Abstract
Introduction
Personalized therapy for patients with COPD requires appropriate choice of drug and delivery device. Inhalers and nebulizers vary in their drug delivery characteristics, particularly the need for passive or active patient inhalation for appropriate drug dispersal and delivery. In this in vitro analysis, we assessed the aerosol performance and drug delivery of two long-acting muscarinic antagonists, glycopyrrolate (GLY; 25 µg solution; 1 ml) and tiotropium (TIO; 18 µg powder) through their respective delivery systems: the eFlow® Closed System (CS) vibrating membrane nebulizer and the HandiHaler® dry-powder inhaler (DPI).
Methods
The aerosol performances of the eFlow® CS nebulizer and the HandiHaler® were determined using the Next Generation cascade Impactor. The delivered dose of GLY and TIO was determined using different breathing patterns, which varied in tidal volume and peak inspiratory flow rate, respectively, to simulate breathing conditions ranging from normal to severe obstruction.
Results
Aerodynamic particle analysis showed generally similar mass median aerodynamic diameter (MMAD, range, 3.6–4.6 µm) and fine particle fraction (FPF, range, 48.2%–63.7%) with GLY delivered using the eFlow® CS nebulizer under all breathing patterns tested. TIO, delivered via the HandiHaler®, showed variations in MMAD (range, 3.8–5.8 µm) and FPF (range, 16.1%–32.4%) under different inspiratory flow rates. The majority of GLY was deposited in stages 2–5 of the impactor, which corresponds with particle sizes in the respirable range (< 5 µm), whereas a large proportion of TIO was deposited in the throat/mouthpiece pre-separator, irrespective of test conditions. The median residual dose of GLY with eFlow® CS was notably lower compared to that of TIO with HandiHaler® (2.4%–4.4% vs. 40%–67%, respectively).
Conclusions
These simulation results highlight the different deposition patterns generated by a DPI device and a vibrating membrane nebulizer, which may help inform device selection and treatment decision in COPD management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent treatment strategies have strongly suggested the need for personalized selection of a drug-delivery device based on individual patient characteristics, as well as appropriate patient training, to ensure optimal management of COPD. |
In this in vitro study, we assess the differences between the HandiHaler® dry-powder inhaler and the eFlow® closed system vibrating membrane nebulizer delivering tiotropium and glycopyrrolate, respectively, using different breathing simulations designed to resemble variations in patient breathing patterns. |
Delivery of glycopyrrolate using the eFlow® closed system nebulizer resulted in consistent drug particle mass within the respirable range (MMAD < 5 µm), high rate of particle delivery, and majority deposition of drug particles within the later stages of the Next Generation cascade Impactor (NGI) under all tidal breathing patterns tested. |
Delivery of tiotropium using the HandiHaler® dry-powder inhaler showed variability of drug particle mass, lower rate of particle delivery, and high proportion of drug deposition within the USP throat section of the NGI under different peak inspiratory flow rates. |
This analysis highlights differences between the eFlow® closed system nebulizer and the HandiHaler® dry powder inhaler in the in vitro delivery of drugs under different, patient-dependent breathing variables, which may help inform clinicians on appropriate device selection for their COPD patients. |

Infographic – this infographic can be downloaded from the article’s associated Figshare page here: https://doi.org/10.6084/m9.figshare.12746840
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the most common pulmonary diseases in the United States and globally [1,2,3]. COPD is most commonly treated by inhalation of bronchodilators, with or without corticosteroids [1]. Inhalation is the preferred route of drug delivery in COPD, reducing the likelihood of adverse events due to lower systemic exposure to the active agents. Therapeutic agents may be delivered by a variety of inhalation devices/systems, including pressurized metered-dose inhalers (pMDI), dry powder inhalers (DPI), soft mist inhalers (SMI), or nebulizer systems [4, 5]. Recent treatment strategies have strongly suggested the need for personalized selection of drug-delivery devices based on individual patient characteristics, as well as appropriate patient training, to ensure optimal management of COPD [1, 4,5,6,7]. Despite numerous advances in inhaler technologies, user error rates have remained high and unchanged over decades [8]. Incorrect use of inhaler devices can lead to errors that prevent effective drug delivery to the lungs, which is associated with non-adherence, poor disease management [9], and increased healthcare utilization cost [10]. Multiple factors have been identified that lead to errors in device use, some related to the device and some related to the patient [9, 11]. Further, patients are often not adequately trained by their healthcare providers in the correct use of their inhalation devices. In an online survey of 205 pulmonologists, only a small fraction were very knowledgeable in teaching patients how to use (43%) or clean and maintain (22%) inhaler devices [12].
