Abstract
This paper investigates the additive manufacturing route for a novel glass-forming titanium-based sulfur-containing alloy of the composition \({\text{Ti}}_{60} {\text{Zr}}_{15} {\text{Cu}}_{17} {\text{S}}_{8}\). This system is a promising candidate for medical devices since the lack of toxic components is combined with a high corrosion resistance and strength. Preliminary experiments and simulations show a general processability of bulk material by powder bed fusion technologies. TEM and XRD reveal an amorphous microstructure of laser-treated surfaces. Ultrasonic atomization is used to fabricate a flowable powder feed stock with spherical morphology and crystalline microstructure, which is suitable for processing in powder bed fusion. Correlations between detected crystalline phase formations and melt pool dynamics are revealed by SEM and EDX. It is shown that bulk samples with a high relative density and partially crystalline microstructure can be manufactured.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction and motivation
The ongoing desire for suitable materials concerns scientists more than ever, and emerging technologies offer novel approaches to enhance application possibilities. In this context, amorphous metals—known since the 1960s [1]—nowadays emerge from their limiting thin ribbon geometry towards applicable substances of bulk shape [2].
Ti-based glass-forming alloys are promising materials for applications in in highly corrosive surroundings [3], although rare, pricey and toxic elements are present in these alloys [4, 5]. Novel \({\text{Ti}}_{{75 - {\text{x}}}} {\text{Zr}}_{{\text{x}}} {\text{Cu}}_{17} {\text{S}}_{8}\) bulk metallic glasses (BMG), discovered by Kuball et al. in 2019, are promising alloys for medical applications. They provide an outstanding yield strength of 2.8 GPa, low density of 5.5 g/cm3, high hardness of 547 (HV5) and an excellent corrosion resistance combined with the renunciation of toxic elements [6, 7]. Albeit, their moderate glass-forming ability (GFA) (critical casting diameter \({D}_{c}\) of up to 3 mm [7]) limits the generation of amorphous bulk material by conventional casting, which restricts their application [8]. At this point, the additive manufacturing method potentially allows a break-through for this alloy system by combining high cooling rates of up to \({10}^{-6}\) K/s with bulk parts manufacturing [9,10,11]. Previous work on amorphous materials such as ZR01 [12] and VIT101 [13] and other Ti-based alloys [14,15,16] in powder bed fusion–laser beam melting (PBF-LB/M) and a previous analytical study on cooling rates [17] inspire the consideration of processing glass-forming alloys with lower GFA.
Due to the novelty of these Ti-based materials, little is known on the processability and there is a shortage of available material for testing. Hence, the present study provides a preliminary investigation on the laser–material interaction and is realized on cast material, followed by an extensive study on the resulting microstructure. These results motivate the elaborate production of powder material necessary for PBF-LB/M.
Eventually, this paper presents the general processability of Ti-based S-containing alloys by PBF-LB/M using the example of \({\text{Ti}}_{60} {\text{Zr}}_{15} {\text{Cu}}_{17} {\text{S}}_{8}\). The investigated processing route covers the steps of pre-alloying, atomization and PBF-LB/M. Material composition and microstructure are investigated for laser surface-treated cast material, powder and additively manufactured specimen, and provide a fundamental basis for the manufacturing of bulk amorphous samples.
2 Methods
This study is composed of two parts: first, a preliminary study was conducted, including the surface laser treatment of cast \({\text{Ti}}_{60} {\text{Zr}}_{15} {\text{Cu}}_{17} {\text{S}}_{8}\) samples and the analysis to assess the general feasibility for further experiments. Second, atomized powder was generated and processed by PBF-LB/M to manufacture solid samples. Finally, the resulting material compositions and microstructures are analyzed and compared.
2.1 Laser remelting of cast material
The impact of laser remelting was investigated using a conventional PBF-LB/M machine (SLM280 HL 2.0 by Nikon SLM Solutions AG, Germany; equipped with 2 × 700 W fiber laser, wavelength of approx. 1075 nm and spot size of approx. 70 µm) to perform a remelting of the surface by laser exposure on a cast material. The cast substrate is made from high-purity elemental materials by pre-alloying Cu and S, and subsequent arc melting according to Kuball [6].
