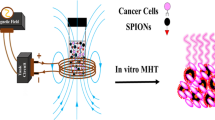
Abstract
Cancer, as the leading cause of death worldwide, has been constantly increasing in mortality every year. Among several therapeutics, nanoscale compounds showed promising results in overcoming cancer diseases. There are numerous types of research on the paramagnetic nanoparticles of iron oxide, which cause apoptosis and cancer cell death. In this study, cobalt/zinc/ferrite nanoferrofluid composites (~ 39 nm) were synthesized and decorated with chitosan to enhance the cell entry for potential applications in cancer therapy. The neat and chitosan-adorned cobalt zinc ferrite nanoferrofluid composites (~ 94 nm) displayed superparamagnetic properties. The nanocomposite exhibited anti-cancer activity against WEHI164 cancer cells in a dose- and time-dependent manner. The chitosan-coated nanocomposite was found to induce oxidative stress in WEHI164 cancer cells, as indicated by reactive oxygen species (ROS) production. Furthermore, DNA damage was indicated in WEHI164 cancer cells after exposure to chitosan-coated nanocomposites. Chitosan-coated nanocomposites promoted dendritic cell maturation by inducing the release of interleukin-6 proinflammatory cytokines. According to the results and ancillary studies, superparamagnetic nanoparticles coated with chitosan can be considered an effective and promising treatment for the destruction of cancer cells.
Graphical Abstract

Summary: Chitosan decorated cobalt zinc ferrite nanoferrofluid composites was fabricated for potential cancer hyperthermia therapy with high biocompatibility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is a malignant disease that results from mutations in DNA sequences that disrupt underlying molecular pathways and mechanisms responsible for regulating cellular growth and migration rates [1,2,3]. Epidemiologically, cancer causes the highest death after cardiovascular diseases, and based on estimates, the number of cancer patients in 2040 will be 27.5 million which is significantly higher compared to 2018 (17 million cases) [4]. The five most frequently diagnosed types of cancer are lung, colorectal, breast, prostate, and stomach cancer [2]. In recent years, there have been many efforts in the application of anti-cancer agents (both synthetic and natural ones) to suppress tumor progression [5]. These compounds mainly stimulate apoptotic cell death and DNA damage decreasing the viability of cancer cells. However, due to the emergence of drug resistance, cancer cells are not sensitive to apoptosis and DNA damage upon exposure to anti-tumor agents. Hence, novel therapeutics including magnetic hyperthermia and microwave ablation for sensitizing cancer cells to apoptosis and DNA damage are of importance [6].
Nanomedicine has provided new opportunities in the diagnosis and treatment of various diseases, particularly cancer [7]. Magnetic nanostructures are capable of deep penetration into normal and tumoral tissues, and they show bioimaging properties after exposure to a magnetic field, turning them into appropriate carriers for MRI [1, 8]. Magnetic ferrite nanoparticles are among the most well-known and promising carriers with applications in both engineering and medicine. In addition to imaging, magnetic ferrite nanostructures can provide hypothermia and provide targeted delivery [9]. The magnetic ferrite nanoparticles have the chemical formula of MFe2O4 and in this formula, M is a divalent metal cation. The addition of cobalt leads to the formation of ferromagnetic nanoparticles with high theoretical coercivity and magnetic properties [1]. On the other hand, the utilization of zinc (Zn) forms non-magnetic structures (ZnFe2O4) [10, 11]. When Zn is introduced to spinel structure, it occupies a tetrahedral site, while iron cations are positioned in octahedral site. When Zn is introduced into the cobalt ferrite crystal lattice, iron cations located in tetrahedral sites displace to octahedral positions. This results in a decrease in the magnetization of the tetrahedral sublattice. Besides, a reduction occurs in compensation between magnetic moments of cations in tetrahedral and octahedral locations. Notably, these alterations promote the overall magnetization of nanostructures [12, 13]. The zinc ferrite (ZnFe2O4) shows ideal characteristics such as high surface area, good biocompatibility, and low resistance. To improve the electromagnetic feature and enhance resistivity and permeability, ZnFe2O4 is doped with cobalt [14].
The cobalt ferromagnetic (CoFe2O4) nanoparticles can be fabricated using various methods such as co-precipitation or chemical precipitation. These nanostructures have a particle size of 10–100 nm and can provide magnetic fluid hyperthermia. Noteworthy, heating efficiency appears to be better in low sizes such as 18 nm [15, 16]. To obtain favorable magnetic hyperthermia, a low Curie temperature in the range of 42–46 °C is suggested [17, 18]. One of the drawbacks of ferromagnetic nanoparticles is the high Curie temperature (793 K for CoFe2O4) which is more than the required one for therapeutic applications [19]. One strategy for increasing the efficacy of magnetic fluid hyperthermia therapy is the fabrication of composite nanoparticles with a low Curie temperature range of 42–46 °C, with the addition of zinc [20]. The introduction of Zn into ferromagnetic nanoparticles diminishes Curie temperature by weakening super-exchange interaction and magneto-crystalline anisotropy in sublattices [20]. The particle size, shape, composition, and cation distribution are determining factors for Curie temperature [21].
The interest in the preparation of magnetic nanocomposites has increased recently. Magnetic nanocomposites can be fabricated using different methods including core–shell, Janus, and a surface modification of neat magnetic nanoparticles and functionalizing them with functional polymers [22, 23]. The surface functionalization of magnetic nanoparticles also imparts new properties such as enhancing loading capacity along with the sustained release of the encapsulated bioactive molecules, e.g., anti-cancer drugs such as doxorubicin [23, 24]. For preparing nanocomposite magnetic NPs, various materials including natural and synthetic polymers (e.g., alginate, chitosan, dextran, polyvinyl alcohol, and polyethylene glycol) have been grafted on these nanomaterials [23].
