Abstract
Oxidative roasting of Nd–Fe‒B permanent magnets prior to leaching improves the selectivity in the recovery of rare-earth elements over iron. However, the dissolution rate of oxidatively roasted Nd–Fe‒B permanent magnets in acidic solutions is very slow, often longer than 24 h. Upon roasting in air at temperatures above 500 °C, the neodymium metal is not converted to Nd2O3, but rather to the ternary NdFeO3 phase. NdFeO3 is much more difficult to dissolve than Nd2O3. In this work, the formation of NdFeO3 was avoided by roasting Nd–Fe‒B permanent magnet production scrap in argon atmosphere, having an oxygen content of \( p_{{{\text{O}}_{2} }} \, \le \,10^{ - 20} \;{\text{atm}}, \) with the addition of 5 wt% of carbon as an iron reducing agent. For all the non-oxidizing iron roasting conditions investigated, the iron in the Nd–Fe‒B scrap formed a cobalt-containing metallic phase, clearly distinct from the rare-earth phase at microscopic level. The thermal treatment was optimized to obtain a clear phase separation of metallic iron and rare-earth phase also at the macroscopic level, to enable easy mechanical removal of iron prior to the leaching step. The sample roasted at the optimum conditions (i.e., 5 wt% carbon, no flux, no quenching step, roasting temperature of 1400 °C and roasting time of 2 h) was leached in the water-containing ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N]. A leaching time of only 20 min was sufficient to completely dissolve the rare-earth elements. The rare-earth elements/iron ratio in the leachate was about 50 times higher than the initial rare-earth elements/iron ratio in the Nd–Fe‒B scrap. Therefore, roasting in argon with addition of a small amount of carbon is an efficient process step to avoid the formation of NdFeO3 and to separate the rare-earth elements from the iron, resulting in selective leaching for the recovery of rare-earth elements from Nd–Fe‒B permanent magnets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Production scrap of Nd–Fe‒B permanent magnets, or just Nd–Fe‒B production scrap, is currently considered as the most valuable secondary resource of neodymium and dysprosium [1]. Several flowsheets have been developed for the recovery of rare-earth elements (REEs) from Nd–Fe‒B scrap, via direct or indirect recycling [1,2,3,4,5,6,7,8]. Roasting of Nd–Fe‒B permanent magnets is known to improve the selectivity and the dissolution rate of the consequent leaching step [9,10,11]. However, when roasting is carried out in air at temperatures above 500 °C, neodymium is converted to a ternary neodymium-iron oxide phase, NdFeO3, and not to the binary neodymium oxide phase, Nd2O3 [12, 13]. If properly carried out, roasting can convert neodymium to Nd2O3 and separate the rare-earth elements from the less valuable iron. The oxidation mechanism of Nd–Fe‒B permanent magnets in air has recently been well described by Firdaus et al. [14]. The authors suggested NdFeO3 as the preferred form to recover neodymium from the roasted magnets. This statement is surprising since NdFeO3 has been observed to dissolve much slower in acidic lixiviants than the binary Nd2O3 [15, 16]. The dissolution of NdFeO3 can take up to 72 h [12, 17, 18]. The phase stability of the products from the roasting of Nd–Fe‒B permanent magnets depends on the composition of the magnet, the partial oxygen pressure \( \left( {p_{{{\text{O}}_{2} }} } \right) \) and the roasting temperature [13, 19]. The \( p_{{{\text{O}}_{2} }} \) at equilibrium can be controlled by the C/CO equilibrium, where CO is used as the oxidizing agent and carbon as the reducing agent [13]. In particular, the conditions favorable to avoid the formation of NdFeO3 are (1) temperatures between 1350 °C and 2000 °C; and (2) a \( p_{{{\text{O}}_{2} }} \) between 10−15 and 10−25 atm.
To avoid the formation of NdFeO3, Maroufi et al. [20] and Bian et al. [21] carried out a combined process of oxidation at 1000 °C and consequent reduction above 1400 °C of Nd–Fe‒B permanent magnets. In both studies, the product was a mixed rare-earth oxide phase separated from a metal phase mainly containing transition metals (iron, cobalt, and nickel). Maroufi et al. used waste tire rubber-derived carbon as the reducing agent, in an attempt to make optimal use of this waste source [20], whereas Bian et al. employed conventional carbon reduction [21]. In these two studies, no details are disclosed about the method by which the REOs and the metal phases were separated. A different approach was employed by Yoon et al., who first converted neodymium into Nd(OH)3 by grinding Nd–Fe‒B scrap in the presence of NaOH and, subsequently, converted the Nd(OH)3 into Nd2O3 by roasting in air at 400 °C [22].
