Abstract
Objective
Our objectives were to describe the basal insulin treatment regimens most widely used in a real-world setting in France and to estimate the associated treatment costs in people with type 2 diabetes mellitus (T2DM).
Methods
A cross-sectional observational study was conducted (November 2017–February 2018) among adult patients with T2DM requiring basal insulin therapy for their own use in a representative sample of pharmacies. Costs were compared between patients treated with three recently marketed insulins (glargine 300 U/ml [Gla-300], biosimilar glargine 100 U/ml [Gla-100] and a fixed-ratio combination of insulin degludec and liraglutide) and those treated with three established basal or intermediate insulins: branded glargine 100 U/ml, insulin detemir and neutral protamine Hagedorn insulin [NPH]).
Results
Overall, 1933 patients were analysed. Gla-300 accounted for 59.9% of novel basal insulin prescriptions, and branded Gla-100 accounted for 67.9% of established insulin prescriptions. Recent insulins were more frequently associated with glucagon-like peptide-1 (GLP-1) analogues. Results confirmed a lower rate of severe hypoglycaemia with Gla-300 than with Gla-100. On average, weekly total costs of treatment with all basal insulins were not significantly different, except with detemir, where they were higher.
Conclusion
New basal insulins are expected to be integrated into clinical practice. This analysis shows that their use does not impact upon the management cost of insulin therapy in people with T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The recent introduction of new basal insulin products has raised several issues regarding their use and associated costs. |
An observational survey comparing novel and established insulins indicates that the main change observed in insulin treatment regimens for type 2 diabetes mellitus in France was related to the wider use of glucagon-like peptide-1 receptor agonists and the introduction of glargine 300 U/ml. |
This has not led to a marked change in direct treatment cost of basal insulin therapies. |
1 Introduction
Insulin therapy is essential to the treatment of type 1 diabetes mellitus (T1DM) and is also important in type 2 diabetes mellitus (T2DM) to achieve glycaemic targets in people who do not achieve adequate glycaemic control with oral antidiabetic drugs (OADs). Over the last two decades, the place of insulin therapy in T2DM has increased as awareness has grown about the benefits of an early switch from OADs to insulin to improve long-term outcomes. A prescription survey of insulin use in patients with T2DM performed in France in 2015 reported that 24% were prescribed insulin therapy [1]. Current international [2] and French [3] practice guidelines recommend using injectable therapy (insulin or glucagon-like peptide-1 [GLP-1] receptor agonists) quite early in patients who do not reach glycaemic targets using dual oral therapy. Over the same period, the available options for insulin therapy have increased, with the introduction of new basal insulin analogues with a tailored duration of action. These include ultra-long-acting insulins and faster rapid-action insulins, as well as fixed-ratio combinations (FRCs) of basal insulin and GLP-1 analogues. A biosimilar version of insulin glargine 100 U/ml has also recently been marketed in France.
In 2010, a survey of pharmacies was performed in France to describe patterns of insulin use in patients with diabetes in France and to estimate associated treatment costs [4]. This study demonstrated significant heterogeneity in the type of insulin treatment regimens prescribed, dominated by the use of basal insulins alone in T2DM. Insulin acquisition contributed the most to costs in people with T1DM, whereas nurse visits for insulin administration were the most important cost components for those with T2DM.
The probable evolution of treatment practice since the time of the 2010 survey, with the introduction of a wider choice of insulin therapy options and the publication of updated practice guidelines, suggests that it would be timely to reassess how insulin therapy is prescribed and used in everyday clinical practice in France. For this reason, the present study was designed with the objectives of describing the insulin treatment regimens most widely used and of estimating associated treatment costs in patients with diabetes mellitus. In particular, treatment regimens for novel long-acting insulins and FRCs of basal insulin and GLP-1 analogues introduced since 2015 have been compared with those used for established basal/intermediate insulins available since at least 2005. Important differences exist between these T1DM and T2DM treatment patterns, so two separate analyses were performed. However, given the relatively small size of the T1DM population enrolled in the study, we focussed this article on the results observed in the T2DM population.
