Abstract
Root rot is a destructive soil-borne disease of Coptis chinensis, which depends on chemical control at present, and more attention should be paid to biocontrol of disease. In the present research, isolate Y9 isolated from healthy root samples of Coptis, was identified as Bacillus cereus. Further screening and pot experiments showed that B. cereus isolate Y9 inhibited the growth of the main causal agents of coptis root rot disease (Fusarium solani and F. avenaceum) and seven other phytopathogenic fungi. The application of B. cereus isolate Y9 and compatible Trichoderma harzianum, T. atroviride and B. amyloliquefaciens, singly and in combination were found to be effective against Fusarium root rot in vitro and in field experiments. In field experiments, combinations of T. harzianum + B. amyloliquefaciens + Y9 (HYJ, in ratio of 1:1:1) showed the highest control efficacy of 63.85%, which was higher than the expected value (53.18%), indicating synergistic effect on the control of coptis root rot. Therefore, B. cereus isolate Y9 may be a potential biological control agent, and combined use with T. harzianum and B. amyloquefaciens offered even greater potential. The long-term effects of isolate B. cereus Y9 and its combinations on C. chinensis should be assessed in different locations and seasons in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese goldthread (Coptidis rhizoma), a widely used traditional Chinese medicine, is the dried rhizome of Coptis chinensis Franch., C. deltoidea C. Y. Cheng et Hsiao or C.teeta Wall., commonly known as ‘Wei-lian’, ‘Ya-lian’ or ‘Yun-lian’, respectively (Commission 2020) (p. 177). The Shizhu county, Chongqing, produces morethan 60% of the Chinese goldthread, which has been exported to many countries and regions.The main medicinal active ingredients of Chinese goldthread are alkaloids, such as berberine, epiberberine, coptisine, palmatine chloride, columbamine, thaliotrine and jatrorrhizine. These alkaloid compounds are reported to have a wide range of pharmacological activities, such as antimicrobial activity, antiinflammatory, antiatherosclerotic, antidiabetic, antitumor effects, antiviral, dispelling dampness, and detoxification agents (Song et al. 2023). However, root rot significantly reduced the content of these alkaloids (except jatrorrhizine), thus seriously affecting the quality of Chinese goldthread (Song et al. 2023). Root rot is a severe soil-borne disease, and Fusarium spp. (Luo et al. 2014; Cheng et al. 2020; Wu et al. 2020; Mei et al. 2021b), Diaporthe spp. (Mei et al. 2021a), Pythium spp. (Wu et al. 2021b) and Ilyonectria spp. (Wu et al. 2021a) were reported as its causal agents. Among these, F. solani is the main pathogenic fungus, which is responsible for disease on about 100 genera of plants (Coleman et al. 2009).
As one of the most serious diseases in Coptis, the incidence rate of root rot can reach to 40%, leading to about 67% yield loss in Shizhu county (Mei et al. 2021b). Infected coptis plants showed stunted growth, and most of the fibrous and main roots were brown or showed black discoloration, while healthy roots were yellow. Severely infected plants were wilted and necrotic. The prevention and control of these diseases is largely achieved by applying chemical fungicides; however, due to the continuous improvement of public concern about drug safety, chemical residues and environmental pollution, the application of pesticides should be reduced to ensure the quality of the traditional Chinese medicine. Biological controls using beneficial microorganisms are an alternative way to minimize the pollution and chemical residues.
Biocontrol agents (BCAs), in particular Bacillus spp. have showed an antagonistic effect against a wide spectrum of diseases, and are used to control the phytopathogenic fungi, such as Rhizoctonia solani, Botrytis cinerea, Verticillium dahliae, Sclerotinia sclerotiorum, Alternaria spp., and Fusarium spp., in tomato (Gao et al. 2017; Zhao et al. 2022), pepper (Jiang et al. 2018), cotton (Hasan et al. 2020), cannabis (Balthazar et al. 2022), ginseng (Wei et al. 2023) and bean crops (Han et al. 2021; Hashem et al. 2021). Trichoderma spp. are also one of the most commonly used BCAs against a broad spectrum of root, shoot, and postharvest pathogens (Zin and Badaluddin 2020; Poveda and Eugui 2022; Behiry et al. 2023). Considering the limitation of using a single biocontrol strain (Mazzola and Freilich 2017), application of strain mixtures in controlling soil-borne diseases has begun to be widely used in modern agriculture (Ma et al. 2022).
Combined use of Trichoderma and compatible Bacillus is possible to offer even greater potential as plant growth promoters and as biocontrol agents (Poveda and Eugui 2022). It was reported that the co-culture of T. asperellum GDFS1009 and B. amyloliquefaciens 1841 improved the maize and wheat growth and biocontrol activity (Karuppiah et al. 2019a, b). Similarly, mixed culture fermentation of B. amyloliquefaciens and T. longibrachiatum improved the biocontrol efficiency on tomato Fusarium wilt (Ma et al. 2022). Some researchers have studied the fungicide sensitivity of the causal agents and the ecological factors of coptis root rot (Cheng et al. 2020; Yang et al. 2020). An eight year rotation, combined with chemicals, such as metalaxyl·hymexazol 3% AS, can effectively control coptis root rot (Yang et al. 2020).
In China, the control of root rot disease of coptis is primarily based on the use of fungicides, which has resulted in chemical residues. Therefore, it is vital to explore for alternative strategies to manage these pathogens. However, the potential of biological agents for control has not been fully explored. Despite, the biocontrol mechanism of Bacillus spp. and Trichoderma spp. are different, and the combined application of Bacillus spp. and Trichoderma spp. is more effective than using a single strain in controlling soil-borne diseases. Hence, the present study aims to: (i) screen and identify beneficial microorganisms that have antagonistic and control effects on coptis root rot; (ii) assess the effectiveness of a new screened isolate against mycelial growth of the main causal agents of coptis root rot disease (F. solani and F. avenaceum); (iii) confirm its efficacy through pot test and field trials.
Materials and methods
Microorganisms
The Y9 bacterium was isolated from a healthy root sample of Coptis chinensis Franchet in Chongqing, China. The Bacillus amyloliquefaciens (CGMCC 1.15674) was obtained from China General Microbiological Culture Collection Center. The B. subtilis (ATCC 6633), B. cereus (CMCC(B) 63303) and B. thuringiensis (ATCC 10792) were obtained from Shanghai Luwei Technology Co., Ltd. The bacteria were maintained on nutrient agar (NA) medium. The Trichoderma harzianum T22 and T. atroviride T1 cultures were preserved in Chongqing Academy of Chinese Meteria Medica. The fungal pathogens F. solani and F. avenaceum causing root rot of Coptis chinensis were isolated from diseased roots, previously (Mei et al. 2021b). The fungi were maintained on potato dextrose agar (PDA) medium.
