Abstract
Introduction
Second-generation basal insulins like glargine 300 U/mL (Gla-300) have a longer duration of action and less daily fluctuation and interday variability than first-generation ones, such as glargine 100 U/mL (Gla-100). The EF-BI study, a nationwide observational, retrospective study, was designed to compare persistence, acute care complications, and healthcare costs associated with the initiation of such basal insulins (BI) in a real-life setting in France.
Methods
This study was conducted using the French healthcare claims database (SNDS). Adult patients living with type 1 or type 2 diabetes mellitus (T1DM or T2DM) initiating Gla-300 or Gla-100 ± other hypoglycemic medications between January 1, 2016 and December 31, 2020, and without any insulin therapy over the previous 6 months were included. Persistence was defined as remaining on the same insulin therapy until discontinuation defined by a 6 month period without insulin reimbursement. Hospitalized acute complications were identified using ICD-10 codes. Total collective costs were established for patients treated continuously with each basal insulin over 1–3 years. All comparisons were adjusted using a propensity score based on initial patient/treatment characteristics.
Results
A total of 235,894 patients with T2DM and 6672 patients with T1DM were included. Patients treated with Gla-300 were 83% (T1DM) and 44% (T2DM) less likely to discontinue their treatment than those treated with Gla-100 after 24 months (p < 0.0001). The annual incidence of acute hospitalized events in patients with T2DM treated with Gla-300 was 12% lower than with Gla-100 (p < 0.0001) but similar in patients with T1DM. Comparison of overall costs showed moderate but statistically significant differences in favor of Gla-300 versus Gla-100 for all patients over the first year, and in T2DM only over a 3-year follow-up.
Conclusion
Use of Gla-300 resulted in a better persistence, less acute hospitalized events at least in T2DM, and reduced healthcare expenditure. These real-life results confirmed the potential interest of using Gla-300 rather than Gla-100.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
The EF-BI study was designed to compare persistence, insulin-related acute events, and healthcare costs associated with the use of a second-generation basal insulin, glargine 300 U/mL (Gla-300), as compared to glargine 100 U/mL (Gla-100) in a real-life setting in France. |
Second-generation basal insulins are well known to have a longer duration of action and less daily fluctuation and interday variability than first-generation ones, but less data exists about their consequences in real life. |
What was learned from the study? |
A better persistence, reduced frequency of insulin-related hospitalized acute events, and lower overall costs in a collective perspective were observed with Gla-300 compared to Gla-100 in the years following initiation especially in patients with T2DM. |
These findings confirm the interest of prescribing second-generation long-acting insulins as compared to first-generation ones. |
Introduction
Insulin therapy is a necessary step in achieving optimal glycemic targets in type 1 diabetes (T1DM), but also in most advanced patients with type 2 diabetes (T2DM) when oral antidiabetic agents and non-insulin injectable treatments have failed to achieve glycemic targets. There are many different insulin regimens available to meet the varying needs of different people with diabetes. Insulin regimens range from once-daily insulin (basal-only regimen) for T2DM to multi-injection daily regimens and insulin pump therapy for people with T1DM or T2DM. In most of these insulin regimens, a long-acting insulin is required to cover glucose secretion by the liver throughout the whole day.
The need for basal insulin can be met by a variety of insulins. Second-generation basal insulins (2BI), insulin glargine 300 U/mL (Gla-300) or insulin degludec (IDeg), have demonstrated a longer duration of action and more suitable pharmacokinetic/pharmacodynamic profiles than first-generation insulins, such as insulin glargine 100 U/mL (Gla-100) or insulin detemir (IDet), with low intraday fluctuation and interday variability [1,2,3].
If Gla-300 and IDeg seem to have similar glycemic control improvements with relatively low hypoglycemia risk [4, 5], further trials of direct comparisons of 2BI with first-generation basal insulins (1BI) [6] are probably needed. The use of real-life databases may answer some of the questions that arise in this field concerning, for example, the maintenance over time of treatment, or the comparative frequency of the main acute events linked to diabetes treatment (i.e. hypoglycemia, ketoacidosis, acute hyperglycemia) with 2BI versus 1BI in both T1DM and T2DM. In addition, costs associated with insulin therapy are also an important criterion to be considered in such comparisons.
