Abstract
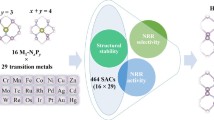
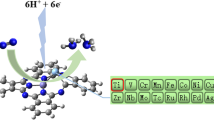
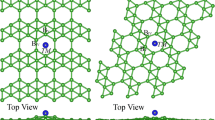
Designing highly selective and efficient single-atom electrocatalysts is essential for ammonia production under ambient conditions. This paper describes a density functional theory study on exploring the performance trends of transition metal complexes with P-based ligands in nitrogen reduction reaction (NRR) and further develops a design principle for high-performance single-atom catalysts (SACs) of NRR. Among the explored catalysts, W@BP (0.40 eV), Ta@BP (0.47 eV), and Nb@BP (0.53 eV) are identified as remarkable candidates with low free energy change in the potential-limiting step, high stability and high electrical conductivity for NRR. It is worth noting that almost all SACs with P-based ligands exhibit high NRR selectivity, due to the fact that they adsorb *N2 more strongly than *H. The adsorption free energy of *N2H can be considered as a descriptor for the intrinsic activity trends in NRR. Furthermore, by constructing a volcano plot of the activity against the electronic charge on metal centers, it is demonstrated that the metal center with a moderate amount of positive charge can promote the catalytic performance of NRR.
摘要
设计和开发高选择性、高活性的单原子电催化剂是实现在 常规环境条件下合成氨的关键. 本论文利用密度泛函理论对P配体 在N2还原反应(NRR)中的应用前景进行了预测, 并且提出了一种高 性能NRR单原子催化剂的设计准则. 理论计算结果显示, W@BP (0.40 eV)、Ta@BP(0.47 eV)和Nb@BP(0.53 eV)由于具有低反应自 由能、高稳定性和导电性, 在高效电催化NRR中潜力巨大. 特别是, 几乎所有金属中心对*N2中间体的吸附能力都比*H更强, 这表明以 P为配体的单原子催化剂具有较强的NRR选择性, 且*N2H中间体的 吸附自由能可作为描述符, 反映这一系列催化剂的催化活性. 此外, 计算结果显示, 金属中心向P配体转移的电子数目与NRR活性存在 着火山关系, 带有适度正电荷的金属中心具有优异的电催化NRR 活性. 该发现为高性能单原子催化剂的设计提供了理论指导.
Similar content being viewed by others
References
Foster SL, Bakovic SIP, Duda RD, et al. Catalysts for nitrogen reduction to ammonia. Nat Catal, 2018, 1: 490–500
Erisman JW, Sutton MA, Galloway J, et al. How a century of ammonia synthesis changed the world. Nat Geosci, 2008, 1: 636–639
Chen JG, Crooks RM, Seefeldt LC, et al. Beyond fossil fuel-driven nitrogen transformations. Science, 2018, 360: eaar6611
Hirakawa H, Hashimoto M, Shiraishi Y, et al. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J Am Chem Soc, 2017, 139: 10929–10936
Marnellos G, Stoukides M. Ammonia synthesis at atmospheric pressure. Science, 1998, 282: 98–100
Lan R, Irvine JTS, Tao S. Synthesis of ammonia directly from air and water at ambient temperature and pressure. Sci Rep, 2013, 3: 1145–1151
Licht S, Cui B, Wang B, et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3. Science, 2014, 345: 637–640
Qiu W, Xie XY, Qiu J, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst. Nat Commun, 2018, 9: 3485–3492
Tang C, Qiao SZ. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem Soc Rev, 2019, 48: 3166–3180
Singh AR, Rohr BA, Schwalbe JA, et al. Electrochemical ammonia synthesis—the selectivity challenge. ACS Catal, 2016, 7: 706–709
Yang Y, Zhang L, Hu Z, et al. The crucial role of charge accumulation and spin polarization in activating carbon-based catalysts for electrocatalytic nitrogen reduction. Angew Chem Int Ed, 2020, 59: 4525–4531
Légaré MA, Bélanger-Chabot G, Dewhurst RD, et al. Nitrogen fixation and reduction at boron. Science, 2018, 359: 896–900
Li L, Tang C, Xia B, et al. Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction. ACS Catal, 2019, 9: 2902–2908
Jiao Y, Zheng Y, Jaroniec M, et al. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem Soc Rev, 2015, 44: 2060–2086
Montoya JH, Tsai C, Vojvodic A, et al. The challenge of electrochemical ammonia synthesis: A new perspective on the role of nitrogen scaling relations. ChemSusChem, 2015, 8: 2180–2186
Skúlason E, Bligaard T, Gudmundsdóttir S, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys Chem Chem Phys, 2012, 14: 1235–1245
Shi MM, Bao D, Li SJ, et al. Anchoring PdCu amorphous nanocluster on graphene for electrochemical reduction of N2 to NH3 under ambient conditions in aqueous solution. Adv Energy Mater, 2018, 8: 1800124–1800129
Bao D, Zhang Q, Meng FL, et al. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle. Adv Mater, 2017, 29: 1604799