Phylogenetic analysis of B. cereus Y9
Genomic DNA of the isolate was extracted with TIANamp Bacteria DNA Kit (Tiangen, Beijing, China). The 16S rRNA gene was amplified with universal primers 27f and 1492r (Frank et al. 2008). Specific primers gyrA-f, gyrA-r and rpoB-f, rpoB-r were used for PCR analysis of the DNA gyrase subunit A (gyrA) and RNA polymerase beta subunit (rpoB) gene, respectively (Yan et al. 2022). The primer sequences that have been used for the amplification were listed in Table 1. The PCR mixture (50 µL) contained 1 µL of DNA template, 25 µL of 2×Hieff® Robust PCR Master Mix (Yeasen, Shanghai, China), 20 µL of ddH2O, and 2 µL of each primer (10 µM). The PCR amplification was conducted in a thermal cycler (ABI ProFlex) according to the following protocol: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 10 s, annealing at 55 °C for 20 s, extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR product was purified and sequencing was performed by the Sanger method (Tsingke Biotechnology Co., Ltd.). All sequences were deposited in the GenBank.
Sequences of the same genus for each gene were obtained from GenBank and multilocus phylogeny was reconstructed using PhyloSuite with the help of several plug-in programs (Zhang et al. 2020; Xiang et al. 2023): Three sequences were aligned in batches with MAFFT (Katoh and Standley 2013), then the alignments were refined with MACSE (Ranwez et al. 2018). Ambiguously aligned fragments of three alignments were removed in batches using trimAl (Capella-Gutiérrez et al. 2009). ModelFinder was used to select the best-fit partition model using BIC criterion (Kalyaanamoorthy et al. 2017). Bayesianinference phylogenies were inferred using MrBayes under partition model (200,000 generations, 1000 sampling freq, 0.25 burn-in fraction, default values for the other parameters) (Ronquist et al. 2012). When the average standard deviation of split frequencies falls below 0.01, stop the run and infer the tree. The posterior probability values were used to assess branch support.
The phenotypic and biochemical characteristics of B. cereus Y9
Main physiological and biochemical test was used to distinguish Y9, with the two standard strains (B. cereus, CMCC(B) 63303 and B. thuringiensis, ATCC 10792) as controls. Gram and endospore staining was performed according to the manufacturer’s instructions for Gram stain kit and Spore stain kit (Solarbio, Beijing, China), respectively. Biochemical characterization (e.g. motility, citrate, catalase, Voges-Proskauer, methyl red, nitrate reduction and parasporal crystal) was conducted according to Systemic identification manual of common bacteria (Dong and Cai 2001) (pp. 353–384). The principal distinguishing characteristics of B. cereus and the related species were listed in Table 2. B. thuringiensis can produce the parasporal crystals which were different rhombus and small cuboidal, while B. cereus not.
Compatibility among biocontrol agents
Bacillus and Trichoderma were tested for their compatibility among each other following the method described by Barbosa et al. (2018). To prepare the cell suspensions, two bacterial isolates were grown on NA for 24 h at 30 °C and then transferred to nutrient broth with shaking at 180 rpm at 30 °C for 12 h (OD600 = 0.5, approximately 6 × 106 CFU mL−1), while three fungi were routinely grown on PDA plates at 25 °C and spore suspensions were prepared by scraping the spores from seven-day-old plates (106 conidia mL−1). The cell suspensions were streaked horizontally and vertically to each other on PDA and NA plates. The plates were incubated at 30 °C for 72 h and observed for the inhibition zone. Compatible isolates were able to grow over each other, while the presence of inhibition zone indicated the incompatibility. Three plates per replicate were analyzed, and all experiments were performed in triplicate.
The co‑cultured combinations
The liquid BP broth (beef extract 0.3% and peptone 0.5%) was used in this study for both pure and co-cultivation (Wu et al. 2018). Two strains of Trichoderma spp. (T. harzianum T22 and T. atroviride T1) and two strains of Bacillus spp. (Y9 and B. amyloliquefaciens CGMCC 1.15674) were tested individually and in combination to assess the radial growth of F. solani and F. avenaceum, and the specific combinations with different inoculation ratios were listed in Table 3. Briefly, 1 mL of T. harzianum or T. atroviride inocula (106 conidia mL−1) was pre-cultured for 48 h in 100 mL of BP broth, subsequently the corresponding amount of Y9 or B. amyloliquefaciens inocula (OD600 = 0.5, approximately 6 × 106 CFU mL−1) was inoculated into the preculture medium and incubated at 180 rpm at 30 °C for another 48 h (Karuppiah et al. 2019a). For pure culture, 1 mL of Trichoderma spp. or Bacillus spp. was singly inoculated into 100 mL of BP broth, incubated in shaker for 4 days.
Antifungal activity in vitro
The antagonistic activity of strain Y9 or the co-cultured combinations against the seven fungal pathogens (F. oxysporum, F. graminearum, F. fujikuroi, Clonostachys rosea, Collteotrichum fructicola, Collteotrichum gloeosporiodies, and Pestalotiopsis olivacea) were tested in a double culture assay as described previously (Han et al. 2022). A fungal disc (6 mm in diameter) or a 10-µL aliquot of spore suspension was placed in the center of PDA plate. Bacterial isolates were streaked 2 cm from the center where they crossed. Plates inoculated with fungal pathogens and sterile water were used as controls. Plates were incubated in the dark at 30 °C for 3 days. The colony diameters were determined by applying the ‘crossing method’ and photographed. The antifungal activity was then evaluated by measuring the fungal growth in the presence and absence of biocontrol agents. The experiment was repeated twice with three replications.
Biocontrol assays under controlled conditions
Healthy and consistent two-year-old coptis plants were transplanted at a rate of two plants per pot (12 × 12 × 10.5 cm), and each treatment contained three pots. The spore suspension of F. solani (5 × 106 conidia·mL− 1, 15 mL) was added to the pot. After 7 days, the suspension of Y9 isolate (OD600 = 1.0, 15 mL) was added to the pot of biocontrol group, and then 15 mL of Y9 isolate suspension was added every 10 d for a total of three times. Meanwhile, an equal volume of sterile water was added to the other groups. There were four treatments in this experiment, namely pathogenic fungi (F. solani, Fs), biocontrol (Fs + Y9), control (Y9) and sterile water (negative control). All the treated coptis plants were grown at 24 °C in the greenhouse with weak natural light. After 30 days post inoculation, the plants were rated for root rot severity using a grading system for Panax notoginseng root rot disease with some modifications: 0, healthy; 1, roots were slightly discolored, and discolored roots were less than 10%; 2, roots turned brown, and brown roots were 10–40%, with no rot of fibrous roots; 3, roots turned brown, and discolored roots were 40–50%, with rot of fibrous roots; 4, roots turned dark brown, and discolored roots were 50–70%, with rot of fibrous roots; 5, roots turned black, and discolored roots were more than 70%, with rot of fibrous roots (Li et al. 2020).