Among these polymers, chitosan can efficiently stabilize colloidal dispersions of magnetic nanoparticles in nanofluid and improve biocompatibility and chemical properties [25]. The exoskeleton of crustaceans, insects, and microorganisms is rich in chitin and its N-deacetylation leads to the production of chitosan. Chitosan has a cationic charge and this linear polysaccharide has glycosidic linkages for bridging monosaccharide units in its structure (2-acetamido-d-glucose and 2-amino-d-glucose). Chitosan has been an interesting biopolymer for surface modification of nanoarchitecture due to its characteristics such as biocompatibility, biodegradability, safety profile, ease of modification as well as affordability. Chitosan-based nanostructures have shown high potential for the delivery of anti-cancer agents, gene delivery, and bioimaging [7, 26,27,28,29]. Chitosan/hyaluronan nanogels have promoted the internalization of anti-cancer agents (methotrexate and 5-aminolevulinic acid) and elevated reactive oxygen species (ROS) levels, leading to apoptosis [30]. Furthermore, chitosan-proanthocyanidin nanostructures induce both apoptosis and DNA damage in colorectal tumor suppression [31]. In addition, chitosan has been proven to have adjuvant immune functions, able to drive potent cell-mediated anti-cancer immunity, promoting dendritic cell maturation and antigen-specific T helper 1 (Th1) responses [32].
In previous research, chitosan-coated superparamagnetic iron oxide nanoparticles and ferrites were examined for applications in drug delivery and hyperthermia [33]. However, there has been limited exploration into the potential of cobalt-zinc ferrite in this context. This current study is centered on the fabrication of a chitosan-coated cobalt-zinc ferrite nanocomposite designed for cancer therapy. Chitosan was employed for the coating of ferrite nanoparticles. The cytotoxicity of chitosan-adorned ferromagnetic nanoparticles against WEH164 cells was evaluated. After that, the capability of the fabricated nanocomposites to induce apoptosis was investigated. The ROS production in the cells incubated with nanocomposites and their genotoxicity was then evaluated. Finally, the nanocomposite immunomodulatory effect was investigated. We hypothesize that the chitosan coating will enhance selective apoptosis in cancer cells while maintaining viability of healthy cells. Additionally, we expect the nanocomposites will efficiently enter cells, induce ROS production and DNA damage preferentially in cancer cells, and stimulate immunomodulatory effects through dendritic cell maturation and proinflammatory cytokine production.
2 Experimental
2.1 Preparation of cobalt zinc ferrite adorned chitosan nanoferrofluid
Nanoferrofluid was prepared by decorating water-soluble chitosan as follows: cobalt zinc ferrite (Co0.3Zn0.7Fe2O4) nanoparticles (100 mg) were added to the aqueous solution of sodium citrate (5 mM in 100 mL dH2O) containing water-soluble chitosan (33.3 mg) and well mixed on a stirrer for 24 h and dried by freeze-dried. The nanocomposite is named Cs/CoZnFe2O4 for all experiments in this study. The synthesis procedure is shown in Figure S1.
2.2 Cytotoxicity assessment
The cytotoxicity of the ferrofluid was investigated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2–5 diphenyl tetrazolium bromide) assay. MTT is captured by cells and reduced into a blue formazan product, principally by the activity of the mitochondrial enzymes, cytochrome oxidase, and succinate dehydrogenase. The capacity of cells to reduce MTT reveals their integrity and mitochondrial activity, which serves as a measure of their vitality.
Cytotoxicity of the ferrofluid was assessed on WEHI 164 cells (Iran National Cell Bank, INCB) using a 10 mg/mL ferrofluid stock solution. WEHI 164 cells were seeded in a 96-well plate and then incubated at 37 °C in 5% CO2 to allow cell attachment. Then, the medium was discarded and replenished with fresh medium containing various concentrations of nanoparticles in RPMI-1640 (ranges 0, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1 mg/mL) containing 10% FBS and adequate amount ampicillin and streptomycin antibiotics. After 24 and 48 h, 20 μL of MTT solution (0.5 mg/mL) was added to each well and incubated at 37 °C for 4 h. Then, the remaining supernatant was discarded, and insoluble formazan crystals were dissolved in 150 μL of DMSO. After 30 min, the absorbance of the formazan product was measured at 570 nm with a microplate spectrophotometer. Finally, the cell viability curves were drawn, and the inhibitory concentration against 50% of cells (IC50) was determined.
2.3 Investigation of apoptosis using flow cytometry
The FITC Annexin V Apoptosis Detection Kit (BD Bioscience, USA) was used to evaluate the induction of apoptosis and necrosis by nanoparticle in WEHI 164 cells, according to the manufacturer’s protocol. In summary, 1 × 105 cells were cultured in each well of a 6-well plate for 24, 48, and 72 h in the absence and presence of the nanocomposites. Then, the medium was discarded; cells were washed twice with PBS; and cells were detached from the bottom of the wells using 0.25% trypsin and 1% EDTA solution. Next, cells were centrifuged at 1200 rpm for 5 min, washed with PBS, and resuspended in the 1X Annexin V binding buffer with a concentration of 1 × 106 per mL. Around 100 µL of the cell suspension was transferred into a tube; 5 µL of propidium iodide (PI) and 5 µL of Annexin V-FITC were added to the tube, and incubated at room temperature in the dark for 30 min. Finally, sample fluorescence was acquired on FL1 and FL2 filters after gating the normal alive cells using the Partec CyFlow ML (Partec System, Germany), and results were analyzed using Flow max Partec and Flow Jo software.
2.4 Hemolysis
We tested the efficacy of Cs/CoZnFe2O4 nanocomposites and CoZnFe2O4 nanoferrofluid on human erythrocytes. In order to check the hemolysis property of samples, blood was taken from adult volunteers with consent and placed in heparin tubes. Phosphate-buffered saline and Triton X-100 were used as negative and positive controls, respectively. To separate red blood cells, centrifugation was performed at 1500 rpm for 15 min at 4 °C, and the supernatant was drained and the cells were washed with PBS. This step was repeated up to three times and finally RBC was diluted 1:4 with PBS. About 25 mg of each sample in 1 mL of PBS were incubated at 37 °C for 60 min. Then, diluted RBC was added to each sample at a ratio of 1:4 and incubated at 37 °C for 30 min. Then, the obtained solutions were centrifuged at 1500 rpm for 15 min, and the absorbance (545 nm) of the supernatant was measured by a spectrophotometer (Shimadzu UV-1800). The percentage of hemolysis cells was calculated as follows:
The ODs, ODn, and ODp were the absorbance of samples and negative and positive controls. All hemolysis experiments were performed in triplicate.