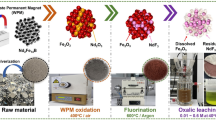
In the present paper, a process for pyrometallurgical separation of REEs from iron by roasting milled Nd–Fe‒B scrap in argon atmosphere at \( p_{{{\text{O}}_{2} }} \) ≤ 10−20 atm with 5 wt% carbon addition for 2 h at 1400 °C was carried out, showing that a first step of oxidation is not necessary to separate iron from the rare-earth elements and to obtain Nd2O3 instead of NdFeO3. Furthermore, the positive effect of the improved roasting process on the leaching rate of neodymium was corroborated by leaching the roasted Nd–Fe‒B scrap in the water-containing functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N] (Fig. 1). This ionic liquid has been found useful for recovery of metals from urban waste and industrial process residues [12, 17, 23,24,25]. The roasting in reductive atmosphere rather than in air can be seen as a form of process intensification since, in comparison to previous studies, the total duration of the roasting and leaching steps is reduced more than tenfold. Eliminating the oxidative roasting step and intensifying the leaching reduced the environmental footprint of the designed flowsheet for the recycling of Nd–Fe‒B permanent magnets.
Experimental
Materials
Bulk, demagnetized Nd–Fe‒B permanent magnets scrap were provided by Magneti Ljubljana d. d. (Ljubljana, Slovenia). The fluxing agent, calcium carbonate, CaCO3 (> 99%), and betainium chloride [Hbet]Cl (> 99%) were supplied by Acros Organics (Geel, Belgium), while lithium bis(trifluoromethylsulfonyl)imide Li[Tf2N] (> 99%) was obtained from Iolitec (Heilbronn, Germany). Carbon was purchased as a high density skeleton activated charcoal (≥ 99.8) from Sigma-Aldrich (Diegem, Belgium). Iron, neodymium, and dysprosium standard solutions (1000 μg mL−1) were obtained from Chem-Lab (Zedelgem, Belgium). The EpoFix epoxy resin was purchased from Struers GmbH Netherlands (Massluis, Netherlands).
Solid Pretreatment and Roasting
The magnets were crushed to a size ≤ 5.0 mm by a hydraulic press and, subsequently, milled in a planetary ball mill (Fritsch Pulverisette 7 premium). The following milling conditions were applied: stainless steel pots and balls; milling medium: ethanol (97% grade); charge ratio balls-to-powder: 4 g g−1; balls diameter: 10.0 and 1.0 mm; milling time: 5 min; pause time: 5 min; cycles (milling + pause): 3. The milling pots were cooled down to room temperature before opening, to avoid spontaneous ignition of the hot milled pyrophoric powder, and the content was transferred to a rotary evaporator to remove the ethanol. The dried powder was stored in argon atmosphere to prevent oxygen capture by the powder itself. The procedure for the elemental analysis of the solid by inductively coupled plasma optical emission spectrometer (ICP-OES, PerkinElmer Optima 8300) has already been described in previous work [12]. Briefly: 50 mg of milled, non-roasted of Nd–Fe‒B permanent magnets scrap was dissolved in 10 mL of 37% HCl at 70 °C for 60 min. Afterwards, the solutions were diluted 100 times in 2% v/v HNO3. Three calibration standards were prepared containing 0.5–5–50 mg L−1 Fe, Nd, B, Dy, Pr, Co, Cu, Ga, and Al in 2% vv. HNO3. Rh was selected as internal standard for Fe, Nd, and Dy; Sr was selected as internal standard for B, Pr, Co, Cu, Ga, and Al.
