Abstract
The cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae), is one of the most important pests and causes major damage to cultivated plants in Turkey. It has recently become a serious problem in Antalya (southwestern Turkey) due to its high resistance to insecticides used. To address this problem, the present study aimed to evaluate the pathogenicity of five indigenous Beuveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes) isolates (BbFn-2, BbKm-2, BbSr-2, BbDs-4, and BbDm-2) with high virulence in previous studies against the pest. All the isolates were tested at five different conidial concentrations (1 × 105, 1 × 106, 1 × 107, 1 × 108 and 1 × 109 conidia mL−1) against the nymphs and adults of A. gossypii using Petri dish and pot trials in the laboratory. The results from the bioassays showed that virulence of the isolates increased significantly with elapsed time up to 10 days after inoculation. All five B. bassiana isolates at the highest concentration (1 × 109 conidia mL−1) caused mortalities ranged from 83.3% to 100% in both nymphs and adults 10 days post-treatment. While the LT50 and LT95 values of the isolates were 1.72–2.12 days and 4.81–8.49 days, respectively, for the nymphs, they were calculated for the adults as 1.65–2.54 days and 4.66–12.93 days, respectively. Results of the phylogenetic analysis showed that these five B. bassiana isolates had high similarities (ranging from 99 to 100%) with the other B. bassiana isolates in GenBank. All the results suggest that above-mentioned five indigenous B. bassiana isolates have significant biocontrol potential against A. gossypii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aphids (Hemiptera: Aphididae) are among the most important insect pests in agricultural areas. There are 4702 species of aphids belonging to 599 genera in the world (Blackman and Eastop 2000). The cotton aphid, Aphis gossypii Glover, is one of the most common aphid pests in the world and has an important place among other species (Cottier 1953). The cotton aphid causes damage to a large number of host plants belonging to a total of 40 plant families, including fruit trees, vegetables, and ornamental plants. Cotton aphid feeds on the stems, young leaves, flowers, and buds of plants by sucking the sap of the plant, causing slow plant development and a change in the shape of the leaves (Murphy et al. 2006). During its feeding on leaves and shoots, it expels excess sugary liquid, on which some saprophytic fungi develop, and the plant has a sooty appearance, calling ‘fumagine’ or ‘sooty mold damage’. This pest also acts as a vector in the spread of more than 100 plant virus diseases, especially cucumber mosaic virus disease (CMV), which causes economic losses in many plants and increases the importance of managing this pest (Kennedy et al. 1962; Blackman and Eastop 1984; Murphy et al. 2006; Von Dohlen et al. 2006).
Management of aphids is generally based on the use of synthetic chemical insecticides. Due to the disadvantages of chemical control, such as resistance problem, cost, and residual problem, the use of alternative control methods has recently increased. The most widely used alternative control method is microbial control (Lacey et al. 2001; Mahr et al. 2001; Khan et al. 2008). This method does not cause residue and resistance problems and is environmentally friendly (Kamp and Bidochka 2002). Among the agents used in microbial control, entomopathogenic fungi (EPFs) are more advantageous because they can be easily developed in their environment and are commercially licensed and used effectively in the control of pests (Roy et al. 2006; Trudel et al. 2007; Lacey et al. 2015). There are approximately 700 species of EPFs belonging to 90 genera, and the most commonly used fungal genera in pest control are Beauveria, Entomophthora, Isaria, Lecanicillium, Metarhizium, and Spipicaria (Goettel et al. 2005). In the virulence of EPFs, it is extremely important to isolate and use those that are generally adapted to the natural conditions of that region. Many previous studies have already indicated that EPF isolates isolated from that region give better results in pest control than EPF isolates isolated from another country or region (Hicks et al. 2001; Beron and Diaz 2005; Eken et al. 2006; Fancelli et al. 2013; Topuz et al. 2016). EPFs could be isolated from both soil and infected insects. However, the soil is a natural source for many EPFs, and many EPF species are mostly isolated from the soil (Ali-Shtayeh et al. 2002; Kamp and Bidochka 2002).
This study aimed to determine the morpho-molecular characterization and phylogenetic relationships of Beuveria bassiana isolates from both soil and infected aphids in Antalya province (southwestern part of Turkey) and to evaluate their effectiveness against A. gossypii.
Materials and methods
Insect material and maintenance
Aphids used in the bioassays were taken from the laboratory culture of A. gossypii that originated with field-collected adults from cotton (Gossypium hirsitum L.) fields in Antalya province. Rearing was carried out on potted pepper (Capsicum annuum L.) plants in a controlled climate room [25 ± 2 ℃ temperature, 60% ± 5% R.H., and 16:8 h L/D photoperiod conditions] in screen cages (40 × 40 × 40 cm) in the Entomology Laboratory of Plant Protection Department (Akdeniz University, Antalya). A. gossypii colonies were grown in the laboratory for several generations before being used in the assays. Healthy nymphs (0–48-h-old nymphs) and wingless adult females (4–5-day-old) were used in all bioassays with EPFs. Each insect and pepper seedling was used only once during the whole study period and then discarded.