2.5 Broth-microdilution method
This method was used to check the MIC of the desired microorganisms in this study. For culture on the microplate, first 100 μL of Mueller Hinton Broth culture medium was added to all the wells; 100 μL of the initial concentration of the Cs/CoZnFe2O4 nanocomposites and CoZnFe2O4 nanoferrofluid were poured into the first well and mixed with the culture medium, and 100 μL was removed from this mixture and added to the next well. This was continued up to column F in each row, and finally 100 μL was discarded from the last well. Next, 100 μL of prepared bacterial suspension was added to each well up to column G and mixed well using a sampler. Column G was considered as a positive control, which contained Mueller Hinton liquid culture medium and bacterial suspension. Column F, which contained only Mueller Hinton’s liquid culture medium, was considered as a negative control. This process was done for all two bacterial strains and specific concentrations separately and with three repetitions. After gently shaking the microplate in order to homogenize suspension in the wells, it was placed in an incubator with a temperature of 37 °C for 24 h. After 24 h, 10 μL of MTT dye was added to all the wells, and after 4 h of incubation at 37 °C, among the wells in which there was no growth, the one with the lowest concentration, was reported as MIC.
2.6 Evaluation the antioxidant properties
The antioxidant activity of the samples was evaluated using the 2,2-dipheny1-1-picrylhydrazyl (DPPH) free radical scavenging method. A concentration of 20 mg/mL of the Cs/CoZnFe2O4 nanocomposites and CoZnFe2O4 nanoferrofluid was prepared in methanol and mixed with DPPH (80 µg/mL) at a ratio of 1:1 as a free radical source. Then, the samples were kept for 30 min in the dark at room temperature (25 ± 1 °C). Lower absorbance of the reaction mixture indicates higher DPPH radical scavenging activity. The neutralization of DPPH was measured against the negative control at 517 nm by a Shimadzu UV-1800 spectrophotometer according to the following equation:
Here, ODn is the absorption of the control reaction, and ODS is the absorption of the sample. The values of SC50 indicate the concentration of the substance that causes 50% inhibition of oxidative processes and were reported for all samples.
2.7 Investigate the amount of ROS using flow cytometry
The amount of ROS produced by lipid peroxidation was measured using the Bodipy 581/591-C11 (D-3861, Life Technologies) marker. 1 × 105 WEHI 164 cells were cultured in each well of a 6-well plate, and after incubating for 72 h (in the absence and the presence of the mentioned concentrations of nanoparticles), cells were washed using PBS. The cells were then adjacent to the bodipy marker at a 1 mg/mL concentration for 30 min at 37 ºC. Then, the cells were washed, and after separating from the plate, were acquired using the Partec CyFlow M flow cytometer. The results were analyzed using Flow max Partec and Flow Jo software.
2.8 Alkaline genotoxicity assay
To investigate the genotoxicity of the nanoparticles, a stock suspension of 2 × 105 per mL of WEHI 164 cells was prepared, and 50 µL of the cell suspension was transferred into microtubes containing serial concentrations of nanoparticles (0, 0.1, 1, 10, 50, and 100 µg/mL). Positive controls consisting of 100 µM hydrogen peroxide and negative comet controls containing phosphate buffer saline were also included in the experiment. After incubation at 4 °C for 30 min and centrifugation for 10 min at 1000 g, the supernatant was discarded, and the cells were resuspended in 50 μL of PBS. Then, 50 μL of pre-melted low-melting agarose (1.5% w/v) was added to each tube and immediately spread on agarose-coated comet slides. A cover slip was placed on top and transferred to the refrigerator for solidification. After congelation, the cell sandwich was prepared by removing the coverslip and pouring 100 μL low melting agarose 0.75% w/v, and putting it back in the refrigerator for 1 h. Next, the slides were immersed in a fresh ice-cold lubricating solution containing 10% v/v methyl sulfoxide and 1% v/v Triton X-100 and refrigerated for 4 h. To perform electrophoresis, the slides were placed in neutralizing buffer for 5 min. Afterward, the slides were placed in a chamber containing 1 mM EDTA solution at pH = 13 and electrophoresed for 45 min at 300 mA on ice. After electrophoresis, the slides were washed three times with 0.4 M tris solution (pH 7.5) and were stained with 50 µg of ethidium bromide (20 µg/mL). Finally, the cell images were taken with a Nikon AZ100 fluorescent microscope. Fifty cells from each of the three repeating slides (randomly selected cells) were obtained using OpenComet software to get various factors such as DNA density in head and tail, head and tail length, DNA percentage in head and tail, etc. The percentage of DNA in the tail was calculated using the following formula:
The anti-genotoxicity of these materials was compared to the PBS and the hydrogen peroxide.
2.9 Statistical analysis
The statistical differences were determined using one-way or two-way analysis of variance (ANOVA) using SPSS software or GraphPad Prism. The results were expressed as mean ± standard deviation (SD), and the significance level was considered based on the probability of p < 0.05.
3 Results and discussion
3.1 Characterizations
The phase study of CoZnFe2O4 nanocomposites is performed by XRD and shown in Fig. 1A. In the XRD pattern of CoZnFe2O4 nanocomposites, all peaks were indexed by the cubic structure of CoZnFe2O4 (COD NO. 96–900-6898), which indicates the high purity of the product. The observed peaks have Miller index (220), (311), (222), (400), (422), (511), (440), (622), and (533).
A XRD pattern of CoZnFe2O4 nanocomposite. B FTIR of (i) CoZnFe2O4 nanocomposite, (ii) chitosan, and (iii) Cs/CoZnFe2O4 nanocomposite. C Representative FESEM images of CoZnFe2O4 (uncoated) and Cs/CoZnFe2O4 nanocomposite (coated). D EDS mapping of CoZnFe2O4 nanoparticles, showing the presence of Fe, Zn, and Co elements. E EDS analysis of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites, respectively
To ascertain that CoZnFe2O4 nanocomposites were coated by chitosan, infrared spectra of pure chitosan, CoZnFe2O4, and Cs/CoZnFe2O4 nanocomposites were performed by a Fourier transform infrared (FTIR) spectrometer. In the FTIR spectrum of CoZnFe2O4 nanocomposites, the absorption band at 540 cm−1 is related to the stretching vibrations of the Co–O, Zn–O, and Fe–O bonds in the octahedral site of cobalt zinc ferrite nanoparticles. The absorption bands at 1730 and 3410 cm−1 are assigned to the stretching vibrations of carbonyl groups of sodium citrate and hydroxyl groups of water molecules adsorbed on the surface of the nanoparticles, respectively. FTIR spectrum of chitosan displayed several peaks at 1164, 1602, 2856, and 3494 cm–1 are related to the stretching vibrations of C–O–C, C = O, CH2, and OH groups, respectively. The presence of characteristic absorption bands corresponding to CoZnFe2O4 and chitosan in the FTIR spectrum of Cs/CoZnFe2O4 nanocomposites indicated success fabrication of nanoferrofluid (Fig. 1B).
