Abstract
Introduction
The selection of inhaler device is of critical importance in chronic obstructive pulmonary disease (COPD) as the interaction between a patient’s inhalation profile and the aerosol characteristics of an inhaler can affect drug delivery and lung deposition. This study assessed the in vitro aerosol characteristics of inhaler devices approved for the treatment of COPD, including a soft mist inhaler (SMI), pressurized metered-dose inhalers (pMDIs), and dry powder inhalers (DPIs).
Methods
High-speed video recording was used to visualize and measure aerosol velocity and spray duration for nine different inhalers (one SMI, three pMDIs, and five DPIs), each containing dual or triple fixed-dose combinations of long-acting muscarinic receptor antagonists and long-acting β2-agonists, with or without an inhaled corticosteroid. Measurements were taken in triplicate at experimental flow rates of 30, 60, and 90 l/min. Optimal flow rates were defined based on pharmacopoeial testing requirements: 30 l/min for pMDIs and SMIs, and the rate achieving a 4-kPa pressure drop against internal inhaler resistance for DPIs. Comparison of aerosol plumes was based on the experimental flow rates closest to the optimal flow rates.
Results
The Respimat SMI had the slowest plume velocity (0.99 m/s) and longest spray duration (1447 ms) compared with pMDIs (velocity: 3.65–5.09 m/s; duration: 227–270 ms) and DPIs (velocity: 1.43–4.60 m/s; duration: 60–757 ms). With increasing flow rates, SMI aerosol duration was unaffected, but velocity increased (maximum 2.63 m/s), pMDI aerosol velocity and duration were unaffected, and DPI aerosol velocity tended to increase, with a more variable impact on duration.
Conclusions
Aerosol characteristics (velocity and duration of aerosol plume) vary by inhaler type. Plume velocity was lower and spray duration longer for the SMI compared with pMDIs and DPIs. Increasing experimental flow rate was associated with faster plume velocity for DPIs and the SMI, with no or variable impact on plume duration, whereas pMDI aerosol velocity and duration were unaffected by increasing flow rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Inhaled delivery of respiratory medicine is the cornerstone of treatment for chronic obstructive pulmonary disease (COPD). |
A multitude of factors can impact the lung deposition and resultant efficacy of an inhaled drug, including the aerosol velocity and spray duration, which can influence oropharyngeal deposition and ease of coordination, respectively. |
What was the aim of the study? |
The objective of this in vitro study was to visualize and measure the aerosol characteristics (velocity and duration) of three inhaler types (SMI, pMDI, and DPI) approved for the treatment of COPD. |
What were the study outcomes/conclusions? |
The Respimat SMI had the slowest plume velocity at 10-cm distance from the inhaler mouthpiece, and longest spray duration compared with pMDIs and DPIs. |
Increasing experimental flow rate was associated with faster plume velocity for DPIs and the SMI, with no or variable impact on plume duration. pMDI aerosol velocity and duration were unaffected by increasing flow rate. |
What was learned from the study? |
This is the first study to compare the aerosol characteristics of the three inhaler types approved for delivery of COPD medication. |
The aerosol characteristics shown in this analysis will help respiratory physicians and nurses understand the differences between inhalers and improve understanding of how to match their patients’ inhalation ability with the most appropriate inhaler. |
Digital Features
This article is published with digital features, including a video showing aerosol plumes, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.24599391
Introduction
Inhaled delivery of respiratory medication is the cornerstone of chronic obstructive pulmonary disease (COPD) management. There are several types of aerosol delivery systems, including the soft mist inhaler (SMI), pressurized metered-dose inhaler (pMDI), and dry powder inhaler (DPI) [1]. For pMDIs and SMIs, aerosol generation is independent of the user (pMDIs rely on propellant, whereas SMIs rely on mechanical energy); for DPIs, generation of the aerosol depends on the patient’s inspiratory airflow [1, 2].
The efficacy of an inhaled drug is largely dependent upon the amount of drug deposited in the lungs and its distribution within the airways [2, 3]. This is affected by factors specific to the patient, such as inhalation maneuver/technique, which can be improved through training [4, 5]; however, some factors, such as airway geometry [6, 7] and disease severity [7], cannot be overcome by training. These can impact a patient’s ability to achieve a sufficient inspiratory flow rate, especially among those of small stature [8, 9], older age [10, 11], female gender [8,9,10], and those who have been recently hospitalized [10].
