Abstract
This research aimed to produce high-entropy alloys (HEA), namely Mn–Fe–Co–Ni + 5Al and Mn–Fe–Co–Ni + 5Al + 5Ti, through the Powder Bed Fusion technique using elemental powders. Alloy composition has been selected to achieve a HEA matrix with strengthening intermetallic precipitates. Thermo-Calc software has been used to predict solidification behavior and phase stability for non-equilibrium conditions. The experiment involved the execution of an additive manufacturing process with a laser working in point-by-point exposure mode to produce samples using varying laser power and exposure time. The samples underwent investigation via macroscopic examination, porosity analysis, scanning electron microscopy, energy-dispersive X-ray spectroscopy, X-ray diffraction, and hardness testing. Results have shown that processing parameters and alloy constituents directly influenced processability and sample traits. What is more, a high-energy laser beam introduction to the material during the process has helped mitigate the formation of large Ti or Al oxides. In addition, EDS analysis indicated that higher Volumetric Energy Density values enhanced the uniformity of chemical composition, indicating that homogeneity can be achieved by selecting appropriate melting parameters. The results clearly show that these alloys can be successfully (by means of porosity and homogeneity) manufactured from elemental powders via the powder bed fusion technique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
High-entropy alloys (HEAs) are metallic materials that have attracted significant attention recently due to their unique properties. These materials are composed of five or more elements, typically in equimolar or near-equimolar proportions, resulting in high configurational entropy that promotes solid solution formation. HEAs may exhibit remarkable mechanical, physical, and chemical properties due to distinct effects, such as lattice distortion or cocktail effect. Thus, they can potentially revolutionize the design and development of new materials for various applications. The most common conventional techniques for producing HEAs are casting and arc melting. However, traditional methods of HEA manufacturing often encounter issues such as intensive evaporation of certain elements or poor homogeneity.[1,2,3]
Additive manufacturing attracted much attention thanks to the unique characteristics of processes and properties of obtained materials. ASTM F2792-12a distinguishes seven main types of AM processes (Figure 1(a)). One of the most promising methods for manufacturing HEAs is powder bed fusion (PBF) additive manufacturing technology, which uses a laser or electron beam to selectively melt layers of metal powder to build up a three-dimensional object. This process offers several advantages, including producing complex geometries, near-net-shape parts, and reduced material waste.[4,5] High-entropy alloys produced by additive manufacturing methods have been the subject of many investigations and comparative analyses, as shown in Figure 1(b).[6,7,8,9,10,11] It has been already shown that in situ alloying during the PBF process provides a unique opportunity to synthesize new HEAs with tailored compositions and microstructures (Figure 1(c)).[12,13,14,15,16,17]
(a) AM methods distinction by ASTM F2792-12a, (b) number of publications on additively manufactured HEAs by various methods, and (c) number of publications on AM HEAs divided by manufacturing method and form of input material. The data come from Ref. [16]
A review of the current literature indicates two main research paths in the in situ alloying of high-entropy alloys using the PBF method. One of them uses alloy or elemental powders to modify the composition and properties of the pre-alloyed HEA. This approach is capable of enhancing HEA parameters through precipitation strengthening, dispersion strengthening (ODS), or by increasing dislocation density.[18,19,20,21,22] On the other hand, the alternative path proposes the use of pure elemental powders for the in situ alloying of HEAs during the AM process. The advantage of the latter lies in the possibility of skipping the conventional alloy powder casting and atomization processes, which require additional effort and make AM processing less cost effective. However, this method is not without its drawbacks. The main issues arise from the increased tendency of cracking, internal defects formation, and elemental segregation. Nevertheless, promising results have been demonstrated in studies involving alloys based on transition metals such as Ni, Co, and Fe,[23,24,25] where good-quality samples have been produced with PBF/AM. So far, several HEAs systems have been successfully synthesized by PBF methods, such as Cr–Fe–Co–Ni and Mn–Cr–Fe–Co–Ni.[26,27,28,29]
For instance, Sun et al.[30] investigated the impact of varying Al concentrations on the microstructure of an AlXCrFeCoNi system. Their findings revealed that the Al addition could alter the microstructure from FCC (for low Al content) to FCC + BCC/B2 and BCC/B2 (above 1 pct). They observed that the formation of the dual-phase FCC + BCC/B2 reduced the crack density of produced samples. Another view is shown by Su et al.,[31] where Al was added to an AlXCrFeNi2Cu system. The CrFeNi2Cu HEA produced by the L-PBF process exhibited a columnar FCC microstructure prone to hot cracks. The addition of Al resulted in a cellular FCC dendrite with a B2 face matrix, reducing crack propagation. The influence of laser energy density on the as-built density of a CrFeCoNi HEA using both mixed elemental and pre-alloyed powders was investigated by Hou et al.[25] They observed that a higher laser energy density was necessary to fabricate parts from mixed elemental powder due to in situ HEA formation. This is in accordance with the results obtained by the authors of this publication during previous studies.[23] In addition, under the same printing conditions (except for laser power), the density of pre-alloyed powder samples was higher compared to mixed elemental powder samples.