Field trials
A field experiment was conducted in a plot of land with naturally occurring root rot disease at Fengmu village (108°29′28″E and 30°13′36″N), Shizhu county, Chongqing, China, with an average altitude of 1545 m. Irrigation root of three co-cultured combinations and four single isolates were conducted every three weeks for a total of three times. Additionally, a commercial formulation of Trichoderma harzianum T22 (WP, BioWorks) was used as a control. The experiment was set up as a completely randomized block design, consisting of nine treatments (Table 4) and three replicates, with a total of 27 plots (5 m2 each). For each plot, 10 L of the 10-fold dilution of the fermentation products or 30 g of T. harzianum (WP) were applied, and equivalent water served as a control. The coptis plants were investigated and classified according to the grading system for P. notoginseng root rot disease in July, when was the peak incidence.
To determine whether there was or not a synergistic interaction in bacterial combinations, Colby’s formula as described (Colby 1967) was used:
Where E is the expected percent inhibition value of the combined biocontrol agents, X, Y and Z are the percent inhibition of corresponding biocontrol agents applied alone, and n is the number of biocontrol agents in the combination. A synergism is determined to be present when the combination produces a value higher than E and when the value is lower there is antagonism in the combination.
Statistical analysis
Unless otherwise indicated, all measurements were carried out in triplicate and were repeated at least twice, with reproducible results. Independent-samples t test was used to estimate the differences of disease index between “Fs” and “Fs + Y9”. Welch ANOVA with Games-Howell post-hoc test was performed to analyze the mycelial growth, inhibition rate and disease index using the SPSS 22.0. All values were given as mean ± SEM, and different letters indicated significant differences at P < 0.05.
Results
Identification of the Y9 isolate
Isolate Y9 formed pink colored colonies with white precipitation ring on mannitol-egg-yolk-polymyxin agar (MYP), indicating that Y9 was mannitol negative and lecithinase positive (Fig. 1a). On NA plates, the colonies of isolate Y9 were white, opaque, extended edges, and surface rough as frosted glass or dissolved wax (Fig. 1b). Through the observation of gram staining and spore staining, the Y9 was a Gram-positive, rod-shaped, and spore producing bacterium (Fig. 1c, d). Parasporal crystals were observed in B. thuringiensis (Fig. 1e), but not in Y9 (Fig. 1f). Morphological characters indicated that isolate Y9 likely belonged to the genus Bacillus.
The morphological characteristics of Bacillus cereus isolate Y9. Colonies of isolate Y9 grown on MYP (a) and NA (b) after 24 h of incubation at 30℃. c Gram staining and d spore staining. Protein toxin crystallization test of B. thuringiensis (e) and Y9 (f) after 4 d of incubation at 30℃. The white arrow marks the precipitation ring and parasporal crystals
Blast results of the 16S rRNA sequence (OP897300) of isolate Y9 showed that isolate Y9 was most closely related to Bacillus cereus (100%) and Bacillus thuringiensis (100%). Similarly, the gyrA (OP909708) sequences of isolate Y9 were 100% identical to B. thuringiensis and B. cereus, and rpoB (OP909710) had 99.87% identity to B. thuringiensis and B. cereus. None of the three genes used alone were able to distinguish isolate Y9 from B. thuringiensis and B. cereus. Then a multilocus phylogeny of isolate Y9 and its relatives based on the three house-keeping genes (Fig. 2) was reconstructed using PhyloSuite, which indicated that Y9 isolate was clustered with Bacillus cereus.
Phylogenetic reconstruction of the isolate Y9 and its close relatives based on concatenated 16S rRNA, gyrA and rpoB nucleotide sequences. Phylogenetic analysis was conducted under Bayesian Inference (BI) criteria, and values above branches indicate BI posterior probabilities (pp). The bar indicates sequence divergence. The isolate Y9 was highlighted in red, and B. cereus (CMCC(B) 63303) and B. thuringiensis (ATCC 10792) were highlighted with an asterisk
Physiological and biochemical characterics (Table 2) also revealed that isolate Y9 was identical with B. cereus, which could not produce the parasporal crystals. Based on the morphological, physiological and biochemical characteristics, and molecular genetic analysis, isolate Y9 was identified as B. cereus, and was preserved in China General Microbiological Culture Collection Center (CGMCC No. 26960), Beijing, China.
Antifungal activity of B. cereus isolate Y9
Antagonistic activity assays showed that B. cereus isolate Y9 inhibited the growth of F. solani and F. avenaceum, which were the pathogens causing root rot of C. chinensis. The results showed that B. cereus isolate Y9 significantly inhibited the growth of F. solani and F. avenaceum, with a reduction in mycelial growth by 51.60% and 54.03%, respectively. The inhibitory effect of B. cereus isolate Y9 was similar to that of B. amyloliquefaciens, and better than that of B. subtilis (Fig. 3). The growth of seven other pathogens (F. oxysporum, F. graminearum, F. fujikuroi, Clonostachys rosea, Collteotrichum fructicola, Collteotrichum gloeosporiodies, and Pestalotiopsis olivacea) was also inhibited by B. cereus isolate Y9 (Fig. 4). The strongest inhibitory effect was against F. graminearum (44.20%), and the inhibition rate against C. fructicola and C. gloeosporiodies was about 40%. Overall, B. cereus isolate Y9 had a broad spectrum of antimicrobial activity, indicating a great potential in biocontrol.
Antagonistic activity of Bacillus cereus isolate Y9 against Fusarium solani and F. avenaceum in vitro. a Fungi treated with B. subtilis (ATCC 6633), B. amyloliquefaciens (CGMCC 1.15674) and B. cereus isolate Y9. Scale bars, 1 cm; b Inhibition rate of the Bacillus strains against F. solani and F. avenaceum. Values are means of three replicates for each treatment and bars indicate SEs. Asterisk indicates statistically significant (P < 0.05)
Antagonistic activity of Bacillus cereus isolate Y9 against seven other pathogens in vitro. a Top row, untreated fungi used as the control; second row, fungi treated with B. cereus isolate Y9. Scale bars, 1 cm; b the inhibition rate of B. cereus isolate Y9 against seven other pathogens. F.o: Fusarium oxysporum, F.g: F. graminearum, F.f: F. fujikuroi, C.r: Clonostachys rosea, C.f: Colletotrichum fructicola, C.g: Colletotrichum gloeosporiodies, P.o: Pestalotiopsis olivacea. Values are means of three replicates for each treatment and bars indicate SEs. Columns with different letters indicate significant difference (P < 0.05)
Efficacy of B. cereus isolate Y9 against coptis root rot disease under controlled conditions
The ability of B. cereus isolate Y9 to inhibit F. solani in coptis plants was evaluated under greenhouse conditions. Disease severity was recorded 30 days post-inoculation by observing root discoloration. The coptis plants treated with B. cereus isolate Y9 only showed no symptoms in the root, and grew better than the mock treated plants. Typical symptoms and the highest disease severity can be observed in the control treatment which was inoculated with F. solani only. The coptis plants treated with B. cereus isolate Y9 in the presence of F. solani showed healthy with fewer symptoms in root compared with the control treatment, and the disease index was significantly reduced (Fig. 5). B. cereus isolate Y9 could effectively control root rot and significantly reduce the disease severity caused by F. solani on coptis plants.