Factors specific to inhaler delivery devices can also affect drug deposition in the lungs, including particle size and aerosol velocity [2, 3]. For maximum alveolar deposition that aligns with muscarinic receptor distribution in the lung, a particle diameter of ~ 3 μm has been suggested [12, 13]. If particles are too small, the aerosol is likely to be exhaled; however, if the particles are too large, they are likely to deposit by impaction in the oropharynx [7]. High aerosol velocity is associated with higher oropharyngeal deposition for pMDIs, as the particles are less able to navigate the approximately right-angled bend at the back of the throat to reach the lung [7, 14, 15]. Conversely, slow aerosol velocity can be problematic for DPIs, limiting drug deposition in the lungs by affecting powder disaggregation and dispersion [7, 16]. A patient’s inspiratory flow rate can influence the velocity of the airborne particles and the probability of their impaction in the upper airways [7]. This is particularly relevant for DPIs, which require a sufficient inspiratory flow rate to disaggregate the drug from its carrier powder to produce particles of optimal size that can then reach the lungs [7]. By contrast, pMDIs and SMIs are less sensitive to inspiratory flow [17, 18].
Aerosol duration can also affect drug delivery [2, 3]. The 2023 Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report guidelines state that it is best practice to assess a patient’s ability to perform the correct specific inhalation maneuver when choosing an inhaler device [19]. A patient’s inhalation maneuver should last longer than the duration of aerosol formation of pMDIs and SMIs and should be long enough to ensure powder dispersion for DPIs [11] (capsule-type DPIs release their dose for a longer time than reservoir or blister-type DPIs [7]). Coordination between actuation and inspiration is required for active inhalers (i.e., pMDIs and SMIs) and therefore a longer aerosol duration may reduce the impact of mismatch and improve the probability of lung deposition [1, 7]. The longer duration over which the dose is expelled from an SMI compared with pMDIs is thought to reduce the impact of poor coordination between actuation and inspiration [1, 2]. Accordingly, the GOLD strategy report acknowledges that SMIs are affected by coordination between actuation and inspiration to a lesser extent than pMDIs [19].
Regarding inhaled therapies, GOLD recommends treatment with long-acting muscarinic antagonist (LAMA) plus long-acting β2-agonist (LABA) for COPD irrespective of exacerbation phenotype, with the addition of inhaled corticosteroids (ICS) considered for patients with eosinophilic inflammation and exacerbations not controlled by LAMA/LABA. GOLD also states that such combinations may be more convenient and effective as a single therapy inhaler than multiple inhalers [19]. Since LABA, LAMA and/or ICS can be delivered in various combinations by pMDI, DPI, and SMI, matching the right device to the patient is important to accommodate a user’s inhalation profile and COPD severity.
To the best of our knowledge, no prior studies have compared the aerosol characteristics of the three inhaler types (SMI, pMDIs, and DPIs) approved for COPD treatment. In this article, we report a novel experimental approach to the visualization of aerosol characteristics for these inhaler types.
Methods
Ethics
This analysis did not involve human participants or animals.
Data Collection
Nine inhalers containing dual or triple fixed-dose combinations approved for the treatment of COPD were examined (Table 1). These included one SMI (Respimat Spiolto), three pMDIs (Bevespi Aerosphere, Trimbow, and Trixeo Aerosphere) and five DPIs (Anoro Ellipta, Brimica Genuair, Trelegy Ellipta, Trimbow NEXThaler, and Ultibro Breezhaler).
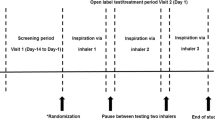
The experimental setup consisted of a lung simulator, dark background, distance measurer, lamp, and a video recorder (Fig. 1). Each inhaler was encapsulated in an airtight container and connected to the lung simulator, which expelled 4 l of air through the inhaler at experimental flow rates of 30, 60, and 90 l/min (taking 8, 4, and 2.667 s, respectively). During patient inhalation from inhalers, ambient air is pulled through vents in the inhaler mouthpiece and/or in the inhaler attached to the mouthpiece. This experimental setup used the same air flow direction, but rather than air being pulled through the mouthpiece, it was pushed via the vents and through the mouthpiece (Fig. 2). All experiments were repeated in triplicate at 20 °C (±2 °C) temperature and 40–60% relative humidity.
To allow comparisons between inhalers, the optimal flow rate for each inhaler was based on pharmacopoeial testing requirements. For pMDIs and SMIs the optimal flow rate was 30 l/min. For DPIs, the flow rate was dependent upon inhaler resistance ([R = square root (pressure drop)/air flow rate] [R = square root (mbar)*minutes/L]) and was back-calculated to create a pressure drop of 4 kPa across the inhaler (square root [40 mbar]/R). The experimental flow rate closest to the calculated optimal flow rate was selected for comparisons (Table S1).