Regarding maintenance, several studies in the USA [7, 8] or in Germany [9] reported a low maintenance rate with Gla-100 within 1 year of initiation. In France a first study [10] showed that only 72% of patients with T2DM were still being treated with BI after 12 months (75% on basal regimen). Regardless of insulin regimen, patients less frequently discontinue Gla-300 insulin than Gla-100 insulin [adjusted odds ratio (OR) 0.39, 95% confidence interval (CI) 0.37–0.41]. Similar results were observed when a basal regimen was considered (adjusted OR 0.38, 95% CI 0.35–0.40).
In this study, patients treated with Gla-100 had higher crude rates of hospitalization for hypoglycemia as compared to those receiving Gla-300 (1.4 per 100 patient-years; OR 0.67, 95% CI 0.55–0.81). However, this difference did not remain statistically significant following adjustment for patient characteristics. A lower frequency of emergency room visits was observed in patients treated with Gla-300.
Several cost-effectiveness modeling studies have compared second-generation to other long-acting insulins. A cost–utility evaluation of Gla-300 versus Gla-100 in patients with T2DM using the Spanish National Health System perspective estimated the Gla-300 incremental cost-effectiveness ratio (ICER) at €5294 per QALY, which is far below on the willingness-to-pay threshold for Spain. Cost-effectiveness results were mainly driven by the lower hypoglycemia rates and insulin-dose flexibility [11]. Similar results were obtained with Ideg in a Swedish prospective observational study of 476 patients switching to Ideg from other Bis and finding life expectancy improvements and lower lifetime healthcare direct costs resulting in the dominance of Ideg in the ICER [12]. A UK study found that Ideg was cost-effective in patients with T1DM and T2DM when using a basal-only regimen as in patients with T2DM treated with a basal-bolus regimen. The lower costs observed in patients with T1DM were the consequences of the lower dose of insulin degludec required. In patients with T2DM lower costs were driven by fewer hypoglycemic events. A French study [13] showed that the use of a newer insulin did not necessarily have an impact on the cost of managing insulin therapy in people with T2DM, but this cross-sectional observational study on a limited sample of patients was conducted only in patients with T2DM.
Evaluating and comparing the maintenance over time of treatment with currently available basal insulins in T1DM and T2DM, as well as the frequency of hospitalizations for all acute events and the costs associated with these insulins in a real-life context, is therefore interesting at a time when second-generation long-acting insulin analogues are increasingly prescribed in France. Because Ideg was marketed later than Gla-300 and data was therefore limited, we restricted the comparison to patients with T1DM or T2DM starting a treatment with Gla-300 versus those starting treatment with Gla-100.
Methods
An observational, retrospective, and comparative study based on a cohort of patients with diabetes (T1DM or T2DM) initiating a treatment with basal insulin was conducted using data from the French national health insurance information system (Système national des données de santé or SNDS) [14]. The SNDS lists all the reimbursed inhosptial and out-of-hospital healthcare resources used by 99% of the French population covered by the national health insurance system. The SNDS also contains anonymous demographic data and data about chronic medical conditions (International Classification of Diseases [ICD], 10th version codes), hospitalizations with ICD-10 codes for primary and associated diagnoses, costs, and dates of death but not any laboratory tests results.
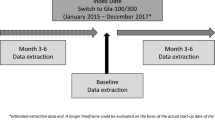
All adult patients (18 years old and over) with diabetes and initiating either a treatment with Gla-300 or Gla-100 (both original and biosimilar) ± oral hypoglycemic agent (OHA) ± other injectable diabetes treatment between 1 January 2016 and 31 December 2020 were selected. The index date (T0) was defined as the starting date of basal insulin. Initiation was defined by a first delivery of insulin, preceded by a period without any insulin therapy reimbursement over the previous 12 months. All patients were categorized as having T1DM or T2DM, using an algorithm described elsewhere [10]. Pregnant women or patients treated with insulin for less than 6 months were excluded.