About 0.5 g (for the screening tests of the roasting conditions) or 2.0 g (for the consequent leaching tests) of milled Nd–Fe‒B powder was mixed with 5 wt% of reagent grade carbon in a magnesia crucible (outer diameter: 30.0 mm, height: 30.0 mm). The roasting experiments were carried out in a vertical tube furnace (impervious recrystallized alumina, 80 mm internal diameter, GERO HTRV 100-250/18) with MoSi2 heating elements, under purified argon atmosphere (flowrate: 0.3 L min−1, \( p_{{{\text{O}}_{2} }} \) ≤ 10−20 atm). A heating rate of 5 °C min−1 was applied to reach the targeted temperature, while a cooling rate of 5 °C min−1 was utilized at the end of the experiment to cool the sample to room temperature, when quenching in water was not applied. The investigated temperatures were 1200 °C, 1400 °C, and 1600 °C. The holding times at the desired temperatures were 2.0, 4.0, and 9.0 h. In selected experiments, CaCO3 was added as a flux in 50 wt% concentration. The calcine was ground to powder by using a hammer and/or a mortar and pestle. Electron-probe microanalysis (EPMA) with five wavelength dispersive spectrometers (JEOL JXA-8530F, JEOL Ltd.) was carried out employing a probe current of 50 nA and an acceleration voltage of 15 kV. Prior to the analysis, the samples were embedded in epoxy resin (EpoFix), following by 1 day of curing under vacuum. Conventional grinding and polishing techniques were used. The polished samples were coated with an approximately 1.0 nm thick platinum film.
Leaching
A first series of leaching experiments was performed with 0.075 g of ground calcine in the ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N], with addition of 5 or 10 wt% water, until complete dissolution of the solid was observed. The ionic liquid was prepared starting from [Hbet]Cl and Li[Tf2N], as described in the literature [26]. The [Hbet][Tf2N] was dried on a rotary evaporator for 3 h and subsequently transferred overnight on a Schlenk line to remove the remaining traces of water. After drying and solidifying the ionic liquid (at room temperature), 5 wt% or 10 wt% of water was added to it. Leaching conditions were temperature: 80 °C; lixiviant/solid (L/S) ratio: 20 mL g−1; mixing speed: 600 rpm. Magnetic stirring bars in polytetrafluoroethylene were used to mix the leaching solutions. The leaching temperature was set at 80 °C to compare the results obtained in this work with results previously obtained in our group. The leaching selectivity S was calculated using Eq. (1):
where \( \% E_{\text{REEs}} \) and \( \% E_{\text{Fe}} \) are the percentage of extraction, respectively, of rare-earth elements (neodymium + dysprosium) and of iron. The %E of any metal M is calculated as in Eq. (2):
where [M]f is the metal concentration in the leachate, while \( \left[ M \right]_{\text{MAX}} \) is the maximum metal concentration in the leachate and corresponds to a total dissolution of the metal from the solid to the lixiviant. The best performing ionic liquid from this first test was further used to study the dissolution rate of the REEs. Temperature, L/S ratio, and mixing speed were kept the same as in the first leaching tests; uptakes were made of 50.0 μL of leachate at 5, 10, 20 min and of 20.0 μL at 30, 45, 60 min. from an initial volume of 10.0 mL of lixiviant. All the leaching samples were diluted 100 times in 2% v/v HNO3 and analyzed via inductively coupled plasma optical emission spectrometry (ICP-OES) on a PerkinElmer Optima 8300 spectrometer. Calibration solutions were prepared with standards of iron, neodymium, and dysprosium in a concentration range of 0.1 to 25 mg L−1; a quality control solution of 1 mg L−1 was used as well. The same experimental and analytical conditions were applied for, respectively, the 5 wt% and 10 wt% water-containing [Hbet][Tf2N] samples.