The abovementioned studies have shown promising properties for high-temperature applications as parts of gas turbines, rocket engines, nuclear reactors, and cryogenic devices. However, there are still several challenges that need to be addressed, such as microstructural homogeneity, porosity control, and mechanical properties.[26,27,28,29,32,33,34] Since the elements used for in situ manufacturing can significantly differ in properties, which may impact the final properties of the prints,[35] this synthetization method can potentially help overcome these issues.
The key benefits of using mixed elemental powder for creating HEAs through PBF for producing multi-component HEAs include flexibility in composition, lower costs for powder manufacturing, and the widespread availability of commercial elemental powders.[13,16,36] The development of HEAs manufactured by PBF methods represents a promising way to design and fabricate new materials with superior properties.
This paper investigates the properties of Mn–Fe–Co–Ni + 5Al and Mn–Fe–Co–Ni + 5Al + 5Ti HEAs manufactured via PBF and compares them to Mn–Fe–Co–Ni + 5Ti alloy investigated in authors’ previous research.[23] The chemical composition of the Mn–Fe–Co–Ni alloy was intentionally enriched in the two elements (Ti and Al) that make up the γ′ phase. In addition, these elements are susceptible to oxidation, forming nanometer-sized oxides in the structure. This paper is a continuation of the author’s previous work on HEAs made by conventional methods[37,38,39,40] as well as the PBF method using an in situ alloying approach.[23] Also, to the best of the authors’ knowledge, this is the first time that Mn–Fe–Co–Ni + 5Al and Mn–Fe–Co–Ni + 5Al + 5Ti alloys have been produced using PBF with the use of elemental powders.
2 Materials and Methods
2.1 Specimen Fabrication
The study involved several stages, including thermodynamic calculations, powder selection and preparation, PBF processing, and testing methods. The thermodynamic calculations were performed using the Thermo-Calc software, utilizing the TCHEA5 database. The calculations were used to predict the alloys' solidification behavior and phase stability under non-equilibrium conditions. Based on previous research,[23] three compositions were selected for extended investigation: Mn–Fe–Co–Ni + 5Ti, Mn–Fe–Co–Ni + 5Al, and Mn–Fe–Co–Ni + 5Al + 5Ti (Table I) and the melting temperatures obtained in the simulations ranged from 1260 °C to 1365 °C (Figure 2). Performed calculations determined that the melting temperature for alloy C is around 1260 °C, alloy D around 1365 °C, and alloy E around 1270 °C. According to the findings, the suggested alloys are expected to exhibit a complicated phase structure, with each alloy consisting of both FCC and BCC phases. Furthermore, all of the alloys are expected to include intermetallic phases.
The powders utilized in the manufacturing process were selected to have a purity of at least 99 pct and included nickel, cobalt, iron, manganese, titanium, and aluminum. The powders had various morphologies and sizes, with an average particle size between 20 and 50 µm. Specifically, the Ni, Co, and Ti powders were spherical, while the Fe, Mn, and Al powders were irregularly shaped. The Jinan Winner 2000 laser particle size analyzer performed a particle size distribution analysis. This technique disperses the powder sample in a liquid medium to prevent particle clumping and then passes the sample through a laser beam. A Turbula® was used to mix each powder batch for 30 minutes without a protective atmosphere, resulting in a batch weight of around 1 kg. Figure 3 illustrates the used elemental powders' different morphologies and size distributions (Figure 3(a)), as well as EDS images of mixtures of elemental powders, after 30 minutes of mixing (Figure 3(b)). There were no changes in the morphology of the powders after mixing. Occasional irregular powders of Fe and Mn were crushed. There was also no pre-alloying of the powders.