2 Methods
This was a cross-sectional observational study in adult patients with diabetes mellitus requiring insulin therapy. The study was performed by community pharmacists in France between November 2017 and February 2018. The study design was very similar to that of a previous pharmacy survey of insulin treatment regimens and costs performed in France in 2010 [4]. Costs were compared between patients treated with three recently introduced (since 2015) basal insulins: glargine 300 U/ml (Gla-300) [Toujeo®], biosimilar glargine 100 U/ml (Gla-100 biosimilar) [Abasaglar®] and FRC insulin degludec and liraglutide (IDegLira) [Xultophy®] and those treated with three established (available since 2005 or earlier) basal or intermediate insulins: glargine 100 U/ml (branded Gla-100) [Lantus®], insulin detemir (IDet) [Levemir®] and neutral protamine Hagedorn insulin (NPH). These six insulin therapies were selected as being the most widely used basal/intermediate insulins in France in 2017, according to the national prescription database of the French national health insurance (MEDICAM) [5]. It should be noted that insulin degludec alone was not available in France when this study was designed in early 2018. The cost analysis was performed from a ‘collective’ perspective limited to direct costs.
2.1 Investigators
Pharmacists were recruited from the Pharmaccess® panel of 6000 pharmacies representative of all pharmacies in France in terms of geographical localisation, size of community served and turnover. A sample of 600 pharmacies was selected randomly and contacted by telephone to invite them to participate in the study. Further random lists were subsequently generated until the target number of 600 participating pharmacists was achieved.
2.2 Patients
During the study period, participating pharmacists invited all adult patients coming to the pharmacy during the study period to fill an insulin prescription for their own use to participate in the study. Patients aged < 18 years and those using insulin pumps were excluded. Assuming a need for actionable information on insulin regimens representing about 5% of patients, it was appropriate to include about 3000 patients. The objective was to recruit 1500 patients prescribed a recent insulin and 1500 prescribed an established insulin to have a minimum sample size of 150 patients in the smaller subgroups of interest.
Recruitment was stopped in each group when this goal was achieved. The patients recruited were retrospectively assigned a diagnosis of T1DM or T2DM using the algorithm used previously in the general population ENTRED epidemiological studies of diabetes in France [6]. Results presented here are limited to T2DM.
2.3 Data Collection
During the pharmacy visit, each patient completed an anonymous questionnaire, with assistance from the pharmacist when necessary. The questionnaire collected data on sociodemographic features (age, sex, weight and height), diabetes treatment (insulin and OAD use, including posology, administration regimens and time since first prescription), time since diagnosis of diabetes, last known glycated haemoglobin (HbA1c) measurement, glucose monitoring, physician type (diabetologist or general practitioner), type of social security coverage and severe hypoglycaemic episodes (defined as requiring hospitalisation, emergency assistance or third-party assistance) on current treatment over the previous 6 months.
Questionnaires were sent to the study’s operational centre for data entry and analysis. The operational centre contacted each participating pharmacist episodically to ensure that questionnaires were returned regularly. All data were reviewed centrally, and potentially illogical or incorrect data were queried with the pharmacist.
2.4 Insulin Treatment Regimens
Five possible treatment regimens were defined for T2DM (Table 1 in the Electronic Supplementary Material [ESM]). Use of OADs was not considered in the allocation of the treatment regimen.
2.5 Cost Analysis
Direct costs of basal insulin treatment regimens were limited to diabetes medication costs, nurse-associated costs and self-monitoring of blood glucose. Weekly treatment costs were evaluated using official French national tariffs. Medication costs included costs of insulins, OADs and GLP-1 receptor agonists. Nurse visit costs for insulin administration or for blood glucose monitoring and the cost of kits for self-monitoring of blood glucose were estimated using official tariffs from the French National Sickness Fund.
2.6 Statistical Analysis
Treatment regimens were described as proportion of patients using them, together with their 95% confidence intervals (CIs). Treatment costs were described as mean ± standard deviation (SD). Costs were compared between treatment regimens in a two-by-two fashion using ordinary least square regression adjusting for other patient variables collected on the questionnaire (age, sex, body mass index [BMI], history of diabetes, history of the insulin therapy, use of GLP-1 analogues) [7]. Data were analysed using SAS software version 9.4 (Cary, NC, USA).
3 Results
3.1 Participants
Overall, 513 pharmacists participated in the study and enrolled at least one patient over the recruitment period. Participating pharmacies appeared to be representative of all French pharmacies in terms of geographical localisation, size of community served and turnover (data not shown).