Results and Discussion
Solid Pretreatment and Roasting
The elemental composition is reported in Table 1, the Nd–Fe‒B scrap has a dysprosium content, currently the most critical REE, higher (circa 8.5 wt%) than the usual dysprosium content in Nd–Fe‒B permanent magnets (3–5 wt%). This confirms the value of the Nd–Fe‒B scrap as a secondary REEs source. The elemental distribution of the milled, not-roasted Nd–Fe‒B scrap is displayed in Fig. 2. From this analysis, the presence of a mixed iron–rare earths phase and of a rare-earth oxides phase are confirmed. The first roasting experiments were carried out on 0.5 g of milled Nd–Fe‒B scrap with 0.025 g of carbon powder at 1400 °C for 2.0, 4.0, and 9.0 h. The reaction was stopped for all the three tests by quenching. It was assumed that an amount of carbon equal to 5 wt% was sufficient for the smelting process of iron. Any excess of carbon would remain unreacted, without influence on the metals, whereas an insufficient amount would lead to the formation of γ-Fe which is not liquid at the operating temperature [27]. The added carbon has two simultaneous effects on the iron‒rare earths separation at the investigated working temperatures: (1) it forms a eutectic with iron, which can form a liquid phase and flow within the sample; (2) it reduces the iron, which may have been partially oxidized to Fe2O3 (according to the reactions in Eqs. (3–6)), but not the rare earths [28].
The elemental distribution in the phases after 2, 4, and 9 h of thermal treatment, followed by quenching, was investigated by electron-probe microanalysis (EPMA). The results are shown in Figs. 3, 4, and 5 with back-scattered electron (BSE) images of the samples.
Two main observations emerged from the EPMA mapping, namely (1) the rare-earth elements are well separated from the iron, also after only 2 h of holding time (Fig. 3) contrarily to what happens in the not-roasted sample (Fig. 2), and (2) iron was in metallic form while the REEs formed a phase with boron. The present results are not in perfect agreement with the modeling performed by Tranell and Jung [13]. In fact, the authors suggested a phase of liquid metal with Nd2O3 as a solid phase; moreover, according to them, boron would form an Fe–B solution. However, Tranell and Jung carried out a qualitative study without quantifying how much Nd2O3 is present in the system after roasting at 1400 °C at \( p_{{{\text{O}}_{2} }} \) ≤ 10−15 atm and a pCO ≤ 10−1 atm. Due to the very low content of oxygen in the roasting atmosphere, it is very likely that such Nd2O3 does not form during the roasting process but represents the Nd2O3 already present in the Nd–Fe‒B scrap (circa 5 wt%). Contamination of the Nd–Fe‒B scrap powder by oxygen during the operations of crushing and milling cannot be excluded either. Nevertheless, the scope of the roasting was to separate the neodymium from the iron and this was successfully achieved. It is important to clarify that we largely omit dysprosium in the discussion on the ternary oxides, since the diffractogram of the roasted Nd–Fe‒B scrap in air only revealed the presence of NdFeO3 and not of any dysprosium compound. Furthermore, the stability of LnFeO3 has been reported to decrease, at high temperatures, with decreasing ionic radius and, thus, the presence of DyFeO3 can be excluded [29].
Nevertheless, it was not possible to separate the metallic iron from the REEs phase, neither magnetically nor mechanically. The magnetic separation was tested first on the roasted sample just after crushing and, subsequently, on the same powder dispersed in ethanol, instead of water, to avoid iron hydrolysis. In both cases, the entire specimen (the metallic iron together with the REEs phase) was attracted to the magnet. The reason is that the iron phase and the REEs phase have not been liberated, and the fineness of the crushed roasted solid also affects the separation [30]. An example is shown in the back-scattered image (Fig. 3), where the iron phase is encapsulated by the REEs phase. Firdaus et al. also stated that the separation of metal from slag is a major challenge in high-temperature processes for the REEs recovery from Nd–Fe‒B permanent magnets [31]. Borra et al. were, instead, able to collect and easily separate the iron in the form of a nugget at the bottom of a bauxite residue sample after reductive smelting [32]. A flux was used in that work, but the main difference is the composition of the two samples. In case of Nd–Fe‒B scrap, the rare-earth elements are about 33 wt% of the sample and their atomic weight is, on average, three times the atomic weight of the iron. On the contrary, bauxite residue comprises only 0.1 wt% of rare-earth elements and about 30 wt% of the solid is made of Al2O3 and CaO, which are about one and half and three times lighter than Fe2O3, respectively. Hence, it is difficult to obtain an easy-to-remove iron nugget at the bottom of selectively roasted Nd–Fe‒B scrap. Different parameters (addition of a flux, roasting temperature, and cooling mode) were investigated to promote the growth of iron agglomerates to collect either at the bottom or dispersed in the sample. CaCO3 was used as a flux, added in 50 wt% to the Nd–Fe‒B scrap. As CaCO3 decomposes to CaO and CO2 at temperatures below the operational temperatures in this study, the actual flux is CaO. In this case, a significant amount of oxygen is introduced into the system. The experiment was carried out at 1400 °C for 2 h, and quenching was once more applied. The sample R2 as it appeared at the end of the roasting, is shown in Table 2, while the EPMA is presented in Fig. 6. The distribution of oxygen actually shows a band that is typical for when a large area is investigated. It is an artifact of the machine/spectrometer curvature. Nonetheless, oxygen is not present in the iron phase.