The SEM micrographs of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites are shown in Fig. 1C. Cs/CoZnFe2O4 nanoparticles showed lower aggregation compared to the CoZnFe2O4. Figure 1D and E show EDS spectra and elemental mapping of nanocomposites. The homogenous distribution of constituent elements can be seen in elemental mapping. The EDS analysis confirms the presence of Zn Fe O and Co in the nanoparticles. Si, Ti, and Cu elements were also visualized as background due to the TEM sample holder and grids. EDS spectrum was also acquired on an empty region of the grid to show that Ti Si and Cu are background signals.
The TEM micrographs of CoZnFe2O4 nanocomposite indicate that the CoZnFe2O4 particles are almost spherical, homogenous in both morphology and crystallite size, besides having agglomeration to some extent attributable to interactions between magnetic particles (Fig. 2A). The TEM micrographs of Cs/CoZnFe2O4 nanocomposite show that the nanocomposite demonstrates a distinct chitosan shell on the CoZnFe2O4 particles. In the most right panel TEM image, the coating via chitosan is visible (Fig. 2A). From the TEM images, the particle size of the CoZnFe2O4 (uncoated) and Cs/CoZnFe2O4 (coated) were also measured with sizes showing 7.9 ± 1.8 nm and 8.1 ± 1.5 nm, respectively. The Cs/CoZnFe2O4 nanocomposite particles displayed increased size compared with CoZnFe2O4 particles, which is confirmed by DLS as well. We have also calculated the particle size of the nanoparticle via XRD. The average crystal size (d) measured from the XRD pattern was ~ 11 nm using the Scherrer Eq. 34 which is in agreement with TEM data. In the Scherrer equation (d = k − λ/β cos θ), K = 0.9 is the shape factor; λ is the X-ray wavelength; θ is the diffraction angle; and β is the broadening of the diffraction line measured at half of its maximum intensity.
The DLS was performed to study the hydrodynamic particle size of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites, as shown in Fig. 2B. As can be observed, the particle size distribution of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites was obtained by DLS. After coating the chitosan on the CoZnFe2O4, the size was enlarged by the ratio of 15–40%, and the aggregations were enhanced as well. The difference between the particle size in TEM and DLS approaches is due to the hydrodynamic diameter in DLS which shows increased particle size expectedly [35]. The colloidal stability of particles was increased by coating the particles from 3 to more than 5 months. An increase in the stability of particles can be related to steric repulsion of particles when chitosan is added between particles [36].
The size of nanoparticles is of importance for drug delivery applications. It has been shown that the internalization of nanoparticles with a size of less than 300 nm is better in enterocytes and M cells compared to nanoparticles with a larger size [37]. Furthermore, nanoparticles with sizes of 50 and 200 nm can be internalized by intestinal cells [38]. In our experiment, we prepared a CoZnFe2O4 nanocomposite with an average particle size of 39.1 ± 3.2 nm and further surface modification with chitosan enhanced their size to an average particle of 93.7 ± 4.8 nm. The resulting nanocarriers demonstrated an appropriate size that is good for internalization by cancer cells and can be considered as a promising carrier for drug delivery. The increase in particle size after chitosan coating is attributed to generation of agglomerates and presence of chitosan on the surface of the nanoparticles.
One of the most important techniques on magnetic materials is studying their magnetic properties using a vibrating sample magnetometer. The hysteresis curves of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites are shown at room temperature in Fig. 2C. The applied magnetic field range is about 20 kOe. As seen in Fig. 2C, the magnetic hysteresis curves of uncoated and coated have an S-like shape without any coercivity (Hc) or remanence (Mr). This confirms their superparamagnetic properties. Consequently, the saturation magnetization (MS) of the CoZnFe2O4 nanocomposites was detected at about 22.23 emu g−1, whereas the Ms value for the Cs/CoZnFe2O4 nanocomposites was measured at approximately 6.11 emu g−1. This decrease can be related to the presence of non-magnetic-adorned chitosan on the CoZnFe2O4 surface.
3.2 Cell toxicity assessment test
The MTT results showed that 24 h after exposure to different concentrations of CoZnFe2O4 to WEHI164 cells, a significant decrease in cell viability was observed in 10, 16, and 20 mg/mL concentrations. A slight increase was observed in 2 mg/mL concentration; however, this increase was not statistically significant. Upon exposure to Cs/CoZnFe2O4 nanocomposites, a significant decrease in cell viability was observed at all concentrations above 4 mg/mL. The results of WEHI164 cells viability after 24 h indicate that Cs/CoZnFe2O4 nanocomposites possess cytotoxicity toward WEHI164 cells at a lower concentration (6 mg/mL) compared to CoZnFe2O4 (10 mg/mL) which is attributable to the variations in the surface properties of the nanocomposites and chitosan anticancer properties (Fig. 3A) [39].
Bar graph and their corresponding heatmap showing the CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites-induced cytotoxicity in WEHI164 cells. The cells were exposed to the nanocomposites at the dosages of 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 20 mg/mL for A 24 and B 48 h. The cytotoxicity of the various dosages was measured using an MTT assay. (* p < 0.05, ** p < 0. 01, *** p < 0.001, **** p < 0.0001, #p < 0.05, ##p < 0. 01, ####p < 0.0001)
After 48 h, CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites possessed significant cytotoxicity toward WEHI164 cells in all concentrations (1–20 mg/mL). The toxicity of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites toward WEHI164 cells showed a proportional increment with the increase in the nanocomposite concentration (Fig. 3B).
The results indicated that CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites possess anti-cancer characteristics. According to the results of the MTT assay, the IC50 concentration of both nanocomposites was considered 12 mg/mL. Furthermore, ineffective concentration was considered as the highest concentration with no significant cytotoxicity toward WEHI164 cells which was 4 mg/mL and 8 mg/mL for CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites, respectively.
MTT assay is indicative of the mitochondrial damage that ultimately causes cell death. This assay provides a sensitive and comprehensive measurement of cell viability and cell growth suppression. According to MTT results, CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites decreased cancer cell viability in a concentration- and time-dependent manner. Our results were commensurate with the previous studies indicating the anti-cancer potential of CoZnFe2O4 nanocomposites by alterations in mitochondrial function [40].