Wang M, Liu S, Qian T, et al. Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential. Nat Commun, 2019, 10: 341
Seefeldt LC, Hoffman BM, Dean DR. Electron transfer in nitrogenase catalysis. Curr Opin Chem Biol, 2012, 16: 19–25
Yandulov DV, Schrock RR. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science, 2003, 301: 76–78
Liu X, Jiao Y, Zheng Y, et al. Building up a picture of the electrocatalytic nitrogen reduction activity of transition metal single-atom catalysts. J Am Chem Soc, 2019, 141: 9664–9672
Eizawa A, Arashiba K, Tanaka H, et al. Remarkable catalytic activity of dinitrogen-bridged dimolybdenum complexes bearing NHC-based PCP-pincer ligands toward nitrogen fixation. Nat Commun, 2017, 8: 1–2
Kuriyama S, Arashiba K, Tanaka H, et al. Direct transformation of molecular dinitrogen into ammonia catalyzed by cobalt dinitrogen complexes bearing anionic PNP pincer ligands. Angew Chem Int Ed, 2016, 55: 14291–14295
Buscagan TM, Oyala PH, Peters JC. N2-to-NH3 conversion by a triphos-iron catalyst and enhanced turnover under photolysis. Angew Chem Int Ed, 2017, 56: 6921–6926
Arashiba K, Kinoshita E, Kuriyama S, et al. Catalytic reduction of dinitrogen to ammonia by use of molybdenum-nitride complexes bearing a tridentate triphosphine as catalysts. J Am Chem Soc, 2015, 137: 5666–5669
Zhang L, Ding LX, Chen G, et al. Ammonia synthesis under ambient conditions: Selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets. Angew Chem Int Ed, 2019, 58: 2612–2616
Qiao B, Wang A, Yang X, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat Chem, 2011, 3: 634–641
Wei Z, Zhang Y, Wang S, et al. Fe-doped phosphorene for the nitrogen reduction reaction. J Mater Chem A, 2018, 6: 13790–13796
Zhang H, Liu G, Shi L, et al. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv Energy Mater, 2018, 8: 1701343
Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat Rev Chem, 2018, 2: 65–81
Ren S, Yu Q, Yu X, et al. Graphene-supported metal single-atom catalysts: a concise review. Sci China Mater, 2020, 63: 903–920
Qin R, Liu P, Fu G, et al. Strategies for stabilizing atomically dispersed metal catalysts. Small Methods, 2018, 2: 1700286
Zhao J, Chen Z. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: A computational study. J Am Chem Soc, 2017, 139: 12480–12487
Yang XF, Wang A, Qiao B, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc Chem Res, 2013, 46: 1740–1748
Zhao W, Zhang L, Luo Q, et al. Single Mo1(Cr1) atom on nitrogen-doped graphene enables highly selective electroreduction of nitrogen into ammonia. ACS Catal, 2019, 9: 3419–3425
Geng Z, Liu Y, Kong X, et al. Achieving a record-high yield rate of \(120.0\mu\rm{g}_{\rm{NH}_{3}}\ \rm{mg}_{\rm{cat}}^{-1}\ \rm{h}^{-1}\) for N2 electrochemical reduction over Ru single-atom catalysts. Adv Mater, 2018, 30: 1803498
Choi C, Back S, Kim NY, et al. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline. ACS Catal, 2018, 8: 7517–7525
Guo X, Huang S. Tuning nitrogen reduction reaction activity via controllable Fe magnetic moment: A computational study of single Fe atom supported on defective graphene. Electrochim Acta, 2018, 284: 392–399
Han L, Liu X, Chen J, et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation. Angew Chem Int Ed, 2019, 58: 2321–2325
He T, Matta SK, Du A. Single tungsten atom supported on N-doped graphyne as a high-performance electrocatalyst for nitrogen fixation under ambient conditions. Phys Chem Chem Phys, 2019, 21: 1546–1551
Ling C, Bai X, Ouyang Y, et al. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions. J Phys Chem C, 2018, 122: 16842–16847
Li Q, Qiu S, Liu C, et al. Computational design of single-molybdenum catalysts for the nitrogen reduction reaction. J Phys Chem C, 2019, 123: 2347–2352
Li Q, Liu C, Qiu S, et al. Exploration of iron borides as electrochemical catalysts for the nitrogen reduction reaction. J Mater Chem A, 2019, 7: 21507–21513
Ma X, Hu J, Zheng M, et al. N2 reduction using single transition-metal atom supported on defective WS2 monolayer as promising catalysts: A DFT study. Appl Surf Sci, 2019, 489: 684–692
