038/nrn3301
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
WX conducted most of the experiments and wrote the manuscript, LY conducted some experiments and collected data, ZW collected and collated the data, LY conducted some experiments, HC was responsible for animal resources, SZ management and coordination responsibility for the research activity planning, ZS provided the software, and ZW designed the experiments. All the authors approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest relevant to this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, W., Yang, L., Wang, Z. et al. Stratum Griseum Periventriculare-mediated Fear Emotion Regulates Motor Behavior in Pigeons. J Bionic Eng 20, 2228–2239 (2023). https://doi.org/10.1007/s42235-023-00382-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42235-023-00382-6