Video Recording
High-speed video recording provides an effective means of characterizing aerosol plumes [14, 20]. A Panasonic HC-X1, 4K Video Camera (Osaka, Japan) was selected for its ability to capture 120 frames per second. MAGIX Movie Edit Pro was used first to manually identify time zero, defined as when the aerosol first emerges from the mouthpiece, and to modify the contrast and brightness. A second video was then superimposed with millisecond timings. SkillSpector is a video motion and skill analysis software used for motion analysis (https://skillspector.software.informer.com/1.3/), which was used to determine aerosol characteristics by digitizing the front of the aerosol cloud.
Aerosol Velocity
The front of the cloud was recorded as a function of time and the fit function used was as given by Hochrainer et al. [2] to derive the velocity as the gradient of the regression line of distance as a function of time.
To allow comparison of the aerosol velocity at inhaler exit versus 10 cm from the mouthpiece (the latter derived from Hochrainer et al. [2] and used as a representation of the back of the throat, where the aerosol has to change direction toward the lower airways), the cross-sectional area of the mouthpiece was measured to enable calculation of the mean exit velocity for air exiting each inhaler. Photographs were taken and the projection of the holes was evaluated using a computer-aided design program (SolidWorks, Dassault Systèmes SolidWorks Corporation, Waltham, MA, USA). As the air stream is known to separate from the surface when there is a strong recess (step or angle larger than ~7 degrees [21]), only the straight projection of the inhaler outlet holes was considered. Using a caliper, the main dimensions of the inhaler mouthpiece openings were determined. The cross-sectional area was expressed in square meters. The mean velocity passing through this cross-sectional area is given as: velocity (m/s) = air flow rate [m3/s]/area [m2].
Aerosol Spray Duration
Using the same video frames as for the aerosol velocity calculation, the aerosol spray duration was estimated by counting the frames on which the aerosol was seen exiting the mouthpiece.
Results
Aerosol Velocity
Figure 3 shows the mean aerosol plume velocities at 10 cm from the mouthpiece for all inhalers. At the optimal experimental flow rate for each inhaler, the slowest mean aerosol velocity was generated by the SMI (0.99 m/s). As air flow rate increased, so did the mean aerosol velocity for the SMI. The mean aerosol velocities of the DPIs ranged between 1.43 and 4.60 m/s at the optimal experimental flow rates. As air flow rate increased, so did the mean aerosol velocity for four out of five DPIs. All pMDI inhalers generated considerably higher mean aerosol velocities (3.65–5.09 m/s) at the optimal experimental flow rate for each device, and this was broadly independent of experimental air flow rate.
Mean aerosol plume velocities (measured 10 cm away from the inhaler mouthpiece) at the air flow rates tested for each inhaler. SMI Spiolto Respimat, pMDI Bevespi Aerosphere, Trimbow, Trixeo Aerosphere, DPI Anoro Ellipta, Brimica Genuair, Trelegy Ellipta, Trimbow NEXThaler, Ultibro Breezhaler. Error bars represent standard deviation. Optimal experimental flow rates determined by pharmacopoeial testing requirements (30 l/min for pMDIs and SMI, or flow to achieve a 4-kPa pressure drop against internal inhaler resistance for DPIs): 30 l/min for Spiolto Respimat, Bevespi Aerosphere, Trimbow and Trixeo Aerosphere, 60 l/min for Anoro Ellipta, Trelegy Ellipta, Brimica Genuair and Trimbow NEXThaler, 90 l/min for Ultibro Breezhaler. DPI dry powder inhaler, pMDI pressurized metered-dose inhaler, SMI soft mist inhaler
The SMI and pMDIs had the slowest calculated mean air velocities at the inhaler mouthpiece exit (1.8–5.9 m/s), compared with the DPIs (5.7–39.0 m/s). Comparing velocities at inhaler exit and at 10 cm from the mouthpiece, these were most similar for the SMI and pMDIs, and least similar for the DPIs. The pMDI aerosol velocity tended to increase between inhaler exit and 10 cm from the mouthpiece, except at the highest flow rate, where it slowed down. DPI aerosol velocity tended to slow down considerably from inhaler exit to 10 cm from the mouthpiece, and SMI aerosol velocity also slowed, reaching a slower mean velocity than that of the DPIs (Table S1).
