Abstract
Cotton crops are routinely threatened by emerging fungal diseases. Fungal endophytes also can be considered latent phytopathogens. In this study we tested the hypothesis that an endophytic strain of Diaporthe, isolated from chlorotic leaves of cotton (Gossypium hirsutum), could trigger physiological effects of biotic stress in this oilseed plant. We also assessed the histopathological aspects of the mycelial interaction of the endophyte with the adaxial surface of G. hirsutum leaves. Thus, we studied the synthesis of photosynthetic pigments, pattern of gas exchange, and photochemistry of cotton plants subjected to inoculation with Diaporthe ueckerae via root and leaf at three different phenological stages (vegetative, reproductive, and maturation). Additionally, we histopathologically analyzed infected leaves using electron microscopy to study the process of leaf colonization by this endophytic fungus. We evidenced that D. ueckerae inoculation negatively affected the synthesis of photosynthetic pigments in plants at vegetative and reproductive stages. Moreover, inoculation also negatively affected the photosynthetic rate and carboxylation efficiency of these plants. We also found that the presence of the endophyte increased transpiration and decreased water use efficiency in the plants. Furthermore, foliar inoculation negatively affected stomatal conductance, whereas inoculation via leaf or root reduced the photochemical performance of cotton. We also observed that D. ueckerae colonizes the leaf tissues of G. hirsutum via glandular trichomes and forces penetration into the epidermis using appressoria, and the plant responds by closing the stomata. The observed physiological alterations are indicative of biotic stress, confirming the hypothesis that D. ueckerae may be an opportunistic phytopathogen for cotton plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cotton (Gossypium hirsutum L.) cultivation is a globally important agricultural activity. The oilseed is used in animal feed and as a raw material for biofuel production (Zhang et al. 2013; Kiranmai et al. 2020). In addition, G. hirsutum is the leading textile plant worldwide, accounting for more than 90% of the global fiber production from cotton species (Cai et al. 2014). However, cotton production is constantly threatened by pathogens that cause considerable economic losses. Numerous cotton plant diseases have been identified worldwide. Fusarium and Verticillium wilt, Alternaria leaf spot and seedling diseases, boll rot, leaf curl disease, and bacterial blight are the major hindrances to cotton lint production (Chohan et al. 2020). Thus, cotton plant diseases emerge routinely, challenging current control or biocontrol strategies. For example, Ramularia has recently been observed as an emerging fungal pathogen in Brazil (Da Silva et al. 2019). Cotton has been intensively cultivated in the Brazilian Cerrado since the early 1980s, and the hot and humid environment of the region favors fungal disease epidemics.
Plant–microorganism interaction studies conducted in a controlled environment are useful for providing early knowledge of disease symptoms and predicting epidemics (Uddin et al. 2003; Gullino et al. 2018). For example, little is known about whether endophytic fungi associated with cultivars or wild crop relatives can become pathogens at developmental stages when the plant is most susceptible.
Species of the genus Diaporthe (former anamorph Phomopsis) are examples of fungi that behave as mutualistic or pathogenic endophytes and also as saprophytes, on a wide range of hosts worldwide (Murali et al. 2006; Garcia-Reyne et al. 2010; Udayanga et al. 2011). Pathogenic Diaporthe species affect a wide variety of host plants, including forest trees (Yang et al. 2018), citrus fruits (Udayanga et al. 2014), pepper (Fang et al. 2020), sunflower (Thompson et al. 2011), and soybean (Udayanga et al. 2015; Mena et al. 2022). Recently, Diaporthe species have been found to be responsible for an emerging citrus disease in Europe (Guarnaccia and Crous 2017) and to produce phytotoxins such as fusicoccin A (Hilario et al. 2022).
Diaporthe has already been identified as asymptomatic (Roy 1983) or pathogenic endophyte in cotton plants, as some strains in an anamorphic state can damage cotton bolls (Palmateer 2003; De Araújo 2008). Becerra-Lopez Lavalle et al. (2005) showed that the stem of G. hirsutum can harbor many species of endophytic fungi pathogenic to cotton, including Diaporthe. Reports of infection of cotton plants by D. ueckerae, or even by its anamorphous form, Phomopsis, are rare, although Phomopsis sp. is associated with cases of premature boll rot (McLean and Lawrence 1998). Isolated from leaves and roots, Phomopsis has already been characterized as an endophyte of G. hirsutum (McGee 2002; Fu et al. 2011). Wang et al. (2007) isolated strains of Phomopsis sp. from native Australian Gossypium species and investigated whether these strains could be pathogenic to cultivated cotton (G. hirsutum). Although they did not observe any apparent leaf disease symptoms during the five-week experimental period by either inoculation method (root dipping or stem puncture), localized discolorations were observed in the stem tissue when inoculation was performed by the puncture method.
Biotic stress conditions in crop plants may be determined by physiological analyses of photosynthetic pigment production, gas exchange, and chlorophyll a fluorescence (Balachandran et al. 1997). Transcriptomic studies have shown that the overall expression of photosynthesis-related genes decreases under pest or pathogen attack (Berger et al. 2007; Bilgin et al. 2010), as metabolism is directed toward defense-related molecules. Chlorophyll a fluorescence analyses have been widely used to discover perturbations in photosynthetic efficiency caused by pathogen incidence (Sandmann et al. 2018; Pérez-Bueno et al. 2019). There is evidence that fungal effectors specifically target chloroplasts during the infection process (Kretschmer et al. 2019), thereby compromising light energy utilization and causing productivity losses (Lu and Yao 2018).
We were also interested in elucidating the primary mechanisms through which D. ueckerae interacts with the epidermis of G. hirsutum to ensure tissue penetration. In the region of contact with host cells, some phytopathogenic fungi develop fairly typical hyphal penetration structures, such as appressoria and transpressoria (Cruz-Mireles et al. 2021; Ryder et al. 2022), or use classical entry ports such as stomata or lenticels and keep them open through the production of phytotoxins and/or effectors (Nemesio-Gorriz et al. 2019; Navarro et al. 2022; Wu and Liu 2022). Understanding histopathological mechanisms involving the plant–microorganism association can assist in developing management techniques, anticipating prevention strategies, and understanding patterns associated with biotic attacks. Thus, since the studies reporting that Diaporthe species are asymptomatic endophytes in cotton plants did not focus on physiological parameters, we decided to assess the hypothesis of latent pathogenicity of an endophytic strain of Diaporthe, on leaves and roots of cotton, in different stages of development, through the study of a comprehensive set of physiological parameters. We also analyzed the histopathological aspects of the mycelial interaction of the endophyte with the adaxial surface of G. hirsutum leaves. We focused on determining opportunistic pathogenicity traits, the most aggressive route of infection (root or leaf), and the most susceptible developmental stage of the plants (vegetative, reproductive, or maturation).
Material and methods
Isolation and identification of the phytopathogenic strain
An anamorphic endophytic strain of Diaporthe ueckerae (syn. Phomopsis ueckerae) isolated from chlorotic leaves of Gossypium hirsutum was used. Leaves showing discoloration damage were randomly sampled from three cotton plants grown in the rural area of Primavera do Leste, MT, Brazil (15° 23′ 12″ S and 45° 23′ 12″ W; 650 m altitude). The leaves were packed in aseptic plastic bags and immediately taken to the Agricultural Microbiology laboratory of IFGoiano, Rio Verde campus, where they underwent superficial asepsis for epiphytic removal. Briefly, the material was immersed in a 2% Tween 80 solution and kept under agitation at 150 rpm for 5 min. The leaves were then washed with water and disinfected with 70% alcohol for 1 min, sodium hypochlorite for 3 min, and again with 70% alcohol for 30 s.
To isolate the endophyte, 1 × 1 cm fragments were removed from the leaf (one leaf per plant) and deposited in solid potato dextrose agar (PDA) medium in Petri plates. The plates were incubated at 35 °C for 3 days, when the development of mycelium was observed emerging from the fragments. The mycelium corresponding to three different fungal isolates was subcultured on PDA supplemented with chloramphenicol at 150 µg mL−1 and subsequently identified.
To identify the endophyte, the genomic DNA of the specimens was extracted according to the methodology of Cheng and Jiang (2006), using a Minirep extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s guidelines. Identification was performed by partial sequencing of the internal transcribed spacer (ITS) as well as calmodulin (CAL) and β-tubulin (TUB2) genes after amplification (PCR conditions and purification, according with Raja et al. (2017)). Sequencing was performed using the Sanger method; for phylogenetic inference, sequences were paired by similarity to sequences in GenBank using BLASTn (Altschul et al. 1990) while considering homology greater than 97%. Sequences were then concatenated and aligned to homologous sequences extracted from GenBank using Clustal Omega software (Sievers and Higgins 2014).
The selection of the evolutionary model of the sequences was performed using the Bayesian information criterion (BIC) implemented in jModelTest 2 software (Darriba et al. 2012). The selected model was HKY, with the following respective base frequencies: A = 0.2250, C = 0.2891, G = 0.2671, and T = 0.2188; the phylogenetic trees were inferred with methods based on Bayesian inference using MrBayes v.3.2.6 software (Ronquist et al. 2012). Four independent runs were performed for each tree by assigning 10 × 106 generations to the chains and sampling the a posteriori probability distribution every 500 generations. The first 2500 sampled trees were discarded before calculating the consensus tree to ensure convergence of the chains. The Bayesian highest likelihood phylogenetic tree was visualized and edited using FigTree v.1.4.4 (Rambaut 2014) software. Sequences from the species Penicillium chrysogenum were used as the outgroup.