Persistence was defined as remaining on the same insulin without discontinuation defined by a 6 month period without insulin reimbursement for 6 months and was estimated using a Cox proportional hazards model over a 24-month period after BI initiation. Hospitalized acute complications were identified using ICD-10 codes. Hospital admissions for ketoacidosis were identified using ICD-10 codes E10.1 (type 1 diabetes mellitus with ketoacidosis) and E11.1 (type 2 diabetes mellitus with ketoacidosis) as principal or related diagnosis. Severe hypoglycemia was identified using ICD-10 codes E16.0 (drug-induced hypoglycemia without coma), E16.1 (other hypoglycemia), E16.2 (unspecified hypoglycemia), and T38.3 (poisoning due to adverse effects of insulin and oral hypoglycemic agents [antidiabetics]). Hospital admissions for comas related to diabetes were also collected considering E10.0 (T1DM with coma), E11.0 (T2DM with coma), and E14.0 (unspecified diabetes with coma) as stays related to hyperglycemia with ICD-10 code R739 (hyperglycemia, unspecified). More than one acute episode could be recorded for the same patient.
This was completed by an analysis on the frequency of emergency room visits, whatever the reason for these visits, as a potential indicator of an acute event experienced by patients treated with insulin.
Total collective costs were established for patients treated continuously with each basal insulin over 1–3 years. Only patients treated during full years and for whom comprehensive healthcare resource use data was available for the whole period were analyzed. Comparisons of total costs were conducted in patients continuously treated with BI for 1, 2, or 3 years, from the perspective of the National Sickness Fund.
Persistence, annual frequency of acute events, and costs comparison were adjusted using a propensity score covariate (by quintile) based on age, gender, geographical area, social deprivation index, deprived people reimbursement status, a simplified Charlson index, diabetes history, previous hospitalized acute event over 3 years prior to T0, diabetes treatment regimen 3 months before and 3 months after T0, and use of continuous glucose monitoring in the year preceding T0.
This study was registered on the Health Data Hub website (T58549122021092). In accordance with current regulations, the study protocol was submitted to the committee for research, studies, and evaluations in the field of health (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé [CEREES]) for approval and was authorized by the French data protection authority (Commission Nationale de l’Informatique et des Libertés [CNIL], [CNIL authorization number MR: 921407).
Results
Over the 5-year inclusion period 6672 patients with T1DM and 235,894 patients with T2DM initiated either Gla-300 or Gla-100 (Table 1). Characteristics of the selected patients are presented in Tables 2 and 3. Those characteristics were grouped according to the treatment administered (Gla-100 or Gla-300) even though they were often statistically significantly different in T1DM and T2DM when initiating a treatment with BI.
Over a 24-month period post-BI initiation, 40.7% of patients with T1DM and 21.7% of patients with T2DM treated with Gla-100 (versus respectively 8.1% and 10.9% with Gla-300) stopped the corresponding BI. Patients with T1DM and with T2DM treated with Gla-300 were respectively 83% (HR 0.169 [0.144–0.198], p < 0.0001) and 44% (HR 0.563 [0.550–0.577], p < 0.0001) less likely to stop this treatment than patients treated with Gla-100 (Fig. 1). After stopping the BI, in T1DM 75.8% of patients treated with Gla-100 and 56.7% of patients treated with Gla-300 were switched to pump therapy. In T2DM, most patients who stopped their BI treatment did not receive any insulin therapy over the next 6 months (respectively 80.1% and 74.5% in Gla-100 and Gla-300 treated patients versus 74.5%).
Over the studied period of exposure to BI, in patients with T1DM, hospitalized hypoglycemic event rates were lower with Gla-300 (HR 0.73 (0.55–0.97), p = 0.028). However, no significant difference was observed between patients treated with Gla-100 and patients treated with Gla-300 in the frequency of overall hospitalized acute events possibly related to insulin therapy (HR 1.16 (0.88–1.54), p = 0.3) nor in the frequency of any cause emergency room visits (HR 0.99 (0.77–1.27), p = 0.94). In patients with T2DM, results were significantly in favor of Gla-300, with a lower frequency of overall hospitalized acute events (HR 0.88 (0.84–0.93), p < 0.001), slightly less frequent emergency room visits (HR 0.92 (0.88–0.96), p < 0.001), but a similar rate of hospitalized hypoglycemia events (HR 0.98 (0.94–1.03), p = 0.5).
Comparisons of adjusted costs (hospital costs and outpatient costs) over the 3 years of follow-up after BI initiation show moderate but statistically significant results in favor of Gla-300 insulin compared to Gla-100 insulin, during the first-year post-BI initiation for patients with T1DM (Fig. 2) and during the whole 3 years of follow-up for patients with T2DM (Fig. 3). An extrapolation of these results for all French patients with diabetes initiating Gla-100 over a 3-year period switching to Gla-300 would have resulted in a reduction of health expenditure by €473 million for the National Sickness Fund alone.