3.3 Hemocompatibility
The study of hemolysis is an essential test for biomaterials. In our study, the hemolytic properties of the Cs/CoZnFe2O4 nanocomposites and Cs/CoZnFe2O4 nanoferrofluid were determined at various concentrations between 1.5625 and 25 mg/mL. As reported in Table 1, the maximum and minimum percentages of hemolysis at concentrations of 25 and 1.5625 mg/mL for CoZnFe2O4 (2.31–1.12%) and Cs/CoZnFe2O4 (1.69–0.72%) were detected after contact with red blood cells during 30 min.
The results of hemocompatibility showed that the Cs/CoZnFe2O4 had a less hemolytic effect on human RBC than the CoZnFe2O4, as compositing with chitosan increased the hemocompatibility of the composite.
3.4 Apoptotic properties of nanocomposites in WEHI164 cells
To investigate the ability of both CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites to induce apoptosis, cells were evaluated after exposure to different concentrations of two types of nanoferrofluid after 24, 48, and 72 h. Cells that were only positive for FITC were considered the primary stage of apoptosis, and those with both FITC and PI were considered the second stage of apoptosis. As shown in Fig. 4A, the cells proceed from the first stage of apoptosis to the secondary stage. The results demonstrate a significant increase of apoptotic cells exposed to IC50 concentration of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites after 24 and 48 h, respectively (Fig. 4B). After 72 h of incubation with IC50 concentration, both CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites led to a significant increase in apoptotic cells compared to the untreated group (Fig. 4B).
Effect of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites on apoptosis induction in WHEI 164 cells, evaluated upon exposure to IC50 and ineffective concentrations after 24, 48, and 72 h. A The results show a continuous process of apoptosis. Alive cells (Q4), cells in the primary stage of apoptosis (Q3), cells in the second stage of apoptosis (Q2), and cells undergone necrosis (Q1) are demonstrated for (a) control, (b) IC50 concentration of uncoated nanoparticle, (c) safe concentration of uncoated nanoparticle, (d) IC50 concentration of coated nanoparticle, and (e) safe concentration of coated nanoparticle. B Graph possessing the effect of different nanoparticles on apoptosis induction after (a) 24, (b) 48, and (c) 72 h. Cells treated with CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites at IC50 concentrations showed a significant increase in the number of cells undergoing apoptosis after 24 and 48 h, respectively. After 72 h CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites at IC50 concentrations demonstrated a significant increase compared to the non-treated control group. (* p < 0.05)
3.5 The effect of nanocomposites on ROS production in WEHI164 cells
In the study on both non-coated and coated nano ferrofluids’ ability to cause ROS production, the cells were evaluated after exposure to different concentrations of nanoparticles and incubation for 72 h, using Bodipy dye. As shown in Fig. 5, both nanoparticles cause ROS production in adjacent Bodipy-colored cells, indicating ROS-dependent apoptosis. The results show a significant increase in ROS production in the treated group with non-coated nanoparticle IC50 concentration (Fig. 5) (p < 0.05). Although the amount of ROS produced in the treated group with Cs/CoZnFe2O4 nanocomposites was close to the non-coated group, this rate was not statistically significant (p > 0.05).
ROS production in the WEHI164 cells incubated 72 h with A control, B IC50 concentration of uncoated nanoparticle, C ineffective concentration of uncoated nanoparticle, D IC50 concentration of coated nanoparticle, and E ineffective concentration of coated nanoparticle. The result shows that all nanoparticles induce ROS production in the treated cells. However, only cells treated with IC50 concentration of uncoated nanoparticles showed a significant increase in ROS production levels (p < 0.05)
Apoptosis is a kind of programmed cell death and its induction is a promising approach for the elimination of cancer cells. Stimulation of apoptosis can occur due to stresses in cells provided by external and internal factors. The reactive oxygen species (ROS) overgeneration can mediate stress in cancer cells and lead to apoptotic cell death. After an increase in ROS production, mitochondrial dysfunction, endoplasmic reticulum stress along with changes in proteins such as Bcl-2 and Bax occur to induce apoptosis [41, 42].
Due to the specialized properties of magnetic nanocomposites, which vary depending on the particle size, particle concentration, and particle distribution in the matrix, interest in the preparation of these materials has increased in recent years. The studies have shown the potential of cobalt-zinc ferrite nanoparticles in cancer suppression. Zinc ferrite nanostructures were prepared using Lawsonia inermis and evaluated its activity against breast tumor cells. Based on the findings, zinc ferrite nanoparticles reduced the viability of MCF-7 cells via apoptosis induction in a concentration-dependent manner (25, 50, 100, 250, and 500 µg/mL). The mechanism was attributed to the release of Zn2+ from nanoparticles that disrupts redox balance, resulting in ROS generation. Then, oxidative stress along with cell membrane rupture can induce apoptosis [43]. Another study has prepared cobalt-zinc ferrite nanohybrids for curcumin delivery in breast cancer suppression and by enhancing ROS generation, the nanostructures stimulate apoptotic cell death [44]. This study revealed that cobalt-Zn ferromagnetic nanostructures can be considered for drug delivery and synergistic cancer therapy [44]. Another experiment prepared Zn ferrite nanostructures with a particle size of 44 nm and used various concentrations in the range of 10–40 µg/mL to evaluate their anti-tumor activity. The nanoparticles elevated ROS generation to mediate oxidative stress and also, and they upregulated Bax, caspase-3, and caspase-9 expressions to induce apoptosis [45]. Furthermore, the Co-Zn ferrite nanoparticles can induce magnetic fluid hyperthermia that is of importance in ROS overgeneration and cell death induction [46].
3.6 Antioxidant activity
The maximum antioxidant property of the Cs/CoZnFe2O4 nanocomposites and CoZnFe2O4 nanoferrofluid in the studied concentration is given in Table 2. As shown, the antioxidant properties of Cs/CoZnFe2O4 decreased slightly compared to the CoZnFe2O4 which is attributed to the composition with chitosan, but still, it has the proper antioxidant properties.
3.7 Comet assay
The comet assay is a visual and sensitive method for evaluating single-strand DNA damage in cells. Induction of DNA damage by chitosan-nanoferrofluid in WEHI164 cells shown in Fig. 6 demonstrates that after exposure to the different concentrations (0.1, 1, 10, 50, and 100 μg/mL) of coated and uncoated nanoferrofluids, the significant DNA damage was recorded in uncoated nanoferrofluids at 100 μg/ mL comparing to the negative control. Positive control (100-μM H2O2) caused the highest DNA migration and proved a standard genotoxic material. CS-nanoferrofluids also induced significant DNA destruction of cancer cells at 100 μg/mL concentration, showing the effectiveness of treatment. A dose-dependent increase in DNA damage at all treatments was seen in the times studied.