Obtaining plant material and inoculum
This experiment was conducted using cotton seeds of the FMT 910 cultivar previously untreated with fungicides or insecticides. The seeds were grown in 5 L pots containing 7.5 kg of dystrophic clayey-sandy loam soil with a particle size distribution of 40.0% sand, 10.0% silt, and 50.0% clay and the following nutritional parameters in the 0–20 cm layer: pH (CaCl2), 5.0; H + Al, 2.40 cmolc.kg−1; P, 120 mg.kg−1; K, 0.17 cmolc.kg−1; S, 5.00 cmolc.kg−1; Ca, 2.50 cmolc.kg−1; Mg, 0.70 cmolc.kg−1; cation exchange capacity, 5.78 cmolc.kg−1; Cu, 5.0 mg.kg−1; Fe, 250.00 mg.kg−1; Mn = 29.00 mg.kg−1; Zn, 0.60 mg.kg−1; and B, 0.26 mg.kg−1. Based on this analysis, the chemical and nutritional characteristics were corrected with macro and micronutrients according to the fifth approximation of Goiás (CFSG 1988).
Two plants were grown per pot and kept in a greenhouse with the atmospheric conditions monitored daily (temperature, 28–36 °C; relative humidity, 30–48%). The average photosynthetically active radiation was 598–1011 μm, and the CO2 concentration was 575–662 ppm. The plants were irrigated daily as needed.
Mycelium of the opportunistic endophytic D. ueckerae were grown on PDA (potato infusion, 200.0; dextrose, 20.0; agar, 15.0 L−1; pH, 5.6 ± 0.2) medium for 7 days at 30 °C. Inoculation of healthy plants was performed using 5 mm mycelial fragments extracted directly from the plates and deposited in contact with plant tissues. In the leaf inoculation treatment, the mycelial fragments were deposited on the adaxial side of the leaf, and five leaves per plant were inoculated (counted downwards from the apical leaf). In the root inoculation treatment, the fragments were deposited in 5 cm deep furrows made in the rhizospheric region attached to the main root. Five furrows were made around the root of each plant. These inoculations were scheduled to occur in three phenological stages of cotton: vegetative, reproductive, and maturation. The plants were inoculated with the phytopathogen in the V2 vegetative stage (phenological stage that begins when the second leaf reaches 2.5 cm in length and ends when the third leaf reaches 2.5 cm in length). The reproductive stage was the stage in which all plants showed flowers or developed flower buds, and the maturation stage was the stage in which all plants showed bolls. The results of induced exposure to the phytopathogen were compared to those observed in control, non-inoculated plants, with data obtained 5 days after inoculation.
Photosynthetic pigment concentration
The photosynthetic pigments were evaluated by obtaining three leaf discs of 5 mm diameter from all sample units. The discs were incubated in amber flasks in a solution of dimethyl sulfoxide saturated with CaCO3. The flasks were kept in a water bath (TECNAL, Banho Dubnoff, TE-053) at 65 °C for 8 h under 30 rpm agitation for pigment extraction. The absorbance of the extract was read using a spectrophotometer (SHIMADZU, UV-Visible Spectrophotometer, UV-1800, Kyoto, Japan), at 665, 649, and 480 nm, corresponding to chlorophyll a, chlorophyll b, and carotenoids, respectively. The concentrations of these pigments were measured according to the methodology of Wellburn (1994).
Chlorophyll a fluorescence
The OJIP transient fluorescence of chlorophyll a was determined using a FluorPen FP 100 portable fluorometer (Photon Systems Instruments, Drasov, Czech Republic). Inoculated leaves from all sample units were previously dark-adapted for 30 min to ensure complete oxidation of the photosynthetic electron transport system. Subsequently, they were subjected to a pulse of 3000 µmol m−2 s−1 of blue light to measure the fluorescence at the O step, which corresponds to minimum fluorescence (F0) at 50 μs, when all PSII reaction centers are open; J step, which corresponds to fluorescence at 2 ms; I step, which corresponds to fluorescence at 30 ms; and P step, which corresponds to the maximum fluorescence (FM), when all PSII reaction centers are closed. These values were used for estimating various bioenergetic PSII indices, according to Strasser et al. (2000). The following values were estimated: specific light absorption flux per reaction center (ABS/RC), captured energy flux per reaction center at t = 0 (TR0/RC), electron transport flux per reaction center (ET0/RC), specific energy dissipation flux at the level of the chlorophylls of the antenna complex (DI0/RC), photosynthetic performance index (PiABS) that incorporates the processes of the energy cascade from the first uptake events to the reduction of PQ, maximum quantum yield of primary photochemistry (PHIPO), probability of an exciton moving an electron through the electron transport chain after the Quinone (PHI0), and quantum yield of electron transport (PHIE0) after adaptation of the leaves to the dark (30 min).
Gas exchange
Gas exchange was assessed at 08:00 using an infrared gas analyzer with an attached fluorometer (model LI-68400XT, LI-COR Inc., Lincoln, USA). Data of inoculated leaves were obtained using photosynthetically active radiation (1000 μmol photons m−2 s−1), block temperature of 25 °C, and relative humidity of ~50%. The net photosynthesis rate (A) (µmol of CO2 m−2 s−1), stomatal conductance (gs) (mol of H2O m−2 s−1), transpiration (E) (mmol of H2O m−2 s−1), internal carbon concentration (Ci) (µmol CO2 mol−1), water use efficiency (WUE) (µmol CO2 mmol H2 O s−1), and instantaneous carboxylation efficiency (A/Ci) were measured.
Scanning electron microscopy
Leaf histopathological assessments were performed only on the leaf-inoculated plants. These were performed through images obtained via scanning electron microscopy. Leaf fragments of 1 cm2 were pre-fixed in Karnovsky solution, post-fixed with osmium tetraoxide, dehydrated in ethyl series, transferred to amyl acetate, and critical point dried in a carbon dioxide dryer. The samples were then coated with gold using an ion jet, and images were obtained using scanning electron microscope (Jeol JSM-IT300LV, JEOL USA, Inc., Peabody, MA, USA).
Experimental design and statistical analysis
The experiment was conducted in randomized blocks considering a double factorial scheme (2 × 3 + 1), that is, two types of phytopathogen infection (foliar and root), three stages of cotton development (vegetative, reproductive, and maturation), and a control treatment consisting of non-inoculated plants. The plants in the control treatment were also assessed in the three stages of plant development. Each treatment was assessed in five replicates, with each replicate comprising two plants per pot, with a total of 10 plants analyzed per treatment.
The data observed for the different physiological parameters were subjected to normality and ANOVA tests, and when the effect of the exploratory variables (infection type and development stage) was verified, the means were compared using the Tukey test at 5% probability. To better understand the correlation between the exploratory and physiological variables, these were analyzed together through a correlation matrix and combined in a principal component analysis (PCA). Correlation PCAs were performed since these variables had different units of measurement and were constructed using standardized data with mean 0 and standard deviation 1. The number of selected components was based on eigenvalues (> 1.0) and explained variance (above 80%). All analyses were conducted using R 4.2.1 software (R Core Team 2021).
Results
Identification of endophytic fungus
Identical gene sequences were acquired for the three endophytic isolates obtained from chlorotic leaves of G. hirsutum, thereby demonstrating that these were the same microorganism. The strains were identified as Diaporthe ueckerae based on their similarity to the strain MN424583.1. A distinct and strongly supported clade was recovered for the relationship between the three endophytic isolates that we obtained from cotton and MN424583.1, which together showed the closest link to the D. ueckerae strain KJ612119.1. As expected, strains from other species of this genus were shown to belong to more distinct clades (Fig. 1).
Similarity tree between species of the genus Diaporthe and an endophytic, opportunistic phytopathogenic strain isolated from Gossypium hirsutum plants. Phylogeny recovered based on ITS and the CAL and TUB2 genes, with Penicillium chysogenum as an outgroup. The numbers on the nodes are the probability and the numbers above the branches are the support. The bar below the tree is the genetic distance
Photosynthetic profile of the Gossypium hirsutum × Diaporthe ueckerae interaction
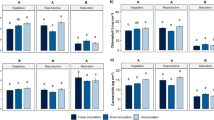
Infection by the endophyte affected the cotton plants differentially, according to the development stage (p < 0.001), and plants in the maturation stage showed the lowest mean chlorophyll a levels in the leaves (Fig. 2a). Meanwhile, the synthesis of this pigment was not affected by the type of inoculation. However, when we analyzed the interaction between developmental stage and type of inoculation, we verified an effect on the synthesis of chlorophyll a (p < 0.001). In plants in the vegetative stage, the highest mean levels were observed in the non-inoculated plants, and the lowest mean levels were observed in the plants exposed to the endophyte inoculated through the leaf. In plants in the reproductive stage, the highest mean chlorophyll a levels were also observed in non-inoculated plants, whereas in the maturation stage, there were no differences between the mean levels obtained via the different method of inoculation.
Concentrations of the photosynthetic pigments chlorophyll a (a), chlorophyll b (b), chlorophyll a/b ratio (c), and carotenoids (d) observed in Gossypium hirsutum plants that underwent infection by Diaporthe ueckerae in leaves or root in three phenological phases (vegetative, reproductive and maturation). Letters above tables compare, by Tukey test at 5% probability, the phenological phases; letters above the bars within the same phase compare the types of inoculation (leaf or root) with non-inoculated plants
Similarly, we also verified the effect of the development stage on the mean chlorophyll b level (p < 0.001). The plants in the maturation stage showed noticeably lower mean levels of this pigment (Fig. 2b). We did not find any isolated effect of the type of inoculation on the concentration of this pigment; however, we found an effect of the interaction between development stage and type of inoculation (p = 0.014), with differences being observed between means only in the reproductive phase, where non-inoculated plants showed the highest concentrations of chlorophyll b.