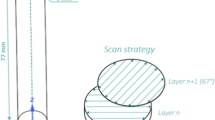
Three single tracks and one area scan were exposed. All scan vectors were oriented orthogonally to the direction of the gas flow. The laser power \({P}_{L}\) was set to 70 W. Scan velocity \({v}_{s}\) was varied from 500 to 2000 mm/s for the single tracks. A hatch distance h of 70 µm in combination with \({P}_{L}\) = 70 W and \({v}_{s}\) = 2000 mm/s was chosen for the exposed area, based on findings according to findings of Wegner [18] related to AM of glass-forming alloys. The setup is drafted in Fig. 1.
The microstructure of the laser melted tracks was first examined by scanning electron microscopy (SEM) using a FEI Helios NanoLab DualBeam 600i dual-beam focused ion beam field emission gun SEM (FIB-FEGSEM), with an accelerating voltage of 5 kV and a beam current of 0.8 nA. Characterization was performed both along the laser-treated surface, which is naturally parallel to the laser tracks, as well as along a cross-section perpendicular to the laser scan direction. The laser-treated surface was examined in the as-processed state, in the absence of any additional surface finishing step. The cross-section was first ground using increasingly finer SiC papers, and then mechanically polished with diamond pastes of particle sizes ranging from 6 µm to 0.25 µm.
Further characterization of the microstructure inside the melt pools, as well as at the melt pool interfaces, was carried out by transmission electron microscopy (TEM) using a FEI Talos F200x microscope with an operation voltage of 200 kV. Examination was performed by bright field (BF) imaging, high angle annular dark field (HAADF) imaging, and selected area electron diffraction (SEAD). For that purpose, rectangular TEM lamellae with their long axis perpendicular to the laser scanning direction and to the laser track width were FIB-milled using the lift-out technique [19] at selected locations as illustrated in Appendix (Fig. 11). An Omniprobe TM micromanipulator, that controls a W-needle, as well as a Pt-gas injection system, was used to extract thin foils in the following way. First, the selected site is coated with a rectangular layer of Pt. The surrounding material is then milled away using the Ga-ion beam with a 30 kV acceleration voltage and with decreasing ion beam currents, ranging from 65 to 0.79 nA, until a thickness of about 1.5 µm is achieved. Second, the lamella is extracted and moved to a V-shaped copper holder using the W-needle. The thin foil is then fixed to the holder by Pt deposition. Finally, both lamella surfaces are polished to achieve a final thickness of about 100 nm using an ion beam current of 16 pA and a voltage of 5 kV.
2.2 Atomization
Based on previous work by Kuball et al. [6], pre-alloyed material is generated by arc melting. Due to the findings of Kuball on a neglectable impact of raw material purity on the resulting performance, industrial grade elemental components are used.
The pre-alloyed material is processed by an AMAZEMET RePowder ultrasonic powder atomizer using arc melting with a W-electrode in an argon atmosphere combined with ultrasonic vibration [20].
XRD measurement of the powder was conducted using a Rigaku Smartlab High Resolution X-ray diffractometer, equipped with a 9 kW rotating Cu-anode (wavelength 1.506 Å) in Bragg–Brentano beam geometry. Diffractograms were recorded by Θ − 2Θ scans in reflection geometry using a Hypix-3000 detector. At this X-ray wavelength, and for an incident angle of Θ = 45°, the attenuation length is less than 8.5 µm.
2.3 PBF-LB/M
The SLM280 HL 2.0 was further used to manufacture samples from the atomized powder feed stock on a custom Ti grade 2 substrate at 150 °C pre-heating temperature. Arcal Prime Argon (99.998%) from Air Liquide was used as shielding gas. The oxygen content within the atmosphere was controlled at 200 ppm by a Linde ADDvanceTM O2 Precision device. If not specified otherwise, a layer thickness of 20 µm, a hatch distance of 70 µm and an alternating rotating scanning strategy (67° shift) combined with 3 contour scans (50 W, 1600 mm/s) are applied.