Commonly used inhaler devices (pMDIs, SMIs, DPIs, and nebulizers) vary in their mechanisms of drug delivery and administration technique [4, 5]. DPIs require active, patient-dependent airflow generation to draw the powder out of the device and create a disaggregated, breathable aerosol plume that will enter the lungs rather than impact in the mouth and throat [4]. In contrast, drug delivery with a nebulizer is a relatively passive process from the patient’s perspective, as the energy required for aerosol generation comes from external sources such as compressed air or a vibrating membrane; it is important to note that nebulization still requires the patient to actively inhale, and thus the process can be considered somewhat active, depending on the nebulizer device and resistance [4, 13]. For effective DPI use, patients need to generate sufficient peak inspiratory flow (PIF) to overcome the unique internal resistance of the device in order to disaggregate and disperse the drug [14, 15]. Drug particle characteristics with a DPI are highly dependent on flow rate through the device, and even devices with low internal resistance, such as the Breezhaler® (Novartis, East Hanover, New Jersey), produce a reduced fine particle fraction (FPF) with low flow rates [16]. Many factors, including inability to breath hold for several seconds, physical or cognitive impairments and suboptimal PIF, may lead to reduced drug delivery and impaired treatment efficacy; all of which may be more common among elderly patients [4, 15, 17]. In such patients, SMIs, pMDI with spacers, and nebulizers are viable options; however, SMIs require some coordination between actuation and inhalation [4,5,6] and error rates with pMDI actuation can reduce drug deposition despite the use of spacers [18]. Nebulizers are a good alternative in these patients as they produce a fine mist and use tidal breathing to deliver the medication, thereby circumventing PIF constraints [19].
Patient characteristics, such as tidal volume, PIF, anatomy of the lungs and inhalation pattern may affect drug deposition in the lower respiratory tract versus the upper airway and oropharyngeal cavity, which impacts treatment efficacy and safety [4,5,6, 13]. In addition, aerosol properties such as particle size (assessed by mass median aerodynamic diameter [MMAD]: the diameter at which 50% of the particles by mass are larger and 50% are smaller) affect deposition. Aerosol particles < 5 µm have the greatest deposition in the lungs; particles > 5 µm are more likely to deposit in the oropharynx and are swallowed. Deposition is also a function of dispersion of particle diameter (assessed by geometric standard deviation [GSD]: the measure of the spread of the aerodynamic particle size distribution) and fine particle levels (assessed by fine particle dose [FPD]: the mass of particles < 5 µm in size within the total delivered dose; FPF: the FPD, expressed as a percentage of the delivered dose). A GSD ≥ 1.22 is ideal for delivery throughout the lungs; most therapeutic aerosols have a GSD between 2 and 3 [13, 20].
In light of the increase in handling errors with age and disease severity in patients using various devices [18, 21,22,23], it is important to study the drug delivery properties of different devices under varying breathing conditions. Long-acting muscarinic antagonists (LAMAs) are bronchodilators that are widely used alone or in combination with long-acting β2-agonists, with or without inhaled corticosteroids. In this study, we compared the in vitro aerosol and drug-delivery properties of two LAMAs, delivered using different devices: tiotropium (TIO) delivered using the HandiHaler® DPI (18 μg powder in single-use capsules; SPIRIVA® HandiHaler®, Boehringer Ingelheim Pharmaceuticals, Ridgefield, CT, USA), one of the most commonly prescribed DPIs, [24] and glycopyrrolate (GLY, 25 µg/ml in 1-ml single-use vials; LONHALA®, Sunovion Pharmaceuticals, Inc., Marlborough, MA, USA) delivered using the eFlow® Closed System (CS) (MAGNAIR®; PARI Pharma GmbH; Starnberg, Germany), a vibrating membrane nebulizer [25, 26]. It is to be noted that the HandiHaler® is a high-resistance device and may not be suited for patients who cannot generate high PIF (e.g., in cases of COPD); [14] GLY delivered by the eFlow® CS may be an alternative for patients with low PIF who prefer a LAMA-based inhalation therapy.