The developmental stages also affected the chlorophyll a/b ratio (p < 0.001), with the highest mean ratios being observed in plants at maturation stage given the lower chlorophyll b levels in relation to chlorophyll a levels (Fig. 2c). We also verified the effect of inoculation type (p = 0.003); non-inoculated plants as well as root-inoculated plants showed higher mean ratios (2.45 and 2.21 µg cm−2, respectively). We also verified the effect of the interaction between developmental phase and type of inoculation (p = 0.020) on this ratio. In the vegetative phase, the lowest mean values were observed in leaf-inoculated plants and in plants in the reproductive phase infected through leaves and roots. In the maturation stage, there were no differences between the mean ratios obtained via the different inoculation routes.
The different developmental stages also affected the synthesis of carotenoids by G. hirsutum leaves (p < 0.001). Plants in the reproductive stage accumulated more amount of this pigment, followed by plants in the vegetative and maturation stages (Fig. 2d). The types of inoculation also affected the concentration of carotenoids (p = 0.001). Thus, root-inoculated plants showed the lowest mean values for this pigment (10.65 µg cm−2), followed by stem-inoculated and non-inoculated plants (11.36 and 12.22 µg cm−2), respectively. We also verified the effect of the interaction between developmental phase and type of inoculation (p = 0.020) on the concentration of this pigment (p < 0.001), so that plants in the vegetative or reproductive phase, inoculated by root or leaf, synthesized less carotenoids than control, non-inoculated plants. For plants in the maturation phase, no differences were observed between the mean levels of carotenoids as a function of the different inoculation routes.
Developmental stages also differentially affected the photosynthetic rate (A) of G. hirsutum plants (p < 0.001). Thus, plants at the maturation stage showed greatly reduced rates for this parameter (Fig. 3a). The inoculation types did not affect this parameter; however, the interaction between developmental phase and inoculation type (p = 0.023) showed that plants infected by the endophyte in the vegetative phase, either through root or leaves, had their photosynthesis reduced. Similarly, the Ci of CO2 was also affected by the phenological stage of G. hirsutum (p < 0.001) so that plants in the maturation stage tended to show lower mean values (Fig. 3b). Ci also varied as a function of the infection route (p = 0.028) as plants inoculated through leaves showed lower mean values for this parameter (321.427 µmol CO2 mol−1). The interaction between developmental phase and type of inoculation also affected Ci (p = 0.035), but only in the reproductive and vegetative phases. In the former phase, the lower average values were observed in control plants, whereas in the latter phase, the lower average values were observed in plants inoculated through the leaves.
Photosynthetic rate, A (a), internal concentration of CO2, Ci (b) and carboxylation efficiency—A/Ci (c) observed in Gossypium hirsutum plants subjected to infection by Diaporthe ueckerae through leaves or root, in three phenological phases (vegetative, reproductive, and maturation). Letters above the boxes compare, by Tukey test at 5% probability, the phenological phases; letters above the bars within the same phase compare the types of inoculation (leaf or root) with non-inoculated plants
Carboxylation efficiency, given by the ratio A/Ci, was also affected by the phenological stage of cotton (p < 0.001) so that the plants in the maturation phase also showed lower mean values for this parameter; the datum was clearly impacted by the high values of Ci and low values of A observed for these plants (Fig. 3c). This efficiency was also affected by the interaction between development stage and inoculation type (p = 0.026) in the vegetative and reproductive phases. Thus, in both the former and the latter, the highest efficiency was observed in the non-inoculated plants.
The developmental stages also affected the stomatal conductance (gs) of G. hirsutum plants (p < 0.001), with plants in the maturation phase showing more reduced values for this parameter (Fig. 4a). Similarly, the types of inoculation affected gs (p = 0.015), with stem-inoculated plants showing the lowest conductance values (0.649 mol H2O m−2 s−1) followed by non-inoculated (0.680 mol H2O m−2 s−1) and root-inoculated (0.798 mol H2 O m−2 s−1) plants. The interaction between developmental stage and inoculation type also affected gs (p = 0.038). Thus, lowest mean conductances were observed in the vegetative and maturation stages in the leaf-inoculated plants. In contrast, in the reproductive phase, the lowest mean gs was observed in control plants, followed by stem-inoculated plants.
Stomatal conductance, gs (a), transpiration rate E (b), and water use efficiency WUE (c) observed in Gossypium hirsutum plants subjected to infection by Diaporthe ueckerae in leaves or root in three phenological phases (vegetative, reproductive, and maturation). Letters above the boxes compare, by Tukey test at 5% probability, the phenological phases; letters above the bars within the same phase compare the types of inoculation (leaf or root) with non-inoculated plants
The transpiration rate (E) of G. hirsutum plants differed according to the development stage (p < 0.001), and was lower in the maturation phase, followed by the reproductive and vegetative phases (Fig. 4b). Meanwhile, inoculation pathways also affected plants differently (p < 0.001), with transpiration being lower in control, non-inoculated plants (8.443 mmol H2O m−2 s−1). However, there was no effect of the interaction between developmental stage and inoculation type on this rate.
The WUE also differed according to the phenological phase (p = 0.001), with the values observed in the maturation stage being higher than those in the other phases analyzed (Fig. 4c). The type of inoculation also affected plants differently (p = 0.014), so that non-inoculated plants showed the highest average efficiencies (2.103 µmol CO2 mmol H2O−1), followed by stem-inoculated (1.756 µmol CO2 mmol H2O−1) and root-inoculated (1.634 µmol CO2 mmol H2O−1) plants. We also found no effect of the interaction between developmental stage and inoculation type on WUE.
Photochemical profile of the G. hirsutum × D. ueckerae interaction
We also analyzed the primary photochemistry of G. hirsutum plants that underwent D. uekerae inoculation and observed that ABS/RC values were efficiently affected by the plant development stage (p < 0.001), with plants in the maturation phase appearing more stressed, and the same is indicated by the higher mean values observed for this parameter (Fig. 5a). ABS/RC was also affected by the type of inoculation (p < 0.001), with root-inoculated plants presenting the most stress (3.257), followed by stem-inoculated (3.10) and non-inoculated plants (2.728). The interaction between developmental phase and type of inoculation also affected ABS/RC (p < 0.001), and in the vegetative and reproductive phases, the lowest mean values were observed in the control, non-inoculated plants. In the maturation stage, the lowest values were observed in control plants, but also in plants where the endophytic infection occurred through leaves.
Absorption flux per reaction center, ABS/RRC (a), electron transport per reaction center ET0/RC (b), energy flux per reaction center at t = 0, TR0/RC (c), and dissipated energy flux per reaction center DI0/RC (d), observed in Gossypium hirsutum plants that were infected by Diaporthe ueckerae in leaves or root, in three phenological phases (vegetative, reproductive, and maturation). Letters above the tables compare, by Tukey test at 5% probability, the phenological phases; letters above the bars within the same phase compare the types of inoculation (leaf or root) with non-inoculated plants
ET0/RC values also differed according to phenological stage (p < 0.001). Thus, plants at maturation stage showed the highest values (Fig. 5b). The types of inoculation also affected this parameter (p < 0.001), with the highest means observed in root-inoculated plants (0.628) followed by leaf-inoculated (0.594) and non-inoculated plants (0.536). We observed no effect of the interaction between the developmental stage and inoculation type on this photochemical parameter. The TR0/RC data followed a similar pattern to that observed for ABS/RC, with the highest values observed in plants at the maturation stage (p < 0.001) (Fig. 5c) and in root-inoculated plants (2.104), followed by stem-inoculated (2.061) and non-inoculated plants (1.973) (p < 0.001). According to the interaction between development stage and type of inoculation (p < 0.001), the lowest mean values were observed in control plants in the vegetative and reproductive phases. In the maturation stage, the lowest values were observed in control plants, but also in the plants where the infection occurred through leaves.
The indicator of energy dissipation as heat, DI0/RC, was also affected by the developmental phase (p = 0.009). Therefore, both the reproductive and vegetative phases showed the highest mean values for this parameter (Fig. 5d). Regarding the effect of inoculation type (p < 0.001), root-inoculated and stem-inoculated plants showed the highest values (1.137 and 1.022, respectively), followed by non-inoculated plants (0.758). The interaction between developmental phase and type of inoculation also affected DI0/RC (p < 0.001), and the pattern was similar to ABS/RC and TR0/RC, i.e., in the vegetative and reproductive phases, the lowest mean values were observed in the non-inoculated plants and in the maturation phase, in the leaf-infected and non-inoculated plants.
The phenological stages of G. hirsutum also affected the photosynthetic performance index, PIABS (p < 0.001), with plants at maturation stage showing higher average index (Fig. 6a). The infection route also affected this index (p < 0.001). Non-inoculated plants showed better performance (0.644), followed by leaf- and root-inoculated plants (0.306 and 0.210, respectively). We also observed the effect of the interaction between developmental stage and inoculation type on PIABS (p = 0.009). The best mean performances during all developmental phases were observed in non-inoculated plants; however, in the maturation phase, it was possible to verify that root-inoculated plants had their performance more affected. The parameter PHIPO was affected by the phenological phase (p < 0.001), with plants in the maturation stage showing the highest values (Fig. 6b) and by the type of inoculation (p < 0.001), with non-inoculated plants showing higher values (0.714) followed by stem- and root-inoculated plants (0.641 and 0.630, respectively). Regarding the interaction between development stage and type of inoculation (p < 0.001), non-inoculated plants in all developmental stages stood out with respect to this parameter. Furthermore, in the reproductive phase, stem-inoculated plants were more affected than root-inoculated plants, but we observed an opposite effect in the maturation phase with root-inoculated plants being more affected than stem-inoculated ones.