Single tracks are laser exposed to assess the general processability of the powder material. The processing parameters are chosen as all possible combinations of the values given in Fig. 2b). These monolayer experiment is applied to a leveling base of ten layers additively manufactured material in order to neglect possible z-leveling inaccuracies within the first layers during PBF/LB-M.
For the generated tracks, the melt pool dimensions (width and depth) are analyzed. Cross-sections (y–z) of selected tracks are examined by SEM. Based on the results, a set of parameters is selected for the production of multilayer samples to investigate the interlayer bonding. The amorphicity by X-ray diffraction (XRD) and differential scanning calorimetry (DSC) is analyzed at the example of a cylinder (5 mm diameter, 1.5 mm height). Further, 3 × 3 × 30 mm beam geometries are manufactured and used to analyze the homogeneity by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). The relative density of the beam geometry is measured using an optical approach (Olympus BX51 microscope combined with an Olympus SC50 digital camera using Stream Essentials software.
Cross-sections are prepared by grinding with SiC paper of up to 1200, polishing with a diamond paste of 9 µm particle size and subsequent chemical polishing using diluted H2O2. Subsequently, Kroll etchant (consisting of 100 ml distilled water, 2–6 ml nitric acid HNO_3 65% and 1–3 ml hydrofluoric acid HF-40%; acc. to [21]) is applied to the cross-section of the beam geometry.
The analysis of additive manufactured samples and powder material was performed using a JEOL JSM-IT500LV SEM (20 kV) in combination with an Ultim Max 100 EDX detector.
XRD measurements on the PBF-LB/M cylinder were conducted using a Bruker D8-A25 diffractometer applying the Kα-radiation of Cu with a wavelength of 1.5406 Å. The attenuation length is less than 8.5 µm, giving access predominantly to structural information in the vicinity of the surface of the manufactured samples. Therefore, the sample surface is ground using SiC paper with granulation of 280 to remove any remaining powder unless otherwise stated.
The DSC temperature scan on the PBF-LB/M sample was performed using a Perkin-Elmer DSC8000 under high-purity Ar flow in copper crucibles. The samples were heated from 333 to 863 K with a heating rate of 0.333 K/s. A second scan of the crystallized sample allows for baseline determination.
3 Results
3.1 Laser remelting of cast material
SEM images of the single scan tracks are shown in Fig. 3a–c. The material is molten in all investigated cases. The melt pool width increases from 71 µm (line A) to 113 µm (line C), increasing line energy density EL = \({P}_{L}/{v}_{s}\) [22] of 35 Ws/m (A) to 140 Ws/m (C). Dark structures in a distinctive V-shaped wave pattern (similar to kelvin ship wave pattern) can be seen in all three weld tracks along their center line, which indicates crystallinity or structural inhomogeneity. The contrast becomes darker at higher scan speeds.
Next, a TEM-lamella was FIB-milled along the y–z plane from the center of line A for further examination in different z-distances to the upper surface. Figure 4a shows the selected area diffraction (SAED) pattern from within the melt pool, 8 µm below the surface, that exhibits a single isotropic circular pattern, indicating the presence of a fully amorphous region. Close to the surface (0.7 µm), as shown in Fig. 4b; however, distinct diffraction spots are present, next to the isotropic scattering signal whose intensity is reduced. These stem from crystals confined close to the surface, which could originate from the natural oxide layer present in Ti-based alloys, and which could give rise to the dark contrast observed in Fig. 3a–c. The reduction of the contrast with increasing energy input might be attributed to the dissolution of the crystallites into the bulk material.
The area scan with 70 W, 2000 mm/s on cast material leads to a surface covered by coherent melt tracks decorated with occasional spatter particles (Fig. 3d). The X-ray diffractogram (Fig. 10a) of the laser-treated surface shows a single, broad peak absent of Bragg reflections, revealing an overall amorphous structure. This together with the amorphous structure derived from the SAED patterns within the single melt track allows to hypothesize that the glass-forming ability of \({\text{Ti}}_{60}{\text{Zr}}_{15}{\text{Cu}}_{17}{\text{S}}_{8}\) is generally sufficient for a PBF-LB/M processing. However, both powder atomization and PBF-LB/M can lead to compositional changes due to evaporation or contamination [20]. In addition, laser melting of powder layers causes repetitive reheating of material, presumably affecting the microstructure within the heat-affected zone [23], which will be further evaluated in the following sections.