Biocontrol of Bacillus cereus isolate Y9 against coptis root rot under controlled conditions. a Representative photographs of the coptis plants grown in pots in four different groups including Control (distilled water), Fs (Fusarium solani), Fs + Y9 (F. solani and B. cereus) and Y9 (B. cereus). The infected roots to show the root rot incidence of b Control, c Fs, d Fs + Y9, e Y9; f disease index of F. solani in mock and treated. Pictures were taken 30 days post inoculation. Values are means of three replicates for each treatment and bars indicate SEs. Columns with different letters indicate significant difference (P < 0.05). Scale bars, 2 m
Compatibility test
To determine the compatibility between Y9 and other biocontrol agents, in vitro assays were performed as previously described (Barbosa et al. 2018). Incompatible isolates showed inhibition halos regardless of the direction they were spread on the plates. In this study, a total of five isolates, including two isolates of Bacillus (B. cereus, Y9; B. amyloliquefaciens, JDF) and three isolates of Trichoderma (T. harzianum, HC; T. atroviride, SL; T. reesei, LS) were tested for the compatibility in all possible combinations of two isolates. Only isolate LS was inhibited by the two Bacillus bacteria, while the other eight combinations were compatible (Fig. 6). The same responses were observed on PDA and NA media and in another independent experiment. Only compatible combinations were tested in further experiments.
Compatibility between biocontrol agents on agar plates. a The plus signal indicates a compatible combination and a minus indicates an incompatible combination; b Compatible and c incompatible combinations on plates. Y9, Bacillus cereus; JDF, B. amyloliquefaciens; HC, Trichoderma harzianum; SL, T. atroviride; LS, T. reesei. Scale bars, 1 cm
Antifungal activity of the combinations in vitro
The B. cereus isolate Y9 (T1) had a similar inhibitory effect on the growth of pathogenic fungi with B. amyloliquefaciens (T2), but lower than T. harzianum (T3) or T. atroviride (T4), while the antifungal activities increased when T. harzianum or T. atroviride was mixed in co-culture (Table 3). All the treatments were effective in reducing the mycelial growth of the pathogens. However, the top five effective combinations against F. solani were T15 (68.23 ± 1.29), T9 (66.68 ± 0.08), T7 (66.58 ± 0.05), T5 (66.18 ± 0.22), T20 (66.13 ± 0.88), and T7 (68.52 ± 2.52), T15 (67.66 ± 2.85), T13 (67.42 ± 3.51), T6 (67.04 ± 2.11), T20 (66.91 ± 2.49) against F. avenaceum (Table 2). Combinations T7 (HY, T. harzianum + Y9, in ratio of 1:1), T15 (HYJ, T. harzianum + B. amyloliquefaciens + Y9, in ratio of 1:1:1) and T20 (HLYJ, T. harzianum + T. atroviride + B. amyloliquefaciens + Y9, in ratio of 2:2:1:1) had high inhibitory effect against both F. solani and F. avenaceum, and were selected for further field experiment.
Antagonistic efficacy of the combinations in the field
The field efficacy assessments showed that all the treatments reduced the disease index of coptis root rot, with a range from 19.26 to 63.85% (Table 4). The control efficacy of Bacillus isolates Y9 and JDF showed no significant difference, but was significantly lower than that of Trichoderma isolates HC and SL. The optimum combinations exhibited significantly higher antagonistic efficacy when compared to individual isolates. HYJ showed the highest control efficacy of 63.85%, which was higher than the expected value (53.18%), indicating synergistic effects on the control of coptis root rot. Interestingly, synergistic effect was only found in HYJ, but not HY or HLYJ. The results indicated the combinations of B. cereus isolate Y9 and Trichoderma spp. can be used as BCAs to control coptis root rot disease, and provided significantly better control efficacy than single T. harzianum (WP).
Discussion
In this study, isolate Y9 was isolated from the healthy fibrous roots of coptis plants, and showed strong antifungal activity against F. solani and F. avenaceum, with the inhibition rate of 51.60% and 54.03%, respectively. By comparing 16S rRNA gene sequences, we found that the isolate Y9 belonged to the genus Bacillus. But 16S rRNA sequences are not sufficient to discriminate Bacillus species and related genera, which share very similar 16S rRNA gene sequences (Xu and Cote 2003; Ehling-Schulz et al. 2019).Housekeeping genes gyrA and rpoB were reported to provide better phylogenetic resolution than 16S rRNA, and can be used to identify Bacillus species (Ki et al. 2009; Masum et al. 2018; Liu et al. 2022). However, we could not determine whether isolate Y9 was B. thuringiensis or B. cereus, no matter which marker genes were used alone. It is difficult to separate the B. cereus group (e.g. B. cereus and B. thuringiensis) by using molecular comparisons. Although the rpoB gene could be used to identify some members of the B. cereus group, but failed to discriminate between B. thuringiensis and B. cereus (Ki et al. 2009). Then, we present a multilocus phylogeny of the isolate Y9 and its relatives based on all of the three molecular markers (16S rRNA, gyrA and rpoB), and found that isolate Y9 was clustered with B. cereus (Fig. 2). To provide more reliable results, we also conducted the protein toxin crystallization test on isolate Y9, with B. cereus (CMCC(B) 63303) and B. thuringiensis (ATCC 10792) as controls. The results showed that isolate Y9 was identical with B. cereus, which could not form the parasporal crystals.
Although some B. cereus isolates are commonly referred to food poisoning agents (Ehling-Schulz et al. 2019), several strains of B. cereus exhibit plant growth-promoting traits and show biocontrol capabilities against various plant pathogens (Kulkova et al. 2023). For example, seed bacterization of maize with B. cereus sensu lato strain B25 not only significantly reduced Fusarium stalk rot and Fusarium ear rot incidence and severity, but also significantly increased grain yield and reduced Fumonisin contamination up to 93.9% (Lizárraga-Sánchez et al. 2015). In this work, the antagonistic traits of B. cereus isolate Y9 against F. solani and F. avenaceum were evaluated (Fig. 3). Antagonism assay on PDA plates revealed the antimicrobial activity of B. cereus isolate Y9 against F. solani and F. avenaceum, with inhibition rates of 51.60% and 54.03%, respectively. The results showed that B. cereus isolate Y9 seemed to be less efficient than T. harzianum and T. atroviride (Table 3). Differences in growth rate might be a factor for relative low antifungal activity of Y9, since the growth rate of Trichoderma spp. on PDA is about 30 mm/d (Tian et al. 2023), while Bacillus spp. grows along the inoculation line (or site) and spreads slowly on PDA (Izquierdo-García et al. 2020). Notably, the antagonistic efficiency of B. cereus isolate Y9 was significantly higher than that of B. subtilis, a well known antagonistic bacterium. Similar results were found by Kushwaha et al. (2020) with B. cereus EPP5 the highest inhibitor for F. solani, followed by B. amyloliquefaciens EPP62 and B.subtilis EPP65. Moreover, the growth of other pathogens was also significantly inhibited (Fig. 4), indicating that B. cereus isolate Y9 exhibited a significant inhibitory effect on plant pathogens. According to Tian (2017), the sterile culture filtrate of B. cereus TJB-8 effectively inhibited the hyphae growth of F. oxysporum, F. graminearum and C. gloeosporioides, which is compatible with the current study. In the present study, B. cereus isolate Y9 displayed a protective effect against coptis root rot under greenhouse conditions (Fig. 5). However, the protective effect of B. cereus isolate Y9 was less effective compared to results reported by Kushwaha et al. (2020) where B. cereus EPP5 protected the pearl millet plants against F. solani under greenhouse conditions by 45.74%. Many factors influenced the control efficiency, including temperature, host plants, the specific strains and population density of BCAs. For example, the population density of B. cereus B8W8 was relatively higher in citrus than in apple at the same condition (Khadiri et al. 2023).