A total of 3061 patients were enrolled, corresponding to 84.7% of all eligible patients (n = 3740) visiting participating pharmacies during the enrolment period. Of these, 2257 (74.4%) could be analysed. The remaining 804 were excluded principally because insufficient information was provided by the patient on the questionnaire to document costs adequately or to attribute the diagnosis of T1DM or T2DM with confidence (Fig. 1 in the ESM). A total of 166 patients were excluded because they were treated only with rapid-acting insulin.
In the analysed population, a diagnosis of T2DM was assigned to 1933 patients (85.6%) and a diagnosis of T1DM to the remainder. Among patients with T2DM, the sample was approximately evenly divided between patients prescribed a novel insulin treatment and those prescribed an established insulin treatment. Gla-300 accounted for the majority (62.2%) of prescriptions of novel insulins, and branded Gla-100 accounted for the majority (69.1%) of established insulin prescriptions.
The demographic and diabetes characteristics of these patients are presented in Table 1. In patients with T2DM, novel insulins were prescribed to patients who were, on average, younger with a more recent diagnosis of diabetes, and more frequently overweight or obese, than those prescribed an established insulin.
Less than 30% of patients with T2DM knew their last HbA1c measurement. This was below the threshold of 7% in 36.0% of patients taking novel insulins and in 45.2% (T2DM) of those taking established insulins.
The mean HbA1c level was significantly different between the two patient populations (p = 0.01) but clinically irrelevant: 7.5 ± SD 1.1% among patients treated with novel insulin versus 7.4 ± 1.1% among patients on ‘old’ insulin. Moreover, this statistical difference disappeared when the distribution by HbA1c level class was compared. The average HbA1c level varied only slightly according to the insulin: Gla-300 7.6 ± 1.1%, biosimilar Gla-100 7.4 ± 0.9%, IDegLira 7.6 ± 1.2%, branded Gla-100 7.2 ± 1.1%, IDet 7.7 ± 1.2%, NPH 7.5 ± 0.9%.
3.2 Insulin Treatment Regimens
The insulin treatment regimens used by patients participating in the study are illustrated in Table 2. Distribution according to basal insulin use differed from those observed in the excluded patients with T2DM (due to missing data) (p = 0.0015), with an over-representation of all established insulin in the analysed sample (49.1 vs. 40.4%).
Regardless of oral agent, most patients with T2DM used their basal insulin alone, with the exceptions of FRC of insulin degludec with liraglutide, insulin detemir and NPH insulin, which were more frequently used in association with rapid insulin.
The average daily dose/kg of insulin varied from 0.34 to 0.36 units/day/kg for Gla-100 and to 0.45 units/day for Gla-300 (Table 3) (descriptive unadjusted analysis). This higher insulin dose with Gla-300 was mainly observed in patients who had received previous insulin treatment before the current treatment. Mean insulin doses were similar for Gla-300 and branded Gla-100 (33.5 vs. 30.0 U/day; p = 0.11) among patients initiating their first insulin treatment with these insulins.
3.3 Diabetes Management
All except 19 patients were registered as having a chronic disease, providing eligibility for full reimbursement of healthcare by public health insurance. Around 73% of those with T2DM were followed by a diabetologist. Around 25% of those with T2DM received aid from a nurse with their insulin injections. Around 90% monitored their own glucose levels, on average twice a day for those with T2DM. The data are presented according to the type of insulin used in Table 4. No significant difference between patients using novel or established insulins was observed except for diabetologist follow-up, which was more frequent in patients using a novel insulin (p<0.0001) in T2DM.
3.4 Hypoglycaemia
Significant differences were observed between patients with T2DM treated with novel insulins and patients treated with established insulins for hypoglycaemia: 22.2% of patients treated with established insulins reported having had hypoglycaemia requiring third-party assistance versus 11.2% of patients receiving novel insulin (p < 0.0001) (data were missing in 0.8% and 1.1%, respectively). The percentage of patients with severe hypoglycaemia requiring third-party assistance with IDet (16.3%; 95% CI 10.6–23.5) was not statistically different from that for Gla-100 or NPH insulin: 22.5% (95% CI 19.3–25.9) and 26.1% (95% CI 19.5–33.6), respectively (p = 0.118). Among novel insulins, the differences were also not statistically significant, with the rate ranging from 9.3% (95% CI 5.6–14.3) for IDegLira to 10.2% (95% CI 6.3–15.3) for biosimilar Gla-100 and 12.2% (95% CI 9.7–15.1) for Gla-300.