3.2 Pre-alloying and atomization of powder feed stock
The atomized powder is screened at 80 µm to ensure a size distribution and spherical morphology suitable for additive manufacturing [24]. The sieved powder feed stock is analyzed by SEM. As typical for ultrasonic atomization, the morphology of the received powder particles is spherical with a rather narrow particle size distribution (Fig. 5) [20, 25].
SEM picture of powder particles. Areas for EDX mapping (Table 1) are highlighted
The composition of the powder particles is determined by EDX and compared to the original composition. A certain variation in the content of Ti and Cu is detected comparing measurement spots 1 and 3. In general, the composition is matching the expectations. The sulfur content is not considerably lowered by the atomization process, whereas the oxygen contamination is noticeable. A certain amount of W (up to 1.08 at.%) is detected, which seems to be related to the atomization with a W-electrode. The X-ray diffractogram (see Fig. 10a) shows Bragg reflections, next to a much weaker amorphous peak, revealing the presence of crystalline microstructure. The main peak agrees well with the reported (TiZr)2Cu phase [6, 7]. Due to the small particle size, cooling rates during atomization are typically adequate to yield amorphous particles if sufficient glass-forming ability of the composition is given. Investigations in Ref. [20] report that Zr01 powder particles below 50 µm vitrify during ultrasonic atomization. Similar findings are published in Ref. [26], where a threshold of 63 µm was found during gas-atomization of Vit101.
However, such crystallization effects in the powder material do not necessarily rule out the possibility to vitrify the material during PBF-LB/M. This projects a mediocre glass-forming ability on the powder material examined here. However, one has to bear in mind that the particles were classified below 80 µm which inherently reduces the amorphous yield. In addition, previous work on Zr01 proved that also partially crystalline powder can be vitrified during PBF-LB/M [27], since the cooling rates during the laser processing can exceed those during atomization [28, 29].
3.3 Additively manufactured samples
First, the laser–material interaction is investigated by analysis of single tracks processed with different energy inputs, as shown in Fig. 6. Subsequently, bulk samples are manufactured and analyzed.
Single tracks were exposed with parameter sets varying between \({P}_{L}\) = 40 to 120 W at 40 W steps, and \({v}_{s}\) = 800 to 2000 mm/s at 400 mm/s steps (see Table 3 in appendix). The scans were conducted first onto a PBF-LB/M processed \({\text{Ti}}_{60} {\text{Zr}}_{15} {\text{Cu}}_{17} {\text{S}}_{8}\) cast substrate without additional powder as remelting and secondly with powder application. The melt track width is measured, and the remolten cross-section is analyzed by SEM.
In Fig. 7, the melt pool width is plotted over the applied line energy density EL. The remelting of the bulk material (red data points) shows an almost linear increase of the track width with rising EL. For PBF (blue data points) in general, a linear increase with stronger variation can be seen, as for low energy densities the melt track width even drops down to zero, meaning that the energy input was not sufficient to fully melt and fuse the powder layer. This is the case for \({P}_{L}<\) 50 W and EL < 50 Ws/m.
Two different parameter sets are chosen for further investigation: I: \({P}_{L,I}\) = 80 W and \({v}_{s,I}\) = 1600 mm/s with minimum energy to melt a powder layer to a fused track (EL,I = 50 Ws/m) and II: \({P}_{L,II}\) = 120 W and \({v}_{s,II}\) = 800 mm/s with the maximum energy within this study (EL,II = 150 Ws/m), in order to display both extreme cases. A SEM image of a cross-section of the respective melt tracks is depicted in Fig. 8a. Two fundamentally different morphologies are displayed: for set I, a high contact angle of 123° and a shallow remolten zone with an approximate melt pool depth of 6 µm and a melt track height of 83 µm (aspect ratio total depth/width = 89/132 = 0.67). Set II leads to a contact angle of 42°, a higher melt pool depth of 63 µm compared to a melt track height of 44 µm and wider melt track of 224 µm (aspect ratio 117/224 = 0.5) but can be still considered as a conduction mode welding due to the semi-circular melt pool geometry [30].