Discussion
Previous economic cost-effectiveness modeling studies comparing Gla-300 to Gla-100 [15,16,17] that were based on available clinical trials data have suggested that Gla-300 is likely to be an efficient and dominant strategy compared to Gla-100 in T2DM. Such results have also been translated in budgetary terms to the USA, showing that savings were achieved when switching patients from long-acting BIs to Gla-300 [18]. However, the highly selected participants and frequent follow-up of the clinical trials could not be truly representative of real-life clinical practice.
The results of the EF-BI observational national study confirm that in real life, the use of Gla-300 was more effective and less costly versus Gla-100 in patients with T1DM and patients with T2DM treated with these basal insulins using a collective perspective in France. Regarding effectiveness, our results are in line with a previous study reporting a better maintenance rate with Gla-300 as compared to Gla-100 within 1 year of initiation in T2DM [10] but expand these positive results to 24 months and to both T1DM and T2DM. They simultaneously confirm a in real-life setting the results obtained throughout the EDITION clinical trial program, demonstrating both a similar glycemic control with Glar-300 and Glar-100, but a lower risk of hypoglycemia when using Gla-300 in both T1DM and T2DM [19]. The improved pharmacokinetic and pharmacodynamic characteristics of 2BI could explain this prolonged duration of action as compared with 1BI [20].
Considering costs, the DELIVER program based on a US healthcare database compared outcomes in people with T2DM receiving either Gla-300 or other BI in a real-world setting. In the DELIVER 2 study [21], people with T2DM who switched to Gla-300 had a significantly lower incidence of healthcare resource utilization related to hypoglycemia than those who were switched to another BI analogue, considering hospitalizations (2.8% vs. 4.3%; p = 0.037), emergency department visits (3.1% vs. 5.1%; p = 0.007), and outpatient visits (12.6% vs. 15.4%; p = 0.011) [17]. Finally, people treated with Gla-300 had an overall saving of US$1439 per person per year in healthcare costs compared with those receiving another BI.
In our study, propensity score-adjusted analysis shows that substituting the use of Gla-100 with Gla-300 over a 3-year period would result in a saving of €1875 in T1DM and €2649 in T2DM from a collective perspective (considering all direct costs regardless of whether they were financed by the national sickness fund or complementary insurances). These savings are the result both of a reduction in hospital costs, partly linked to a reduction in the number of acute events associated with insulin therapy, and of a reduction in outpatient costs, no doubt as a result of a reduction in medical monitoring. Switching all patients treated with Gla-100 to Gla-300 in France at the time of the study would result in a saving in health expenditure of €473 million.
Our study had several limitations. First, because it was conducted using a claims database (French SNDS), which provides only limited information, it considered only some diabetes-related events but could not consider other important factors (e.g., HbA1c levels or diabetes equilibrium, other diabetes complications, etc.) either as an outcome or as a parameter in the propensity score. However, our results were adjusted on all available parameters in the database. Secondly, as a result of the need for sufficient observation time in the analyses, and to the relatively recent marketing of other basal insulins, the number of patients available did not allow us to make other comparisons than between BI Gla-300 and Gla-100. Several articles have been published showing acceptability of the incremental cost-effectiveness ratio or dominance of insulin degludec 100 U/mL (IDeg-100) as compared to Gla-100 or insulin detemir [22,23,24,25,26,27]. Evidence was also provided in Sweden through a prospective observational study using real-world data [12].
Recently, several cost-effectiveness studies have compared the two main second-generation insulin analogues Gla-300 and IDeg-100. Results appeared to be contradictory with some studies concluding that greater use of Gla-300 vs. IDeg-100 for the treatment of patients with T2DM would lead to a relevant reduction of therapy costs in the USA [28], Slovenia [16], and Italy [29] but others suggesting the opposite in the USA [30], the Netherlands [31], Algeria [32], and Japan [33]. Further investigations should be conducted in real life to clarify this point.