Genotoxic properties of CoZnFe2O4 and Cs/CoZnFe2O4 nanocomposites. DNAs were stained with ethidium bromide solution and pictured on the Nikon fluorescent microscope at 20 × magnification on the green filter. About 100 μM of H2O2 and PBS was recorded as standard positive and negative comet controls in all experiments, respectively
3.8 CoZnFe2O4 and chitosan-coated CoZnFe2O4 nanocomposites’ immune activation of dendritic cells
To assess the effect of uncoated or chitosan-coated nanoparticles on dendritic cells, we derived DCs by bone marrow precursors using GM-CSF. Immature BM-DCs were cultivated in the presence of CoZnFe2O4 or Cs/CoZnFe2O4 nanocomposites for 20 h. The effect of both kinds of CoZnFe2O4 nanocomposites on activation of the murine DCs was assessed by measuring the modulation of the expression levels of CD86 costimulatory molecule which is a marker of primary DC maturation. As shown in Fig. 7A and B, the exposure of DCs to both naked and chitosan-coated CoZnFe2O4 induces the upregulation of the CD86 expression. This upregulation is evident at the highest concentrations of nanocomposites tested compared to the untreated cells.
Immune effects of Cs/CoZnFe2O4 nanocomposites on dendritic cells A CD86 expression on Bone marrow-derived dendritic cells (BM-DCs) incubated for 20 h with medium (light blue histograms) or with graded doses of CoZnFe2O4 or Cs/CoZnFe2O4 (dark blue histograms). BM-DCs incubated with E. coli lipopolysaccharides (LPS) were used as a positive control. Mean fluorescence intensity (MFI) of one of the representative experiments. B Percentage of CD86high positive BM-DCs, after 20-h treatment with CoZnFe2O4, Cs/CoZnFe2O4, or medium. C IL-6 release by BM-DCs pulsed with CoZnFe2O4 or Cs/CoZnFe2O4 nanoparticles. BM-DCs were incubated with graded doses of CoZnFe2O4 or Cs/CoZnFe2O4 nanocomposites for 20 h, and then culture supernatants were assayed by ELISA in duplicate. Mean + SEM is reported, and one representative experiment of two is shown. Differences are significant by two-way ANOVA (*** p < 0.001)
Moreover, we also analyzed the proinflammatory cytokine IL-6 release, as an indicator of the maturation status of the DCs. Surprisingly, the exposure of DCs to naked CoZnFe2O4 does not induce the release of proinflammatory cytokine IL-6 (Fig. 7C). On the contrary, Cs/CoZnFe2O4 treatment showed a significant dose-dependent effect on the release of the IL-6 cytokine, as compared to untreated (medium-incubated) DCs. These findings demonstrate that CoZnFe2O4 nanoparticles’ surface modification with chitosan can induce dendritic cell activation, with subsequent release of the proinflammatory cytokine IL-6 in a dose-dependent manner that in turn can stimulate immune anti-cancer responses.
Previous studies demonstrated that chitosan has also immune stimulatory effects on antigen-presenting cells, being able to induce a significant augment of pro-inflammatory cytokine production and thus influencing the subsequent anti-cancer immune responses [47, 48]. In this work, the immunomodulatory capacity of chitosan-coated nanoparticles on bone marrow-derived dendritic cells was analyzed in cytokine release studies on DC incubated with nanoparticles. We found that DC exposure to chitosan-coated nanoferrofluid enhances the activation status of the dendritic cells, inducing the release of the proinflammatory cytokine IL-6 in a dose–response manner. Dendritic cells are the most professional antigen-presenting cells of the immune system and play a pivotal role in coordinating innate and adaptive anti-cancer immune responses. Activating dendritic cells by nanotechnology is a promising strategy in anti-cancer immunotherapy, and nanoparticles can modulate the DC immune responses altering their maturation, migration, and antigen processing and presentation.
Even though the magnetic fluid agents for hyperthermia have improved, they still have difficulty achieving excellent biocompatibility despite having great properties for tumor targeting and rapid temperature rise. Aside from the cytotoxic behavior of some nanoparticles, particle size and surface characteristics are critical properties that must be precisely controlled. The modification of nanostructures with organic or inorganic biocompatible materials, namely, polyethylene glycol, chitosan, hydroxyapatite, gold, and silica, has been extensively investigated as a means of preventing the action of reticuloendothelial systems and assuring safety for bio applications [44]. Functionalized nanocomposites have shown promising results in biological applications. Due to its ideal characteristics, including biocompatibility, biodegradability, and bioactivity, chitosan has been extensively employed as a molecular surfactant to prevent nanoparticle aggregation, improve colloidal stability, and increase in vivo blood circulation. Surface modification of nanoscale materials provides some benefits in the biomedical field. For instance, it improves colloidal stability in the surrounding medium and the formation of ferrofluids by creating positive charges, biocompatibility, efficient biodistribution, prevention of their quick clearance, and increasing cellular uptake. In hyperthermia, these nanocomposites prevent nanoparticle aggregation, which can cause burns as a result of overheating and reduce magnetic efficiency [49, 50]. The anti-cancer activity and potential application of the fabricated nanocomposite are shown in Fig. 8. Such surface functionalization of magnetic nanostructures may improve cancer treatment alongside reducing side effects on healthy tissues.
3.9 MIC determination
In this investigation, the anti-bacterial effect of certain concentrations of the Cs/CoZnFe2O4 nanocomposites and CoZnFe2O4 nanoferrofluid on the growth of Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) strains was carried out by broth microdilution method to measure MIC with three repetitions against control antibiotics ampicillin and penicillin, as shown in Table 3. The results showed that both samples had specific anti-bacterial effects. the MIC concentration for both samples against E. coli was 25 mg/mL, but Cs/CoZnFe2O4 showed better anti-bacterial properties against S. aureus was 3.125 mg/mL compared to CoZnFe2O4 which was 6.25 mg/mL, and it was attributed to anti-bacterial properties of chitosan.