Varying gray values of the SEM images in swirl-like patterns indicate density variations along the cross-section which can correspond to inhomogeneities such as chemical segregation or partial crystallization. For parameter set I, a bell-shaped structure appears which may originate from the solidification front during cooling. It can be assumed that the outer and lower zone solidified faster due to the convective heat transfer. The mid- and top regions (pear-shaped region) on the other hand depend on the mostly convective heat dissipation which is significantly lower in PBF-LB/M (REF) which leads to slower cooling. Here crystal-like dark shapes are visible as shown in the magnified image in Fig. 8b. Similar shapes of a smaller scale can be seen in the heat-affected zone surrounding the melt track. The size differences in these structures are likely caused by the crystallization mechanisms. High temperatures in the liquid phase favor crystal growth (on the top of I) while a devitrification at low temperatures leads to smaller crystallites (heat-affected zone of I).
Opposed to scan track I, no clear solidification front is observed on the top of Scan track II indicating a mostly amorphous structure. However, a crystalline powder particle with a diameter of ~ 50 µm is partly fused to the melt track, featuring a dendritic structure as depicted in the magnified image Fig. 8d). This underlines that the PBF-LB/M processing enhances the amorphous fraction compared to the atomized material.
Within the melt track, small spots indicate the presence of crystallites of a size below < 1 µm. The structures are below the resolution limits of the SEM. Further evaluation will be part of future work.
Bulk samples (3 × 3 × 30 mm) were processed with parameter set I for this study, since set II leads to elevated edges and an instable manufacturing process. The resulting samples have a relative density of up to 99.95% and are prone to cracking, as can be seen in Fig. 9. The swirl-shaped gray value variations can be connected to inhomogeneously distributed elements, which can be seen in Fig. 9. To quantify the elements variations, EDX spectra given in Table 2 are taken at specific points marked in Fig. 9.
SEM image of polished and etched bulk sample with regions of interest for EDX spectra (see Table 2) plus area mapping. Inset: PBF-LB/M bulk samples (80 W, 1600 mm/s), as build
In addition, a cylinder of 5 mm diameter is manufactured and used for further testing of the microstructure. The resulting spectrum presented in Fig. 10a shows a combination of amorphous and crystalline spectrum. The low amount of tungsten detected by EDX seems to have no detectable effect on the microstructure, since distinct peaks at 40.4° (110), 58.4° (200) and 73.3° (211) [31] cannot be observed. The DSC scan presented in Fig. 10b) shows the typical trace of a metallic glass, including a glass transition starting at Tg = 672 K, followed by a crystallization event at Tx = 724 K. Both temperature values are determined by the tangent method, as indicated by the dashed lines. The enthalpy of crystallization ΔHx is determined by integrating over the crystallization event (dashed line) and yields a value of 2.74 kJ/g-atom.
a Comparison of XRD measurements: pre-studied laser-treated high-purity cast material, PBF-LB/M fabricated material and atomized powder feedstock. b DSC heating scan with 20 K/min heating rate of the PBF-LB/M sample. The glass transition Tg and the crystallization temperature Tx resemble those of an as-cast reference [6, 7], but the crystallization enthalpy ΔHx is significantly decreased (by about 50%) in reference to a fully amorphous as-cast reference [7]
3.4 Comparison
In the following section, the properties of the additively manufactured Ti60Zr15Cu17S8 specimen are compared to the powder feed stock and the preliminary analyzed surface-treated cast material.
The EDX mappings indicate a stable elemental stoichiometry within the detectable range. Further, if oxygen is neglected, the sulfur content seems to remain stable throughout the process. In this context, it should be mentioned that an EDX analysis is not accurate for the exact determination of S and O as these are lighter elements, and therefore, a detailed chemical analysis by Carrier hot gas extraction reveals an oxygen content of less than 0.05 wt% of the high-purity cast material compared to 0.18 wt% of additively manufactured bulk material from industrial grade raw elements. The atomized powder material inhered 0.17 wt% oxygen, thus it can be concluded that the PBF-LB/M process is not the major cause for oxygen intake. The sulfur content is detected by combustion analysis is detected as 4.3 wt% for cast material vs. 4.73 wt% for powder feed stock and 4.9 wt% for the PBF-LB/M bulk material.