Indigenous EPF isolates
First of all, a preliminary study was conducted to determine effective EPF isolates to be included in bioassay tests. For this purpose, 19 isolates from soil samples and 14 isolates from infected aphids were screened for their virulence against adult A. gossypii in Petri dishes at a concentration of 1 × 107 conidia mL−1. Based on the preliminary tests, five B. bassiana isolates were included in this evaluation. Their codes, sampling locations, isolation areas, and geographic coordinates are listed in Table 1.
Preparation of conidial suspensions
Five B. bassiana isolates selected for inclusion in the assays were first grown on SDA (Sabouraud Dextrose Agar) in Petri dishes (9 cm in diameter) and then kept in the dark at 26 ± 2 ℃ and 65 ± 5 RH for 10 days to complete sporulation. Conidia were then collected by gently scraping the culture surface of each fungal isolate with an inoculation needle and placed in vials containing 50 ml of sterile distilled water + 0.03% Tween-80 (Sigma Chemical, St. Louis, Mo, USA). Prepared stock suspensions were filtered using a sieve (60 mesh) to remove hyphae and growing substrate and then homogenized for 3 min using a vortex. The conidial concentration of stock suspensions was determined by direct counting using a Neubauer hemocytometer (Fancelli et al. 2013). Serial dilutions (105–109 conidia mL−1) were prepared in sterile distilled water containing Tween-80 and stored at 4 ℃ until used in the assays. Five different concentrations (1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia mL−1) of each isolate were tested using the spray method.
The conidia viability of each isolate was determined before the assays using the method developed by Goettel and Inglis (1997). The percent conidia germination of each isolate was determined by counting the germinated conidia (a germ tube twice the advancing diameter) from 100 randomly counted spores over the surface area under the light microscope (400 ×). Three replications were used for each isolate. The isolates with > 95% viability were used in the assays.
Pathogenicity assays against A. gossypii
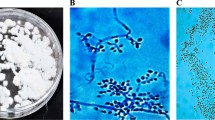
In all pathogenicity tests, all the isolates were tested at five different conidial concentrations (1 × 105, 1 × 106, 1 × 107, 1 × 108, and 1 × 109 conidia/mL) against nymphs and adults of A. gossypii in Petri and pot trials (Gabarty et al. 2014). All experiments were carried out in a climate room with a temperature of 25 ± 1 ℃ and a relative humidity of 70 ± 5%. Pepper plants (3 weeks old) were used in pot trials. Pepper production was carried out in 250-mL plastic contaniers containing forest soil. In Petri experiments, first, three layers of blotter paper were placed in Petri dishes (9 cm in diameter), and then the pepper leaf was placed with the bottom side up. Prepared conidial supensions of each fungal isolate were applied onto the leaves in petri dishes with a hand-held sprayer three times (~ 100 µL of aqueous conidial suspension per dish) from a distance of 15–20 cm. After the leaves were air-dried, the nymphs and adult females of A. gossypii obtained from the culture were placed in each Petri dish with the help of a fine-tipped brush. In the pot trials, a single leaf of a plant with 5–6 leaves was used. Each of conidial suspensions of each fungal isolate was applied using the spray method to the leaves from a distance of 15–20 cm with a hand-held sprayer three times. Then, ten individuals (nymphs or adult females) obtained from the A. gossypii culture were transferred to each of these plants with the help of a fine-tipped brush. Ziplock bags (6 × 9 cm) were used to prevent pest escape from the applied leaves. To prevent excess moisture in the ziplock bags, they were pierced 20 times with the help of a needle. As a negative control, sterile distilled water containing 0.03% Tween 80 was used. All the experiments consisted of three replications and were carried out according to a randomized plot design. Treatment efficacies were determined by live/dead counts 3, 5, 7, and 10 days after treatment. In the counts under the stereomicroscope, all insects were checked, and it was taken into account whether there was any fungal growth on them. Since each fungal entomopathogen has mycelial growth of a certain structure and color (Fig. 1), natural deaths were excluded from the experiment. In all bioassays, all dead aphids were placed in Petri dishes containing moist blotting paper and kept for 14 days for spore growth. To confirm that the deaths were caused by the applied fungal isolate, reisolation was made from the dead individuals for confirmation, and Koch’s postulates were confirmed.