Prior comparisons of inhaler devices have been performed and showed differences between inhalers [27,28,29,30,31], but comparisons of the aerosol performance and drug deposition with nebulizers are limited. In addition, the previous analysis of the aerosol performance and drug deposition of GLY delivered using the eFlow® CS nebulizer assessed characteristics at constant flow or a single tidal volume, consistent with normal patients [32]. The current in vitro study was designed to assess the differences between a DPI and a vibrating membrane nebulizer system, delivering two LAMAs, using different breathing patterns in a Next Generation cascade Impactor (NGI) system. The in vitro conditions tested in this study were designed to resemble breathing patterns or PIF variations frequently observed in patients with COPD. In addition, these analyses aimed to extend the observations on aerosol properties of GLY delivered using the eFlow® CS nebulizer, under simulated condition to mimic the range of breathing patterns observed among COPD patients.
Methods
Aerosol Performance Characterization
For each device type, three unique devices were used (three eFlow® CS nebulizers and three HandiHaler® DPIs) and three replicate measurements were made for each breathing pattern or flow rate in each device (a total of nine replicates per breathing pattern or flow rate across the three devices). All analyses were performed between January and May 2019 at PPD® Laboratories (Middleton, WI, USA).
The aerosol performance of the eFlow® CS nebulizer was determined by particle size distribution via NGI and delivered dose using a breathing simulator with three different breathing patterns (Table 1). The aerosol performance of the HandiHaler® was determined by particle size distribution via NGI and delivered dose analysis at three different constant flow rates.
This study is based on in vitro data and does not contain any studies with human participants or animals performed by any of the authors.
Aerodynamic Size Distribution by NGI
eFlow® CS Nebulizer
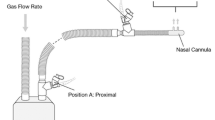
The aerodynamic particle size distribution was determined using an NGI, USP induction port, NGI collection cups, breathing simulator (Copley BRS 2100), mixing inlet, and compressed air inlet manifold (all from Copley Scientific Ltd, Colwick, Nottingham, United Kingdom). The USP induction port model was used for the in vitro testing because it was used throughout the development of Lonhala® Magnair® and is the USP standard for aerosol testing (USP < 601 >). For the GLY delivery test, the large, solid NGI collection cup under the micro-orifice collector stage of the NGI was replaced with an internal filter holder cup (Copley Scientific Ltd) fitted with a glass microfiber filter (GE Healthcare Bio-Sciences, Marlborough, MA, USA). To minimize errors in determination of particle size due to evaporation of aerosols as a result of heat-transfer from the NGI, the NGI and components were all chilled at 5°C for a minimum of 90 min prior to collection [33]. All collections were initiated within 5 min of removal from 5°C. The breathing simulator was used to generate three different breathing profiles which varied tidal volume, breath frequency, PIF, and inspiratory:expiratory ratios (Table 1). The breathing pattern 3 (tidal volume, 500 ml; 15 breaths/min; inhalation: exhalation ratio of 1:1) is standardized adult breathing pattern described in the USP < 1601 >. This was also the same breathing pattern used in a prior publication where the aerosol performance characteristics of the Lonhala® Magnair® device was described [32]. Two tidal volumes (200 and 350 ml) below the normal volume of 500 ml were chosen as they are representative of patients with obstructive lung diseases such as COPD.
Using the mixing inlet and compressed air inlet manifold, a 30 l/min vacuum flow through the impactor was balanced with a compressed air flow rate allowing for the breathing patterns to be replicated at the induction port. Prior to initiation of each collection, the flow rate at the NGI outlet was set to 30 l/min and the flow rate at the induction port was verified as 0 l/min. A constant flow of 30 l/min was utilized to accommodate the upper range of PIF under test, ensuring that the applied breathing pattern withdrew flow only from the compressed air line during the inspiratory phase of the applied breathing pattern. Aerosol was collected until at least 10 s after the nebulizer automatically shut off, indicated by an audio indicator (i.e., beep). The eFlow® CS nebulizer and NGI components were recovered and analyzed by high-performance liquid chromatography (HPLC) per previously verified procedures [32].