For hypoglycaemia requiring emergency assistance, rates were 8.1 versus 3.2% (p < 0.0001), respectively (data were missing for 2% of both populations). For hypoglycaemia requiring hospitalization, these rates were, respectively, 9.1 versus 3.9% (p < 0.0001) (data were missing for 2% of both populations). Such crude differences may be a consequence of the insulin treatment duration.
The percentages of patients with hypoglycaemia requiring urgent rescue intervention on IDet and branded Gla-100 tended to be lower than for those receiving NPH insulin: 5.7% (95% CI 2.5–10.9) and 7.1% (95% CI 5.2–9.4), respectively, versus 13.9% (95% CI 8.9–20.3), although the CIs overlapped (overall test p = 0.0103). A similar situation was observed with the new insulins: the rate of patients with hypoglycaemia requiring emergency medical intervention on Gla-300 was slightly lower than for those on biosimilar Gla-100 or IDegLira: 2.1% (95% CI 1.1–3.6) versus 5.6% (95% CI 2.8–9.8) and 4.2% (95% CI 1.8–8.1), respectively (CIs overlap, but the overall test was statistically significant, p = 0.0384).
The percentage of patients with hypoglycaemia requiring hospitalisation differed between those on NPH insulin and those on branded Gla-100 or IDet: 16.3% (95% CI 10.9–22.9) versus 7.6% (95% CI 5.6–9.9) and 7.9% (95% CI 4.0–13.6), respectively (overall test p = 0.0026). No significant difference was found between the novel insulins (p = 0.4458). The percentage of patients reporting hospitalisation for hypoglycaemia on IDegLira was 2.6% (95% CI 0.9–6.0) versus 4.0% (95% CI 2.5–5.9) on Gla-300 and 5.2% (95% CI 2.5–9.3) on biosimilar Gla-100.
Adjusted comparisons confirmed that the occurrence of hypoglycaemia requiring hospitalization in patients with T2DM was less frequent in patients treated with Gla-300 than in those treated with all other basal insulins (odds ratio 2.151; 95% CI 1.10–4171; p = 0.0233).
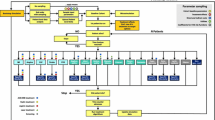
The multivariate analysis describing potential factors associated with severe hypoglycaemia requiring hospitalization is summarized in Fig. 1.
Factors associated with severe hypoglycaemia requiring hospitalization (multivariate analysis). *Basal with GLP-1 or enhanced insulin therapy with GLP-1 may use a fixed-ratio combination (insulin degludec and liraglutide [IDegLira]) or a free combination. BMI body mass index, CI confidence interval, Gla glargine, GLP-1 glucagon-like peptide-1, OR odds ratio, WO without
3.5 Treatment Costs
The mean weekly per capita cost of insulin therapy was €60.7 for patients treated with a novel insulin and €64.6 for those treated with an established insulin for T2DM (p = 0.13) (Table 5).
In patients with T2DM, the average total weekly cost did not differ between novel insulins and established insulins (Table 5). However, the acquisition costs for insulins (p < 0.0001) and for associated OAD and GLP-1 analogue treatments (p = 0.0022) were significantly lower in patients using established insulins, whereas costs for nurse visits for injections and glucose monitoring were significantly higher. Some differences appeared by product. Although the number of insulin injections was similar across the treatment groups, nursing costs were significantly higher in the biosimilar Gla-100 group: the weekly average cost of nurse referral for insulin injections was €27.1 ± 42.0 versus €17.2 ± 37.2 with Gla-300 (p = 0.003) or €18.9 ± 38.0 with branded Gla-100 (p=0.014). Cost of drug was more important in the IDegLira group: €32.3 ± 19.0 versus €11.6 ± 8.6 with Gla-300 (p < 0.0001) or €10.7 ± 7.6 with branded Gla-100 (p < 0.0001). The average total weekly cost in the IDet group was €80.5 ± 90.5 compared with €73.4 ± 89.2 in the biosimilar Gla-100 group (p = 0.47), €68.1 ± 79.7 in the NPH insulin group (p = 0.2), €60.2 ± 75.5 in the branded Gla-100 group (p = 0.01) and €57.4 ± 69.8 in the Gla-300 group (p = 0.005). When cost data were adjusted according to patients’ characteristics (insulin scheme, treatment duration, insulin duration, BMI, age, sex), average weekly costs were similar whatever the basal insulin with the exception of patients treated with IDet, where the costs were higher (+€23.5 per week vs. Gla-300; p = 0.005).