The PBF process was conducted utilizing a commercially available Renishaw AM400 machine, which featured a laser beam power of 400 W operating at a wavelength of 1064 nm in a point-by-point working mode. The procedure did not involve heating the powder bed. To gain a comprehensive understanding of the alloys’ behavior, the variables in this study were the laser power and exposure time, while other parameters such as laser point distance, layer thickness, and hatch distance remained constant. The melting temperatures determined through Thermo-Calc calculations were compared to literature data with a focus on Ni, Co, and Fe alloy systems.[15,22,25] These materials, along with their process parameters, served as reference points for selecting the parameters in this study for the HEA alloys. The temperature of molten material during the process was not measured due to hardware limitations. A summary of the relevant parameters can be found in Table II. Printing was carried out using a meander scanning strategy, with a change in scanning direction between layers set at 67 deg. The printing process was conducted under protective argon atmosphere conditions, ensuring that the oxygen level remained below a maximum of 1000 atomic PPM. The Volumetric Energy Density (VED) was calculated using the following equation[41]:
2.2 Testing Methods
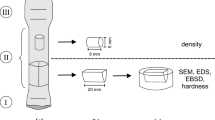
Twenty-four samples measuring 10 × 12 × 5 mm (8 samples per process) were produced. With the use of electrical discharge machining (EDM), samples were cut in a parallel to the printing direction. The material was embedded in a conductive resin and polished for further investigation. For SEM/SE/BSE analysis, samples were etched electrolytically. This study employed different techniques to characterize the fabricated samples' macrostructure and determine the sample porosity and hardness. For quantitative composition analysis, inductively coupled plasma atomic emission spectroscopy (ICP-AES) was used. Determination of the oxygen content was carried out by inert gas fusion technique on a LECO ON836 analyzer. Light microscopy (LM) with a NIKON Eclipse MA100 and QIMAGING Micropublisher 3.3 RTV camera was used for imaging the samples' macrostructure, and sample porosity was quantified through the ImageJ software.[42] Scanning electron microscopy (SEM) using secondary electron (SE) and backscattered electron (BSE) detectors with an FEI VERSA 3D instrument was utilized for microstructure and morphology characterization. The SEM fitted with an EDS detector (Oxford Instruments Ultim Max) was used to analyze the samples’ homogeneity. Furthermore, the microstructure analysis was complemented by X-ray diffraction (XRD) measurements, performed in Bragg–Brentano geometry with a PANalytical Empyrean diffractometer using Co Kα radiation at room temperature. Vickers hardness tests were conducted on the samples' cross sections using a TUKON 2500 hardness tester with an automatic measurement system and a 4.9 N load. The average hardness of each sample was determined from 40 measurements, with values affected by porosity being excluded.
3 Results
The quantitative analysis of three manufactured samples was conducted as presented in Table III. For the test, a sample was selected from each alloy produced using 250 W of laser power and 145 μs of exposure time. It should be noted that both manganese and aluminum exhibited a noticeable reduction in their respective contents. Anomaly was observed in the case of alloy C, in the form of iron deficiency in favor of nickel.
For all alloys and parameters’ variants oxygen content was investigated. Figure 4 shows oxygen content decreased for shorter exposure time with increasing laser power in alloys C and E. Slight increase was observed for alloy D. For longer exposure time and with increasing laser power across all alloys, a tendency was observed for the oxygen content to converge toward a value of 0.15 pct.
Based on the average hardness results (Figure 5), it is evident that the VED affects the material properties. Among the manufactured alloys, Alloy E showed the highest hardness. The hardness increased with increasing laser power for an exposure time of 74 μs. However, for an exposure time of 145 μs, the hardness decreased with increasing laser power. It may suggest a stress reduction caused by the introduction of a significant amount of energy, which caused an increase in print temperature and lowering cooling rate. The lowest hardness was observed in alloy D, which contains aluminum. The scatter of the results is also noticeably smaller for samples manufactured with an exposure time of 145 μm. It can be correlated with the porosity results or the homogeneity of the alloys.
The macrostructure of the metallic prints and their porosity are presented in Figures 6 and 7. Results indicate that the laser power and exposure time greatly affect the porosity of the prints. Analysis of the macrostructures revealed that lower laser powers resulted in unmelted powder particles. An increase in laser power decreases the porosity of all alloys. Notably, low porosity was achieved for all alloys with longer exposure time due to significantly higher VED. Except for one sample, for a longer exposure time, all other samples achieved porosity below 0.6 pct. Although the VED of the samples labeled as “2” and “7” (150 W/145 µs and 300 W/74 µs) is comparable, slight differences can be observed in the properties of the obtained samples, while significant difference is seen by means of porosity for alloy D. It may indicate that the VED can only serve as a general simplification in predicting the properties of the produced materials.