HandiHaler® DPI
The aerodynamic particle size distribution of TIO using the HandiHaler® DPI was determined at three different PIF (20, 30, and 60 l/min) using an NGI, USP induction port, NGI preseparator, NGI collection cups, and critical flow controller (all Copley Scientific Ltd, Colwick, Nottingham, United Kingdom) in accordance with USP < 601 >. NGI collection cups were coated with 1% polysorbate 20 in methanol. Testing was performed in a controlled environment of 23 ± 2°C and 50% ± 5% relative humidity. Collections were performed at constant flow rates of 20, 30, and 60 l/min, which had previously been shown to result in similar delivery of TIO using a HandiHaler® [34]. Collection times were adjusted such that 4 l of air passed through the device during collection. The NGI components and capsule were recovered and TIO levels analyzed by HPLC.
Individual component results for each replicate of each device type were entered into validated Copley Inhaler Testing Data Analysis Software (Copley Scientific Ltd) to calculate the GSD, FPD, and FPF.
Delivered Dose Analysis
The simulated delivered dose of GLY using the eFlow® CS nebulizer was determined at all three different breathing patterns (Table 1). Aerosol was collected until the nebulizer automatically shut off; the nebulizer handset, filter pad, and holder were recovered and analyzed by HPLC. The simulated delivered dose of TIO using the HandiHaler® DPI was determined using a USP dose unit sampling apparatus (DUSA) and critical flow control (both Copley BRS 2100, Copley Scientific Ltd). The DUSA was prepared by placing a 47-mm metal mesh screen support and a 47-mm GF/A glass fiber filter (GE Healthcare Bio-Sciences, Marlborough, MA, USA) on the filter holder of the DUSA. Testing was performed in a controlled environment of 23 ± 2 °C and 50 %± 5% relative humidity. Collections were performed at constant flow rates of 20, 30, and 60 l/min with collection times adjusted such that 2 l of air passed through the device during collection. The capsule and DUSA were recovered and analyzed by HPLC.
Results
Aerosol Performance—Drug Particle Mass
The mean MMAD of GLY delivered using the eFlow® CS nebulizer was relatively similar under all simulated breathing patterns tested, and was in the respirable range. The mean (range) MMAD for GLY was 4.1 (3.6–4.6), 4.2 (4.0–4.3) and 3.9 (3.7–4.1) µm under breathing patterns 1, 2, and 3 (corresponding tidal volumes are 200 ml, 350 ml, and 500 ml), respectively (Fig. 1a). The mean MMAD of TIO using the HandiHaler® DPI changed with the inspiratory flow rates, and exceeded the respirable range cutoff (5 µm) at 20 l/min inspiratory flow rate. The mean (range) MMAD for TIO was 5.3 (4.9–5.8), 4.7 (4.5–5.0) and 3.9 (3.8–4.0) µm at 20, 30, and 60 l/min inspiratory flow rates, respectively (Fig. 1b). The GSD of GLY delivered using the eFlow® CS nebulizer was in the optimal range for pulmonary drug delivery (2.0–2.4 across the three breathing patterns tested), while that of TIO using the HandiHaler® DPI was lower (~ 1.8 across the three flow rates).
Comparison of the mean MMAD with a nebulized GLY 25 µg using the eFlow® CS and b TIO 18 µg using the HandiHaler® assessed using the NGI and under different breathing patterns. The dotted line represents the respirable range cut-off of 5 µm [13, 20]. CS closed system, GLY glycopyrrolate, MMAD mass median aerodynamic diameter, NGI Next Generation cascade Impactor, TIO tiotropium
Aerosol Performance—FPF and FPD
The mean (range) FPF of GLY using the eFlow® CS nebulizer was 52.0% (48.2%–56.6%), 57.2% (54.2%–59.7%), and 61.6% (58.7%–63.7%) with breathing patterns 1, 2, and 3, respectively (Fig. 2a). The mean (range) FPF of TIO using the HandiHaler® DPI was 19.9% (16.1%–23.2%), 24.2% (20.6%–27.7%), and 29.2% (26.4%–32.4%) with 20 l/min, 30 l/min, and 60 l/min inspiratory flow rates, respectively (Fig. 2b).
The mean (range) FPD of GLY using the eFlow® CS nebulizer was 3.6 (2.3–4.2), 7.1 (6.4–8.1), and 8.7 (7.9–9.0) µg with breathing patterns 1, 2, and 3, respectively. The mean (range) FPD of TIO using the HandiHaler® DPI was 1.1 (0.2–1.4), 2.2 (1.7–2.8), and 2.9 (2.2–3.8) µg with 20 l/min, 30 l/min, and 60 l/min inspiratory flow rates, respectively (Table 2).