Since EDX measurements are used for qualitative analysis, here it is assumed that compositional relation of the main elements Ti, Zr, Cu, and S matches the target and minor losses occur during the processing [32].
Finally, the amorphicity is analyzed by XRD measurements. The diffractograms of laser-treated cast material, powder and PBF-LB/M-manufactured samples are given in Fig. 10a. The laser-treated surface is X-ray amorphous. In contrast, the powder sample shows a dominant Bragg reflection at 38°, as well as a broad amorphous peak of lower intensity at a similar position, and distinct reflections at higher angles, revealing the presence of both crystalline and amorphous structures. The crystal phase can be attributed to a (Ti, Zr)2Cu phase, as previously observed by Kuball et al. [6]. This corresponds to the observation in the SEM imaging of Fig. 8a), where a clear crystalline microstructure is observed within a powder particle in line II. For the additively manufactured sample, crystal formation is detected as, next to the amorphous halo, Bragg reflections are observed, indicating an overall partially crystalline structure. This correlates to the observed crystal shapes detected by SEM (Fig. 9a).
To further quantify the amorphicity of the PBF-LB/M sample, the heat flow determined by the DSC scan is shown in Fig. 10b. The temperatures of the glass transition and the crystallization onset, Tg and Tx, are in good agreement with the as-cast reference measured by Kuball in [7]. Yet, the enthalpy of crystallization features with 2.74 kJ/g-atom only about half the value observed in the fully amorphous cast reference [7]. Hence, the PBF-LB/M sample features an amorphous volume fraction of about 50%, which supports the qualitative XRD results in Fig. 10a.
4 Conclusion and outlook
This study reveals that Ti-based sulfur-containing bulk metallic glass (composition: Ti60Zr15Cu17S8) renouncing toxic components can be used for atomization and additive manufacturing by PBF-LB/M. A relative density of 99.95% is achieved. Summarized, it is found that:
-
Surface laser treatment of cast material leads to an amorphous microstructure as shown by XRD and TEM.
-
Flowable powder feed stock material with spherical morphology but crystalline structure results from ultrasonic atomization.
-
Compositional deviations and high-oxygen intake occur in powder.
-
PBF-LB/M bulk material is successfully produced and reaches a high relative density of 99.95%.
-
Low energy density leads to poor wettability and increased crystallinity in single tracks due to unfavorable weld track morphology
-
Produced material is prone to cracking
-
The amorphous fraction is significantly increased after PBF-LB/M processing compared to the powder particles.
These findings promote further research on the processability in commercial PBF-LB/M machines focusing on optimizing the purity of powder material, in-situ crack stability and amorphicity. A detailed analysis of possibly occurring beneficiary effects of pre-heating temperatures and optimized scan strategies as well as an analysis of the laser–material interaction by experimental and computational approaches are planned. Further, the adaptation for other material classes with comparable glass-forming ability can be approached. An energy input between I (50 Ws/m—elevated edges) and II (150 Ws/m—in-situ cracking) is assumed to be favorable for the production of dense, amorphous devices.
Data availability
Data available on request from the authors.