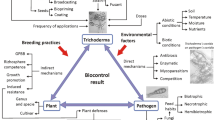
One of the important biocontrol mechanisms against phytopathogens present in Bacillus spp. is the production of antimicrobial substances. B. cereus genomes show a high abundance of cyclic lipopeptides gene clusters (e.g., iturin A, C, D; fengycin D; and surfactin A, C) (Kulkova et al. 2023). It was repotred that B. cereus XZ30-2 culture filtrate could produce hydrolases and lipopeptides (including iturin, surfactin and fengycin) and led to the intracellular leakage and nucleic acids release, and reactive oxygen species (ROS) accumulation in Aspergillus niger (Yi et al. 2023). Moreover, fengcin was found to play a vital role in inhibiting growth of F. graminearum, by damaging the plasma membranes and cell walls, and also inhibiting DNA synthesis of F. graminearum (Hanif et al. 2019). The activity of lytic enzymes (e.g. glucanases, proteases, cellulases, chitinases and chitosanases), which efficiently hydrolyze the main components of fungal cell walls, is another biocontrol mechanism of fungi in Bacillus spp. (Kulkova et al. 2023). For instance, 10% cell free supernatant of B. amyloliquefaciens FX2 damaged the cell ultrastructure, resulting in a remarkable increase in cellular leakage in Botryosphaeria dothidea mycelia, and the antifungal constituents were composed mainly of antifungal proteins, such as serine protease, metallopeptidase, hydrolase, and flagellin (Li et al. 2022). To date, many volatile organic compounds (VOCs) with antifungal effects produced by B. cereus have been identified. For instance, the VOCs of B. cereus strain B8W8 have high efficacy to inhibit the growth of Monilinia laxa and M. fructigena (Khadiri et al. 2023). The VOCs in B. cereus MH778713 promoted tomato seedling growth and showed antifungal activity against F. oxysporum (Ramírez et al. 2022). And five major VOCs released by B. cereus CF4-51 damaged the Sclerotinia sclerotiorum hyphae and inhibited the formation of sclerotia (Hu et al. 2023). In B. cereus B25, the chitinase and chitosanase activities were induced to inhibit the growth of the phytopathogen F. verticillioides P03 (Báez-Astorga et al. 2022).Another biocontrol mechanism of fungi present in B. cereus strains is by triggering induced systemic resistance (ISR), which requires salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) signaling pathways (Nie et al. 2017). For example, B. cereus AR156 triggered ISR against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis by simultaneously activating the SA- and JA/ET-signaling pathways in an NPR1 dependent manner (Niu et al. 2011). AR156 were able to increase the average biomass of the tomato by 47.7%, and decrease bacterial speck disease severity by 1.6-fold, via simultaneous activation of the SA- and JA-signaling pathways (Niu et al. 2012). However, JA/ET-signaling pathways and NPR1, but not SA-, were activated by AR156-induced ISR against B. cinerea in Arabidopsis (Nie et al. 2017). Therefore, we hypothesized that the main biocontrol mechanism of B. cereus isolate Y9 is also antibiotics and ISR in plants (Fig. 7).
Combined use of BCAs is more effective than using a single strain (Poveda and Eugui 2022). Almost all the combinations tested improved the inhibition rate in dual culture assay (Table 3). However, only in 10 of the total 465 published treatments was there evidence for synergistic effects among BCAs (Xu et al. 2011a). In most cases, combined use of two BCAs with different biocontrol mechanisms only achieved biocontrol efficacies similar to those achieved by the more efficacious one alone (Xu et al. 2011b). It seemed that antagonistic interactions were more likely to occur among BCAs than synergistic interactions. However, it is worth noting that, plant defense can be more effective when combined application of several biocontrol agents are used, compared with the application of a single biocontrol strain, although no statistical analysis is done to prove the existence of synergism numerically. When the inoculation proportion between B. amyloliquefaciens ACCC11060 and T. asperellum GDFS1009 was 1.9:1, it could produce more specific antimicrobial substances than single inoculation, and exhibited the highest antifungal effect on B. cinerea (66.86%) (Wu et al. 2018). Similarly, co-cultivation of T. asperellum GDFS1009 and B. amyloliquefaciens 1841 significantly enhanced biocontrol activity against F. graminearum, F. oxysporum and B. cinerea, and improved the wheat growth (Karuppiah et al. 2019a). Single treatment of B. cereus (EPP5), B. amyloliquefaciens (EPP62) or B. subtilis (EPP65) offered protection ranging from 35.68 to 45.74% under greenhouse conditions. However, the co-culture (EPP5 + EPP62 + EPP65) provided the highest protection (71.96%) against F. solani with significant increase in pearl millet (Pennisetum glaucum) biomass (Kushwaha et al. 2020). It was reported that Bacillus spp. and Trichoderma spp. act synergistically to enhance root colonization rate for each other. Root colonization of T. harzianum OMG16 increased up to 10-fold in the presence of B. velezensis FZB42, and the presence of OMG16 increased the root colonization rate of FZB42 significantly, too (Hafiz et al. 2022). In this study, more fine roots were induced by HYJ and HLYJ treatment (data not shown) demonstrated that Bacillus spp. and Trichoderma spp. were able to stimulate fine-root formation, which subsequently provides increased potential sites for plants and fungi to interact (Fig. 7).
In this study, Colby’s formula was used to evaluate the expected synergism in combination of two to four BCAs. Colby’s formula was developed from Limpel’s formula (Richer 1987), which was widely used in studies of synergy with fungicide mixtures and biocontrol of plant diseases (Barbosa et al. 2018; Yaderets et al. 2021; Öztekin and Karbancioglu-Guler 2023). Thus, we think Colby’s formula seems equally applicable for fungicides. Our results showed that only the combination of HYJ, but not HLYJ, exhibited synergistic effects on the control of coptis root rot. Antagonistic activity of the BCAs in HLYJ treatment might be affected by each other, but not in HYJ treatment. The inoculation ratio of HLYJ was 2:2:1:1, and the high concentration of antimicrobial compounds produced by Trichoderma spp. might negatively affected the growth and biocontrol activity of Bacillus. Since it was reported that exposing B. velezensis Bs006 to T. virens Gl006-supernatant negatively affected the growth of the bacteria, biofilm formation and biocontrol activity, in a concentration dependent manner, although its viability and antagonistic ability was not affected (Izquierdo-García et al. 2020). A synergistic effect, forming more stable rhizosphere communities and occupying different or complementary niches may be the antagonistic mechanisms of the co-culture of Bacillus-Trichoderma (Poveda and Eugui 2022). It was reported that BCAs with different mechanisms have a higher chance of acting synergistically, as compared to BCAs with the same mechanisms, and BCAs with both mycoparasitism and competition mechanisms was more effective over the range of parameter values evaluated (Xu et al. 2011b).