Although, the CaCO3 flux did not affect the settling behavior of the REEs phase from the metallic iron, boron preferentially reported to the Ca‒O phase, indicating that boron can be separated from both the rare-earth elements and iron. Both the B‒Ca‒O phase and the REEs phase did not contain iron. The effect of the cooling mode on the growth of iron agglomerates was investigated, by replacing the quenching with a slow cooling at − 5 °C min−1 in the furnace under inert atmosphere. A flux was again used. The resulting sample R3 is shown in Table 2. Small iron spheres were observed for the first time: it is assumed that the slow cooling provides sufficient time for the metallic iron to migrate within the sample and uniformly grow to iron agglomerates. It was decided to continue the roasting study without adding a flux, since the flux does not mix with the REEs phase to increase the settling of the iron phase and since, even worse, the calcium contaminates the leachate of the subsequent leaching step. Rather than a calcium-based fluxing agent, the use of a fluorine- or boron-containing fluxing agent could decrease the melting point of the slag, but this was not investigated further. In the next experiment, about 0.5 g of Nd–Fe‒B scrap was roasted at 1400 °C for 2 h, without flux and without quenching (R4, in Table 2). Finally, iron spheres of about 1.0–3.0 mm diameter were obtained, which could be mechanically removed with ease, by sieving after crushing the specimen.
The effect of the roasting temperature on the iron behavior was evaluated as well. An experiment was performed with the same conditions of the R4 test, albeit with a roasting temperature of 1600 °C. It was expected that a higher roasting temperature would decrease the viscosity of the melt, allowing the iron particles to migrate more freely and to agglomerate at a higher extent in the melt. The resulting sample R5 is shown in Table 2. Although iron pearls were obtained, a higher temperature is not favorable to the process: when the liquid sample solidified, the sample ended up to be very hard and the slag solidified as a thin coating on the crucible and on the iron particles. Therefore, the slag can neither be separated from the iron particles, nor from the magnesia crucible. In the previous experiments (pictures R2, R3, and R4 in Table 2), the samples had the appearance of a sintered powder, meaning that a liquid melt did not really form. At this stage, in the perspective of the absence of NdFeO3 formation and a clear microscopic separation of the iron particles from the REEs phase, the optimum conditions for roasting were defined as 5 wt% carbon, no flux, no quenching, and a roasting temperature of 1400 °C of 2 h.
Leaching
Once the pyrometallurgical step was optimized, the effect of the separation of the rare-earth elements phase from the iron phase on the leaching was investigated. The leaching was carried out in the [Hbet][Tf2N] ionic liquid and the yields as well as the rates of the process were investigated. Previous work by the present authors has already shown that the leaching efficiency of roasted Nd–Fe‒B scrap is affected by (1) the water content in the ionic liquid, since water saturates the first coordination sphere of the metals, and (2) the presence of the ternary oxide NdFeO3 [12]. About 2.0 g of powder was successfully roasted under optimal conditions (no flux and no quenching, a roasting temperature of 1400 °C and roasting time of 2 h). Most of the iron was removed manually, resulting in variations of iron content in samples from different experiments and affecting the calculated selectivity. 5 wt% or 10 wt% water content was added to the [Hbet][Tf2N]. It is known that water-containing ionic liquid is a much better solvent for metal oxides than the dry ionic liquid [26]. Most of the solid was dissolved after only 60 min in [Hbet][Tf2N] containing 10 wt% of water and about 90 min in [Hbet][Tf2N] containing 5 wt%. of water. The residue left behind in the leachate was undissolved metallic iron. The results of the leaching tests, in terms of selectivity S (Eq. 1), are summarized in Table 3. The selectivity S is approximately 50 for both ionic liquid samples. Furthermore, the water content in the ionic liquid has a significant influence on the leaching rate of the Nd–Fe‒B permanent magnets in [Hbet][Tf2N], which is in agreement with previous observations [12]. The ionic liquid [Hbet][Tf2N] with 10 wt% water was selected as the most favorable solvent system for further studies.