References
Klement W, Willens RH, Duwez P (1960) Non-crystalline structur in solidified gold-silicon alloys. Nature 187:869–870
Suryanarayana C, Inoue A (2017) Bulk metallic glasses, 2nd edn. CRC Press, Milton
Gong P, Deng L, Jin J et al (2016) Review on the research and development of Ti-based bulk metallic glasses. Metals 6:264. https://doi.org/10.3390/met6110264
Lin XH, Johnson WL (1995) Formation of Ti–Zr–Cu–Ni bulk metallic glasses. J Appl Phys 78:6514–6519. https://doi.org/10.1063/1.360537
Kim Y, Kim W, Kim D (2004) A development of Ti-based bulk metallic glass. Mater Sci Eng A 375–377:127–135. https://doi.org/10.1016/j.msea.2003.10.115
Kuball A, Gross O, Bochtler B et al (2019) Development and characterization of titanium-based bulk metallic glasses. J Alloy Compd 790:337–346. https://doi.org/10.1016/j.jallcom.2019.03.001
Kuball A. Development, characterization and processing of a novel family of bulk metallic glasses: sulfur-containing bulk metallic glasses, Saarländische Universitäts- und Landesbibliothek
Laws KJ, Gun B, Ferry M (2009) Influence of casting parameters on the critical casting size of bulk metallic glass. Metall Mater Trans A 40:2377–2387. https://doi.org/10.1007/s11661-009-9929-7
Pauly S, Wang P, Kühn U et al (2018) Experimental determination of cooling rates in selectively laser-melted eutectic Al-33Cu. Addit Manuf 22:753–757. https://doi.org/10.1016/j.addma.2018.05.034
Zhang P, Tan J, Tian Y et al (2021) Research progress on selective laser melting (SLM) of bulk metallic glasses (BMGs): a review. Int J Adv Manuf Technol. https://doi.org/10.1007/s00170-021-07990-8
Sohrabi N, Jhabvala J, Kurtuldu G et al (2021) Additive manufacturing of a precious bulk metallic glass. Appl Mater Today 24:101080. https://doi.org/10.1016/j.apmt.2021.101080
Wegner J, Kleszczynski S, Hechler S et al. (2017) Parameter study about processing Zr-based bulk metallic glass with laser beam melting. In: Witt G (ed) Rapid tech - international trade show & conference for additive manufacturing: proceedings of the 15th rapid tech conference. Carl Hanser Verlag GmbH & Co. KG, München
Frey M, Wegner J, Barreto ES et al (2023) Laser powder bed fusion of Cu-Ti-Zr-Ni bulk metallic glasses in the Vit101 alloy system. Addit Manuf 66:103467. https://doi.org/10.1016/j.addma.2023.103467
Jang J-H, Kim H-G, Kim H-J et al (2021) Crystallization and hardness change of the Ti-based bulk metallic glass manufactured by a laser powder bed fusion process. Metals 11:1049. https://doi.org/10.3390/met11071049
Deng L, Wang S, Wang P et al (2018) Selective laser melting of a Ti-based bulk metallic glass. Mater Lett 212:346–349. https://doi.org/10.1016/j.matlet.2017.10.130
Chen P-S, Tsai P-H, Li T-H et al (2023) Development and fabrication of biocompatible Ti-based bulk metallic glass matrix composites for additive manufacturing. Materials. https://doi.org/10.3390/ma16175935
Schonrath H, Wegner J, Frey M et al. (2023) Analytical modeling of cooling rates in PBF-LB/M of bulk metallic glasses. In: SFF Symposium Preceedings
Wegner J (2023) Funktionalisierung der prozessinhärenten Abkühlraten des Laser-Strahlschmelzens zur Verarbeitung metallischer Massivgläser. Dissertationsschrift
Cepeda-Jiménez CM, Castillo-Rodríguez M, Pérez-Prado MT (2019) Origin of the low precipitation hardening in magnesium alloys. Acta Mater 165:164–176. https://doi.org/10.1016/j.actamat.2018.11.044
Żrodowski Ł, Wróblewski R, Choma T et al (2021) Novel cold crucible ultrasonic atomization powder production method for 3D printing. Materials. https://doi.org/10.3390/ma14102541
Fleißner-Rieger C, Pogrielz T, Obersteiner D et al (2021) An additively manufactured titanium alloy in the focus of metallography. Pract Metallogr PM 58:4–31. https://doi.org/10.1515/pm-2020-0001
Wang L, Wei QS, Shi YS et al (2011) Experimental investigation into the single-track of selective laser melting of IN625. Adv Mater Res 233–235:2844–2848. https://doi.org/10.4028/www.scientific.net/AMR.233-235.2844