According to the investigation in the field, Coptis root rot generally started to occur from March to April, and the peak incidence was from May to July, then gradually decreased after September. Based on the occurrence of Coptis root rot, the B. cereus isolate Y9 and its combinations were first applied in April for a total of three times, at three weeks intervals, and the incidence of the disease was investigated in July. The long-term effects of B. cereus isolate Y9 and its combinations on the coptis root rot need to be assessed in more seasons and locations, under different environmental conditions and disease pressure.
Conclusions
Isolate Y9 which was isolated from the healthy roots of coptis, was identified as B. cereus based on the morphological, physiological and biochemical characteristics, and molecular genetic analysis. The B. cereus isolate Y9 had inhibitory effects on F. solani and F. avenaceum and seven strains of other pathogenic fungi. In this study, combined use of T. harzianum, B. amyloliquefaciens and B. cereus isolate Y9 (HYJ, in ratio of 1:1:1) led to a synergistic effect in controlling coptis root rot in the field. The underlying mechanism and more field application deserve further investigation.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Báez-Astorga PA, Cázares-Álvarez JE, Cruz-Mendívil A, Quiroz-Figueroa FR, Sánchez-Valle VI, Maldonado-Mendoza IE (2022) Molecular and biochemical characterisation of antagonistic mechanisms of the biocontrol agent Bacillus cereus B25 inhibiting the growth of the phytopathogen Fusarium verticillioides P03 during their direct interaction in vitro. Biocontrol Sci Technol 32:1074–1094. https://doi.org/10.1080/09583157.2022.2085662
Balthazar C, Novinscak A, Cantin G, Joly DL, Filion M (2022) Biocontrol activity of Bacillus spp. and Pseudomonas spp. against Botrytis Cinerea and other cannabis fungal pathogens. Phytopathology 112:549–560. https://doi.org/10.1094/phyto-03-21-0128-r
Barbosa LO, Lima JS, Magalhães VC, Gava CAT, Soares ACF, Marbach PAS, Souza JT (2018) Compatibility and combination of selected bacterial antagonists in the biocontrol of sisal bole rot disease. Biocontrol 63:595–605. https://doi.org/10.1007/s10526-018-9872-x
Behiry S, Soliman SA, Massoud MA, Abdelbary M, Kordy AM, Abdelkhalek A, Heflish A (2023) Trichoderma pubescens elicit induced systemic resistance in tomato challenged by Rhizoctonia solani. J Fungi 9:167. https://doi.org/10.3390/jof9020167
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Cheng HH, Yang F, Gao J, Tan DM, Zhang MX, Ding HX (2020) Identification and fungicide sensitivity of pathogen causing root rot of goldthread. Acta Phytopathologica Sinica 50:377–380. https://doi.org/10.13926/j.cnki.apps.000329
Colby SR (1967) Calculating synergistic and antagonistic responses of herbicide combinations. Weeds 15:20–22. https://doi.org/10.2307/4041058
Coleman JJ, Rounsley SD, Rodriguez-Carres M, Kuo A, Wasmann CC, Grimwood J, Schmutz J, Taga M, White GJ, Zhou S, Schwartz DC, Freitag M, Ma LJ, Danchin EG, Henrissat B, Coutinho PM, Nelson DR, Straney D, Napoli CA, Barker BM, Gribskov M, Rep M, Kroken S, Molnár I, Rensing C, Kennell JC, Zamora J, Farman ML, Selker EU, Salamov A, Shapiro H, Pangilinan J, Lindquist E, Lamers C, Grigoriev IV, Geiser DM, Covert SF, Temporini E, Vanetten HD (2009) The genome of Nectria Haematococca: contribution of supernumerary chromosomes to gene expansion. PLoS Genet 5:e1000618. https://doi.org/10.1371/journal.pgen.1000618
Commission CP (2020) Pharmacopoeia of the people’s republic of China, 2020 edn. vol. I. China Medical Science Press, Beijing, pp 177
Dong XZ, Cai MY (2001) Systemic identification manual of common bacteria. Science, Beijing, pp 353–384
Ehling-Schulz M, Lereclus D, Koehler TM (2019) The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol Spectr 7. https://doi.org/10.1128/microbiolspec.GPP3-0032-2018.:GPP3-0032-2018
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74:2461–2470. https://doi.org/10.1128/AEM.02272-07
Gao Z, Zhang B, Liu H, Han J, Zhang Y (2017) Identification of endophytic Bacillus velezensis ZSY-1 strain and antifungal activity of its volatile compounds against Alternaria solani and Botrytis Cinerea. Biol Control 105:27–39. https://doi.org/10.1016/j.biocontrol.2016.11.007
Hafiz FB, Moradtalab N, Goertz S, Rietz S, Dietel K, Rozhon W, Humbeck K, Geistlinger J, Neumann G, Schellenberg I (2022) Synergistic effects of a root-endophytic Trichoderma fungus and Bacillus on early root colonization and defense activation against Verticillium Longisporum in rapeseed. Mol Plant Microbe Interact 35:380–392. https://doi.org/10.1094/mpmi-11-21-0274-r
Han SY, Chen JX, Zhao YJ, Cai HS, Guo CH (2021) Bacillus subtilis HSY21 can reduce soybean root rot and inhibit the expression of genes related to the pathogenicity of Fusarium oxysporum. Pestic Biochem Physiol 178:104916. https://doi.org/10.1016/j.pestbp.2021.104916
Han PJ, Liu TR, Zheng Y, Song RQ, Nan TG, Yang XL, Huang LQ, Yuan Y (2022) A mycorrhizal bacteria strain isolated from Polyporus umbellatus exhibits broad-spectrum antifungal activity. Front Plant Sci 13:954160. https://doi.org/10.3389/fpls.2022.954160
Hanif A, Zhang F, Li PP, Li CC, Xu YJ, Zubair M, Zhang MX, Jia DD, Zhao XZ, Liang JG, Majid T, Yan JYA, Farzand A, Wu HJ, Gu Q, Gao XW (2019) Fengycin produced by Bacillus amyloliquefaciens FZB42 inhibits Fusarium graminearum growth and mycotoxins biosynthesis. Toxins 11:295. https://doi.org/10.3390/toxins11050295
Hasan N, Farzand A, Heng Z, Khan IU, Moosa A, Zubair M, Na Y, Ying S, Tang CM (2020) Antagonistic potential of novel endophytic Bacillus strains and mediation of plant defense against verticillium wilt in upland cotton. Plants-Basel 9:1438. https://doi.org/10.3390/plants9111438
Hashem AH, Abdelaziz AM, Askar AA, Fouda HM, Khalil AMA, Abd-Elsalam KA, Khaleil MM (2021) Bacillus megaterium-mediated synthesis of selenium nanoparticles and their antifungal activity against Rhizoctonia solani in faba bean plants. J Fungi 7:195. https://doi.org/10.3390/jof7030195