4 Conclusion
Cobalt zinc ferrite (CoZnFe2O4) nanoparticles were successfully synthesized and then coated with chitosan. The chitosan-coated nanocomposites showed a mean diameter of ~ 94 nm with minimal agglomeration observed by TEM. The chitosan-coated nanocomposites showed anti-cancer cell activity. The chitosan-coated nanocomposites were demonstrated to induce oxidative stress in cancer cells by ROS production. Furthermore, genotoxicity and DNA damage were indicated in cells treated with chitosan-coated nanocomposites. Additionally, chitosan-coated nanocomposites elicited stimulation of dendritic cells, leading to a dose-dependent production of the proinflammatory cytokine IL-6, which can induce immunological anti-cancer responses. Recent inquiries on cancer treatment focused on the lowest side effects of it and using chitosan as a coating material showed it could be effective, with more apoptosis of cancer cells and healthy cell viability. Proper cell entry, DNA damage of cancer cell induction, and suitable physical properties made the nanoferrofluid an effective candidate for drug delivery and targeted cell elimination like cancers. After further investigation and studies, using chitosan-nanoferrofluid can be preferable for cancer therapy.
References
Jia W et al (2021) Facile fabrication of monodisperse CoFe2O4 nanocrystals@dopamine@DOX hybrids for magnetic-responsive on-demand cancer theranostic applications. Adv Compos Hybrid Mater 4:989–1001
Lopes et al (2023) Cell membrane-coated biomaterials for bone cancer-targeted diagnosis and therapy: a critical update on osteosarcoma applications. Mater Chem Horizons 2(1):65–79
Ghomi M et al (2023) A multifunctional bioresponsive and fluorescent active nanogel composite for breast cancer therapy and bioimaging. Adv Compos Hybrid Mater 6:51
Dabbagh Moghaddam F, Romana Bertani F (2022) Application of microfluidic platforms in cancer therapy. Mater Chem Horizons 1(1):69–88
Mondal SK, Perumal V, Das R, Roymahapatra G, Mandal SM (2022) Antimicrobial and anticancer activity of a novel peptide (Musterolysin) extracted from slurry of mustard oil refinery industry. ES Food Agrofor. https://doi.org/10.30919/esfaf776
Preechaphonkul W, Rattanadecho P (2022) The effects of dielectric & thermal property functions on the thermal response during the focused microwave ablation treatment in the liver cancer model: numerical investigation. Eng Sci. https://doi.org/10.30919/es8e788
Ghovvati M, Bolouri K, Guo L, Kaneko N, Jin X, Xu Y, Hua Z, Lei Y (2023) Harnessing the power of electroconductive polymers for breakthroughs in tissue engineering and regenerative medicine. Mater Chem Horizons 2(3):195–206. https://doi.org/10.22128/mch.2023.693.1042
Wang X et al (2022) Fe3O4@PVP@DOX magnetic vortex hybrid nanostructures with magnetic-responsive heating and controlled drug delivery functions for precise medicine of cancers. Adv Compos Hybrid Mater 5:1786–1798
Tatarchuk T et al (2021) Green synthesis, structure, cations distribution and bonding characteristics of superparamagnetic cobalt-zinc ferrites nanoparticles for Pb(II) adsorption and magnetic hyperthermia applications. J Mol Liq 328:115375
Hatami Kahkesh K et al (2023) Synthesis, characterization, antioxidant and antibacterial activities of zinc ferrite and copper ferrite nanoparticles. Mater Chem Horizons 2(1):49–56
Ghayem F, Mirani Nezhad S, Hosseini S, Pourmousavi SA (2023) ZnFe2O4@Fe3O4 nanocatalyst for the synthesis of the 1,8-dioxooctahydroxanthene: antioxidant and antimicrobial studies. Mater Chem Horizons 2(3):207–224. https://doi.org/10.22128/mch.2023.704.1046
Tiwari P, Verma R, Kane SN, Tatarchuk T, Mazaleyrat F (2019) Effect of Zn addition on structural, magnetic properties and anti-structural modeling of magnesium-nickel nano ferrites. Mater Chem Phys 229:78–86
Khanvilkar MB et al (2023) Effect of divalent / trivalent doping on structural, electrical and magnetic properties of spinel ferrite nanoparticles. Eng Sci. https://doi.org/10.30919/es8d850
Mang C et al (2021) Electromagnetic wave absorption properties of cobalt-zinc ferrite nanoparticles doped with rare earth elements. J Rare Earths 39:1415–1426
Salunkhe AB, Khot VM, Ruso JM, Patil SI (2016) Water dispersible superparamagnetic cobalt iron oxide nanoparticles for magnetic fluid hyperthermia. J Magn Magn Mater 419:533–542
Al Lehyani SH, Hassan RA, Alharbi AA, Alomayri T, Alamri H (2017) Magnetic hyperthermia using cobalt ferrite nanoparticles: the influence of particle size. Int J Adv Technol 8
Arulmurugan R, Jeyadevan B, Vaidyanathan G, Sendhilnathan S (2005) Effect of zinc substitution on Co–Zn and Mn–Zn ferrite nanoparticles prepared by co-precipitation. J Magn Magn Mater 288:470–477
Zhang W, Wu C, Silva SRP (2018) Proposed use of self-regulating temperature nanoparticles for cancer therapy. Expert Rev Anticancer Ther 18:723–725
Shaterabadi Z, Nabiyouni G, Soleymani M (2018) Physics responsible for heating efficiency and self-controlled temperature rise of magnetic nanoparticles in magnetic hyperthermia therapy. Prog Biophys Mol Biol 133:9–19
Appa Rao P et al (2019) A systematic study of cobalt-zinc ferrite nanoparticles for self-regulated magnetic hyperthermia. J Alloys Compd 794:60–67
Brusentsova TN, Brusentsov NA, Kuznetsov VD, Nikiforov VN (2005) Synthesis and investigation of magnetic properties of Gd-substituted Mn–Zn ferrite nanoparticles as a potential low-TC agent for magnetic fluid hyperthermia. J Magn Magn Mater 293:298–302
Liu Y et al (2021) Synthesis of Janus Fe3O4&mSiO2 nanocarriers for chemo-microwave therapy of cancer cells. ES Energy Environ. https://doi.org/10.30919/esee8c503
Dhavale RP et al (2021) Chitosan coated magnetic nanoparticles as carriers of anticancer drug Telmisartan: pH-responsive controlled drug release and cytotoxicity studies. J Phys Chem Solids 148:109749