In the SEM–SE images of electrolytically etched samples (Figure 8), a dendritic structure can be observed, which is indicative of non-equilibrium solidification caused by rapid cooling from the liquid. Within the dendrites, two distinct types of precipitates can be identified. The first type consists of spherical precipitates ranging in size from 0.5 to 3 μm, while the second type consists of smaller precipitates with irregular shapes measuring around 100 nm. SEM–BSE imaging reveals that the larger spherical precipitates contain elements of lower atomic number compared to the surrounding material, while the smaller irregular precipitates exhibit a composition similar to that of the matrix, giving almost no contrast. Quantitative analysis of dendritic structures shows that geometric characteristics such as dendrite arm spacing do not change significantly with changing process parameters; therefore, no definitive conclusions can be drawn on that matter.
EDS analysis was carried out to investigate the distribution of oxygen within the material (Figure 9). The results indicated that regions with elevated oxygen concentration displayed irregular shapes and sizes, approximately around 50 μm, particularly for lower VED values. Higher VED values had a beneficial effect on the fragmentation and dispersion of these precipitates, resulting in a reduction in their proportion within the matrix material. This suggests that a VED value influences the distribution of oxygen within the material, with higher VED contributing to a more uniform dispersion of oxygen-rich regions.
The XRD outcomes (depicted in Figure 10) for samples produced with the greatest VED (C8, D8, and E8) indicate the existence of the γ phase. Moreover, small amounts of (CoNi)3Ti, (CoNi)3Al, and (NiCo)3AlTi were detected in all alloys (respectively alloy C, D, and E) as well as oxides of titanium and aluminum.
EDS was utilized to investigate the influence of laser power and exposure time on the homogeneity of printed samples (Figure 11). Lower laser powers resulted in poor homogeneity and inadequate powder fusion, whereas higher powers achieved better homogeneity. For the highest VED, alloy C exhibited uniformly distributed Ti oxides. Accordingly, aluminum oxides were observed in alloy D, and in alloy E, both titanium and aluminum oxides were present. No Mn oxides were spotted in the structure of examined samples.
4 Results and Discussion
The thermodynamic calculations conducted using Thermo-Calc simulations demonstrate good agreement with the experimental results, confirming the correlation between the predicted melting point and the tendency for homogenization. In the case of alloy C (specifically, sample C8 produced with the highest VED), a solid solution with an FCC structure and the precipitation of the Co3Ti phase and Ti oxides were observed. It suggests that the rapid crystallization during PBF significantly restricts phase transformations. Despite variations in the liquidus and solidus temperatures of the examined alloys (C: ~ 250 °C, D: ~ 195 °C, E: ~ 255 °C), high homogeneity was observed in samples produced with sufficient VED. According to the solidification range theory, the likelihood of hot cracking is expected to be proportional to the solidification range.[43] Among the investigated samples, the highest occurrence of cracks was observed at the lower range of process parameters. However, it is unclear whether these cracks were caused by stress or insufficient melting, resulting in elongated voids resembling fractures. More distinct single cracks were observed for the highest VED in all examined alloys. Hence, it appears that a strong relationship with the solidification range theory was not observed for PBF prints. This observation may be attributed to the small melting pool and high cooling rates, which effectively limit transformations during crystallization.
The quantitative analysis showed that both manganese and aluminum may be prone to evaporation from melted metal pool. Such behavior was observed for both these elements under vacuum during smelting.[44,45] Although in our case, the atmospheric pressure was used, the laser power and exposure time were adequate to melt all elements, thus, significantly exceeding the required levels for manganese and aluminum due to their lower melting temperatures. Evaporation from alloys during L-PBF is well recognized for low melting metals and a countermeasure for such losses involves altering the content of elements or usage of additives.[46] Other anomalies in alloys’ compositions (especially lower Fe content in alloy C) can be attributed to measurement errors during powder preparation, uneven powder mixing or segregation during the powder spreading process on the working area.