Hu JH, Dong BZ, Wang D, Meng HW, Li XJ, Zhou HY (2023) Genomic and metabolic features of Bacillus cereus, inhibiting the growth of Sclerotinia sclerotiorum by synthesizing secondary metabolites. Arch Microbiol 205:8. https://doi.org/10.1007/s00203-022-03351-5
Izquierdo-García LF, González-Almario A, Cotes AM, Moreno-Velandia CA (2020) Trichoderma virens Gl006 and Bacillus velezensis Bs006: a compatible interaction controlling Fusarium wilt of cape gooseberry. Sci Rep 10:6857. https://doi.org/10.1038/s41598-020-63689-y
Jiang CH, Liao MJ, Wang HK, Zheng MZ, Xu JJ, Guo JH (2018) Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis Cinerea. Biol Control 126:147–157. https://doi.org/10.1016/j.biocontrol.2018.07.017
Kalyaanamoorthy S, Minh BQ, Wong TKF, Haeseler AV, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589. https://doi.org/10.1038/NMETH.4285
Karuppiah V, Sun JN, Li TT, Vallikkannu M, Chen J (2019a) Co-cultivation of Trichoderma Asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 causes differential gene expression and improvement in the wheat growth and biocontrol activity. Front Microbiol 10:16. https://doi.org/10.3389/fmicb.2019.01068
Karuppiah V, Vallikkannu M, Li T, Chen J (2019b) Simultaneous and sequential based co-fermentations of Trichoderma Asperellum GDFS1009 and Bacillus amyloliquefaciens 1841: a strategy to enhance the gene expression and metabolites to improve the bio-control and plant growth promoting activity. Microb Cell Fact 18:185. https://doi.org/10.1186/s12934-019-1233-7
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Khadiri M, Boubaker H, Askarne L, Ezrari S, Radouane N, Farhaoui A, El Hamss H, Tahiri A, Barka EA, Lahlali R (2023) Bacillus cereus B8W8 an effective bacterial antagonist against major postharvest fungal pathogens of fruit. Postharvest Biol Technol 200:112315. https://doi.org/10.1016/j.postharvbio.2023.112315
Ki JS, Zhang W, Qian PY (2009) Discovery of marine Bacillus species by 16S rRNA and rpoB comparisons and their usefulness for species identification. J Microbiol Methods 77:48–57. https://doi.org/10.1016/j.mimet.2009.01.003
Kulkova I, Dobrzynski J, Kowalczyk P, Belzecki G, Kramkowski K (2023) Plant growth promotion using Bacillus cereus. Int J Mol Sci 24:9759. https://doi.org/10.3390/ijms24119759
Kushwaha P, Kashyap PL, Srivastava AK, Tiwari RK (2020) Plant growth promoting and antifungal activity in endophytic Bacillus strains from pearl millet (Pennisetum glaucum). Brazilian J Microbiol 51:229–241. https://doi.org/10.1007/s42770-019-00172-5
Li YB, Liu YX, Zhu SS, Luo LX, Li JQ (2020) Grading system for Panax notoginseng root rot disease. Acta Phytopathologica Sinica 50:450–461. https://doi.org/10.13926/j.cnki.apps.000468
Li Q, Hou ZQ, Zhou DQ, Jia MY, Lu SP, Yu JP (2022) Antifungal activity and possible mechanism of Bacillus amyloliquefaciens FX2 against the postharvest apple ring rot pathogen. Phytopathology 112:2486–2494. https://doi.org/10.1094/phyto-02-22-0047-r
Liu Y, Štefanič P, Miao Y, Xue Y, Xun W, Zhang N, Shen Q, Zhang R, Xu Z, Mandic-Mulec I (2022) Housekeeping gene gyrA, a potential molecular marker for Bacillus ecology study. AMB Express 12:133. https://doi.org/10.1186/s13568-022-01477-9
Lizárraga-Sánchez GJ, Leyva-Madrigal KY, Sánchez-Peña P, Quiroz-Figueroa FR, Maldonado-Mendoza IE (2015) Bacillus cereus sensu lato strain B25 controls maize stalk and ear rot in Sinaloa, Mexico. Field Crops Res 176:11–21. https://doi.org/10.1016/j.fcr.2015.02.015
Luo XM, Li JL, Dong JY, Sui AP, Sheng ML, Yang XY (2014) First report of Fusarium solani causing root rot on Coptis chinensis in southwestern China. Plant Dis 98:1273. https://doi.org/10.1094/pdis-02-14-0164-pdn
Ma QF, Cong YZ, Feng LT, Liu CY, Yang WH, Xin Y, Chen KS (2022) Effects of mixed culture fermentation of Bacillus amyloliquefaciens and Trichoderma longibrachiatum on its constituent strains and the biocontrol of tomato fusarium wilt. J Appl Microbiol 132:532–546. https://doi.org/10.1111/jam.15208
Masum MMI, Liu L, Yang M, Hossain MM, Siddiqa MM, Supty ME, Ogunyemi SO, Hossain A, An Q, Li B (2018) Halotolerant bacteria belonging to operational group Bacillus amyloliquefaciens in biocontrol of the rice brown stripe pathogen Acidovorax oryzae. J Appl Microbiol 125:1852–1867. https://doi.org/10.1111/jam.14088
Mazzola M, Freilich S (2017) Prospects for biological soilborne disease control: application of indigenous versus synthetic microbiomes. Phytopathology 107:256–263. https://doi.org/10.1094/phyto-09-16-0330-rvw
Mei PY, Song XH, Zhu ZY, Li LY (2021a) First report of Diaporthe eres causing root rot of Coptis Chinensis Franchet. Plant Dis 105:1854. https://doi.org/10.1094/PDIS-12-20-2564-PDN
Mei PY, Song XH, Zhu ZY, Li LY (2021b) First report of root rot caused by Fusarium avenaceum on Coptis chinensis in Chongqing, China. Plant Dis 105:496. https://doi.org/10.1094/PDIS-05-20-1110-PDN
Nie PP, Li X, Wang SN, Guo JH, Zhao HW, Niu DD (2017) Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis. Front Plant Sci 8:238. https://doi.org/10.3389/fpls.2017.00238
Niu DD, Liu HX, Jiang CH, Wang YP, Wang QY, Jin HL, Guo JH (2011) The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways. Mol Plant Microbe Interact 24:533–542. https://doi.org/10.1094/MPMI-09-10-0213
Niu DD, Wang CJ, Guo YH, Jiang CH, Zhang WZ, Wang YP, Guo JH (2012) The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces resistance in tomato with induction and priming of defence response. Biocontrol Sci Technol 22:991–1004. https://doi.org/10.1080/09583157.2012.706595
Öztekin S, Karbancioglu-Guler F (2023) Biological control of green mould on mandarin fruit through the combined use of antagonistic yeasts. Biol Control 180:105186. https://doi.org/10.1016/j.biocontrol.2023.105186