Farhang B, Ravichander BB, Venturi F et al (2020) Study on variations of microstructure and metallurgical properties in various heat-affected zones of SLM fabricated Nickel-Titanium alloy. Mater Sci Eng A 774:138919. https://doi.org/10.1016/j.msea.2020.138919
Brika SE, Letenneur M, Dion CA et al (2020) Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit Manuf 31:100929. https://doi.org/10.1016/j.addma.2019.100929
Phala N, Polese C, Choma T et al (2023) Atomisation of Ti-6Ta-1.5Zr-0.2Ru-5Cu (wt%) for additive manufacturing for biomedical applications. MATEC Web Conf 388:8003. https://doi.org/10.1051/matecconf/202338808003
Soares Barreto E, Uhlenwinkel V, Frey M et al (2021) Influence of processing route on the surface reactivity of Cu47Ti33Zr11Ni6Sn2Si1 metallic glass. Metals 11:1173. https://doi.org/10.3390/met11081173
Wegner J, Frey M, Piechotta M et al (2021) Influence of powder characteristics on the structural and the mechanical properties of additively manufactured Zr-based bulk metallic glass. Mater Des 209:109976. https://doi.org/10.1016/j.matdes.2021.109976
Hooper PA (2018) Melt pool temperature and cooling rates in laser powder bed fusion. Addit Manuf 22:548–559. https://doi.org/10.1016/j.addma.2018.05.032
Ciftci N, Ellendt N, Coulthard G et al (2019) Novel cooling rate correlations in molten metal gas atomization. Metall Mater Trans B 50:666–677. https://doi.org/10.1007/s11663-019-01508-0
Eagar TW, Tsai NS (1983) Temperature fields produced by traveling distributed heat sources. Weld J 62:346–355
Fayed A, Sallam M, Abdo G, Abdallah A (2017) Phases identifications by X-ray analysis for liquid phase sintered tungsten heavy alloys. IJRET 6:69–79. https://doi.org/10.15623/ijret.2017.0605012
Goldstein JI, Newbury DE, Michael JR et al (2018) Energy dispersive X-Ray microanalysis checklist. In: Goldstein JI, Newbury DE, Michael JR et al (eds) Scanning electron microscopy and X-Ray microanalysis. Springer, New York, pp 461–470
Acknowledgements
The authors want to thank Tobias Deckers and Pierre Foret from Linde AG for providing the ADDVance O2 precision system. The authors express their sincere thanks to Silke Rink/Prof. Dr.-Ing. Rüdiger Deike from the institute for technologies and metals at the University Duisburg-Essen for their support carrying out SEM measurements. In addition, the authors would like to thank Prof. Stefanie Hanke for their support in metallographic analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research was supported by the German Research Foundation (DFG) through Grant No. KL 3357/1-1. The PBF-LB/M system SLM 280 Hl was funded by the German Research Foundation (DFG) through Grant No. INST 20876/341-1. The XRD data were collected using the Rigaku Smartlab High Resolution X-ray diffractometer of the Nanoparticle Processing Technology (NPPT) group at the University Duisburg-Essen. The instrument is funded through the DFG (German Research Foundation) Instrument proposal INST 20876/395-1 FUGG and the state of North Rhine-Westphalia, Germany. Funding from Spanish Ministry of Science, Innovation and Universities under project PID2022-139008OB-I00 is acknowledged. We further acknowledge support from the Open Access Publication Fund of the University of Duisburg-Essen.
Author information
Authors and Affiliations
Contributions
Analysis: Hanna Schönrath, Jan Wegner, Maximilian Frey, Martin A. Schroer, Xueze Jin. Writing: Hanna Schönrath. Review and editing: Jan Wegner, Maximilian Frey, Martin A. Schroer, María Teresa Pérez-Prado, Stefan Kleszczynski. Resources: Stefan Kleszczynski. Conceptualization: Stefan Kleszczynski, Ralf Busch.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Figs. 11, 12, 13, and 14 and Tables 3, 4, and 5.
Single tracks without powder application. Parameter combinations acc. to Table 3
Single tracks with prior powder application. Parameter combinations acc. to Table 3
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schönrath, H., Wegner, J., Frey, M. et al. Novel titanium-based sulfur-containing BMG for PBF-LB/M. Prog Addit Manuf 9, 601–612 (2024). https://doi.org/10.1007/s40964-024-00668-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40964-024-00668-z