To evaluate the hardness of investigated alloy samples C8, D8, and E8, a comparison was made with previously reported results of PBF samples featuring similar chemical compositions (as presented in Table IV). The analysis of hardness variations reveals a noticeable trend: shorter exposure times (74 μs) exhibit a wider range of hardness values than longer exposure times (145 μs). It can be attributed to the uneven melting of the elemental powders at lower volumetric energy density (VED). Due to the introduction of a substantial amount of energy, resulting in a decrease in crystallization rate, the hardness of the samples in all alloys slightly decreased. It suggests a possible reduction in stress caused by introducing a significant energy input. The hardness of sample C8 (Mn–Fe–Co–Ni + 5Ti) closely resembles that of the equimolar Cr–Mn–Fe–Co–Ni (Cantor) alloy (pre-alloyed powder).[47] Initially, the C alloy may appear different from Cantor's alloy due to the absence of Cr and the addition of Ti; however, significant variations in the composition of individual alloying elements exist within the C alloy. Alloy E exhibited the highest hardness, aligning with expectations given the higher entropy of this six-component alloy. The higher hardness obtained for alloys containing titanium may also result from the formation of secondary phases providing precipitation strengthening, similar to that observed in Reference 48.
Conversely, the lowest hardness was observed in alloy D. According to the literature, it was suggested that aluminum, in some scenarios, can negatively affect printability and promote segregation formation.[49] The oxide film on aluminum powder particles can have a negative effect on homogenization during in situ alloying in the PBF process. The presence of oxide films can hinder the diffusion of aluminum atoms into the surrounding matrix, leading to incomplete alloying and segregation effects within the printed part.
The results demonstrate a strong dependency between laser power, exposure time, and porosity of the printed components (Figure 12). Examination of the macrostructures revealed that lower laser powers led to unmelted powder particles while increasing the laser power reduced porosity across all alloys. It is important to highlight that longer exposure times, which correspond to higher volumetric energy density (VED), resulted in significantly lower porosity for all alloys—except, for sample D2, longer exposure times yielded porosity levels below 0.6 pct. Unlike in the authors’ previous study,[23] there was no observed increase in porosity at the highest VED values. However, the authors speculate that this outcome is not specific to the studied materials and that a broader range of process parameters enabling higher energy input would yield similar results. Moreover, Chen et al.,[22] presented a similar phenomenon, where the pre-alloyed Cr–Fe–Co–Ni powder, in situ alloyed with the Mn, increased its density with the rising VED up to 259 J/mm3. The study proposed that the presence of Mn contributes to restraining the defect formation in parts produced by PBF at higher VED values, but no explanation was presented for such influence. This claim does not coincide with the results shown in our previous study,[23] where Mn–Fe–Co–Ni alloy does not exhibit such an effect. However, when considering all these results combined, it could be hypothesized that increasing entropy by introducing additional elements to the composition could be responsible for the upward shift in the optimal VED values. VED values frequently reported in the literature[22,25] are typically above 200 J/mm3. The variation in our results may come from the specific point-by-point working mode of the Renishaw AM400, which provides different thermal conditions and, therefore could not be directly comparable to values obtained from devices with lasers operating in continuous mode.[50]
EDS analyses and SEM–BSE imaging indicated the existence of oxides in the investigated alloys. Specifically, titanium oxides were observed in alloys C and E, while aluminum oxides were found in alloys D and E. The EDS maps revealed areas where oxygen overlapped with elements prone to oxidation. It is worth noting that despite the expectation that Al2O3 would be the dominant oxide in alloy E based on the Ellingham diagram (Figure 13), the presence of titanium was frequently detected in the same areas as aluminum and oxygen. Interestingly, no significant oxidation of manganese was observed in the examined alloys. In addition, important factor in oxide formation is oxygen content dependence on process parameters. It was observed that oxygen content tends to approach the equilibrium value, most likely determined by the oxygen traces present in the protective atmosphere. Longer exposure time and higher laser powers provided more opportunities to equalize the oxygen content of the liquid metal with the environment. This explains the increase in oxygen content for alloy D, while alloys C and E exhibited a decrease, eventually reaching very similar values.