In Vitro Delivered Dose
NGI stage-by-stage deposition profiles of GLY, under different tidal volumes, and TIO, using different inspiratory flow rates, are shown in Fig. 3. The deposition profiles show greater drug deposition in the later stages of the NGI representative of the respirable range with GLY delivered using the eFlow® CS nebulizer [35]. However, consistent with the more variable MMAD observed with breathing pattern 1 (tidal volume of 200 ml), the deposition of GLY in the later stages of the NGI was lower in breathing pattern 1 compared with breathing patterns 2 and 3, which may be due to the low tidal volume. In contrast, the greatest proportion of TIO deposition using the HandiHaler® DPI is within the USP throat under all three inspiratory flow rates tested (Fig. 3). The mean delivered dose of GLY using the eFlow® CS nebulizer was greater within later NGI compartments with breathing patterns 2 and 3 compared with breathing pattern 1; however, all 3 breathing patterns showed similar aerosol deposition, with the greatest proportion being in the stages with lower cutoff sizes (Fig. 3a). Similarly, the deposition profile of aerosols generated with the HandiHaler® showed greater mean delivered dose with higher inspiratory flow rates (30 l/min and 60 l/min), but the overall deposition pattern was similar to all inspiratory flow rates assessed (Fig. 3b).
NGI stage-by-stage deposition profile of a GLY 25 µg using the eFlow® CS nebulizer and b TIO 18 µg using the HandiHaler® aerosols with effective size cutoff. CS closed system, GLY glycopyrrolate, IP/MA induction port/mouthpiece adaptor, MOC micro-orifice collector, NGI Next Generation cascade Impactor, TIO tiotropium
Nebulization Time
The nebulization time of GLY using all three eFlow® CS nebulizers was similar across all breathing patterns tested, with median time to nebulization of 118 or 119 s (Table 3).
Residual Dose
The median residual dose of GLY in the ampule following nebulization was low across all breathing patterns tested, ranging from 2.4% to 4.4% (Table 4). The residual dose of TIO using the HandiHaler® DPI was markedly greater than that of GLY with the eFlow® CS nebulizer, with median residual dose of 67, 42 and 40% at flow rates of 20, 30, and 60 l/min, respectively.
Discussion
Recent guidelines for the treatment of COPD recommend the personalization of therapeutic agent and delivery device, with the goal of optimal management of the disease. In this analysis, we assessed the impact of varying breathing patterns and inspiratory flow rates on drug delivery and pulmonary deposition with a vibrating membrane nebulizer and a DPI. The results show the majority of MMAD < 5 µm, high FPF, and, importantly, the majority of drug was within the respirable range, using the eFlow® CS nebulizer under all breathing patterns tested. We observed some variability in the MMAD of GLY under breathing pattern 1, in which the tidal volume was low (200 ml); this breathing pattern is representative of patients with severe COPD [36]. In contrast, the HandiHaler® DPI showed variations in the MMAD and FPF with different PIF, and a majority of drug deposition within the USP throat under all flow rates tested. These in vitro results support the use of the eFlow® CS nebulizer in patients with COPD with breathing patterns ranging from ‘normal’ to ‘severe’, although it must be noted that patients with very low tidal volumes (e.g., 200 ml) may experience reduced nebulizer performance, in terms of aerosol properties, which suggests that these patients require careful consideration regarding their treatment choice and attention to treatment outcomes. These results highlight the importance of device selection based on patient characteristics and needs. However, it is important to note that the testing of the eFlow® CS nebulizer and the HandiHaler® were performed under different conditions, with varying breathing patterns (including tidal volumes and PIF) for the nebulizer and varying PIF alone for the HandiHaler®. This is reflective of the variation in the drug deposition using these devices, whereby the nebulizer creates a mist that can be inhaled during normal tidal breathing whereas the HandiHaler® requires a sharp intake of breath representative of the PIF. Thus, while our results do not represent an exact comparison of the two devices, the data provide insight into the aerosol properties of both devices under conditions characteristic of COPD patients.