Aerosol Spray Duration
The mean aerosol spray durations of all inhalers examined are shown in Fig. 4. At the optimal experimental flow rate for each inhaler, the longest spray duration was generated by the SMI (1447 ms) (Fig. 4). All pMDI inhalers produced aerosol sprays of short duration (227–270 ms). For both the SMI and pMDIs, there was very little difference in spray duration as the air flow rate increased. The DPIs produced aerosol sprays of very short duration (blister-type: 60–70 ms; reservoir-type: 100–163 ms) as well as longer duration (capsule-type: 757 ms). There was little difference in spray duration as air flow rate increased for the blister and reservoir-type DPIs. The capsule-type DPI (Ultibro Breezhaler) produced its longest spray duration (1213 ms) at the lowest air flow rate.
Mean aerosol spray duration at the air flow rates tested for each inhaler. SMI Spiolto Respimat, pMDI Bevespi Aerosphere, Trimbow, Trixeo Aerosphere, DPI Anoro Ellipta, Brimica Genuair, Trelegy Ellipta, Trimbo NEXThaler, Ultibro Breezhaler. Error bars represent standard deviation. Optimal experimental flow rates determined by pharmacopoeial testing requirements (30 l/min for pMDIs and SMI, or flow to achieve a 4-kPa pressure drop against internal inhaler resistance for DPIs): 30 l/min for Spiolto Respimat, Bevespi Aerosphere, Trimbow and Trixeo Aerosphere, 60 l/min for Anoro Ellipta, Trelegy Ellipta, Brimica Genuair and Trimbow NEXThaler, 90 l/min for Ultibro Breezhaler. DPI dry powder inhaler, pMDI pressurized metered-dose inhaler, SMI soft mist inhaler
Aerosol plumes can be viewed in Video 1. Further details are provided in Table S1 in the Supplementary Materials.
High-speed video recording of aerosol plumes from each inhaler tested with milliseconds superimposed (MP4 49,853 KB)
Discussion
This is the first study to compare the aerosol characteristics of the three inhaler types approved for delivery of COPD medication. Of the nine devices tested (one SMI, three pMDIs, and five DPIs), the SMI delivered the slowest plume velocity and longest spray duration compared with pMDIs and DPIs. The SMI delivered the longest spray duration irrespective of experimental flow rate, but plume velocity was susceptible to change in flow rate.
The Respimat SMI showed similar velocities at mouthpiece exit versus 10 cm from the mouthpiece. Although the inhaler exit velocities for the pMDIs were largely similar to the exit velocity of the Respimat SMI, the aerosol velocities 10 cm away from the mouthpiece were higher than the velocity of the SMI aerosol at the same distance, consistent with previous findings [2]. This may be influenced by the initially larger particle diameters and the pressurized propellants inside pMDIs, which push the drug out very quickly and evaporate during flight, causing particles to shrink [2]. As particles with a larger diameter take a longer time and distance until they reach equilibrium velocity with ambient air compared with smaller particles, this may account for the observed difference between the SMI and pMDIs [2]. The Respimat SMI uses a fine nozzle system, which aerosolizes more than 60% of the metered dose of drug solution into particles with an aerodynamic diameter of < 5 μm to facilitate lung deposition [11, 22]. The mechanics of the device are also designed to slow aerosol velocity and increase aerosol duration to minimize the impact of the patient’s inhalation profile and reduce the need for precise co-ordination between actuation and inhalation [11, 22, 23].
In contrast with the SMI and pMDIs, the DPIs demonstrated a high exit velocity that slowed down with flight distance. DPIs require a minimum level of inspiratory flow to disaggregate the drug particles from the carrier molecules; therefore, they are susceptible to changes in a patient’s airflow rate and rely on small particle size to ensure slowing [4, 7]. Achieving sufficient early airflow acceleration is particularly critical for reservoir- and blister-type DPIs as they emit their dose early and produce shorter aerosol sprays compared with capsule devices. Patients who are unable to accelerate airflow fast enough at the start of inhalation may have reduced drug delivery to the lung and suboptimal clinical benefit [4, 7, 24]. The prevalence of suboptimal peak inspiratory flow rates in hospitalized patients with COPD is shown to vary according to the internal resistance of DPI inhalers [25]. As studies of DPI use have shown that suboptimal peak inspiratory flow is associated with poorer health status, increased hospitalization and healthcare resource utilization, [26, 27] as well as lower treatment adherence [28], it is important to assess whether sufficient peak inspiratory flow is consistently achieved by patients to ensure they are matched with an appropriate inhaler [26,27,28].