4 Discussion
This study evaluated the treatment regimens used by patients with T2DM prescribed insulin therapy and their associated costs. Approximately half of the insulin-treated patients evaluated were taking one of the three novel basal insulins (a second-generation basal insulin Gla-300 [Toujeo®], Gla-100 biosimilar [Abasaglar®] or FRC of IDegLira [Xultophy®], marketed since 2015, whereas the others were taking one of the established insulins (Gla-100 [Lantus®], insulin detemir [Levemir®] or NPH). In both groups, insulin glargine formulations accounted for the majority of prescriptions. An FRC of basal insulin with a GLP-1 receptor agonist was prescribed to around 20% of subjects with T2DM. The characteristics of patients and their management varied according to whether these patients benefited from novel or established insulin, according to the insulin used and according to the insulin strategy. In addition, patients receiving novel insulins were more frequently followed by a diabetologist.
Treatment regimens and costs of established insulins can be compared to those reported in the previous pharmacy survey of 2010 [4]. In 2010, basal insulin analogues had largely replaced NPH insulins in the treatment of diabetes, and this trend has continued, with 8.4% of patients with T2DM receiving NPH insulins in 2018. Treatment regimens with established insulins are similar to those observed in 2010, although the use of basal insulins as the only insulin therapy has declined from 42 to 38%, and associations with GLP-1 receptor agonists (either in free or fixed combinations) now account for nearly one-third of prescriptions. However, patients excluded due to missing data were less frequently treated by established insulin than the analysed sample.
Worryingly, with respect to glycaemic control, only a minority of patients were aware of their last HbA1c measurement, regardless of treatment group. Among the patients who were able to provide their last HbA1c level, HbA1c was < 7% in around one-third of patients taking novel insulins and in nearly one-half of those taking established insulins. Given that information was missing for the majority of patients and that the profiles of patients prescribed novel and established insulins differed, no conclusions can be drawn on the quality of glycaemic control in these patients.
The reported incidence over time of severe hypoglycaemic events, and notably the incidence of events requiring hospitalisation, was over twice as high in patients prescribed an established insulin as in those using a novel insulin. Although more contrasted than during phase III clinical trials, a lower incidence of hypoglycaemia in patients treated with Gla-300 [8] than in those receiving Gla-100 was also observed in this real-life setting after adjusting for patients’ characteristics.
In our study, insulin daily doses in T2DM were higher for Gla-300 than for Gla-100, considering the overall population of patients naïve of insulin or already treated with another basal insulin. These results were confirmed after multivariate adjustments (data not shown). A need for a slightly higher daily dose to achieve adequate glycaemic control with Gla-300 compared with branded Gla-100 has been documented in randomised clinical trials [6], and this could explain the difference in doses observed in the present study. The Gla-300 summary of product characteristics indicates that a higher dose (approximately 10–18%) may be needed to achieve target ranges for plasma glucose levels when switching from Gla-100 to Gla-300. Ritzel et al. [9] also described the dose in a T2DM pooled analysis of the EDITION 1, 2 and 3 studies. The mean (± SD) basal insulin dose at month 6 was 0.85 ± 0.36 U/kg/day with Gla-300 and 0.76 ± 0.32 U/kg/day with Gla-100, representing a 12% higher dose with Gla-300 insulin. A number of analyses of the French medico-administrative database have been conducted on costs of diabetes treated with insulin [10,11,12]. In 2013, the cost of total healthcare consumption by patients with T2DM treated with insulin was €12,509, which was €8630 higher than for matched control subjects without diabetes [9]. Another study reported a total spend on healthcare by patients starting insulin therapy in 2011–2012 of €11,600, an increase of €5025 compared with the pre-insulin period [8].