Ellingham diagram, in which Mn, Ti, and Al elements were marked in green. The graphic is adapted from Ref. [51] under the terms of the Creative Commons Attribution—NonCommercial-ShareAlike 4.0 International (https://creativecommons.org/licenses/by-nc-sa/4.0/). The data come from Ref. [52] (Color figure online)
The regions with high oxygen concentration in EDS results exhibit irregular shapes and sizes of approximately 50 μm for lower VED values. However, higher VED values have a favorable impact on the fragmentation and distribution of these precipitates, leading to a decrease in their proportion within the matrix material. Furthermore, the SEM–BSE imaging shows the contrast between the darker spherical precipitates, ranging in size from 0.5 to 3 μm, and the surrounding matrix. The presence of lower atomic number areas suggests the potential existence of small spherical oxides and larger ones, which were identified using the EDS method. In the presence of titanium and aluminum, both of these elements may form oxides at the same time. A related study[53] observed that the IN718 alloy produced through additive manufacturing (AM) had residual oxides predominantly composed of Al2O3. The distribution and morphology of these oxides were influenced by the specific processing and the cooling rates during solidification. Similarly, our results suggest that the morphology of the oxides can be controlled with process parameters such as laser power and exposure time. Moreover, inert gas fusion analysis shows that volume fraction may be influenced by oxygen level in a protective atmosphere. Unfortunately, some sources[22,34] lack information on the permitted oxygen content in the process chamber and do not endeavor to create a connection between the final oxygen content and this variable.
The X-ray diffraction results obtained for samples manufactured with the highest volumetric energy density (C8, D8, and E8) provide strong evidence for the presence of the face-centered cubic phase. However, the XRD results alone cannot be considered conclusive evidence for the presence of (CoNi)3Ti, (CoNi)3Al, TiO2, and Al2O3 due to the limited resolution of the method and the small quantities of these phases in the manufactured materials.
Furthermore, the results of EDS studies demonstrated a significant impact of process parameters on the homogenization of the alloy. More uniform chemical composition was achieved using higher VED values, indicating that homogeneity can be attained by selecting appropriate parameters. Important to note is that employing additional heat treatment to achieve similar results is common practice. In the research conducted by Hou,[25] high-temperature heat treatment was utilized to eliminate microstructure segregation and achieve homogeneity in their Cr–Fe–Co–Ni HEA samples. Therefore heat treatment is another area we plan to investigate.
5 Summary
This study investigated and compared the influence of laser power and exposure time on the quality and properties of 5-, and 6- component alloys fabricated via powder bed fusion (PBF) using elemental powders. To the best of the authors’ knowledge, this is the first time that Mn–Fe–Co–Ni + 5Al and Mn–Fe–Co–Ni + 5Al + 5Ti alloys have been produced using PBF from elemental powders. The key findings and conclusions of this research may be summarized as follows:
-
The thermodynamic calculations using Thermo-Calc simulations demonstrated good agreement with experimental results, confirming the correlation between predicted melting points and the tendency for homogenization in the investigated alloys.
-
The applied VED has a direct impact on the porosity of the printouts—low VED results in high porosity and unmelted elemental powders.
-
Titanium oxides were observed in alloys C and E, while aluminum oxides were observed in alloys D and E. High energy should be introduced to the material during the process to reduce the occurrence of large Ti or Al oxides because longer exposure time and higher laser powers reduce oxygen content.
-
EDS studies revealed that higher VED values led to more uniform chemical composition, indicating that homogeneity can be achieved by selecting appropriate process parameters. The results clearly show that this type of alloys can be produced from elemental powders using PBF.
-
Appropriate chemical composition of the alloy allows for obtaining a high-entropy matrix with strengthening precipitations of intermetallic phases. It gives the possibility of obtaining various properties. The highest hardness was observed in alloy E due to (NiCo)3AlTi precipitations.
-
Process parameters can be utilized for the design and control of oxides morphology and their volume fraction.
-
Bearing in mind all the advantages of in situ HEA additive manufacturing we believe that extensive research in this area is critical. Utilizing elemental powders readily available in the market, we successfully demonstrate an approach to bypass the costly and time-consuming procedure of manufacturing alloy powders from high-entropy alloys.
References
E.P. George, D. Raabe, and R.O. Ritchie: Nat. Rev. Mater., 2019, vol. 4(8), pp. 515–34.
J.W. Yeh, Y.L. Chen, S.J. Lin, and S.K. Chen: Mater. Sci. Forum, 2007, vol. 560, pp. 1–9.
B. Cantor: Entropy, 2014, vol. 16(9), pp. 4749–68.
Y. Zhang, L. Wu, X. Guo, S. Kane, Y. Deng, Y. Jung, J. Lee, and J. Zhang: J. Mater. Eng. Perform., 2018, vol. 27(1), pp. 1–13.
T.D. Ngo, A. Kashani, G. Imbalzano, K.T.Q. Nguyen, and D. Hui: Composites B, 2018, vol. 143(February), pp. 172–96.