Although not measured in this work, aerodynamic particle diameter is one of the most important factors affecting aerosol deposition [7] (details provided in Table 1). All inhalers tested produce particles with a mass median aerodynamic diameter (MMAD) < 5 μm, which is important as particles > 5 μm are most likely to result in oropharyngeal deposition by impaction [7]. As such, fine particle fraction is a key determinant of the proportion of the emitted dose delivered to the lungs. It has been confirmed both in vivo and in vitro that the Respimat SMI provides high lung deposition and low oropharyngeal deposition of drug particles compared with pMDIs and DPIs [12, 29,30,31], and clinically, lung function outcomes are consistent irrespective of the peak inspiratory flow a patient can generate [32]. There is also evidence that the Respimat SMI is able to deliver a higher dose to the lungs compared with DPIs and pMDIs, irrespective of inhaler position, which is important when patients do not follow instructions regarding correct inhaler orientation [33]. DPIs have a low MMAD; however, the aerosol particles are less homogeneous, resulting in a lower fine particle fraction (Table 1), which can negatively impact deposition of particles in the lungs [34, 35]. If particles are too small, this can result in a greater percentage of particles being exhaled by a patient, resulting in less efficient drug deposition [36]. Most particles of 0–1 μm diffuse by Brownian motion and deposit when they collide with the airway wall, highlighting the importance of the breath-hold after inhalation to enhance deposition in the peripheral airways [7]. As disease severity worsens, maximal breath-holding time reduces [37], which, in turn, limits the lung deposition of the smaller drug particles and emphasizes the importance of optimizing breath-holding technique, especially for effective DPI use [7, 38].
Matching each patient to the right inhaler is critical because if patients do not perceive their inhaler to be intuitive or effective, it can negatively impact treatment adherence and lead to an increase in symptom burden, poorer clinical outcomes, and increased healthcare costs [11, 26, 39]. Inhalers are often perceived as being easy to use with little training required; however, it has been estimated that up to 68% of patients with COPD do not use their inhaler correctly, as many patients inhale too fast with a pMDI or SMI and too slowly with a DPI [40, 41]. To overcome problems with suboptimal inhalation, breath-actuated pMDIs can be used to ensure a predetermined threshold in inspiratory flow is achieved before the drug is released [7]. Additionally, there is evidence that assessments of a patient’s inspiratory flow rate, and inhaler education guided by this, can significantly reduce the incidence of exacerbations compared with conventional inhaler education, especially for those using multiple inhalers [5]. Training in inhaler technique, including practicing with a placebo formulation, can help patients achieve an optimal inspiratory flow, but the effect of training to ensure lung deposition may vary by inhaler type [42]. Studies suggest that up to 90% of healthcare professionals are unable to adequately describe or perform critical steps of inhaler use [40, 43, 44], which is likely to translate into suboptimal patient education [43]. Myriad other factors contribute to inhaler misuse, though focusing on patient-centered device development and ease of use may improve inhaler use in clinical practice [43]. In a study where the same therapy was delivered via an SMI (5 µg tiotropium) or DPI (18 µg tiotropium, providing the same systemic exposure [45]), the SMI users had significantly fewer COPD-related exacerbations and lower odds of hospital readmissions – an indication that device choice is important [46].
Our analysis has several limitations. The ex vivo nature of the experiment, though a strength in that it allowed for all inhaler types to be compared, could only be considered descriptive because it did not include multiple inhaler batches with robust statistical analysis of within- or between-inhaler differences. Additionally, although the aerosol characteristics are informative regarding the inter-inhaler class differences, assessment of lung deposition (through imaging) and clinical evaluation of lung function and other outcomes would be required to extrapolate in vitro findings to in vivo effects. On this point, it is important to consider all results from this analysis in the context of the clinical efficacy data associated with each inhaler (all inhalers are approved for the treatment of COPD based on their proven effects on lung function and other clinical outcomes).
Although the use of high-speed laser imaging is a more common technique for analyzing plume geometry (as discussed in a White Paper by the International Pharmaceutical Aerosol Consortium on Regulation and Science [IPAC-RS] [20]), our novel experimental setup did not use a laser light sheet as this would have prevented full visualization of the plume. Instead, our methodology was similar to that used by Hochrainer et al. in their plume analysis of the Respimat SMI and nine different pMDIs [2]. As we were interested in the front velocity of the plume, using white light to achieve full illumination of the entire plume front was the technique most suited to our research objective.
In summary, the effectiveness of inhaled therapies, independent of the constituent molecules, is reliant on many patient- and device-related factors. Even with good adherence and training in inhaler technique, aerosol characteristics of specific inhaler types for COPD vary widely, with implications for drug deposition in the lungs and consequently, medication effectiveness. Although this study was experimental in nature, it offers an insight into the characteristics of the aerosols produced by different inhaler types and the effects of speed of inhalation.