Molecular identification and phylogenetic placement of B. bassiana isolates tested
The genomic DNA of the tested EPF isolates was extracted through the CTAB method described by Doyle and Doyle (1990). PCR primers of the ITS gene region are used in the molecular identification of many organisms as well as for the diagnosis of EPF isolates (White et al. 1990), including isolates of B. bassiana species Specific EF3F/EF5R classical PCR primers used in molecular analysis, and EF4F/EF4R nested PCR primers (McKinnon et al. 2018) were used in the molecular characterization of B. bassiana isolates. The classic PCR was conducted in a Peqlab Thermocycler Primus 96 device using five different primer sets, sequences, and PCR conditions, as shown in Table 2.
The PCR amplification products were sequenced with the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, CA) and the ABI 3730XL Sanger sequencing device (Applied Biosystems, CA) in the Macrogen laboratory in the Netherlands. The DNA sequences of B. bassiana isolates were performed using the Bioedit program with the ClustalW algorithm (Thompson et al. 1994; Hall 1999).
Molecular phylogenetic analyses were executed through the maximum likelihood (ML) method based on the Tamura 3-parameter model using MEGA7 software (Biodesign Institute, Arizona) (Tamura et al. 2011). The phylogenetic analyses were performed using the ITS region sequence of B. bassiana isolates and the nucleotide sequence of the other isolates of the respective species retrieved from GenBank (Altschul et al. 1990). Maximum Likelihood dendrogram using the Tamura 3-parameter model, phylogenetic trees were drawn with 1000 repetitions of bootstrapping (Tamura 1992; Tamura et al. 2011).
Molecular identification of A. gossypii
For this purpose, DNA isolation was made from a single adult female taken from the aphid culture using the CTAB method (Doyle and Doyle 1990). In the amplification of the mitochondrial COI (Cytochrome Oxidase Subunit I) gene region of A. gossypii, the COI deg F1/R1 primers and PCR program given in Table 3 were used (Timm et al. 2008). As a result of the evaluation, it was sent for DNA sequencing analysis. The DNA sequence of A. gossypii was determined using the ClustalW algorithm in the Bioedit program and the species identification was confirmed molecularly by uploading it to the NCBI GenBank (Thompson et al. 1994; Hall 1999).
Data analysis
In laboratory pathogenicity tests, treatment efficiencies of the EPF isolates tested were calculated with the help of the formula developed by Henderson and Tilton (1955). Percent mortalities from both Petri dish and pot trials were normalized with arcsine square-root transformation and subjected to analysis of variance (ANOVA). Duncan’s multiple range test (DMRT; P ≤ 0.01) was used to determine the significant differences between treatments (SPSS® 17.0). In addition, for all B. bassiana isolates, LT50 and LT95 (lethal time) values with 95% confidence limits were determined using Probit analysis and the Log-probit method (SPSS® 23.0).
Results
Results of Petri dish trials
Results of the Petri dish trials with A. gossypii nymphs showed that all B. bassiana isolates tested were significantly different in terms of mortality rates compared to the control group (P ≤ 0.01) (Table 4). However, mortality rates from fungal isolates have varied over time. Differences in mortality rates per count day were generally significant between different fungal isolates (P ≤ 0.01). Of the fungal isolates tested, BbSr-2 and BbKm-2 were statistically more pathogenic than others (BbDm-2, BbFn-2, and BbDs-4) and caused 100% mortality of A. gossypii nymphs 10 days post-treatment. However, as seen in Table 4, different conidial concentrations of the isolates were found to be statistically different. In Petri trials, the most effective concentrations were 1 × 108 and 1 × 109 conidia/mL, while the least effective one was 1 × 105 conidia/mL.
The results from Petri trials with A. gossypii adults showed that the difference between mortality rates of all fungal isolates and the control group was statistically significant (P ≤ 0.01) (Table 5). However, mortality rates from fungal isolates varied over time. Differences in mortality rates per count day were generally significant between different fungal isolates (P ≤ 0.01). Of the five B. bassiana isolates tested, two isolates (BbDm-2 and BbSr-2) caused 100% mortality in A. gossypii adults at a lower concentration (1 × 106 conidia/mL) 10 days after treatment. However, as seen in Table 5, isolates BbSr-2, BbKm-2, and BbDm-2 were statistically more effective than other two isolates (BbFn-2 and BbDs-4). Different conidial concentrations of the isolates were found to be statistically significantly different. In Petri trials, the most effective concentrations were 1 × 108 and 1 × 109 conidia/mL, while the least effective one was 1 × 105 conidia/mL. There was no statistical difference between two concentrations, 1 × 107 and 1 × 108 conidia/mL (P ≤ 0.01).