A previous analysis of the aerosol properties of GLY using the eFlow® CS nebulizer was performed with a generally accepted adult normal breathing pattern with 500 ml tidal volume [32]. The current analysis extends the available data and shows drug particle size distribution similar to the previously published data under breathing conditions consistent with COPD patients [32]. Whereas a previous study had shown that MMAD with an ultrasonic nebulizer was within the respirable range using tidal volumes between 300 and 500 ml but not at tidal volumes between 150 and 250 ml [36], our data show consistent MMAD within the respiratory range using the eFlow® CS vibrating membrane nebulizer across tidal volumes between 200 and 500 ml. The minimum PIF required for the HandiHaler® DPI is 20 l/min [34], whereas optimal PIF for other DPIs are between 30 and 60 l/min [37]. We tested the HandiHaler® at both the minimal and optimal PIF for DPIs, and observed PIF-dependent changes in MMAD, which was above or just at the respirable fraction size. These in vitro data suggest that drug deposition in the lungs may be compromised in patients who use DPIs and cannot generate and sustain the optimal PIF during the inspiratory maneuver.
In this study, use of the HandiHaler® DPI resulted in drug deposition in the USP throat, independent of inspiratory flow rate, whereas use of the eFlow® CS nebulizer led to drug deposition mostly in the later stages of the NGI, under all breathing patterns tested. The high in vitro deposition of GLY within the respirable range using the eFlow® CS nebulizer is consistent with previous analyses [32], and highlights the potential for efficient drug delivery to the lungs with this device. The in vitro deposition of TIO within the USP throat using the HandiHaler® DPI is consistent with a previous analysis, which showed that the HandiHaler® resulted in lower pulmonary drug deposition compared to other inhalers [27, 38]. These results provide support for benefits of nebulization in a wider set of patients who may show variable tidal volumes and breathing patterns, whereas DPIs may be more beneficial among patients who are capable of generating the required, device-specific PIF consistently. It is important to note that TIO is also available for delivery using an SMI, which may provide greater advantages for patients having similar breathing patterns to those tested in this study, as SMIs have been shown to have a higher in vitro deposition compared to other inhalers [29, 39, 40]. The timing of this study in the summer of 2019 coincided with a period in which the HandiHaler® was predominantly used for delivery of TIO, and as such led to the use of the HandiHaler® as a comparator device in the current analysis. However, clinical outcomes were similar with TIO delivered using the HandiHaler® and the Respimat® (5 µg dose), suggesting that patient-dependent factors are the key consideration for selection between the two devices [30]. While there are no direct comparisons of drug delivery by SMIs, DPIs, and nebulizers, drug aerosol properties and delivery tend to be improved with nebulizers and SMIs compared with DPIs [41].
The results of this analysis confirm the nebulization times of approximately 2 min using the eFlow® CS nebulizer, independent of breathing patterns assessed, and consistent with previous studies [13, 25, 32, 42]. In addition, the median residual dose of GLY in the nebulizer was markedly lower than that of TIO using the HandiHaler® DPI. These in vitro data further support the high efficiency of drug delivery with a vibrating membrane nebulizer, and highlight the dependence of drug delivery by DPI on patient PIF.
Proper and personalized device selection for patients with COPD is essential, as it is associated with optimal efficacy and importantly adherence to treatment [1]. Errors (being either patient- or device-related) associated with bronchodilators lead to poor disease control and clinical outcomes [17] and nonadherence to long-term therapy [4, 43, 44]. To date, there have been no head-to-head comparisons between DPIs and nebulizers with respect to patient adherence. Future studies in COPD are needed to assess patient adherence to DPI versus nebulizer therapy.
Conclusions
Personalization of therapy for patients with COPD can have a major impact on the success of treatment. However, there is limited guidance on prescribing devices [20], with selection largely driven by prescriber familiarity, rather than patient demographic and disease characteristics. Our analysis highlights potential differences in the two delivery systems tested, which may help inform clinicians on appropriate device selection for their COPD patients.
Change history
31 March 2021
A peer-reviewed infographic was retrospectively added to this publication.
References
Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/ Accessed Jan 31 2020
COPD. https://www.cdc.gov/dotw/copd/index.html. Accessed 1 Oct 2019.
Wheaton AG, Cunningham TJ, Ford ES, Croft JB. US Centers for Disease Control and Prevention employment and activity limitations among adults with chronic obstructive pulmonary disease—United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64:289–95.
Depietro M, Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease. Postgrad Med. 2018;130:83–97.
Hanania NA, Braman S, Adams SG, Adewuya R, Ari A, Brooks J, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5:111–23.