Wang Z, Yu Z, Zhao J. Computational screening of a single transition metal atom supported on the C2N monolayer for electrochemical ammonia synthesis. Phys Chem Chem Phys, 2018, 20: 12835–12844
Chen Z, Zhao J, Cabrera CR, et al. Computational screening of efficient single-atom catalysts based on graphitic carbon nitride (g-C3N4) for nitrogen electroreduction. Small Methods, 2018, 3: 1800368
Kresse G, Furthmüller J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci, 1996, 6: 15–50
Blöchl PE. Projector augmented-wave method. Phys Rev B, 1994, 50: 17953–17979
Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B, 1999, 59: 1758–1775
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77: 3865–3868
Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys, 2010, 132: 154104
Dudarev SL, Botton GA, Savrasov SY, et al. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys Rev B, 1998, 57: 1505–1509
Hashmi A, Hong J. Transition metal doped phosphorene: first-principles study. J Phys Chem C, 2015, 119: 9198–9204
Nørskov JK, Rossmeisl J, Logadottir A, et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B, 2004, 108: 17886–17892
Henkelman G, Uberuaga BP, Jónsson H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys, 2000, 113: 9901–9904
Yang B, Wan B, Zhou Q, et al. Te-doped black phosphorus field-effect transistors. Adv Mater, 2016, 28: 9408–9415
Wang X, Tang C, Zhu W, et al. A new effective approach to prevent the degradation of black phosphorus: The scandium transition metal doping. J Phys Chem C, 2018, 122: 9654–9662
Cui X, Tang C, Zhang Q. A review of electrocatalytic reduction of dinitrogen to ammonia under ambient conditions. Adv Energy Mater, 2018, 8: 1800369
Liu X, Jiao Y, Zheng Y, et al. Isolated boron sites for electroreduction of dinitrogen to ammonia. ACS Catal, 2020, 10: 1847–1854
Liu C, Li Q, Wu C, et al. Single-boron catalysts for nitrogen reduction reaction. J Am Chem Soc, 2019, 141: 2884–2888
Yu X, Han P, Wei Z, et al. Boron-doped graphene for electrocatalytic N2 reduction. Joule, 2018, 2: 1610–1622
Rahman MZ, Kwong CW, Davey K, et al. 2D phosphorene as a water splitting photocatalyst: Fundamentals to applications. Energy Environ Sci, 2016, 9: 709–728
Li L, Yang F, Ye GJ, et al. Quantum Hall effect in black phosphorus two-dimensional electron system. Nat Nanotech, 2016, 11: 593–597
Acknowledgements
We thank Prof. Dr. Lyudmila Moskaleva for her valuable comments on the revision. This work was supported by the National Natural Science Foundation of China (21525626 and 21761132023), and the Program of Introducing Talents of Discipline to Universities (BP0618007).
Author information
Authors and Affiliations
Contributions
Zhao ZJ conceived and coordinated the research. Lin X designed the models and performed the calculations. Lin X and Zhao ZJ wrote the manuscript. All authors participated in the discussions of the research and the revision of manuscript.
Corresponding author
Additional information
Conflict of interest
The authors declare no conflict of interest.
Supplementary information
Supporting data are available in the online version of the paper.
Xiaoyun Lin is currently a MSc candidate at the School of Chemical Engineering and Technology, Tianjin University. She received her BSc degree from Beijing University of Chemical Technology in 2018. Her current interest lies in the molecular level understanding of electrocatalytic reactions.
Zhi-Jian Zhao received his BSc and MSc degrees in chemistry from Zhejiang University and his PhD degree from Technische Universität München in 2012. After working as a Postdoc with Prof. Greeley in Purdue University and with Dr. Studt and Prof. Nørskov at Stanford University, now he is a professor at Tianjin University. His current research focuses on mechanistic studies on heterogeneous catalysts using multi-scaling simulation methods.
Supporting Information
Rights and permissions
About this article
Cite this article
Lin, X., Li, L., Chang, X. et al. Black phosphorus-hosted single-atom catalyst for electrocatalytic nitrogen reduction. Sci. China Mater. 64, 1173–1181 (2021). https://doi.org/10.1007/s40843-020-1522-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1522-y