Results of pot trials
The results of pot experiments with A. gossypii nymphs showed that there were statistically significant differences between the fungus isolates tested and the control group in terms of mortality rates (P ≤ 0.01) (Table 6). However, mortality rates from fungal isolates varied over time. Differences in mortality rates per count day were generally significant between different fungal isolates (P ≤ 0.01). Isolate BbSr-2 caused 100% mortality in the nymphs of A. gossypii at all the conidial concentrations tested 10 days post-treatment. However, as seen in Table 6, BbSr-2 and BbDs-4 were statistically more effective than the other isolates tested (BbFn-2, BbDm-2 and BbFn-2). Different conidial concentrations of the isolates were found to be significantly different. There was no statistical difference between the most effective concentrations (1 × 107, 1 × 108 and 1 × 109 conidia/mL) in pot trials. However, the least effective concentration was 1 × 105 conidia/mL (P ≤ 0.01).
Result of the pot trials with A. gossypii adults showed that the difference between the mortality rates of all the fungal isolates tested and the control group was statistically significant (P ≤ 0.01) (Table 7). However, mortality rates from the fungal isolates varied over time. Differences in mortality rates per count day were generally significant between different fungal isolates (P ≤ 0.01). Of the five isolates tested, BbFn-2 caused 100% mortality of A. gossypii adults at a lower concentration (1 × 106 conidia mL−1) 10 days after treatment. However, as seen in Table 7, isolate BbKm-2 was statistically more effective than all other ones. In terms of percent mortality, different conidial concentrations of the isolates were found to be significantly different. In pot trials, the most effective concentration was 1 × 109 conidia/mL, followed by the concentrations 1 × 108, 1 × 107, and 1 × 106 conidia/mL. The least effective one was 1 × 105 conidia/mL (P ≤ 0.01).
The LT50 and LT95 values (days) of the five B. bassiana isolates tested at the highest concentration (1 × 109 conidia mL−1) to nymphs and adults of A. gossypii in pot experiments are given in Table 8. While the LT50 values calculated for the nymphs of A. gossypii varied between 1.72 and 2.12 days, the LT95 values ranged from 4.81 and 8.49 days. The calculated LT50 and LT95 values for A. gossypii adults ranged from 1.65 to 2.54 and 4.66 to 12.93 days, respectively. The lowest LT50 and LT95 values were calculated for isolate BbSr-2 against both nymphs and adults of A. gossypii.
Molecular identification and phylogenetic placement of B. bassiana isolates
Molecular characterization of the B. bassiana isolates is used in the study, and they were successfully amplified with ITS1/ITS4 primers used in the amplification of the ITS region in Classical PCR, and the PCR products (~ 560 bp) were visualized in agarose gel electrophoresis (Fig. 2a) (White et al. 1990). Specific EF3F/EF5R, and EF4F/EF4R (McKinnon et al. 2018) were also tested in the study, and PCR products were visualized in agarose gel electrophoresis. In the PCR process of EPF isolates with EF3F/EF5R primers, ~ 406 bp (Fig. 2b) of the product was obtained, and in the nested PCR process using EF4F/EF4R primers together with the obtained PCR products, ~ 176 bp (Fig. 2c) product was obtained. At the same time, ~ 350 bp product was obtained in the Classical PCR process performed with COIdegF1/COIdegR1 primers from single-individual adults taken from A. gossypii cultures used in efficacy trials, and the obtained PCR products were visualized in agarose gel electrophoresis (Fig. 2d) (Timm et al. 2008).
ITS1/ITS4 PCR products of BbSr-2, BbDs-4, BbDm-2, BbFn-2, and BbKm-2 isolates from the ITS region and PCR products obtained from A. gossypii individuals using COIdegF1/COIdegR1 primers BM Labosis-Macro Gene (Çankaya, Ankara) (www.bmlabosis.com) at the Macrogen Netherlands laboratory using the ABI 3730XL Sanger sequencing instrument (Applied Biosystems, Foster City, CA) and the Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). The DNA sequences of the isolates were determined using the ClustalW algorithm in the Bioedit program, and their accession numbers were obtained by uploading them to the NCBI GenBank database (Thompson et al. 1994; Hall 1999). Accession numbers of the isolates provided by NCBI GenBank are given in Table 9. The sequence data of isolates were compared with similar strains in GenBank using NCBI BLAST analysis. As a result of BLAST analysis, each species showed 99–100% similarity to other species in GenBank, thus confirming the species-level molecular identification of fungus isolates and A. gossypii (Altschul et al. 1990).