Conclusions
The SMI produced the slowest plume velocity and longest spray duration compared with pMDIs and DPIs. The DPIs produced aerosol sprays of the shortest duration except for a capsule-type DPI, which generated the second-longest spray duration of all inhalers. Given the expected global increase in disease burden of COPD [47], it will be essential to ensure that healthcare practitioners are aware of the characteristics of each inhaler type. Matching the patient’s inhalation profile and COPD severity to their inhaler has the potential to allow for a more personalized approach to COPD therapy and greater clinical benefit.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manage. 2019;15:461–72.
Hochrainer D, Holz H, Kreher C, Scaffidi L, Spallek M, Wachtel H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J Aerosol Med. 2005;18(3):273–82.
Cheng YS. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech. 2014;15(3):630–40.
Peche R, Attar-Zadeh D, Scullion J, Kocks J. Matching the inhaler to the patient in COPD. J Clin Med. 2021;10(23):5683.
Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12: 704316.
Demoly P, Hagedoorn P, de Boer AH, Frijlink HW. The clinical relevance of dry powder inhaler performance for drug delivery. Respir Med. 2014;108(8):1195–203.
Laube BL, Janssens HM, de Jongh FH, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–31.
Ghosh S, Pleasants RA, Ohar JA, Donohue JF, Drummond MB. Prevalence and factors associated with suboptimal peak inspiratory flow rates in COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:585–95.
Mahler DA, Waterman LA, Gifford AH. Prevalence and COPD phenotype for a suboptimal peak inspiratory flow rate against the simulated resistance of the diskus? Dry powder inhaler. J Aerosol Med Pulm Drug Deliv. 2013;26(3):174–9.
Sharma G, Mahler DA, Mayorga VM, Deering KL, Harshaw O, Ganapathy V. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4(3):217–24.
Iwanaga T, Tohda Y, Nakamura S, Suga Y. The Respimat® Soft Mist inhaler: Implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39(11):1021–30.
Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat(®) inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulm Dis. 2017;12:1565–77.
Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37(4):337–62.
Dhand R. Aerosol plumes: slow and steady wins the race. J Aerosol Med. 2005;18(3):261–3.
Levy ML, Dekhuijzen PNR, Barnes PJ, et al. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim Care Respir Med. 2016;26(1):16017.
Dal Negro RW. Dry powder inhalers and the right things to remember: a concept review. Multidiscip Respir Med. 2015;10(1):13.
Baloira A, Abad A, Fuster A, et al. Lung deposition and inspiratory flow rate in patients with chronic obstructive pulmonary disease using different inhalation devices: a systematic literature review and expert opinion. Int J Chron Obstruct Pulmon Dis. 2021;16:1021–33.
Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161: 105857.
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for prevention, diagnosis and management of COPD (2023 report). 2023. Available from https://goldcopd.org/2023-gold-report-2/. Aaccessed 4 Dec 2022.
Baxter S, Myatt B, Stein S, et al. Spray pattern and plume geometry testing and methodology: an IPAC-RS working group overview. AAPS PharmSciTech. 2022;23(5):145.
Chandavari V. Diffuser angle control to avoid flow separation. Int J Tech Res Appl. 2014;2(5):16–21.
Wachtel H, Kattenbeck S, Dunne S, Disse B. The Respimat® development story: patient-centered innovation. Pulm Ther. 2017;3(1):19–30.
Dalby RN, Eicher J, Zierenberg B. Development of Respimat® Soft Mist™ Inhaler and its clinical utility in respiratory disorders. Med Dev (Auckland, NZ). 2011;4:145–55.
Ferguson GT. Suboptimal peak inspiratory flow (PIF) in patients with severe COPD does not impact effective drug delivery of an inhaled triple therapy dry powder inhaler (DPI). Chest. 2022;162(4):A1881–3.
Mahler DA, Demirel S, Hollander R, et al. High prevalence of suboptimal peak inspiratory flow in hospitalized patients with COPD: a real-world study. Chronic Obstr Pulm Dis. 2022;9(3):427–38.
Kocks WH, Wouters H, Bosnic-Anticevich S, et al. Factors associated with health status and exacerbations in COPD maintenance therapy with dry powder inhalers. NPJ Prim Care Respir Med. 2022;32(1):18.