Conclusion
The national EF-BI study confirmed that the use of Gla-300 resulted in a better persistence and fewer acute hospitalized events at least in T2DM compared to Gla-100 in real life in France. It also resulted in reduced overall healthcare expenditure during the whole 3 years of follow-up for patients with T2DM and over the first year post BI initiation for patients with T1DM. These data confirm the potential interest of using second-generation insulins rather than first-generation ones.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to regulatory reasons as they were based on the SNDS French national administrative healthcare claims database.
References
Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 units·mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 units·mL−1. Diabetes Care. 2015;38:637–43.
Becker RH, Nowotny I, Teichert L, et al. Low within- and between-day variability in exposure to new insulin glargine 300 U/ml. Diabetes Obes Metab. 2015;17:261–7.
Heise T, Nosek L, Bottcher SG, et al. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–50.
Rosenstock J, Cheng A, Ritzel R, et al. More similarities than differences testing insulin glargine 300 units/ml versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41(10):2147–54. https://doi.org/10.2337/dc18-0559.
Buzzetti R, Fadini GP, Nicolucci A, et al. Comparative effectiveness of glargine 300 U/mL vs. degludec 100 U/mL in patients with type 2 diabetes switching from 1° generation basal insulins [published correction appears in Nutr Metab Cardiovasc Dis. 2023 Apr;33(4):920-921]. Nutr Metab Cardiovasc Dis. 2022;32(9):2255–63.
Battelino T, Edelman SV, Nishimura R, Bergenstal RM. Comparison of second-generation basal insulin analogs: a review of the evidence from continuous glucose monitoring. Diabetes Technol Ther. 2021;23(1):20–30. https://doi.org/10.1089/dia.2020.0180.
Wei W, Buysman E, Grabner M, et al. A real-world study of treatment patterns and outcomes in US managed-care patients with type 2 diabetes initiating injectable therapies. Diabetes Obes Metab. 2017;19(3):375–86. https://doi.org/10.1111/dom.12828.
Baser O, Tangirala K, Wei W, Xie L. Real-world outcomes of initiating insulin glargine-based treatment versus premixed analog insulins among US patients with type 2 diabetes failing oral antidiabetic drugs. Clinicoecon Outcomes Res. 2013;3(5):497–505. https://doi.org/10.2147/CEOR.S49279.
Pscherer S, Chou E, Dippel FW, Rathmann W, Kostev K. Treatment persistence after initiating basal insulin in type 2 diabetes patients: a primary care database analysis. Prim Care Diabetes. 2015;9(5):377–84. https://doi.org/10.1016/j.pcd.2015.01.011.
Roussel R, Detournay B, Boultif Z, Bahloul A, Teissier C, Charbonnel B. Persistence with basal insulin and frequency of hypoglycemia requiring hospitalization in patients with type 2 diabetes. Diabetes Ther. 2020;11(8):1861–72. https://doi.org/10.1007/s13300-020-00874-2.
Delgado M, Rubio M, Gasche D, Fournier M, Monereo S. Cost-utility evaluation of insulin glargine 300 (GLA-300) versus insulin glargine 100 (GLA-100) in patients with type 2 diabetes mellitus (T2DM). Value Health. 2016;19(7):A675. https://doi.org/10.1016/j.jval.2016.09.1887.
Landstedt-Hallin L, Gundgaard J, Ericsson A, Ellfors-Zetterlund S. Cost-effectiveness of switching to insulin degludec from other basal insulins: evidence from Swedish real-world data. Curr Med Res Opin. 2017;33(4):647–55. https://doi.org/10.1080/03007995.2016.1277194.
Detournay B, Boultif Z, Bahloul A, Jeanbat V, Robert J. Treatment costs of basal insulin regimens for type 2 diabetes mellitus in France. Pharmacoecon Open. 2021;5(2):211–9. https://doi.org/10.1007/s41669-020-00237-4.
Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Pub. 2017;65(Suppl. 4):S149–67.
Delgado M, Rubio M, Gasche D, Fournier M, Monereo S. Cost-utility evaluation of insulin glargine 300 (gla-300) versus insulin glargine 100 (gla-100) in Patients with type 2 diabetes mellitus (T2DM). Value Health. 2016;19(7):A675.
Bogdanovic M, Rados Krnel S, Fournier M. Cost-utility evaluation of insulin glargine (300 U/ML) versus insulin glargine (100 U/ML) and insulin degludec (100 U/ML) in patients with type 2 diabetes mellitus in Slovenia. Value Health. 2017;20(9):A481.