As a result of the phylogenetic analysis, the isolates were found to be in the same groups as the isolates in GenBank (Fig. 3). In the dendrogram drawn using the ITS gene regions of the isolates of B. bassiana (Fig. 3), the indigenous EPF isolates of BbKm-2, BbSr-2, BbDm-2, BbFn-2, and BbDs-4 and B. bassiana AP18, R13, TR-52–003, JEF-573, A1032B, ERL293, and NBAII-Bb-70 isolates obtained from Gen Bank were included in the same group. As a result of the analysis, the highest BootStrap (select and distribute test) value (1000 repetitions) among Beauveria species was determined to be 99% for B. bassiana native and other isolates.
The Maximum Likelihood tree based on the Tamura 3-parameter model showing the phylogenetic relationship between the three Beauveria bassiana isolates (BbKm-2, BbSr-2, BbDm-2, BbFn-2, and BbDs-4) found to have high virulence in the present study and other B. bassiana isolates from GenBank based on ITS region sequence
Discussion
The results from the study showed that indigenous isolates of B. bassiana with high pathogenicity have the potential to be used in the control of A. gossypii. Studies on EPFs around the world have gained momentum in the last fifteen years, and researchers have reported that EPFs are effective in controlling different agricultural pests. Similarities were observed between the results obtained from previous studies and the results obtained from the present study in term of activity of EPFs on aphids (Hatting et al. 1999; Satar et al. 1999; Yeo et al. 2003; Perdikis et al. 2004; Scorsetti et al. 2006; Kim et al. 2010; Jandricic et al. 2014; Castillo et al. 2014; Wang et al. 2016; Ebadollahi et al. 2017). In parallel with the results obtained from this study, some previous studies have shown that B. bassiana isolates are highly virulent against A. gossypii and can be used in the control of this aphid species (Vu et al. 2007; Gurulingappa et al. 2010; Castillo et al. 2014; Jandricic et al. 2014). Herlinda et al. (2010) tested ten B. bassiana and 15 Metarhizium anisopliae isolates from infected insects against A. gossypii at a concentration of 1 × 106 spores/mL. They reported that the most virulent B. bassiana isolate (BPM) resulted in 100% mortality in A. gossypii in 2.54 days and the M. anisopliae isolate (MaAgIn) in 2.81 days, and they can be used in controlling A. gossypii popoulations. In another study, Castillo et al. (2014) tested one isolate of P. lilacinum and one isolate of B. bassiana against A. gossypii on cotton plants both in greenhouse and open-field. Their results indicate that both isolates can be used in controlling the aphid pest. Mohammed et al. (2018) evaluated indigenous isolates of Lecanicillium lecanii, B. bassiana, Isaria fumosorosea, Chaetomium globosum (Kunze, Chaetomiaceae), and M. anisopliae against the aphids, M. persicae, A. gossypii, Aphis fabae, and Macrosiphum euphorbiae (Thomas) under greenhouse conditions. B. bassiana, M. anisopliae, and L. lecanii isolates caused 100% mortality of M. persicae, and A. gossypii at a concentration of 108 spores/mL on day 7. Vu et al. (2007) evaluated 12 different fungal isolates belonging to L. lecanii, Paecilomyces farinosus, B. bassiana, M. anisopliae, Cordyceps scarabaeicola (Kobayasi), at 4 different conidial concentrations (1 × 105, 1 × 106, 1 × 107, and 1 × 108 spores/mL) against A. gossypii and M. persicae and observed a decrease in LT50 value as spore concentration increased. They also reported that the most virulent isolate (41,185) belonged to the L. lecanii species. The results obtained in the aforementioned concentration and efficacy studies show parallelism with the findings obtained in this study. Gurulingappa et al. (2010) investigated the colonization of B. bassiana, L. lecanii, and Aspergillus parasiticus (Speare, Aspergillaceae) species in different plants and their effects against A. gossypii and Chortoicetes terminifera (Walker, Orthoptera, Acrididae). Their results indicated that all tested species could be used in the biological control of aforementioned aphid and locust species. In another study by Gurulingappa et al. (2010), the duration and causes of the effects of L. lecanii and B. bassiana isolates on A. gossypii were investigated. The results revealed that methanolic fractions of B. bassiana mycelium caused the significant death of A. gossypii. The results of Gurulingappa et al. (2010) have also showed that A. gossypii is affected by contact with both conidia and fungal metabolites. This broad effect indicates that EPFs may have an important role in controlling pest populations. In a more recent study, Ebadolahi et al. (2017) similarly investigated the effectiveness of Lecanicillium muscarium isolate against A. gossypii and reported that the tested isolate could be used in the control of A. gossypii. However, L. longisporum and L. muscarium isolates with high activity on M. persicae and A. gossypii could not be obtained in the current study. The results found in all above-mentioned studies are consistent with the findings obtained in this study.