Houshiar M, Zebhi F, Razi ZJ, Alidoust A, Askari Z (2014) Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: a comparison study of size, structural, and magnetic properties. J Magn Magn Mater 371:43–48
Pu L et al (2022) N-doped MXene derived from chitosan for the highly effective electrochemical properties as supercapacitor. Adv Compos Hybrid Mater 5:356–369
Karimi S, Salahinejad E, Sharifi E, Nourian A, Tayebi L (2018) Bioperformance of chitosan/fluoride-doped diopside nanocomposite coatings deposited on medical stainless steel. Carbohydr Polym 202:600–610
Zafari M et al (2020) Physical and biological properties of blend‐electrospun polycaprolactone/chitosan‐based wound dressings loaded with N‐decyl‐N, N‐dimethyl‐1-decanaminium chloride: an in vitro and in vivo study. J Biomed Mater Res Part B Appl Biomater 108:3084–3098
Kushwaha R, Sharma S, Kumar S, Kumar A (2023) Biopolymers as topical haemostatic agents: current trends and technologies. Mater Chem Horizons 2(1):11–39
Bagheri M, Validi M, Gholipour A, Makvandi P, Sharifi E (2022) Chitosan nanofiber biocomposites for potential wound healing applications: antioxidant activity with synergic antibacterial effect. Bioeng Transl Med 7
Wang Y et al (2022) Chitosan/hyaluronan nanogels co-delivering methotrexate and 5-aminolevulinic acid: a combined chemo-photodynamic therapy for psoriasis. Carbohydr Polym 277:118819
Mani S et al (2020) Synthesis and characterization of proanthocyanidin-chitosan nanoparticles: an assessment on human colorectal carcinoma HT-29 cells. J Photochem Photobiol B Biol 210:111966
Carroll EC et al (2016) The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 44:597–608
Anila I, Lahiri BB, Mathew MJ, Philip J (2022) Synthesis and magneto-structural properties of chitosan coated ultrafine cobalt ferrite nanoparticles for magnetic fluid hyperthermia in viscous medium. Ceram Int 48:22767–22781
Patterson AL (1939) The Scherrer Formula for X-ray particle size determination. Phys Rev 56:978–982
Yousefiasl S et al (2023) Chitosan/alginate bionanocomposites adorned with mesoporous silica nanoparticles for bone tissue engineering. J Nanostructure Chem 13:389–403
Sharifi I, Shokrollahi H, Amiri S (2012) Ferrite-based magnetic nanofluids used in hyperthermia applications. J Magn Magn Mater 324:903–915
He C, Yin L, Tang C, Yin C (2012) Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 33:8569–8578
Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S (2016) Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J Control Release 238:176–185
Mahmoudi M et al (2010) A new approach for the in vitro identification of the cytotoxicity of superparamagnetic iron oxide nanoparticles. Colloids Surfaces B Biointerfaces 75:300–309
Ahamed M, Akhtar MJ, Alhadlaq HA, Alshamsan A (2016) Copper ferrite nanoparticle-induced cytotoxicity and oxidative stress in human breast cancer MCF-7 cells. Colloids Surfaces B Biointerfaces 142:46–54
Amini P et al (2021) Resveratrol Induces Apoptosis and attenuates proliferation of MCF-7 cells in combination with radiation and hyperthermia. Curr Mol Med 21:142–150
Koklesova L et al (2020) Carotenoids in cancer apoptosis—the road from bench to bedside and back. Cancers (Basel) 12:2425
Sarala E, Madhukara Naik M, Vinuth M, Rami Reddy YV, Sujatha HR (2020) Green synthesis of Lawsonia inermis-mediated zinc ferrite nanoparticles for magnetic studies and anticancer activity against breast cancer (MCF-7) cell lines. J Mater Sci Mater Electron 31:8589–8596
Sawant VJ, Bamane SR, Shejwal RV, Patil SB (2016) Comparison of drug delivery potentials of surface functionalized cobalt and zinc ferrite nanohybrids for curcumin in to MCF-7 breast cancer cells. J Magn Magn Mater 417:222–229
Alhadlaq HA, Akhtar MJ, Ahamed M (2015) Zinc ferrite nanoparticle-induced cytotoxicity and oxidative stress in different human cells. Cell Biosci 5:55
Bohara RA, Thorat ND, Chaurasia AK, Pawar SH (2015) Cancer cell extinction through a magnetic fluid hyperthermia treatment produced by superparamagnetic Co–Zn ferrite nanoparticles. RSC Adv 5:47225–47234
Koppolu B, Zaharoff DA (2013) The effect of antigen encapsulation in chitosan particles on uptake, activation and presentation by antigen presenting cells. Biomaterials 34:2359–2369
Xu C et al (2021) The immunostimulatory effects of hydroxypropyltrimethyl ammonium chloride chitosan-carboxymethyl chitosan nanoparticles. Int J Biol Macromol 181:398–409
Salmanian G, Hassanzadeh-Tabrizi SA, Koupaei N (2021) Magnetic chitosan nanocomposites for simultaneous hyperthermia and drug delivery applications: a review. Int J Biol Macromol 184:618–635
Shende P, Shah P (2021) Carbohydrate-based magnetic nanocomposites for effective cancer treatment. Int J Biol Macromol 175:281–293
Funding
Open access funding provided by Istituto Italiano di Tecnologia within the CRUI-CARE Agreement. E. Sharifi was supported by the Research and Technology Deputy of the Shahrekord University of Medical Sciences (Grant number 2086).
Author information
Authors and Affiliations
Contributions
E. Sharifi and I. Sharifi: conceptualization, methodology, and writing—original draft preparation, writing—review, and editing. F. Elahian, S. Parchami Barjui, E. N. Zare, R. Sartorius, P. De Berardinis, N. Fattahi, P. Parlanti, M. Gemmi, P. Makvandi, V. Mattoli: methodology, writing—review, and editing. S. Yousefiasl, N. Rabiee, Pahlevani-Gazi, A. C. Paiva-Santos, M. R. Mobini, M. Hashemzadeh-Chaleshtori: writing— review, and editing; S. Yousefiasl and P. Makvandi: figure preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sharifi, E., Reisi, F., Yousefiasl, S. et al. Chitosan decorated cobalt zinc ferrite nanoferrofluid composites for potential cancer hyperthermia therapy: anti-cancer activity, genotoxicity, and immunotoxicity evaluation. Adv Compos Hybrid Mater 6, 191 (2023). https://doi.org/10.1007/s42114-023-00768-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-023-00768-4