Photosynthetic performance index, PIABS (a), maximum quantum yield of primary photochemistry, PHIPO (b), probability that a trapped exciton moves an electron into the electron transport chain beyond Quinone (Qa), PHI0 (c), and total performance index, PITOTAL (d), observed in Gossypium hirsutum plants that underwent infection by Diaporthe ueckerae in leaves or root, in three phenological phases (vegetative, reproductive and maturation). Letters above the tables compare, by Tukey test at 5% probability, the phenological phases; letters above the bars within the same phase compare the types of inoculation (leaf or root) with non-inoculated plants
The PHI0 also suffered the effect of the developmental stages of cotton (p < 0.001), with plants at maturation showing the highest average indices (Fig. 6c). Regarding infection type (p < 0.001), the control plants showed the highest values for this parameter (0.339), followed by those infected by leaves and root (0.217 and 0.192, respectively). The interaction between developmental phase and type of inoculation also affected this photochemical parameter (p = 0.027), following a pattern similar to that observed for PHIPO, that is, the non-inoculated plants stood out in all developmental phases. However, the stem-inoculated plants were more affected in the reproductive phase than root-inoculated ones, whereas root-inoculated plants were more affected in the maturation phase.
However, the total performance index, PITOTAL, was not differentially affected by cotton plant phenology, although it was affected by inoculation type (p < 0.001) with non-inoculated plants showing superior performance (0.453) compared to stem- and root-inoculated plants (0.289 and 0.261, respectively) (Fig. 6d). The total performance was also affected by the interaction between developmental phase and inoculation type (p < 0.001). Thus, the non-inoculated plants performed better during all phases of development; however, it was possible to observe a less pronounced performance during the reproductive phase in the leaf-inoculated plants and poorer performance was observed during the maturation phase in case of the root-inoculated plants.
Analysis of the interaction between variables
Principal component analysis recovered a strong correlation of plants in vegetative development stage with photosynthetic variables such as A, Ci, E, gs, and A/Ci, so that such variables defined the differences observed between these plants and plants in maturation stage (Fig. 7a). Meanwhile, plants in the reproductive stage appeared more correlated to high concentrations of the chlorophyll a, b pigments as well as carotenoids, although these plants were differentiated from others by the high values of DI0 /RC. However, plants in the maturation phase differentiate themselves from the plants in other stages by their superior photochemical performance indices as given by PIABS, PHIPO, PHI0 and PITOTAL, and the tendency to accumulate more chlorophyll b than chlorophyll a (Chla/Chlb) as well. The higher WUE means define the difference between plants of this phase and those of other phases analyzed.
Principal component analysis of photosynthetic pigment concentrations, gas exchange, and chlorophyll a fluorescence in Gossypium hirsutum plants at three phenological stages (vegetative, reproductive, and maturation) and subjected to Diaporthe ueckerae infection in leaves or root. Chla = chlorophyll a and Chlb = chlorophyll b
When we assessed the dispersion of variables in relation to inoculation methods, we found that control plants concentrated more pigments (chlorophyll a, b, and carotenoids), thereby showing the best photosynthetic rates (A), carboxylation efficiency (A/Ci), and WUE, as well as the best photochemical performance as indicated by the highest mean values of PIABS, PHIPO, PHI0, and PITOTAL (Fig. 7b). However, the root-inoculated plants differed from others due to the high values of stomatal conductance (gs), internal CO2 concentration (Ci), and transpiration rate (E). High ET0/RC means, which is one of the indicators of photochemical stress, were also observed in these plants. Other indicators of photochemical stress (ABS/RC, TR0/RC, and DI0/RC) were also shown to be related to these plants, as well as to plants that interacted with the phytopathogen through stem.
Leaf histopathology
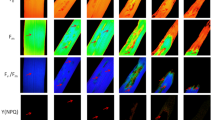
Histopathology examination showed the effective development of D. ueckerae hyphae along the adaxial surface of sampled cotton plants at all developmental stages, in addition to the frequent presence of asexually reproducing propagules, α-conidia and β-conidia (Fig. 8a–c), dispersed, clumped, and also at the germination stage. Hyphae of D. ueckerae were continuously observed locating close to stomata and trichomes (Fig. 8d).
Microscopic aspects of Diaporthe ueckerae colonization on the adaxial surface of Gossypium hirsutum plant leaves, observed in three phenological phases (vegetative, reproductive, and maturation). Development of hyphae and conidia (a–c) and interaction of hyphae with stomata and glandular trichome (d) are shown
Many of the hyphae were closely associated with the contact region of the dorsal wall of the stomata and adjacent epidermal cells, including the formation of appressoria (Fig. 9a). However, no direct penetration via stomatal pore was observed despite the proximity of the fungal hyphae to the stomata (see Fig. 8c, top).
Microscopic aspects of Diaporthe ueckerae colonization on the adaxial surface of Gossypium hirsutum plant leaves, observed in three phenological phases (vegetative, reproductive, and maturation). Interaction of hyphae with cells adjacent to stomata, with formation of appressoria (a), interaction of hyphae and α-conidia with the base of glandular trichome (b), and non-glandular trichome (c), deterioration of glandular trichome associated with hyphae (d), and partially closed and closed stomata in leaf region with presence of hyphae (d) are shown
The association of hyphae with the basal region of glandular trichomes was frequently observed, and the presence of α-conidia next to hyphae was also verified (Fig. 9b). These glandular trichomes associated with hyphae appeared at different stages of decay, from mild to acute (see Figs. 8d and 9b, d). The hyphae of D. ueckerae also tended to associate with non-glandular trichomes, although no direct damage to these structures was observed (Fig. 9c). We also noticed an overall tendency towards stomatal closure, given the presence of D. ueckerae on the limbus (Fig. 9e).
Discussion
Physiological damage caused by endophytic Diaporthe ueckerae on Gossypium hirsutum plants shows opportunistic pathogenicity
We did not identify visual symptoms of necrosis or wilt when inoculating an endophytic strain of D. ueckerae on leaves and roots. However, we did observe physiological changes indicative of stress, thereby suggesting that D. ueckerae may be an opportunistic phytopathogen for cotton plants. i.e., despite the endophytic condition, D. ueckerae may relate antagonistically to cotton leaves in situations favorable to infection, thereby colonizing tissues and triggering stress symptoms that may affect growth and productivity.
Phytopathogenic Diaporthe species are associated with a number of diseases affecting other agronomically important plants (Thompson et al. 2018) such as soybean, including seed rot, pod and stem rot, and stem canker, which lead to considerable losses in crop production worldwide (Udayanga et al. 2015; Zaw et al. 2019; Mena et al. 2020). Thus, we also caution against cross-contamination between crops, as species of the Diaporthe/Phomopsis complex occurring as phytopathogens of grasses, or even of other Malvaceae may pose a real threat to the cotton crop. Leaf spot symptoms caused by Diaporthe spp. were observed on Pachira glabra seedlings, for example, in a Brazilian forest nursery. This member of Malvaceae naturally occurs in the Atlantic Forest of Brazil and is used to restore degraded areas of permanent preservation (Milagres et al. 2018).
Diaporthe ueckerae inoculation negatively affects synthesis of photosynthetic pigments in cotton plants at vegetative and reproductive stages, while plants at maturation stage naturally tend to concentrate less pigments
Overall, fungal pathogens seem to alter the synthesis of chlorophylls in G. hirsutum. Khotamov et al. (2020) showed that cotton plants infected by Verticillium dahliae had reduced leaf content of chlorophylls a and b, as well as decreased proportion of these pigments. Similarly, cotton leaves showing leaf spot symptoms caused by Alternaria macrospora have reduced chlorophyll a, b, and chlorophyll total contents, compared to leaves of uninfected plants. Chlorophyll a and b contents appear to decrease with increasing severity of fungal diseases (Chen et al. 2013; Ahmad et al. 2019). Thus Tork et al. (2021) show that the chlorophyll index in cotton plant leaves can negatively correlate with the severity of Verticillum infection. Indeed, leaf photosynthetic pigment contents have been used as indicators of biotic stress, and consequently have been applied in monitoring diseases in cotton (Yang et al. 2022).
However, the observed reduction in photosynthetic pigment contents at the maturation stage appears to be a common physiological response of the cotton plant, and is induced by metabolic changes and the differential reallocation of biomass to the fruits, which is more intense at maturation than at other stages. Throughout the early vegetative growth phase, most of the photo-assimilates produced by the cotton plant leaves are sent to the root system. Later, however, most of the sugars are directed to the developing bolls, and the rate of root and aboveground growth tends to decrease (Ritchie et al. 2007).