Today, molecular identification studies are needed in addition to morphological identification in the characterization of EPFs. Rehner and Buckley (2005) reported that the diagnosis of Beauveria species is difficult to make morphologically and showed that molecularly based sequences based on a single region can be misleading in determining species diversity. Using the multiple gene sequencing approach, the researchers determined the taxonomy of Beauveria species. In the genus Beauveria, new species are often described based on genotypic data. For example, morphological identification of Beauveria brongniartii ((Sacc.) Petch) and Beauveria caledonica (Bissett & Widden) species is not possible. Both form a cylindrical cone. In molecular characterization studies (PCR using ITS regions), two different species were determined (Glare and Inwood 1998; Glare 2004; Rehner and Buckley 2005; Glare et al. 2008). In the world, Beauveria australis species were isolated from locusts and soil only in Australia, Beauveria kipukae was isolated from Homoptera in Hawaii, and Beauveria pseudobassiana and Beauveria varroae species were isolated from Varroa destructor and defined molecularly (Rehner et al. 2011). In this study, only B. bassiana species belonging to the genus Beauveria were isolated.
Recently, in the molecular identification of EPFs, PCR using general identification primers of the ITS, TEF, and EF1 alpha gene regions and identification and characterization processes based on sequencing of the obtained PCR products and comparing them with the isolates in NCBI GenBank are performed. In this study, the ITS region was also used to compare with other species in the GenBank and to perform phylogenetic analysis, and similar results were obtained with other studies (Fisher et al. 2011; Castillo et al. 2014; Perez-Gonzalez et al. 2014; Muniz-Reyes et al. 2014; Clifton et al. 2018). At the same time, different researchers have designed primers to perform specific molecular identification of fungi at the species level without the need for DNA sequence analysis of PCR products. In this study, EF3F/EF5R and EF4F/EF4R (McKinnon et al. 2018) primer sets were also used in molecular characterization to specifically diagnose B. bassiana isolates with high efficiency and to form a source for future studies. PCR products of similar size were obtained with the results in the reference article.
Based on the results of this study, it was concluded that five indigenous isolates (BbKm-2, BbSr-2, BbDm-2, BbFn-2, and BbDs-4) of B. bassiana can be used as potential alternatives for the management of A. gossypii. Further studies should be conducted under field conditions to better understand the efficacy of these five B. bassiana isolates and their potential as effective biocontrol agents within the framework of an integrated pest management (IPM) program. It has been reported that the role of Beauveria would be better as one of many tools in integrated pest management rather than as a stand-alone management approach and needs to be better developed across the range of crop systems (Mascarin and Jaronski 2016). However, in order for the five isolates tested to be included in a sustainable IPM system to minimize synthetic chemicals, it is extremely important that they be easily produced in mass by IPM-appliers/growers or easily accessible to them in the form of ready-made commercial preparations (Galli et al. 2024). Mass production of Beauveria is required by the typical inundative use strategy, requiring a cost-effective production and stabilization process that delivers a large number of viable, infective propagules (Jackson et al. 2010). Mascarin and Jaronski (2016) reported that aerial conidia are the primary infective propagule of Beauveria and solid-substrate fermentation is very well suited for low technology production of aerial conidia of Beauveria. They also indicated that solid-substrate fermentation could even be implemented on the basis of producer associations.
Finally, we believe there is a need for improving education and extension of growers and IPM practitioners about the utility and availability of Beauveria in order to foster its widespread adoption into comprehensive pest management strategies.