Clark B, Wells BJ, Saha AK, et al. Low peak inspiratory flow rates are common among COPD inpatients and are associated with increased healthcare resource utilization: a retrospective cohort study. Int J Chron Obstruct Pulm Dis. 2022;17:1483–94.
Leving MT, Bosnic-Anticevich S, van Cooten J, et al. Clinical recommendations for dry powder inhaler use in the management of COPD in primary care. NPJ Prim Care Respir Med. 2022;32(1):59.
Newman SP, Brown J, Steed KP, Reader SJ, Kladders H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest. 1998;113(4):957–63.
Pitcairn G, Reader S, Pavia D, Newman S. Deposition of corticosteroid aerosol in the human lung by Respimat® Soft Mist™ inhaler compared to deposition by metered dose inhaler or by Turbuhaler® dry powder inhaler. J Aerosol Med. 2005;18(3):264–72.
Anderson P. Use of Respimat Soft Mist inhaler in COPD patients. Int J Chron Obstruct Pulm Dis. 2006;1(3):251–9.
Mahler DA, Ludwig-Sengpiel A, Ferguson GT, et al. TRONARTO: a randomized, placebo-controlled study of tiotropium/olodaterol delivered via Soft Mist Inhaler in COPD patients stratified by peak inspiratory flow. Int J Chron Obstruct Pulm Dis. 2021;16:2455–65.
Strohe S, Wachtel H, Emerson-Stadler R, Usmani O. The effects of inhaler type and orientation at inhalation on dosing efficiency—a study of the SMI, pMDI and DPI. 2023. Available from https://www.inhalationmag.com/article/the-effects-of-inhaler-type-and-orientation-at-inhalation-on-dosing-efficiency-a-study-of-the-smi-pmdi-and-dpi/. Accessed 30 June 2023.
Ciciliani AM, Langguth P, Wachtel H. In vitro dose comparison of Respimat® inhaler with dry powder inhalers for COPD maintenance therapy. Int J Chron Obstruct Pulm Dis. 2017;12:1565–77.
Lavorini F, Janson C, Braido F, Stratelis G, Lokke A. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther Adv Respir Dis. 2019;13:1753466619884532.
de Boer AH, Gjaltema D, Hagedoorn P, Frijlink HW. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. 2015;96:143–51.
Hedhli A, Slim A, Ouahchi Y, et al. Maximal voluntary breath-holding tele-inspiratory test in patients with chronic obstructive pulmonary disease. Am J Men’s Health. 2021;15(3):15579883211015856.
Horvath A, Balashazy I, Tomisa G, Farkas A. Significance of breath-hold time in dry powder aerosol drug therapy of COPD patients. Eur J Pharm Sci. 2017;104:145–9.
Miravitlles M, Soler-Cataluna JJ, Alcazar B, Viejo JL, Garcia-Rio F. Factors affecting the selection of an inhaler device for COPD and the ideal device for different patient profiles. Results of EPOCA Delphi consensus. Pulm Pharmacol Ther. 2018;48:97–103.
Haidl P, Heindl S, Siemon K, Bernacka M, Cloes RM. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65–75.
Haughney J, Price D, Barnes NC, Virchow JC, Roche N, Chrystyn H. Choosing inhaler devices for people with asthma: current knowledge and outstanding research needs. Respir Med. 2010;104(9):1237–45.
Brand P, Hederer B, Austen G, Dewberry H, Meyer T. Higher lung deposition with Respimat Soft Mist inhaler than HFA-MDI in COPD patients with poor technique. Int J Chron Obstruct Pulm Dis. 2008;3(4):763–70.
Volerman A, Carpenter D, Press V. What can be done to impact respiratory inhaler misuse: exploring the problem, reasons, and solutions. Expert Rev Respir Med. 2020;14(8):791–805.
Plaza V, Giner J, Rodrigo GJ, Dolovich MB, Sanchis J. Errors in the use of inhalers by health care professionals: a systematic review. J Allergy Clin Immunol Pract. 2018;6(3):987–95.
van Noord JA, Cornelissen PJ, Aumann JL, Platz J, Mueller A, Fogarty C. The efficacy of tiotropium administered via Respimat Soft Mist Inhaler or HandiHaler in COPD patients. Respir Med. 2009;103(1):22–9.
Singer D, Bengtson LGS, Elliott C, Buikema AR, Franchino-Elder J. Healthcare resource utilization, exacerbations, and readmissions among Medicare patients with chronic obstructive pulmonary disease after long-acting muscarinic antagonist therapy initiation with soft mist versus dry powder inhalers. Int J Chron Obstruct Pulm Dis. 2020;15:3239–50.