Dolovich MB, Ahrens RC, Hess DR, Anderson P, Dhand R, Rau JL, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines: American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127:335–71.
The Society for Post-Acute and Long-Term Care Medicine. COPD management in the post-acute and long-term care setting clinical practice guideline. Columbia: AMDA; 2016.
Sanchis J, Gich I, Pedersen S. Systematic review of errors in inhaler use: Has patient technique improved over time? Chest. 2016;150:394–406.
Molimard M, Raherison C, Lignot S, Balestra A, Lamarque S, Chartier A, et al. (2017) Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 49
Roggeri A, Micheletto C, Roggeri DP. Inhalation errors due to device switch in patients with chronic obstructive pulmonary disease and asthma: critical health and economic issues. Int J Chron Obstruct Pulmon Dis. 2016;11:597–602.
Sulaiman I, Cushen B, Greene G, Seheult J, Seow D, Rawat F, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1333–43.
Braman SS, Carlin BW, Hanania NA, Mahler DA, Ohar JA, Pinto-Plata V, et al. Results of a pulmonologist survey regarding knowledge and practices with inhalation devices for COPD. Respir Care. 2018;63:840–8.
Pleasants RA 2nd. Glycopyrrolate/eFlow CS: the first nebulized long-acting muscarinic antagonist approved to treat chronic obstructive pulmonary disease. Ann Pharmacother. 2019;53:285–93.
Ghosh S, Pleasants RA, Ohar JA, Donohue JF, Drummond MB. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585–95.
Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14:1103–7.
Abadelah M, Chrystyn H, Bagherisadeghi G, Abdalla G, Larhrib H. Study of the emitted dose after two separate inhalations at different inhalation flow rates and volumes and an assessment of aerodynamic characteristics of Indacaterol Onbrez Breezhaler((R)) 150 and 300 mug. AAPS PharmSciTech. 2018;19:251–61.
Price DB, Roman-Rodriguez M, Mcqueen RB, Bosnic-Anticevich S, Carter V, Gruffydd-Jones K, et al. Inhaler errors in the CRITIKAL study: type, frequency, and association with asthma outcomes. J Allergy Clin Immunol Pract. 2017;5(1071–81):e9.
Dhand R, Dolovich M, Chipps B, Myers TR, Restrepo R, Farrar JR. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9:58–72.
Tashkin DP. A review of nebulized drug delivery in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2585–96.
Laube BL, Janssens HM, De Jongh FH, Devadason SG, Dhand R, Diot P, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–31.
Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010;35:913–22.
Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration. 2008;75:18–25.
Giraud V, Roche N. Misuse of corticosteroid metered-dose inhaler is associated with decreased asthma stability. Eur Respir J. 2002;19:246–51.
Spiriva HandiHaler [package insert]. Boehringer Ingelheim, Ingelheim am Rhein, Germany; 2004.
LONHALA® MAGNAIR® (glycopyrrolate) inhalation solution [package insert]. Sunovion Pharmaceuticals, Inc., Marlborough, MA; June 2019.
Kerwin E, Ferguson GT. An overview of glycopyrrolate/eFlow(R) CS in COPD. Expert Rev Respir Med. 2018;12:447–59.
Asakura Y, Nishimura N, Maezawa K, Terajima T, Kizu J, Chohnabayashi N. Effect of switching tiotropium HandiHaler(R) to Respimat(R) Soft Mist Inhaler in patients with COPD: the difference of adverse events and usability between inhaler devices. J Aerosol Med Pulm Drug Deliv. 2013;26:41–5.
Chrystyn H, Safioti G, Keegstra JR, Gopalan G. Effect of inhalation profile and throat geometry on predicted lung deposition of budesonide and formoterol (BF) in COPD: An in-vitro comparison of Spiromax with Turbuhaler. Int J Pharm. 2015;491:268–76.
Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat((R)) inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulmon Dis. 2017;12:1565–77.
Dahl R, Kaplan A. A systematic review of comparative studies of tiotropium Respimat(R) and tiotropium HandiHaler(R) in patients with chronic obstructive pulmonary disease: does inhaler choice matter? BMC Pulm Med. 2016;16:135.
Ninane V, Vandevoorde J, Cataldo D, Derom E, Liistro G, Munghen E, et al. New developments in inhaler devices within pharmaceutical companies: a systematic review of the impact on clinical outcomes and patient preferences. Respir Med. 2015;109:1430–8.