References
Ali-Shtayeh MS, Marai AB, Jamous RM (2002) Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian Area. Mycopathologia 156(3):35–244
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Mol Biol 215:403–410
Beron CM, Diaz BM (2005) Pathogenicity of hyphomycetous fungi against Cyclocephala signaticollis. Biocontrol 50:143–150
Blackman RL, Eastop VF (1984) Aphids on the World’s Crops. Wiley, Chichester, p 466
Blackman RL, Eastop VF (2000) Aphids on The World’s Crops: An Identification quide, 2nd edn. Wiley, London, p 414
Castillo LD, Zhu-Salzman K, Ek-Ramos MJ, Sword GA (2014) The entomopathogenic fungal endophytes Purpureocillium lilacinum (Formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 9(8):e103891
Clifton EH, Jaronski ST, Coates BS, Hodgson EW, Gassmann AJ (2018) Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 13(3):e0194815
Cottier W (1953) Aphids of New Zealand. N.Z. Am J Sci Ind Research 106:1–382
Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Ebadollahi A, Davari M, Razmjou J, Naseri B (2017) Separate and combined effects of Mentha piperata and Mentha pulegium essential oils and a pathogenic fungus Lecanicillium muscarium against Aphis gossypii (Hemiptera: Aphididae). J Econ Entomol 110(3):1025–1030
Eken C, Tozlu G, Dane E, Çoruh S, Demirci E (2006) Pathogenicity of Beauveria bassiana (Deuteromycotina: Hypomycetes) to larvae of the small poplar longhorn beetle, Saperda populnea (Coleoptera: Cerambycidae). Mycopathologia 162:69–71
Fancelli M, Dias A, Delalibera IJ, Cerqueira de Jesus S, Souza do Nascimento A, Oliveira e Silva S (2013) Beauveria bassiana strains for biological control of Cosmopolites sordidus (Germ.) (Coleoptera: Curculionidae) in Plantain. Biomed Res Int 1:184756
Fisher JJ, Stephen A, Rehner D, Bruck J (2011) Diversity of rhizosphere associated entomopathogenic fungi of perennial herbs, shrubs and coniferous trees. J Invertebr Pathol 106:289–295
Gabarty A, Salem HM, Fouda MA, Abas AA, Ibrahim AA (2014) Pathogencity induced by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in Agrotis ipsilon (Hufn.). J Radiat Res Appl Sci 7:95–100
Galli M, Feldmann F, Vogler UK, Kogel KH (2024) Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J Plant Dis Protect 131:265–291
Glare TR (2004) Molecular characterization in the entomopathogenic fungal genus Beauveria. Laimburg Journal 1:286–298
Glare TR, Inwood A (1998) Morphological and genetic characterization of Beauveria spp. from New Zealand. Mycol Res 102:250–256
Glare TR, Reay SD, Nelson TL, Moore R (2008) Beauveria caledonica is a naturally occurring pathogen of forest beetles. Mycol Res 112:352–360
Goettel MS, Inglis GD (1997) Fungi Hyphomycetes: Manual of Techniques in Insect Pathology. Academic Press, San Diego, pp 213–248
Goettel MS, Eilenberg J, Glare T (2005) Entomopathogenic fungi and their role in regulation of insect populations. Comprehensive Molecular Insect Science, Elseiver, Amsterdam, pp 361–405
Gurulingappa P, Sword GA, Murdoch G, Mcgee PA (2010) Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control 55:34–41
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hatting JL, Humber RA, Poprawski TJ, Miller RM (1999) A survey of fungal pathogens of aphids from South Africa with special reference to cereal aphids. Biol Control 16:1–12
Henderson CF, Tilton EW (1955) Tests with acaricides against the brown wheat mite. J Econ Entomol 48:157–161
Herlinda S, Irsan C, Mayasari R, Septariani S (2010) Identification and selection of entomopathogenic fungi as biocontrol agents for Aphis gossypii from South Sumatra. Microbiol Indones 4:137–142
Hicks BJ, Watt AD, Cosens D (2001) The potential of Beauveria bassiana (Hyphomycetes: Moniliales) as a biological control agent against to pine beauty moth, Panolis flammea (Lepidoptera: Noctuidae). For Ecol Manag 149:275–281
Jackson MA, Dunlap CA, Jaronski S (2010) Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. Biocontrol 55:129–145
Jandricic SE, Filotas M, Sanderson JP, Wraight SP (2014) Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J Invertebr Pathol 118:34–46
Kamp AM, Bidochka M (2002) Conidium production by insect pathogenic fungi on commercially viable agars. Lett Appl Microbiol 35:74–77
Kennedy JS, Day MF, Eastop VF (1962) A conspectus of aphids as vector of plant viruses commonwealth. Inst Ent, London, England, p 114
Khan ZR, James DG, Midega CAO, Pickett JA (2008) Chemical ecology and conservation biological control. Biol Control 45(2):210–224
Kim JJ, Goettel MS, Gillespie DR (2010) Evaluation of Lecanicillium longisporum, Vertalec® against the cotton aphid, Aphis gossypii, and cucumber powdery mildew, Sphaerotheca fuliginea in a greenhouse environment. Crop Protect 29:540–544
Lacey LA, Frutos R, Kaya HK, Vail P (2001) Insect pathogens as biological control agents: do they have a future. Biol Control 21:230–248