Diaporthe ueckerae inoculation negatively affects photosynthetic rate (A) and carboxylation efficiency (A/Ci) in cotton plants at vegetative and reproductive stage, while mature plants naturally tend to photosynthesize less
Overall, photosynthesis can be negatively affected by a variety of abiotic (Chaves et al. 2008; Hou et al. 2016) or biological, such as insects and pathogens (Meyer and Whitlow 1992) stressors. Yang and Luo (2021) hypothesized that photosynthetic changes during the phytopathogenesis process may be related to responses to stressors. It is well documented that pathogen invasions lead to decline in the photosynthesis rate of the host (Kretschmer et al. 2019). However, several authors have monitored photosynthetic changes during pathogen infection in a host and detected a remarkable phenomenon: in the early stages of infection by a large number of different pathogens, the photosynthetic capacity of the host always decreases (Chen et al. 2015; Hu et al. 2020). As found for several other plant species interacting with a wide range of pathogens, the decrease in chlorophyll concentration is often accompanied by a reduced photosynthetic activity, we found that the impact of D. ueckerae on photosynthesis in cotton plants during the vegetative and reproductive phases was related to decreases in chlorophyll a and b concentrations as well as carotenoid contents.
The photosynthetic rate in cotton leaves decreased by 74.5% as a result of Verticillium infection (Haijun et al. 1995) and, therefore, the activities of the oxidative stress enzymes, SOD and POD, decreased considerably while the content of malondialdehyde, which is an indicator of lipid peroxidation, increased. Hampton et al. (1990) also assessed photosynthesis along the canopy of Verticillum-infected cotton plants and found that photosynthesis was reduced in diseased lower canopy leaves by more than 65% compared to controls, and the reduction was associated with chlorophyll loss and greatly reduced stomatal conductance. Leaves from the upper canopy of diseased plants, although not showing visible symptoms of Verticillium wilt, exhibited a 20% decrease in photosynthetic activity. Similarly to findings shown in this work, Ayele et al. (2020) observed that the photosynthetic rate of plants from different cotton genotypes tends to decrease throughout development, and drops dramatically after 86 days of cultivation.
Diaporthe ueckerae inoculation increases transpiration (E) of cotton plants and decreases water use efficiency (WUE), and foliar inoculation negatively affects stomatal conductance (gs)
It is commonly reported that pathogens are able to compromise leaf transpiration during infection, as stomata are key players in the innate immunity of plants (Barón et al. 2016). Pathogens often have the ability to manipulate signaling pathways, thereby interfering with plant defense. As a result, some pathogens are able to prevent stomatal closure after their detection by the plant, or even reopen them (Melotto et al. 2006, 2008; Zeng et al. 2010). This helps to partially explain the high transpiration rates we found in plants inoculated with D. ueckerae, as our results suggest that the putative pathogen decreased photosynthesis through non-stomatic processes as is evidenced by the high gs rates. In contrast, the photosynthesis in leaf-inoculated plants was reduced directly due to decreased stomatal conductance, thereby indicating that closing the stomata may have been an important measure for these plants to avoid fungal infection. However, the same did not incur an increase in WUE. This is because transpiration remained high in these plants, even under reduced gs, which indicates that non-stomatic water loss mechanisms are perhaps associated with pathogen-induced damage and restructuring in cell walls (Vorwerk et al. 2004; Bellimcampi et al. 2014).
Diaporthe ueckerae inoculation via leaf or root negatively affects photochemical performance of cotton plants
Overall, the presence of phytopathogenic fungi affects the functioning of photosystems as fungal toxins induce changes in PSI and PSII, therefore, compromising electron transport which subsequently leads to the accumulation of reactive oxygen species and cell death (Manning et al. 2009). Similarly, Khotamov et al. (2020) evaluated cotton plants infected by Verticillium dahliae and concluded that observed changes in the induced chlorophyll a fluorescence parameter may indicate symptoms caused by wilt. We found a negative correlation between D. ueckerae infection and photochemical performance in G. hirsutum plants, with this low performance being associated with low photosynthetic pigment concentrations.
Studies reveal that species of the genus Diaporthe can harbor a wide variety of genes encoding pathogenicity-related proteins including carbohydrate-active enzymes (CAZymes), necrosis-inducing proteins, oxidoreductases, proteases, and effector candidates (Mena et al. 2022). Cellular transporters involved in the transport of toxins, ions, sugars, effectors, and genes implicated in pathogenicity were identified in the genomes of D. amygdali and D. eres. Hydrolases and oxidoreductases were the most prevalent CAZymes (Hilário et al. 2022). Diaporthe helianthi produces polyketide phytotoxins that play an important role in host colonization (Ruocco et al. 2018). Polyketides have also been identified in D. breyniae culture (Kemkuignou et al. 2022). Indeed, mycotoxins appear to increase the production of reactive oxygen species in host plant tissues (Iqbal et al. 2021a, 2021b). Oxidative stress directly affects electron flow and the working efficiency of PSI and PSII (Shujun 2005; Iqbal et al. 2021a, 2021b, 2022). This explains the higher levels of photochemical stress and lower performance observed in plants induced to interact with D. ueckerae.
Diaporthe ueckerae colonizes the leaf tissues of Gossypium hirsutum through glandular trichomes and forces penetration using appressoria, while plant responds by closing the stomata
One of the infection strategies used by D. ueckerae to access the inner leaf epidermal tissue in cotton plants is the penetration of hyphae into the contact region of the dorsal wall of the stomata and adjacent epidermal cells. There is strong evidence for differential thickening of guard cell walls in different plants, with the outer, inner, and ventral walls being thicker than the dorsal wall (Merced and Renzaglia 2013, 2014). The wall reaches a maximum of 2 μm in typical epidermal cells, and since the organization of wall components in guard cells (cellulose, xyloglucan, and pectins) is different from epidermal cells (see Rui et al. 2018), it is possible that the contact region may become more fragile. Meanwhile, the continuous mechanics of the dorsal wall may also weaken the region of conjunction with adjacent epidermal cells and, therefore, render it more vulnerable to penetration by fungal hyphae. This occurs because increased turgor pressure in guard cells forces their thin dorsal walls to elongate and project into subsidiary or neighboring cells (Pautov et al. 2017).
Another region of the adaxial epidermis used by D. ueckerae as a gateway to the internal tissues of the leaf are the glandular trichomes. Both non-glandular and glandular trichomes are found in the hairy leaves of cotton. The latter are composed of a small peduncular cell and a multicellular head and may be globose or have ornamented surfaces (Bondada 2000). These trichomes occupy a special position as a first line of defense and management tool against sucking insects, pests of cotton (Ahmed et al. 2020). These structures produce pest- or pollinator-interacting phytochemicals that are stored or volatilized on the plant surface and are specific sites for biosynthesis and excretion of secondary metabolites (Venditti et al. 2014). Thus, the observation of glandular trichomes associated with hyphae of D. ueckerae, and in different stages of deterioration, evidences the release of defense antifungal substances directly by these structures or the deteriorating effect of fungal toxins acting on these trichomes.
Studies show that G. hirsutum trichomes synthesize gossypol and related compounds such as dimeric disesquiterpenes that are natural pesticides (Dayan and Duke 2003) and have strong antifungal activity (Mellon et al. 2012). Despite the metabolites secreted by the glandular trichomes on the adaxial surface of cotton leaves, D. ueckerae colonizes these structures, thereby showing resistance and insensitivity to these active compounds. In contrast, in the contact regions of the hyphae with the plant tissue, D. ueckerae is able to eliminate toxins that will affect the host and increase the colonization capacity of the fungus. In fact, D. helianthi production of polyketide phytotoxins has already been reported, with such production playing an important role in host colonization (Ruocco et al. 2018). Huang et al. (2022) identified azafilones or azafilonoids, which are a structurally diverse family of cytotoxic polyketide metabolites, and are produced by an endophytic strain of Diaporthe. Additionally, an important mycotoxin, fomopsin A, has been reported to be produced by the parasitic and saprophytic fungus Diaporthe toxica, which infects Lupinus spp. causing crop losses (Schloß et al. 2017).
Stomatal closure seems to be one of the strategies used by cotton plants to inhibit D. ueckerae penetration into leaf tissues, although we did not observe any direct penetration of hyphae into stomatal pores. The chitin oligosaccharide of the fungal cell wall induces stomatal closure through its CERK1 receptor, Ca2+, and S-type anion channel SLAC1, which prevent fungal penetration (Ye et al. 2020). This action can lead to a hypersensitivity response, thereby causing guard cell death and eventually preventing fungal infection (Shafiei et al. 2007). However, fungal chitinases may convert chitin to chitosan in order to bypass stomatal immunity, which does not interact with immune response-inducing receptors (El Gueddari et al. 2002; Cord-Landwehr et al. 2016). Following this pathway, we are studying whether trichome death could also constitute a hypersensitivity response effect in G. hirsutum plants. We suggest future work that can lay the groundwork to better understand the strategies used by this species as a defense against fungal infection. Park et al. (2020) revealed that in diseased leaves of Quercus acutissima, epidermal shrinking of trichomes was quite frequent. Transmission electron microscopy analyses have revealed the presence of fungal hyphae in naturally infected trichomes of this species. This suggests that hyphae growth may dissolve the non-glandular trichomes on the abaxial surface of the leaf. Non-glandular trichomes are able to act as a physical barrier that traps pathogens, thereby preventing them from reaching the leaf surface (Łaźniewska et al. 2012). We observed the interaction of D. ueckerae hyphae with non-glandular trichomes on the adaxial surface of G. hirsutum leaves. The strong colonization of trichomes by D. ueckerae helps explain the large non-stomatic water loss observed in leaf inoculated plants, as is indicated by the reduced gs.