J. Kim, A. Wakai, and A. Moridi: J. Mater. Res., 2020, vol. 35(15), pp. 1963–83.
C. Han, Q. Fang, Y. Shi, S.B. Tor, C.K. Chua, and K. Zhou: Adv. Mater., 2020, vol. 32(26), pp. 1–41.
J.M. Torralba and M. Campos: Metals, 2020, vol. 10(5), pp. 1–15.
A. Ostovari Moghaddam, N.A. Shaburova, M.N. Samodurova, A. Abdollahzadeh, and E.A. Trofimov: J. Mater. Sci. Technol., 2021, vol. 77, pp. 131–62.
S. Chen, Y. Tong, and P.K. Liaw: Entropy, 2018, vol. 20(12), p. 937.
W. Zhang, A. Chabok, B.J. Kooi, and Y. Pei: Mater. Des., 2022, vol. 220, p. 110875.
S.L. Sing, S. Huang, G.D. Goh, G.L. Goh, C.F. Tey, J.H.K. Tan, and W.Y. Yeong: Prog. Mater. Sci., 2021, vol. 119(March), p. 100795.
M.H. Mosallanejad, B. Niroumand, A. Aversa, and A. Saboori: J. Alloys Compd., 2021, vol. 872, p. 159567.
Y. Brif, M. Thomas, and I. Todd: Scripta Mater., 2015, vol. 99, pp. 93–96.
S. Ewald, F. Kies, S. Hermsen, M. Voshage, C. Haase, and J.H. Schleifenbaum: Materials, 2019, vol. 12(10), p. 1706.
T. Ron, A. Shirizly, and E. Aghion: Materials, 2023, vol. 16(6), p. 2454.
M. Tokarewicz and M. Grądzka-Dahlke: Metals, 2021, vol. 11(8), p. 1302.
P. Chen, C. Yang, S. Li, M.M. Attallah, and M. Yan: Mater. Des., 2020, vol. 194, p. 108966.
D. Vogiatzief, A. Evirgen, M. Pedersen, and U. Hecht: J. Alloys Compd., 2022, vol. 918, p. 165658.
S. Yan, X. He, M. Krüger, Y. Li, and Q. Jia: Mater. Des., 2024, vol. 238, p. 112676.
P. Chen, X. Yao, M.M. Attallah, and M. Yan: J. Mater. Sci. Technol., 2022, vol. 123, pp. 123–35.
P. Chen, S. Li, Y. Zhou, M. Yan, and M.M. Attallah: J. Mater. Sci. Technol., 2020, vol. 43, pp. 40–43.
R. Bardo, R. Dziurka, K. Fryzowicz, G. Cios, M. Marciszko-Wiąckowska, M. Gajewska, C. Hain, and P. Bała: J. Alloys Compd., 2024, vol. 972, p. 172862.
F. Huber, D. Bartels, and M. Schmidt: Materials, 2021, vol. 14(11), p. 3095.
Y. Hou, H. Su, H. Zhang, X. Wang, and C. Wang: Metals, 2021, vol. 11(6), p. 942.
Y.K. Kim, J. Choe, and K.A. Lee: J. Alloys Compd., 2019, vol. 805, pp. 680–91.
A. Piglione, B. Dovgyy, C. Liu, C.M. Gourlay, P.A. Hooper, and M.S. Pham: Mater. Lett., 2018, vol. 224, pp. 22–25.
D. Lin, L. Xu, Y. Han, Y. Zhang, H. Jing, L. Zhao, and F. Minami: Intermetallics, 2020, vol. 127(August), p. 106963.
R. Li, P. Niu, T. Yuan, P. Cao, C. Chen, and K. Zhou: J. Alloys Compd., 2018, vol. 746, pp. 125–34.
Z. Sun, X. Tan, C. Wang, M. Descoins, D. Mangelinck, S.B. Tor, E.A. Jagle, S. Zaefferer, and D. Raabe: Acta Mater., 2021, vol. 204, p. 116505.
Y. Su, S. Luo, and Z. Wang: J. Alloys Compd., 2020, vol. 842, p. 155823.
A. Amar, J. Li, S. Xiang, X. Liu, Y. Zhou, G. Le, X. Wang, F. Qu, S. Ma, W. Dong, and Q. Li: Intermetallics, 2019, vol. 109, pp. 162–66.