The cost estimation provided in this study is only focused on insulin treatment overall costs considering costs of pharmacological treatment, nurses involved and monitoring of blood glucose. Although some differences were observed in acquisition costs of insulin (with significantly higher costs observed with IDegLira and IDet but also with Gla-300 because of higher doses), overall costs did not differ significantly between novel insulins and established insulins in T2DM. This is partly the consequence of French pricing policies that limit the price of new insulins based on the price of established insulins. In the real-life setting, in the French context, average weekly costs were similar, except for IDet, in T2DM after adjusting for patients’ characteristics (insulin scheme, treatment duration, insulin duration, BMI, age, sex). Differences in acquisition costs were outweighed by differences in costs associated with nurse visits, which were higher for established insulins and for the biosimilar of insulin glargine 100 U/ml. This situation could be the consequence of differences in patient’s profiles but also in the prescribers’ profiles as the use of home nurses is the sole responsibility of the prescriber. The use of fully reimbursed private nurses to give injections or monitor blood sugar levels for old or deprived people in their homes is not a current practice all over the world but a rather frequent practice in France.
Curiously, considering that the branded Gla-100 and its biosimilar are essentially the same molecule, the average costs seemed to differ, but this difference was not statistically significant (before and after statistical adjustment, data not shown).
One finding of a 2010 survey [4] was the relatively large proportion of patients with T2DM who required a nurse visit for their insulin injection or for glucose monitoring (28%), which was a major cost driver. This proportion has not decreased significantly over the intervening period. Whilst insulin acquisition costs and the cost of glucose self-monitoring have remained relatively stable, the costs of nurse visits have doubled. Together with acquisition of GLP-1 receptor agonists, not considered in 2010, this accounts for the increased total cost in 2018 compared with 2010.
The study has several limitations. First, the extent to which the study sample is representative of all patients with diabetes taking insulin in France is unknown. Participating pharmacies were representative of all pharmacies in France in terms of geographic distribution, turnover and size of community served. No other indicators were available for comparison. The patients enrolled correspond to 85% of all eligible patients, which is a high proportion and should help minimise inclusion bias. Nonetheless, a significant portion of the enrolled patients could not be assessed because of incomplete data on costs. In addition, the information was provided by the patients, with help from the pharmacist if required, and therefore declarative. Third, no information is available on other medical costs of diabetes, notably hospitalisations for hypoglycaemia (the study did not plan to link the data collection with hospital data) and management of complications, which may vary between different insulin treatment regimens. Since severe hypoglycaemia rates are lower with novel insulins than with established insulins, hospital costs may well be lower for patients using novel insulins. Some clinical trial results and US Home Medicines Review databases on patients with T2DM in real-life settings [13, 14] have shown that fewer patients treated with Gla-300 experienced a severe hypoglycaemia episode than patients treated with older insulin therapies. We did not consider the use of insulin secretagogue as a potential confounding factor with regards to hypoglycaemia in our analysis because of the low frequency of the association between such OAD classes and insulin therapy in France. Finally, we also note that the wholesale price of most insulin products has reduced since the time of the study.
In conclusion, this study of insulin delivery in pharmacies indicates that the main change observed in insulin T2DM treatment regimens in France is related to the wide use of GLP-1 receptor agonists and the introduction of new basal insulins such as Gla-300. New insulins are increasingly being integrated into professional practice. From an economic viewpoint, the introduction of these novel insulins has not led to a marked change in direct treatment costs, whereas novel insulins could help physicians and patients overcome, at least partially, the hypoglycaemic risk associated with insulin therapy.
References
Roussel R, Fontaine P, Gouet D, Serusclat P, Martinez L, Detournay B, et al. Le traitement du diabète de type 2 en France est dynamique plutôt qu’inerte: analyse des prescriptions de 847 122 patients. Méd Malad Métabol. 2018;12:346–52.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669–701.
Darmon P, Bauduceau B, Bringer J, Chabrier G, Charbonnel B, Detournay B, et al. Prise de position de la Société Francophone du Diabète (SFD) sur la prise en charge médicamenteuse de l’hyperglycémie du patient diabétique de type 2. Méd Malad Métabol. 2017;11:577–93.