Pham S, Ferguson GT, Kerwin E, Goodin T, Wheeler A, Bauer A. In vitro characterization of the eFlow closed system nebulizer with glycopyrrolate inhalation solution. J Aerosol Med Pulm Drug Deliv. 2018;31:162–9.
Dennis J, Berg E, Sandell D, Ali A, Lamb P, Tservistas M, et al. Cooling the NGI—an approach to size a nebulised aerosol more accurately. Pharmeur Sci Notes. 2008;2008:27–30.
Chodosh S, Flanders JS, Kesten S, Serby CW, Hochrainer D, Witek TJ Jr. Effective delivery of particles with the HandiHaler dry powder inhalation system over a range of chronic obstructive pulmonary disease severity. J Aerosol Med. 2001;14:309–15.
Miscetti G, Garofani P, Gargarella LR, Pickard D. Chemical risk assessment from exposure to fine dusts in workers of the feed industry. It J Occup Environ Hyg. 2011;2:213–9.
Riedler J, Robertson CF. Effect of tidal volume on the output and particle size distribution of hypertonic saline from an ultrasonic nebulizer. Eur Respir J. 1994;7:998–1002.
Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381–7.
Chapman KR, Fogarty CM, Peckitt C, Lassen C, Jadayel D, Dederichs J, et al. Delivery characteristics and patients' handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:353–63.
Brand P, Hederer B, Austen G, Dewberry H, Meyer T. Higher lung deposition with Respimat soft mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulmon Dis. 2008;3:763–70.
Iwanaga T, Tohda Y, Nakamura S, Suga Y. The Respimat((R)) soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021–30.
Fink JB, Colice GL, Hodder R. Inhaler devices for patients with COPD. COPD. 2013;10:523–35.
Leaker BR, Barnes PJ, Jones CR, Tutuncu A, Singh D. Efficacy and safety of nebulized glycopyrrolate for administration using a high efficiency nebulizer in patients with chronic obstructive pulmonary disease. Br J Clin Pharmacol. 2015;79:492–500.
Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence. 2017;11:1205–12.
Sriram KB, Percival M. Suboptimal inhaler medication adherence and incorrect technique are common among chronic obstructive pulmonary disease patients. Chron Respir Dis. 2016;13:13–22.
Acknowledgements
This analysis was supported by funding from Sunovion Pharmaceuticals Inc.
Funding
Funding from Sunovion Pharmaceuticals Inc. was used to support the rapid review service and open-access fees associated with publication of this manuscript.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Hashem Dbouk, PhD, and Dhivya Ramalingam, PhD, of FireKite, an Ashfield company, part of UDG Healthcare plc, and funded by Sunovion Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
AB, SSh, and SSa contributed to protocol development and design. AB, SSh, and SSa were involved in data acquisition; JO, AB, SSh, and SSa were involved in data analysis and interpretation. JO, AB, SSh, and SSa were involved at all stages of manuscript development, writing, and revision.
Disclosures
Jill A. Ohar has participated in advisory boards for Sunovion Pharmaceuticals Inc., AstraZeneca, Boehringer Ingelheim, Verona, GlaxoSmithKline, Novartis, Mylan, and Theravance, and has received grant funding from Sunovion Pharmaceuticals, Inc. and Boehringer Ingelheim. Shahin Sanjar, Andrea Bauer, and Sanjay Sharma are employees of Sunovion Pharmaceuticals, Inc.
Compliance with Ethics Guidelines
This study is based on in vitro data and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Sunovion Pharmaceuticals, Inc. is part of a clinical trial data-sharing consortium that facilitates access for qualified researchers to selected anonymized clinical trial data. For up-to-date information on data availability, please visit https://www.clinicalstudydatarequest.com/Study-Sponsors.aspx and click on Sunovion.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Author information
Authors and Affiliations
Corresponding author
Additional information
Digital Features
To view digital features for this article go to https://doi.org/10.6084/m9.figshare.12746840
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ohar, J.A., Bauer, A., Sharma, S. et al. In Vitro Effect of Different Airflow Rates on the Aerosol Properties of Nebulized Glycopyrrolate in the eFlow® Closed System and Tiotropium Delivered in the HandiHaler®. Pulm Ther 6, 289–301 (2020). https://doi.org/10.1007/s41030-020-00125-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-020-00125-6