R. Zhou, Y. Liu, C. Zhou, S. Li, W. Wu, M. Song, B. Lin, X. Liang, and P.K. Liaw: Intermetallics, 2017, vol. 94, pp. 165–71.
Z.G. Zhu, Q.B. Nguyen, F.L. Ng, X.H. An, X.Z. Liao, P.K. Liaw, S.M.L. Nai, and J. Wei: Scripta Mater., 2018, vol. 154, pp. 20–24.
M.S. Knieps, W.J. Reynolds, J. Dejaune, A.T. Clare, and A. Evirgen: Mater. Sci. Eng. A, 2021, vol. 807, p. 140849.
C. Haase, F. Tang, M.B. Wilms, A. Weisheit, and B. Hallstedt: Mater. Sci. Eng. A, 2017, vol. 688, pp. 180–89.
K. Górecki, P. Bała, T. Kozieł, and G. Cios: Arch. Metall. Mater., 2017, vol. 62(4), pp. 2141–45.
P. Bała, K. Górecki, W. Bednarczyk, M. Watroba, S. Lech, and J. Kawałko: J. Mater. Res. Technol., 2020, vol. 9(1), pp. 551–59.
K. Górecki, P. Bała, W. Bednarczyk, and J. Kawałko: Mater. Sci. Eng. A, 2019, vol. 745, pp. 346–52.
P. Bała, K. Górecki, R. Dziurka, and T. Kozieł: Materials, 2023, vol. 16(18), p. 6297.
J.A. Cherry, H.M. Davies, S. Mehmood, N.P. Lavery, S.G.R. Brown, and J. Sienz: Int. J. Adv. Manuf. Technol., 2015, vol. 76(5), pp. 869–79.
C.A. Schneider, W.S. Rasband, and K.W. Eliceiri: Nat. Methods, 2012, vol. 9(7), pp. 671–75.
H. Ding, H. Fu, Z. Liu, R. Chen, B. Liu, and Z. Zhong: Acta Metall. Sin.Sin., 1997, vol. 33, pp. 921–26.
L. Blacha, B. Olesiak, A. Smalcerz, and T. Matuła: Arch. Mater. Sci. Eng., 2012, vol. 58, pp. 28–32.
B. Olesiak, L. Blacha, G. Siwiec, and A. Smalcerz: Adv. Mater. Res., 2014, vol. 1036, pp. 422–27.
L. Deillon, F. Jensch, F. Palm, and M. Bambach: J. Mater. Process. Technol., 2022, vol. 300, p. 117416.
R. Savinov, Y. Wang, J. Wang, and J. Shi: Procedia Manuf., 2021, vol. 53, pp. 435–42.
M. Cagirici, P. Wang, F.L. Ng, M.L.S. Nai, J. Ding, and J. Wei: J. Mater. Sci. Technol., 2021, vol. 94, pp. 53–66.
E.O. Olakanmi, R.F. Cochrane, and K.W. Dalgarno: Prog. Mater. Sci., 2015, vol. 74, pp. 401–77.
P. Tang, S. Wang, M. Long, H. Duan, S. Yu, D. Chen, and S. Fan: Metall. Mater. Trans. B, 2019, vol. 50B, pp. 2804–14.
J. Chivall and D. Brook. The Ellingham Diagram. University of Cambridge, Department of Materials Science & Metallurgy. https://www.doitpoms.ac.uk/tlplib/ellingham_diagrams/ellingham.php. Accessed 5 Nov 2023.
H.J.T. Ellingham: J. Soc. Chem. Ind., 1944, vol. 63(5), pp. 125–33.
H. Yu, S. Hayashi, K. Kakehi, and Y.-L. Kuo: Metals, 2019, vol. 9(1), p. 19.
Acknowledgments
This work was financially supported by the Polish Ministry of Science and Higher Education as part of the “Implementation Doctorate Programme” realized by the AGH University of Science and Technology and the Company Progresja S.A. The research results presented in this paper have been developed with the use of equipment financed from the funds of the “Excellence Initiative - Research University” Program at AGH University of Krakow. Thanks to Ph.D. Marianna Marciszko-Wiąckowska for her assistance in XRD investigations.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bardo, R., Dziurka, R., Fryzowicz, K. et al. Exploring Elemental Powder Approach for Making Al and Ti Containing High-Entropy Alloys by Powder Bed Fusion. Metall Mater Trans A 55, 2840–2854 (2024). https://doi.org/10.1007/s11661-024-07439-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-024-07439-z