Charbonnel B, Penfornis A, Varroud-Vial M, Kusnik-Joinville O, Detournay B. Insulin therapy for diabetes mellitus: Treatment regimens and associated costs. Diabetes Metab. 2012;38:156–63.
French National Sickness Fund. MEDIC’AM database. https://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/donnees-statistiques/medicament/medicaments-pharmacies-de-ville-par-prescripteur/medic-am-2018.php. Accessed 4 Dec 2019.
Romon I, Fosse S, Weill A, Varroud-Vial M, Fagot-Campagna A. Prévalence des maladies cardiovasculaires et niveau de risque vaculaire des diabétiques en France, étude Entred 2001. Bull Epidemiol Hebd. 2005;12–13:46–8.
Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320(7243):1197–200.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Jarvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:541–8.
Ritzel R, Roussel R, Bolli GB, Vinet L, Brulle-Wohlhueter C, Glezer S, Yki-Järvinen H. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859–67.
Hanaire H, Attali C, Lecointre B, Fraysse M, Gouet D, Babel MR, et al. Determinants of the cost of initiation of insulin therapy type 2 diabetic patients in France: possible approaches to optimization. Sante Publique. 2016;28:781–9.
Charbonnel B, Simon D, Dallongeville J, Bureau I, Dejager S, Levy-Bachelot L, et al. Direct medical costs of type 2 diabetes in France: an insurance claims database analysis. Pharmacoecon Open. 2018;2(2):209–19.
Detournay B, Bureau I, Gourmelen J. Le coût de l’insulinothérapie chez les patients diabétiques de type 2, en France. Méd Malad Métabol. 2015;9:S30–3.
Zhou FL, Nicholls C, Xie L, Wang Y, Vaidya N, Meneghini LF. Hypoglycaemia and treatment patterns among insulin-treated patients with type 2 diabetes whoswitched to insulin glargine 300 units/mL versus other basal insulin in a real-world setting. Endocrinol Diabetes Metab. 2019;2(3):e00073.
Pettus J, Roussel R, Liz Zhou F, Bosnyak Z, Westerbacka J, Berria R, Jimenez J, Eliasson B, Hramiak I, Bailey T, Meneghini L. Rates of hypoglycemia predicted in patients with type 2 diabetes on insulin glargine 300 U/ml versus first- and second-generation basal insulin analogs: the real-world LIGHTNING Study. Diabetes Ther. 2019;10(2):617–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Sanofi France, manufacturers of glargine 300 U/ml and branded glargine 100 U/ml.
Conflicts of interest
VJ, JR and BD are employed by CEMKA, a research and consulting firm providing studies and advisory services for private and public organizations. BD has served as an advisor, consultant or member of a speaker bureau for Merck Sharpe & Dohme Corp., Novo Nordisk, Pfizer Inc. and Sanofi-Aventis. ZB and AB are employees of Sanofi France.
Ethics approval
The study was performed in accordance with relevant international and national legislation for medical research and with good epidemiological practice. The survey protocol was submitted for evaluation to the Comité de Protection des Personnes Nord-Ouest (Ethics Advisory Board) and have respected the obligations defined by the French Law ‘Informatique et Liberté’, which ensures that all medical information is kept confidential and anonymous. All patient data were anonymised before transmission by the pharmacist to the operational centre for entry into the study database, in which each patient was identified by a number.
Availability of data and material
European regulations mean that the original database is no longer available. However, full calculations are available from the authors upon request.
Consent
Informed consent was obtained from all individual participants.
Consent to participate
Patients’ consent to participate was obtain by the participating pharmacist (investigators). Data were collected and analyzed on a on a strictly anonymous basis.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
BD conceived and designed the analysis, interpreted the results and wrote the paper. ZB and AB revised the study protocol and contributed to the paper. VJ collected the data and conducted the data management. JR performed all statistical and economic analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Detournay, B., Boultif, Z., Bahloul, A. et al. Treatment Costs of Basal Insulin Regimens for Type 2 Diabetes Mellitus in France. PharmacoEconomics Open 5, 211–219 (2021). https://doi.org/10.1007/s41669-020-00237-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41669-020-00237-4