Poveda J, Eugui D (2022) Combined use of Trichoderma and beneficial bacteria (mainly Bacillus and Pseudomonas): development of microbial synergistic bio-inoculants in sustainable agriculture. Biol Control 176:105100. https://doi.org/10.1016/j.biocontrol.2022.105100
Ramírez V, Martínez J, Bustillos-Cristales MD, Catañeda-Antonio D, Munive JA, Baez A (2022) Bacillus cereus MH778713 elicits tomato plant protection against Fusarium oxysporum. J Appl Microbiol 132:470–482. https://doi.org/10.1111/jam.15179
Ranwez V, Douzery EJP, Cambon C, Chantret N, Delsuc F (2018) MACSE v2: toolkit for the alignment of coding sequences accounting for frameshifts and stop codons. Mol Biol Evol 35:2582–2584. https://doi.org/10.1093/molbev/msy159
Richer DL (1987) Synergism—a patent view. Pest Sci 19:309–315. https://doi.org/10.1002/ps.2780190408
Ronquist F, Teslenko M, Mark PVD, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Song XH, Mei PY, Dou T, Liu QD, Li LY (2023) Multi-omics analysis reveals the resistance mechanism and the pathogens causing root rot of Coptis Chinensis. Microbiol Spectr 11:e04803–04822. https://doi.org/10.1128/spectrum.04803-22
Tian A (2017) Antagonistic strain of TJB-8 and its antifungal substances. J Zhejiang Agric Forestry Univ 34:1071–1078. https://doi.org/10.11833/j.issn.2095-0756.2017.06.015
Tian M, Peng Y, Lu H, Qin N, Ren L, Yin H, Zhao X (2023) Trichoderma Afroharzianum LMNS-M9: identification, biological characteristics, and growth-promoting effect on quinoa. Microbiol China 50:3848–3865. https://doi.org/10.13344/j.microbiol.china.230144
Wei J, Zhao J, Suo M, Wu H, Zhao M, Yang H (2023) Biocontrol mechanisms of Bacillus velezensis against Fusarium oxysporum from Panax ginseng. Biol Control 182:105222. https://doi.org/10.1016/j.biocontrol.2023.105222
Wu Q, Ni M, Dou K, Tang J, Ren JH, Yu CJ, Chen J (2018) Co-culture of Bacillus amyloliquefaciens ACCC11060 and Trichoderma asperellum GDFS1009 enhanced pathogen-inhibition and amino acid yield. Microb Cell Fact 17:155. https://doi.org/10.1186/s12934-018-1004-x
Wu XL, Wang Y, Liu F, Chen DX, Li LY (2020) Identification of Coptis chinensis root rot disease pathogenic fusarium spp. fungi. China J Chin Materia Med 45:1323–1328. https://doi.org/10.19540/j.cnki.cjcmm.20200112.102
Wu X, Chen D, Liu F, Wang Y, Li L (2021a) Identification of Coptis chinensis root rot disease pathogenic fungi belonging to Ilyonectria spp. Southwest China J Agricultural Sci 34:299–305. https://doi.org/10.16213/j.cnki.scjas.2021.2.011
Wu X, Chen D, Liu F, Wang Y, Li L (2021b) Identification of the Pythium pathogen responsible for root rot of Coptis Chinensis. J Southwest Univ (Natural Sci Edition) 43:37–43. https://doi.org/10.13718/j.cnki.xdzk.2021.06.005
Xiang CY, Gao FL, Jakovlić I, Lei HP, Hu Y, Zhang H, Zou H, Wang GT, Zhang D (2023) Using PhyloSuite for molecular phylogeny and tree-based analyses. iMeta 2:e87. https://doi.org/10.1002/imt2.87
Xu D, Cote JC (2003) Phylogenetic relationships between Bacillus species and related genera inferred from comparison of 3’ end 16S rDNA and 5’ end 16S-23S ITS nucleotide sequences. Int J Syst Evol MicroBiol 53:695–704. https://doi.org/10.1099/ijs.0.02346-0
Xu XM, Jeffries P, Pautasso M, Jeger MJ (2011a) Combined use of biocontrol agents to manage plant diseases in theory and practice. Phytopathology 101:1024–1031. https://doi.org/10.1094/PHYTO-08-10-0216
Xu XM, Jeffries P, Pautasso M, Jeger MJ (2011b) A numerical study of combined use of two biocontrol agents with different biocontrol mechanisms in controlling foliar pathogens. Phytopathology 101:1032–1044. https://doi.org/10.1094/PHYTO-10-10-0267
Yaderets VV, Karpova NV, Glagoleva EV, Ovchinnikov AI, Petrova KS, Dzhavakhiya VV (2021) Inhibition of the growth and development of Sclerotinia sclerotiorum (Lib.) de Bary by combining azoxystrobin, Penicillium chrysogenum VKM F-4876d, and Bacillus strains. Agronomy 11:2520. https://www.mdpi.com/2073-4395/11/12/2520
Yan JT, Qiao K, Cai YF (2022) Application of rpoB, gyrA and cheA genes in identifying Bacillus genus. Acta Agriculturae Zhejiangensis 34:128–140. https://doi.org/10.3969/j.issn.1004-1524.2022.01.16
Yang CQ, Yu ZL, Yang J, Lei MY, Quan J, Yang TJ, Song XH (2020) Ecological actors and control strategies of root rot of Coptis chinensis in Shizhu County, Chongqing city. Plant Prot 46:160–166. https://doi.org/10.16688/j.zwbh.2019332
Yi YJ, Hou ZP, Yang Q, Cui LQ, Lu H, Li RF, Liu Y, Zhang YQ, Chen Y (2023) Antimicrobial mechanism and biocontrol effect of Bacillus cereus XZ30-2 on Aspergillus Niger. Qual Assur Saf Crops Foods 15:77–88. https://doi.org/10.15586/qas.v15i4.1379
Zhang D, Gao F, Jakovli I, Zou H, Wang GT (2020) PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol Ecol Resour 20:348–355. https://doi.org/10.1111/1755-0998.13096
Zhao HH, Wang XB, Wang W (2022) Bacillus amyloliquefaciens SN16-1-induced resistance system of the tomato against Rhizoctonia solani. Pathogens 11:35. https://doi.org/10.3390/pathogens11010035
Zin NA, Badaluddin NA (2020) Biological functions of Trichoderma spp. for agriculture applications. Annals Agricultural Sci 65:168–178. https://doi.org/10.1016/j.aoas.2020.09.003
Funding
This work was supported by the Technological Innovation and Application Development Project of Traditional Chinese Medicine (No. 2021ZY3814), Key Project for Technological Innovation and Application Development in Chongqing (cstc2021jscx-dxwtBX0014) and the earmarked fund for CARS-21.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The authors declare that ethical standards have been followed and that no human participants or animals were involved in this research.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mei, P., Dou, T., Song, X. et al. Control of coptis root rot by combination of Bacillus cereus isolate Y9 and other antagonistic microorganisms. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01685-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01685-1