We observed that D. ueckerae forces tissue penetration through the formation of appressoria. The appressorium constitutes an infection structure developed by phytopathogenic fungi in order to invade host tissues (Talbot 2019; Ryder et al. 2022). These structures assist hyphae in attaching to and breaking through the host cuticle (Deising et al. 2000). The appressorium has been thoroughly studied in plant pathogen species such as Magnaporthe oryzae or Ustilago maydis (Ryder and Talbot 2015) and has also been described in entomopathogenic fungi such as Beauveria bassiana, in mutualistic arbuscular mycorrhizal fungi of the phylum Glomeromycota, and in phylogenetically distant Oomycetes including Phytophthora infestans (Harrisson 1999; Hardham 2001; Ortiz-Urquiza and Keyhani 2013). Thus, we consider that the development of this structure constitutes an important mechanism used by D. ueckerae to access the internal tissues of G. hirsutum.
Our work opens perspectives for other phytopathology studies involving the evolution of opportunism in endophytic species. On the other hand, we suggest that studies be carried out with other strains of D. ueckerae, in order to define whether the susceptibility of G. hirsutum is only to this strain that we evaluated, or also to other existing strains. We also suggest that studies monitoring infections caused by D. ueckerae be routinely conducted given the risk of cross-contamination between crops, since species of the Diaporthe/Phomopsis complex are phytopathogens of important plants such as soybeans, thereby constituting a threat to both cotton specifically and to major crops collectively.
Conclusions
We identified physiological changes during an artificially caused infection by the endophytic D. ueckerae in G. hirsutum. We found that this fungus can colonize plant tissues during different phenological phases, thereby compromising the production of photosynthetic pigments, gas exchange parameters, and photochemical efficiency, which may culminate in losses of crop productivity. This colonization occurs through the contact region between guard cells and adjacent epidermal cells, interaction with glandular trichomes, and development of appressoria. The observed physiological alterations are indicative of biotic stress, thereby confirming the hypothesis that D. ueckerae may be an opportunistic phytopathogen for cotton plants. i.e., despite the endophytic condition, in situations favorable for infection, D. ueckerae may relate antagonistically to cotton leaves, thereby colonizing the tissues and triggering stress symptoms that may affect growth and productivity.
Data availability
All the data relevant to this manuscript are available on request from the corresponding author.
References
Ahmad L, Siddiqui ZA, AbdAllah EF (2019) Effects of interaction of Meloidogyne incognita Alternaria dauci and Rhizoctonia solani on the growth chlorophyll carotenoid and proline contents of carrot in three types of soil. Acta Agric Scand B Soil Plant Sci 69(4):324–331. https://doi.org/10.1080/09064710.2019.1568541
Ahmed H, Nazir MF, Pan Z, Gong W, Iqbal MS, He S, Du X (2020) Genotyping by sequencing revealed QTL hotspots for trichome-based plant defense in Gossypium hirsutum. Genes 11(4):368. https://doi.org/10.3390/genes11040368
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/s0022-2836(05)80360-2
Ayele AG, Wheeler TA, Dever JK (2020) Impacts of Verticillium wilt on photosynthesis rate, lint production and fiber quality of greenhouse-grown cotton (Gossypium hirsutum). Plants 9(7):857. https://doi.org/10.3390/plants9070857
Balachandran S, Hurry VM, Kelley SE, Osmond CB, Robinson SA, Rohozinski J, Seaton GGR, Sims DA (1997) Concepts of plant biotic stress Some insights into the stress physiology of virus-infected plants from the perspective of photosynthesis. Physiol Plant 100(2):203–213. https://doi.org/10.1111/j.1399-3054.1997.tb04776.x
Barón M, Pineda M, Pérez-Bueno ML (2016) Picturing pathogen infection in plants. Zeitschrift Für Naturforschung C 71(9–10):355–368. https://doi.org/10.1515/znc-2016-0134
Becerra-lopezlavalle LA, Saleeba JA, Lyon BR (2005) Molecular identification of fungi isolated from stem tissue of upland cotton (Gossypium hirsutum). Aust J Bot 53(6):571. https://doi.org/10.1071/BT04092
Bellincampi D, Cervone F, Lionetti V (2014) Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci 5:228. https://doi.org/10.3389/fpls.2014.00228
Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant pathogen interactions. J Exp Bot 58(15–16):4019–4026. https://doi.org/10.1093/jxb/erm298
Bilgin DD, Zavala JA, Zhu J, Clough SJ, Ort DR, Delucia EH (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ 33(10):1597–1613. https://doi.org/10.1111/j.1365-3040.2010.02167.x
Bondada B (2000) Comparative epidermal ultrastructure of cotton (Gossypium hirsutum L) leaf bract and capsule wall. Ann Bot 86(6):1143–1152. https://doi.org/10.1006/anbo.2000.1283
Cai C, Ye W, Zhang T, Guo W (2014) Association analysis of fiber quality traits and exploration of elite alleles in upland cotton cultivars/accessions (Gossypium hirsutum L). J Integr Plant Biol 56(1):51–62. https://doi.org/10.1111/jipb.12124
CFSG - Comissão de Fertilidade de Solos de Goiás (1988) Recomendações de corretivos e fertilizantes para Goiás: 5a aproximação. Universidade Federal de Goiás EMGOPA. http://www.nutricaodeplantas.agr.br/site/downloads/RECOMENDACOES_DE_CORRETIVOS_E_FERTILIZANTES_PARA_GOIAS.pdf. Accessed 19 Jan 2023
Chaves MM, Flexas J, Pinheiro C (2008) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103(4):551–560. https://doi.org/10.1093/aob/mcn125
Chen B, Han H, Wang F, Zheng L, Fu-Jun D, Hai L, Yu Y, Shao-Kun L, Ke-Ru W, Chun-Hua X (2013) Monitoring chlorophyll and nitrogen contents in cotton leaf infected by Verticillium wilt with spectra red edge parameters. Acta Agron Sin 39(2):319. https://doi.org/10.3724/sp.j.1006.2013.00319
Chen Y, Cui J, Su Y, Yuan S, Yuan M, Zhang H (2015) Influence of stripe rust infection on the photosynthetic characteristics and antioxidant system of susceptible and resistant wheat cultivars at the adult plant stage. Front Plant Sci 6:779. https://doi.org/10.3389/fpls.2015.00779
Cheng H, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett 28(1):55–59. https://doi.org/10.1007/s10529-005-4688-z
Chohan S, Perveen R, Abid M, Tahir MN, Sajid M (2020) Cotton diseases and their management. In: Ahmad S, Hasanuzzaman M (eds) Cotton production and uses. Springer, Singapore, pp 239–270. https://doi.org/10.1007/978-981-15-1472-2_13
Cord-Landwehr S, Melcher RL, Kolkenbrock S, Moerschbacher BM (2016) A chitin deacetylase from the endophytic fungus Pestalotiopsis sp. efficiently inactivates the elicitor activity of chitin oligomers in rice cells. Sci Rep 6(1). https://doi.org/10.1038/srep38018
Cruz-Mireles N, Eseola AB, Osés-Ruiz M, Ryder LS, Talbot NJ (2021) From appressorium to transpressorium – defining the morphogenetic basis of host cell invasion by the rice blast fungus. PLOS Pathog 17(7):e1009779. https://doi.org/10.1371/journal.ppat.1009779
Da Silva JC, Bettiol W, Suassuna ND (2019) Ramularia leaf spot: an emergent disease of cotton in Brazil. Trop Plant Pathol 44(6):473–482. https://doi.org/10.1007/s40858-019-00308-w
Darriba D, Taboada GL, Doallo R, Posada D (2012) JModelTest 2: more models new heuristics and parallel computing. Nat Methods 9(8):772–772. https://doi.org/10.1038/nmeth.2109
Dayan FE, Duke SO (2003) Trichomes and root hairs: natural pesticide factories. Pestic Outlook 14(4):175. https://doi.org/10.1039/b308491b
De Araújo AE (2008) Podridão de maçãs do algodoeiro: principais causas e manejo. Embrapa Algodão-Documentos (INFOTECA-E). https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPA-2009-09/22289/1/DOC212.pdf. Accessed 19 Jan 2023
Deising HB, Werner S, Wernitz M (2000) The role of fungal appressoriain plant infection. Microbes Infect 2(13):1631–1641. https://doi.org/10.1016/s1286-579(00)01319-8
El Gueddari NE, Rauchhaus U, Moerschbacher BM, Deising HB (2002) Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytol 156(1):103–112. https://doi.org/10.1046/j.1469-8137.2002.00487.x
Fang X, Qin K, Li S, Han S, Zhu T, Fang X, Qin K (2020) Whole genome sequence of Diaporthe capsici a new pathogen of walnut blight. Genomics 112(5):3751–3761. https://doi.org/10.1016/j.ygeno.2020.04.018
Fu J, Zhou Y, Li HF, Ye YH, Guo JH (2011) Antifungal metabolites from Phomopsis sp. By254 an endophytic fungus in Gossypium hirsutum. Afr J Microbiol Res 5(10):1231–1236. https://doi.org/10.5897/ajmr11.272
Garcia-Reyne A, López-Medrano F, Morales JM, García Esteban GC, Martín I, Eraña I, Meije Y, Lalueza A, Alastruey-Izquierdo A, Rodríguez-Tudela JL, Aguado JM (2010) Cutaneous infection by Phomopsis longicolla in a renal transplant recipient from Guinea: first report of human infection by this fungus. Transplant Infect Dis 13(2):204–207. https://doi.org/10.1111/j.1399-3062.2010.00570.x