Thongsri W, Rattanawongsawad S, Fournier M. One-year cost-utility evaluation of insulin glargine 300 μ/ml (Gla-300) versus insulin glargine 100 μ/ml (Gla-100) in patients with type 2 diabetes (T2dm) in Thailand. Value Health. 2018;21(S2):S39.
Ponomareva E, Schmerold L, Srinivas SS, et al. The economic value of insulin glargine 300 U/mL (Gla-300) in people ≥18 years of age with type 2 diabetes mellitus: a value-based economic model from a U.S. payer perspective. J Med Econ. 2023;26(1):1469–78. https://doi.org/10.1080/13696998.2023.2277058.
Vargas-Uricoechea H. Efficacy and safety of insulin glargine 300 U/mL versus 100 U/mL in diabetes mellitus: a comprehensive review of the literature. J Diabetes Res. 2018;12(2018):2052101. https://doi.org/10.1155/2018/2052101.
Blonde L, Bailey T, Sullivan SD, Freemantle N. Insulin glargine 300 units/mL for the treatment of individuals with type 2 diabetes in the real world: a review of the DELIVER programme. Diabetes Obes Metab. 2021;23(8):1713–21. https://doi.org/10.1111/dom.14405.
Zhou FL, Ye F, Berhanu P, et al. Real-world evidence concerning clinical and economic outcomes of switching to insulin glargine300 units/mL vs other basal insulins in patients with type 2 diabetes using basal insulin. Diabetes Obes Metab. 2018;20:1293–7. https://doi.org/10.1111/dom.13199.
Jendle J, Ericsson Å, Ekman B, et al. Real-world cost-effectiveness of insulin degludec in type 1 and type 2 diabetes mellitus from a Swedish 1-year and long-term perspective. J Med Econ. 2020;23(11):1311–20. https://doi.org/10.1080/13696998.2020.1805454.
Haldrup S, Lapolla A, Gundgaard J, Wolden ML. Cost-effectiveness of switching to insulin degludec from other basal insulins in real-world clinical practice in Italy. J Med Econ. 2020;23(3):271–9. https://doi.org/10.1080/13696998.2019.1669613.
Pollock RF, Valentine WJ, Marso SP, et al. Long-term cost-effectiveness of insulin degludec versus insulin glargine U100 in the UK: evidence from the basal-bolus subgroup of the DEVOTE trial (DEVOTE 16). Appl Health Econ Health Policy. 2019;17(5):615–27. https://doi.org/10.1007/s40258-019-00494-3.
Russel-Szymczyk M, Valov V, Savova A, Manova M. Cost-effectiveness of insulin degludec versus insulin glargine U100 in adults with type 1 and type 2 diabetes mellitus in Bulgaria. BMC Endocr Disord. 2019;19(1):132. https://doi.org/10.1186/s12902-019-0460-6.
Pollock RF, Heller S, Pieber TR, et al. Short-term cost-utility of degludec versus glargine U100 for patients with type 2 diabetes at high risk of hypoglycaemia and cardiovascular events: a Canadian setting (DEVOTE 9). Diabetes Obes Metab. 2019;21(7):1706–14. https://doi.org/10.1111/dom.13730.
Evans M, Mehta R, Gundgaard J, Chubb B. Cost-effectiveness of insulin degludec vs. insulin glargine U100 in type 1 and type 2 diabetes mellitus in a UK setting. Diabetes Ther. 2018;9(5):1919–30. https://doi.org/10.1007/s13300-018-0478-1.
Shao H, Shi L, Fonseca V, Alsaleh AJO, Gill J, Nicholls C. Cost-effectiveness analysis of once-daily insulin glargine 300 U/mL versus insulin degludec 100 U/mL using the BRAVO diabetes model. Diabet Med. 2023;40(9):e15112. https://doi.org/10.1111/dme.15112.
Napoli R, Fanelli F, Gazzi L, Larosa M, Bitonti R, Furneri G. Using 2nd generation basal insulins in type 2 diabetes: costs and savings in a comparative economic analysis in Italy, based on the BRIGHT study. Nutr Metab Cardiovasc Dis. 2020;30(11):1937–44. https://doi.org/10.1016/j.numecd.2020.07.005.