Lopez-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23.
European Medicines Agency. Spiolto Respimat 2.5 microgram/2.5 microgram, inhalation solution. 2020. Available from https://www.medicines.org.uk/emc/product/6902/smpc#gref. Accessed 3 Jan 2023.
Wachtel H, Ziegler J. Improved assessment of inhaler device performance using laser diffraction. Respir Drug Deliv. 2002;2:379–82.
Bevespi Aerosphere. Summary of Product Characteristics. European Medicines Agency. Updated November 2022. Accessed 4 Jan 2023.
Usmani OS, Roche N, Jenkins M, Stjepanovic N, Mack P, De Backer W. Consistent pulmonary drug delivery with whole lung deposition using the aerosphere inhaler: a review of the evidence. Int J Chron Obstruct Pulm Dis. 2021;16:113–24.
European Medicines Agency. Trimbow pMDI 87 micrograms/5 micrograms/9 micrograms pressurised inhalation, solution. 2021. Available from https://www.medicines.org.uk/emc/product/761/smpc. Accessed 3 Jan 2023
Usmani OS, Mignot B, Kendall I, et al. Predicting lung deposition of extrafine inhaled corticosteroid-containing fixed combinations in patients with chronic obstructive pulmonary disease using functional respiratory imaging: an in silico study. J Aerosol Med Pulm Drug Deliv. 2021;34(3):204–11.
European Medicines Agency. Trixeo Aerosphere 5 micrograms/7.2 micrograms/160 micrograms pressurised inhalation, suspension. 2020. Available from https://www.medicines.org.uk/emc/product/12028. Accessed 3 Jan 2023.
European Medicines Agency. ANORO ELLIPTA 55 micrograms/22 micrograms inhalation powder, pre-dispensed. 2014. Available from https://www.ema.europa.eu/en/documents/product-information/anoro-ellipta-epar-product-information_en.pdf. Aaccessed 4 Jan 2023.
Brimica Genuair. Summary of product characteristics. European Medicines Agency. Updated June 2021. Accessed 3 Jan 2023.
Trelegy Ellipta. Summary of product characteristics. European Medicines Agency. Updated July 2022. Accessed 3 Jan 2023.
Virchow JC, Poli G, Herpich C, et al. Lung deposition of the dry powder fixed combination beclometasone dipropionate plus formoterol fumarate using NEXThaler® device in healthy subjects, asthmatic patients, and COPD patients. J Aerosol Med Pulm Drug Deliv. 2018;31(5):269–80.
European Medicines Agency. Ultibro Breezhaler 85 micrograms/43 micrograms inhalation powder hard capsules: summary of product characteristics. 2013. Available from https://www.ema.europa.eu/en/documents/product-information/ultibro-breezhaler-epar-product-information_en.pdf. Accessed 4 Jan 2023.
Medical writing/editorial assistance.
Medical writing support was provided by Hannah Avery of Meditech Media, a medical communications agency, and funded by Boehringer Ingelheim.
Funding
Boehringer Ingelheim funded study conduct, medical writing support, video production, and the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). Herbert Wachtel designed and conducted the experiments. All authors (Herbert Wachtel, Rachel Emerson-Stadlet, Peter Langguth, Jens M. Hohlfeld, and Jill Ohar) were involved in analysis and interpretation of the data, and in drafting and critically revising the content of the manuscript. All authors approved the final version for publication. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy, as well as intellectual property considerations.
Corresponding author
Ethics declarations
Conflict of Interest
Herbert Wachtel and Rachel Emerson-Stadler are full-time employees of Boehringer Ingelheim. Peter Langguth has nothing to disclose. Jens M. Hohlfeld has received honoraria for board services, consultancy, and lectures outside the scope of this work from Boehringer Ingelheim Pharma & Co KG, CSL Behring GmbH, HAL Allergy Group, Merck & Co., Inc., Nocion Therapeutics, Novartis AG, and Roche. Jill Ohar reports consulting fees from AstraZeneca, Boehringer Ingelheim, Mylan, Sunovion, and Verona, and has received grants from Boehringer Ingelheim, Sunovion, Teva, and the Society to Improve Diagnosis in Medicine (SIDM) under a grant from the Gordon and Betty Moore Foundation.
Ethical Approval
This analysis did not involve human participants or animals.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wachtel, H., Emerson-Stadler, R., Langguth, P. et al. Aerosol Plumes of Inhalers Used in COPD. Pulm Ther 10, 109–122 (2024). https://doi.org/10.1007/s41030-023-00249-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-023-00249-5