Guarnaccia V, Crous PW (2017) Emerging citrus diseases in Europe caused by species of Diaporthe. IMA Fungus 8(2):317–334. https://doi.org/10.5598/imafungus.2017.08.02.07
Gullino ML, Pugliese M, Gilardi G, Garibaldi A (2018) Effect of increased CO2 and temperature on plant diseases: a critical appraisal of results obtained in studies carried out under controlled environment facilities. J Plant Pathol 100(3):371–389. https://doi.org/10.1007/s42161-018-0125-8
Haijun G, Zhiqiang D, Yongzeng L, Zhengshan L, Junlan L, Guocun H, Siping C, Xuebiao P (1995) Effect of infection of verticillium wilt on the SOD, POD activities and photosynthetic character in cotton leaves. Sci Agric Sin 28(6):40–46
Hampton R, Wullschleger S, Oosterhuis D (1990) Impact of verticillium wilt on net photosynthesis respiration and photorespiration in field-grown cotton (Gossypium hirsutum L.). Physiol Mol Plant Pathol 37(4):271–280. https://doi.org/10.1016/0885-5765(90)90076-a
Hardham AR (2001) The cell biology behind Phytophthora pathogenicity. Australas Plant Pathol 30(2):91. https://doi.org/10.1071/ap01006
Harrison MJ (1999) Molecular and cellular aspects of the arbuscular mycorrhizal symbiosis. Annu Rev Plant Physiol 50(1):361–389. https://doi.org/10.1146/annurev.arplant.50.1.361
Hilário S, Gonçalves MF, Fidalgo C, Tacão M, Alves A (2022) Genome analyses of two blueberry pathogens: diaporthe amygdali CAA958 and Diaporthe eres CBS 160.32. J Fungi 8(8):804. https://doi.org/10.3390/jof8080804
Hou W, Sun AH, Chen HL, Yang FS, Pan JL, Guan MY (2016) Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol Plant 60(1):148–154. https://doi.org/10.1007/s10535-015-0575-1
Hu Y, Zhong S, Zhang M, Liang Y, Gong G, Chang X, Tan F, Yang H, Qiu X, Luo L, Luo P (2020) Potential role of photosynthesis in the regulation of reactive oxygen species and defence responses to Blumeria graminis F. sp. tritici in wheat. Int J Mol Sci 21(16):5767. https://doi.org/10.3390/ijms21165767
Huang F, Lin X, Lu Q (2022) Azaphilones from the endophyte Diaporthe sp. and their toxicity. Chem Biodivers 19(11). https://doi.org/10.1002/cbdv.202200849
Iqbal N, Czékus Z, Ördög A, Poór P (2021a) Ethylene-dependent effects of fusaric acid on the photosynthetic activity of tomato plants. Photosynthetica 59(2):337–348. https://doi.org/10.32615/ps.2021.029
Iqbal N, Czékus Z, Poór P, Ördög A (2021b) Plant defence mechanisms against mycotoxin Fumonisin B1. Chem Biol Interact 343:109494. https://doi.org/10.1016/j.cbi.2021.109494
Iqbal N, Czékus Z, Angeli C, Bartók T, Poór P, Ördög A (2022) Fumonisin B1-induced oxidative burst perturbed photosynthetic activity and affected antioxidant enzymatic response in tomato plants in ethylene-dependent Manner. J Plant Growth Regul 1–14. https://doi.org/10.1007/s00344-022-10665-7
Kemkuignou BM, Schweizer L, Lambert C, Anoumedem EG, Kouam SF, Stadler M, Marin-Felix Y (2022) New polyketides from the liquid culture of Diaporthe breyniae sp. Nov. (Diaporthales Diaporthaceae). MycoKeys 90:85–118. https://doi.org/10.3897/mycokeys.90.82871
Khotamov MM, Agishev VS, Akhmedzhanov IG (2020) Influence of verticillium wilt infection on the functional activity of the cotton photosynthetic apparatus. Микология И Фитопатология 54(5):340–346. https://doi.org/10.31857/s0026364820050062
Kiranmai V, Ghai D, Kumar S (2020) A review on classification of land use/land cover change assessment based on normalized difference vegetation index. J Crit Rev 7(14):2416–2431
Kretschmer M, Damoo D, Djamei A, Kronstad J (2019) Chloroplasts and plant immunity: where are the fungal effectors? Pathogens 9(1):19. https://doi.org/10.3390/pathogens9010019
Łaźniewska J, Macioszek VK, Kononowicz AK (2012) Plant-fungus interface: the role of surface structures in plant resistance and susceptibility to pathogenic fungi. Physiol Mol Plant Pathol 78:24–30. https://doi.org/10.1016/j.pmpp.2012.01.004
Lu Y, Yao J (2018) Chloroplasts at the crossroad of photosynthesis pathogen infection and plant defense. Int J Mol Sci 19(12):3900. https://doi.org/10.3390/ijms19123900
Manning VA, Chu AL, Steeves JE, Wolpert TJ, Ciuffetti LM (2009) A host-selective toxin of Pyrenophora tritici-repentis Ptr ToxA induces Photosystem changes and reactive oxygen species accumulation in sensitive wheat. Mol Plant-Microbe Interact 22(6):665–676. https://doi.org/10.1094/mpmi-22-6-0665
McGee PA (2002) Reduced growth and deterrence from feeding of the insect pest Helicoverpa armigera associated with fungal endophytes from cotton. Aust J Exp Agric 42(7):995. https://doi.org/10.1071/ea01124
McLean KS, Lawrence GW (1998) First report of premature boll rot associated with cotton in Louisiana. Plant Dis 82(6):711–711. https://doi.org/10.1094/pdis.1998.82.6.711a
Mellon JE, Zelaya CA, Dowd MK, Beltz SB, Klich MA (2012) Inhibitory effects of gossypol Gossypolone and Apogossypolone on a collection of economically important filamentous fungi. J Agric Food Chem 60(10):2740–2745. https://doi.org/10.1021/jf2044394
Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126(5):969–980. https://doi.org/10.1016/j.cell.2006.06.054
Melotto M, Underwood W, He SY (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annu Rev Phytopathol 46(1):101–122. https://doi.org/10.1146/annurev.phyto.121107.104959
Mena E, Garaycochea S, Stewart S, Montesano M, Ponce De León I (2022) Comparative genomics of plant pathogenic Diaporthe species and transcriptomics of Diaporthe caulivora during host infection reveal insights into pathogenic strategies of the genus. BMC Genom 23(1). doi:https://doi.org/10.1186/s12864-022-08413-y
Mena E, Stewart S, Montesano M, Ponce de León I (2020) Soybean stem canker caused by Diaporthe caulivora; Pathogen diversity colonization process and plant defense activation. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01733
Merced A, Renzaglia K (2014) Developmental changes in guard cell wall structure and pectin composition in the moss Funaria: implications for function and evolution of stomata. Ann Bot 114(5):1001–1010. https://doi.org/10.1093/aob/mcu165
Merced A, Renzaglia KS (2013) Moss stomata in highly elaborated Oedipodium (Oedipodiaceae) and highly reduced Ephemerum (Pottiaceae) sporophytes are remarkably similar. Am J Bot 100(12):2318–2327. https://doi.org/10.3732/ajb.1300214
Meyer GA, Whitlow TH (1992) Effects of leaf and sap feeding insects on photosynthetic rates of goldenrod. Oecologia 92(4):480–489. https://doi.org/10.1007/bf00317839
Milagres CA, Belisário R, Silva MA, Lisboa DO, Pinho DB, Furtado GQ (2018) A novel species of Diaporthe causing leaf spot in Pachira glabra. Trop Plant Pathol 43(5):460–467. https://doi.org/10.1007/s40858-018-0242-0
Murali TS, Suryanarayanan TS, Geeta R (2006) Endophytic Phomopsis species: host range and implications for diversity estimates. Can J Microbiol 52(7):673–680. https://doi.org/10.1139/w06-020
Navarro BL, Molina JPE, Júnior AFN (2022) Penetration by Botryosphaeriaceae species in avocado guava and persimmon fruit during postharvest. J Phytopathol 170(1):57–68. https://doi.org/10.1111/jph.13055
Nemesio-Gorriz M, McGuinness B, Grant J, Dowd L, Douglas G (2019) Lenticel infection in fraxinus excelsior shoots in the context of ash dieback. iForest Biogeosci Forestry 12(2):160–165. https://doi.org/10.3832/ifor2897-012
Ortiz-Urquiza A, Keyhani N (2013) Action on the surface: entomopathogenic fungi versus the insect cuticle. Insects 4(3):357–374. https://doi.org/10.3390/insects4030357
Palmateer AJ (2003) Concerning Phomopsis gossypii the causal organism of boll rot of cotton. Mycotaxon 87:157–172
Park J, An H, Kim KW (2020) Visualization of fungal hyphae in the trichomes of sawtooth oak leaves. EurJ Plant Pathol 156(4):1119–1133. https://doi.org/10.1007/s10658-020-01970-6
Pautov A, Bauer S, Ivanova O, Krylova E, Sapach Y, Gussarova G (2017) Role of the outer stomatal ledges in the mechanics of guard cell movements. Trees 31(1):125–135. https://doi.org/10.1007/s00468-016-1462-x
Pérez-Bueno ML, Pineda M, Barón M (2019) Phenotyping plant responses to biotic stress by chlorophyll fluorescence imaging. Front Plant Sci 10. https://doi.org/10.3389/fpls.2019.01135
R Core Team (2021) R: a language and environment for statistical computing. R: the R project for statistical computing Vienna Austria. https://www.R-project.org/. Accessed 19 Jan 2023
Raja HA, Miller AN, Pearce CJ, Oberlies NH (2017). Fungal identification using molecular tools: a primer for the natural products research community. J Nat Prod 80(3):756–770. https://doi.org/10.1021/acs.jnatprod.6b01085
Rambaut A (2014) FigTree v1.4.2 a graphical viewer of phylogenetic trees. University of Edinburg, Edinburgh UK. http://tree.bio.ed.ac.uk/software/figtree/rightanglebracket. Accessed 19 Jan 2023
Ritchie GL, Bednarz CW, Jost PH, Brown SM (2007) Cotton growth and development. The University of Georgia Bulletin 1252.