Lacey LA, Grzywacz D, Shapiro-Ilan DI, Frutos R, Brownbridge M, Goettel MS (2015) Insect pathogens as biological control agents: back to the future. J Invertebr Pathol 132:1–41
Mahr SER, Cloyd RA, Mahr DL, Sadof CS (2001) Biological control of insectsand other pests of greenhouse crops. North Cent Reg Public 581:102
Mascarin GM, Jaronski ST (2016) The production and uses of Beauveria bassiana as a microbial insecticide. World J Microbiol Biotechnol 32:177
McKinnon AC, Glare TR, Ridgway HJ, Mendoza-Mendoza A, Holyoake A, Godsoe WK, Bufford JL (2018) Detection of the entomopathogenic fungus Beauveria bassiana in the rhizosphere of wound-stressed Zea mays plants. Front Microbiol 9:1161
Mohammed AA, Kadhim JH, Kamaluddin ZN (2018) Selection of highly virulent entomopathogenic fungal isolates to control the greenhouse aphid species in Iraq. Egypt J Biol Pest Control 28:71
Muniz-Reyes E, Guzman-Franco AW, Sanchez-Escudero J, Nieto-Angel R (2014) Occurrence of entomopathogenic fungi in tejocote (Crataegus mexicana) orchard soils and their pathogenicity against Rhagoletispomonella. J Appl Microbiol 117:1450–1462
Murphy G, Ferguson G, Shipp L (2006) Aphids in greenhouse crops. Factsheet. In: Ontario ministry of agriculture, food and rural affairs, Canada, pp 1198–1712
Perdikis DC, Lykouressis DP, Garantonakis NG (2004) Instar preference and parasitization of Aphis gossypii and Myzus pesicae (Hemiptera: Aphididae) by the parasitoid Aphidius colemani (Hymenoptera: Aphididae). Eur J Entomol 101:333–336
Perez-Gonzalez VH, Guzman-Franco AW, Alatorre-Rosas R, Hernandez-Lopez J, Hernandez-Lopez A, Carrillo-Benitez MG, Baverstock J (2014) Specific diversity of the entomopathogenic fungi Beauveria and Metarhizium in Mexican agricultural soils. J Invertebr Pathol 119:54–61
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Rehner SA, Minnis AM, Sung GH, Luangsa-ard JJ, Devotto L, Humber RA (2011) Phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103:1055–1073
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357
Satar S, Kersting U, Uygun N (1999) Development and fecundity of Aphis gossypii Glover (Homoptera: Aphididae) on three malvaceae hosts. Turk J Agric for 23:637–644
Scorsetti A, Garcia J, Lastra C (2006) Natural occurrence of entomopathogenic fungi (Zygomycetes: Entomophthorales) of aphid (Hemiptera: Aphididae) pests of horticultural crops in Argentina. Biocontrol 52:641–655
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Timm J, Figiel M, Kochzius M (2008) Contrasting patterns in species boundaries and evolution of anemonefishes (Amphiprioninae, Pomacentridae) in the centre of marine biodiversity. Mol Phylogenet Evol 49:268–276
Topuz E, Erler F, Gümrükcü E (2016) Survey of indigenous entomopathogenic fungi and evaluation of their pathogenicity against the carmine spider mite, Tetranychus cinnabarinus (Boisd.), and the whitefly, Bemisia tabaci (Genn.) biotype B. Pest Manag Sci 72(12):2273–2279
Trudel R, Lavalle C, Guertin C, Cote C, Todorova SI, Alfar R, Kope H (2007) Potential of Beauveria bassiana (Hyphomycetes: Moniliales) for controlling the white pine weevil, Pissodes strobi (Col., Curculionidae). J Appl Entomol 131(2):90–97
Von Dohlen CD, Rowe CA, Heie OE (2006) A test of morphological hypotheses for tribal and subtribal relationships of Aphidinae (Insecta: Hemiptera: Aphididae) using DNA sequences. Mol Phylogenet Evol 38:316–329
Vu VH, Hongb S, Kima K (2007) Selection of entomopathogenic fungi for aphid control. J Biosci Bioeng 104(6):498–505
Wang L, Zhang S, Luo JY, Wang CY, Li-Min L, Zhu XZ, Li CH, Cui JJ (2016) Identification of Aphis gossypii Glover (Hemiptera: Aphididae) biotypes from different host plants in north China. PLoS ONE 11(1):e0146345
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocols: a Guide to Methods and Applications. Academic Press, New York, pp 315–322
Yeo H, Pell JK, Alderson PG, Clark SJ, Pye BJ (2003) Laboratory evaluation of temperature effects on the germination and growth of entomopathogenic fungi and on their pathogenicity to two aphid species. Pest Manag Sci 59(2):156–165
Acknowledgements
This study was financially supported by the Scientific Projects Coordination Unit of Akdeniz University (Antalya, Turkey). Also, special thanks to the TUBITAK BIDEB 2228-B Domestic Doctoral Fellowship Program.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was funded by the Scientific Projects Coordination Unit (SPCU) of Akdeniz University (Antalya, Turkey) under grant number BAP FDK-2019–4859.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baki, D., Erler, F. Evaluation of indigenous isolates of Beuveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes) against the cotton aphid Aphis gossypii Glover. J Plant Dis Prot (2024). https://doi.org/10.1007/s41348-024-00952-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41348-024-00952-8