Evans M, Chubb B, Gundgaard J. Cost-effectiveness of insulin degludec versus insulin glargine in adults with type 1 and type 2 diabetes mellitus. Diabetes Ther. 2017;8(2):275–91. https://doi.org/10.1007/s13300-017-0236-9.
Evans M, Moes RGJ, Pedersen KS, Gundgaard J, Pieber TR. Cost-effectiveness of insulin degludec versus insulin glargine U300 in the netherlands: evidence from a randomised controlled trial. Adv Ther. 2020;37(5):2413–26. https://doi.org/10.1007/s12325-020-01332-y.
Abotaleb A, Semrouni M, Malek R, et al. Cost-effectiveness analysis of for insulin degludec (TRESIBA®) vs Insulin glargine U300 (TOUJEO®) in Algerian setting. Value Health. 2022;19(7):S40.
Martin ZY, Takagi T, Tian YS. Safety, efficacy, and cost-effectiveness of insulin degludec U100 versus insulin glargine U300 in adults with type 1 diabetes: a systematic review and indirect treatment comparison. Int J Clin Pharm. 2022;44(3):587–98. https://doi.org/10.1007/s11096-022-01410-x.
Funding
This study was funded by Sanofi France, including the journal’s Rapid Fee.
Author information
Authors and Affiliations
Contributions
Pierre Gourdy, Patrice Darmon, Isabelle Bureau, Amar Bahloul, Noemie Allali and Alfred Penfornis, contributed equally to the Design of the study, Methodology, Data interpretation, Review & Editing. Corinne Emery and Isabelle Borget, conducted the formal analysis and contributed to Methodology, Data interpretation, Review & Editing. Bruno Detournay contributed to Study design, Methodology, Formal analysis, Data interpretation, and conducted the original draft writing.
Corresponding author
Ethics declarations
Conflict of Interest
Pierre Gourdy declares that he has received occasional fees, on a personal or institutional basis, for activities as a speaker, scientific advisor or clinical researcher, from the following companies: Abbott, Abbvie, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, Intercept, LVL Médical, Merck Sharp & Dohme, Mundipharma, Organon, Novartis, Novo Nordisk, Pfizer, Sanofi, Servier. Patrice Darmon received personal compensation for his contribution to clinical trials, scientific work, consultancy work, conferences or symposia) from Abbott, AstraZeneca, Menarini, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, Sanofi, Eli Lilly, Bayer, Boehringer Ingelheim, Bastide Médical, LVL Médical. Isabelle Borget has no conflict of interest to declare. Corinne Emery and Isabelle Bureau are employed by CEMKA, a consulting team specializing in health economics, epidemiology, and outcomes research. Bruno Detournay declares that he has received fees for one-off services (scientific work, consultancy, conferences or symposia) for the following companies Cemka, Boehringer Ingelheim, Janssen, Merck Sharp & Dohme, Novo Nordisk, Sanofi, Pfizer, Eli Lilly, Cerba expert, IPSOS, Lumanity, AstraZeneca, Tillots Pharma. Bruno Detournay is an Editorial Board member of Diabetes Therapy and was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Amar Bahloul and Noemie Allali, and Aymeric Mahieu are employed by SANOFI Winthrop Industrie, France. Alfred Penfornis declares having received fees for occasional interventions (clinical trials, scientific work, advisory activity, conferences, or symposiums) from the companies Abbott, Amgen, AstraZeneca, Dexcom, Diabeloop, Johnson & Johnson, Insulet, Isis, Merck Sharp & Dohme, Medtronic, Medtrum, Novo Nordisk, Sanofi, Eli Lilly, Ypsomed.
Ethical Approval
This study was registered on the Health Data Hub website (T58549122021092). In accordance with current regulations, the study protocol was submitted to the committee for research, studies, and evaluations in the field of health (Comité d’Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé [CEREES]) for approval and was authorized by the French data protection authority (Commission Nationale de l’Informatique et des Libertés [CNIL], [CNIL authorization number MR: 921407).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Gourdy, P., Darmon, P., Borget, I. et al. Basal Insulinotherapy in Patients Living with Diabetes in France: The EF-BI Study. Diabetes Ther 15, 1349–1360 (2024). https://doi.org/10.1007/s13300-024-01577-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01577-8