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61(3):539–542. https://doi.org/10.1093/sysbio/sys029
Roy KW (1983) Soybean stem canker incited by isolates of Diaporthe and Phomopsis spp. from cotton in Mississippi. Plant Dis 67(2):135. https://doi.org/10.1094/pd-67-135
Rui Y, Chen Y, Kandemir B, Yi H, Wang JZ, Puri VM, Anderson CT (2018) Balancing strength and flexibility: how the synthesis organization and modification of guard cell walls govern stomatal development and dynamics. Front Plant Sci 9. https://doi.org/10.3389/fpls.2018.01202
Ruocco M, Baroncelli R, Cacciola SO, Pane C, Monti MM, Firrao G, Vergara M, Di San Lio GM, Vannacci G, Scala F (2018) Polyketide synthases of Diaporthe helianthi and involvement of DhPKS1 in virulence on sunflower. BMC Genom 19(1). https://doi.org/10.1186/s12864-017-4405-z
Ryder LS, Talbot NJ (2015) Regulation of appressorium development in pathogenic fungi. Curr Opin Plant Biol 26:8–13. https://doi.org/10.1016/j.pbi.2015.05.013
Ryder LS, Cruz-Mireles N, Molinari C, Eisermann I, Eseola AB, Talbot NJ (2022) The appressorium at a glance. J Cell Sci 135(14). https://doi.org/10.1242/jcs.259857
Sandmann M, Grosch R, Graefe J (2018) The use of features from fluorescence thermography and NDVI imaging to detect biotic stress in lettuce. Plant Dis 102(6):1101–1107. https://doi.org/10.1094/pdis-10-17-1536-re
Schloß S, Hackl T, Herz C, Lamy E, Koch M, Rohn S, Maul R (2017) Detection of a toxic methylated derivative of Phomopsin a produced by the legume-infesting fungus Diaporthe toxica. J Nat Prod 80(6):1930–1934. https://doi.org/10.1021/acs.jnatprod.6b00662
Shafiei R, Hang C, Kang J, Loake GJ (2007) Identification of loci controlling non-host disease resistance in arabidopsis against the leaf rust pathogen Puccinia triticina. Mol Plant Pathol 8(6):773–784. https://doi.org/10.1111/j.1364-3703.2007.00431.x
Shujun J (2005) The effect of the mycotoxin of αβ-dehydrocurvularin from Curvularia eragrostidis on PS II in Digitaria sanguinalis. Sci Agri Sin 38:1373–1378
Sievers F, Higgins DG (2014) Clustal omega. Curr Protoc Bioinformatics 48(1):3–13. https://doi.org/10.1002/0471250953.bi0313s48
Strasser RJ, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Probing photosynthesis: mechanisms regulation and adaptation, pp 445–483
Talbot NJ (2019) Appressoria. Curr Biol 29(5):R144–R146. https://doi.org/10.1016/j.cub.2018.12.050
Thompson SM, Tan YP, Neate SM, Grams RM, Shivas RG, Lindbeck K, Aitken EA (2018) Diaporthe novem isolated from sunflower (Helianthus annuus) and other crop and weed hosts in Australia. EurJ Plant Pathol 152(3):823–831. https://doi.org/10.1007/s10658-018-1515-7
Thompson S, Tan Y, Young A, Neate S, Aitken E, Shivas R (2011) Stem cankers on sunflower (Helianthus annuus) in Australia reveal a complex of pathogenic Diaporthe (Phomopsis) species. Pers Mol Phylogeny Evol Fungi 27(1):80–89. https://doi.org/10.3767/003158511D7617110
Tork DB, Panjehkeh N, Alizadeh H, Razi-Nataj M, Fatemi SM (2021) Selection of genotypes susceptible to Verticillium wilts cotton using morphological and physiological characteristics in greenhouse conditions. J Crop Breed 13(40):181–191. https://jcb.sanru.ac.ir/browse.php?a_id=1279%26sid=1%26slc_lang=en. 19 January 2023.
Udayanga D, Castlebury L, Rossman A, Hyde K (2014) Species limits in Diaporthe: molecular re-assessment of D. citri D. cytosporella D. foeniculina and D. rudis. Pers Mol Phylogeny Evol Fungi 32(1):83–101. https://doi.org/10.3767/003158514D7679984
Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD (2015) The Diaporthe sojae species complex: phylogenetic re-assessment of pathogens associated with soybean cucurbits and other field crops. Fungal Biol 119(5):383–407. https://doi.org/10.1016/j.funbio.2014.10.009
Udayanga D, Liu X, McKenzie EH, Chukeatirote E, Bahkali AH, Hyde KD (2011) The genus Phomopsis: biology applications species concepts and names of common phytopathogens. Fungal Divers 50(1):189–225. https://doi.org/10.1007/s13225-011-0126-9
Uddin W, Serlemitsos K, Viji G (2003) A temperature and leaf wetness duration-based model for prediction of gray leaf spot of perennial Ryegrass turf. Phytopathol 93(3):336–343. https://doi.org/10.1094/phyto.2003.93.3.336
Venditti A, Bianco A, Nicoletti M, Quassinti L, Bramucci M, Lupidi G, Vitali LA, Papa F, Vittori S, Petrelli D, Bini LM, Giuliani C, Maggi F (2014) Characterization of secondary metabolites biological activity and glandular trichomes of Stachys tymphaea Hausskn. from the Monti Sibillini National Park (Central Apennines Italy). Chem Biodivers 11(2):245–261. https://doi.org/10.1002/cbdv.201300253
Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science 9(4):203–209. https://doi.org/10.1016/j.tplants.2004.02.005
Wang B, Priest MJ, Davidson A, Brubaker CL, Woods MJ, Burdon JJ (2007) Fungal endophytes of native gossypium species in Australia. Mycol Res 111(3):347–354. https://doi.org/10.1016/j.mycres.2006.11.004
Wellburn AR (1994) The spectral determination of chlorophylls a and B as well as total carotenoids using various solvents with spectrophotometers of different resolution. J Plant Physiol 144(3):307–313. https://doi.org/10.1016/s0176-1617(11)81192-2
Wu J, Liu Y (2022) Stomata–pathogen interactions: over a century of research. Trends Plant Sci 27(10):964–967. https://doi.org/10.1016/j.tplants.2022.07.004
Yang H, Luo P (2021) Changes in photosynthesis could provide important insight into the interaction between wheat and fungal pathogens. Int J Mol Sci 22(16):8865. https://doi.org/10.3390/ijms22168865
Yang M, Huang C, Kang X, Qin S, Ma L, Wang J, Zhou X, Lv X, Zhang Z (2022) Early monitoring of cotton Verticillium wilt by leaf multiple “Symptom” characteristics. Remote Sens 14(20):5241. https://doi.org/10.3390/rs14205241
Yang Q, Fan X, Guarnaccia V, Tian C (2018) High diversity of Diaporthe species associated with dieback diseases in China with twelve new species described. MycoKeys 39:97–149. https://doi.org/10.3897/mycokeys.39.26914
Ye W, Munemasa S, Shinya T, Wu W, Ma T, Lu J, Kinoshita T, Kaku H, Shibuya N, Murata Y (2020) Stomatal immunity against fungal invasion comprises not only chitin-induced stomatal closure but also chitosan-induced guard cell death. Proc Natl Acad Sci USA 117(34):20932–20942. https://doi.org/10.1073/pnas.1922319117
Zaw M, Aye SS, Matsumoto M (2019) Colletotrichum and Diaporthe species associated with soybean stem diseases in Myanmar. J Gen Plant Pathol 86(2):114–123. https://doi.org/10.1007/s10327-019-00902-5
Zeng W, Melotto M, He SY (2010) Plant stomata: a checkpoint of host immunity and pathogen virulence. Curr Opin Biotechnol 21(5):599–603. https://doi.org/10.1016/j.copbio.2010.05.006
Zhang H, Lan Y, Suh CP, Westbrook J, Clint Hoffmann W, Yang C, Huang Y (2013) Fusion of remotely sensed data from airborne and ground-based sensors to enhance detection of cotton plants. Comput Electron Agric 93:55–59. https://doi.org/10.1016/j.compag.2013.02.001
Acknowledgments
The authors would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Foundation for Research Support of the State of Goiás (FAPEG) for the Master’s grant awarded to Matheus Mendonça de Souza Marques, and the IFGoiano, Rio Verde campus for the infrastructure and students involved in the study.
Funding
The research that yielded these results was funded with resources from Public Notice nº 19 at July 09, 2021, from the Pro-Rectory of Research, Post-Graduation and Innovation (PROPPI - IFGoiano).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research involving human participants and/or animals
The research did not involve either human participants or animals.
Conflict of interest
There is no conflict of interest (financial or non-financial).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marques, M., Silva, I., Bessa, L. et al. Opportunistic pathogenicity observed for the endophytic fungus Diaporthe ueckerae on Gossypium hirsutum plants. J Plant Pathol (2024). https://doi.org/10.1007/s42161-024-01637-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42161-024-01637-9