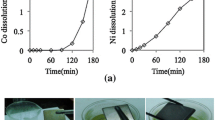
Next, the leaching rates of neodymium, dysprosium, and iron from the roasted sample were investigated by determining the metal concentrations in the ionic liquid as a function of time (Fig. 7).
The parameter %E was calculated based on the initial amount of metal in the Nd–Fe‒B scrap and, thus, it includes the results of both the roasting-manual separation step and of the leaching. A more appropriate definition of %E would be based on the actual material processed in the leaching, after the manual separation of iron. Nevertheless, the unavoidable losses of material in this former step made impossible to identify the actually leached material. On the other hand, the results so presented in Fig. 7 give a global overview of the performance of the recovery process. Nevertheless, the value of S after 20 min was 15.8, i.e., about three times lower than the values in Table 3, showing how crucial the step of mechanical iron removal is. Such step can be more easily optimized at industrial scale for larger volumes with an automated system for the mechanical treatment (crushing, sieving, magnetic or gravity separation) of the roasted product, compared to the process in the lab where the operations of crushing the roasted specimen and removing the iron were achieved manually. Furthermore, iron recovery is not the objective of this work and the sample losses during the mechanical removal of iron were not minimized.
The concentration of the REEs stabilized after 20 min, indicating that this period of time is sufficient to fully dissolve the REEs phase. The leaching rates obtained in this work on the roasted sample are significantly higher than the leaching rates obtained in previous research [17]. Dupont and Binnemans were able to leach Nd–Fe‒B permanent magnets in water-containing [Hbet][Tf2N], but a complete dissolution of 6 μm particles took at least 24 h when using 10 wt% [Hbet][Tf2N] and 72 h in 5 wt% [Hbet][Tf2N] [17]. In that work, the permanent magnets were pretreated by roasting at 950 °C in air for 15 h, resulting in an optimal total treatment time of 39 h, compared to the 2.3 h treatment time required in this work. The improved leaching rate is explained by the absence of any mixed neodymium-iron phase due to the roasting in argon atmosphere at \( p_{{{\text{O}}_{2} }} \) ≤ 10−20 atm with 5 wt% carbon, whereas roasting in air results in the conversion of neodymium mainly as ternary oxide NdFeO3. In particular, the NdFeO3 content of the sample Nd–Fe‒B magnets roasted in air is about 25 wt%, and the Nd2O3 content is only 0.44 wt% [12]. The recovery of the rare-earth elements from the ionic liquid leachate was outside the scope of this work and can be done, according to methods previously published, by precipitation stripping of the REEs with an aqueous H2C2O4 solution [17, 33]. A suggested flowsheet is presented in Fig. 8: the green lines represent the solvometallurgical steps, while the red lines are pyrometallurgical steps and the blue lines are hydrometallurgical steps. Moreover, dashed lines represent steps which have not been tested, but assumed from the literature: in particular, the 10 wt% [Hbet][Tf2N] ionic liquid is assumed to be reused at the end of the process for many times since the contact with aqueous H2C2O4 does not degrade it. The limited input of chemicals in the flowsheet is in agreement with the principles of Green Chemistry [34]. The flowsheet was simplified, considering only the main elements iron, neodymium, and dysprosium in the process; as for other elements, cobalt would be separated together with iron, with which it forms a phase (see Figs. 3, 4, and 5), boron and praseodymium would follow the route of the REEs, traces elements (copper, gallium) were not considered.
Conceptual flowsheet for the combined pyrometallurgical and solvometallurgical recycling of Nd–Fe‒B scrap. The green lines represent the solvometallurgical steps, the red lines the pyrometallurgical steps, and the blue lines the hydrometallurgical steps. The dashed lines represent steps which have not been tested but assumed from the literature (Color figure online)
Conclusion
The leaching rate of Nd–Fe–B permanent magnets has been observed to be affected by the formation of the ternary oxide NdFeO3. Roasting of Nd–Fe‒B scrap in argon atmosphere at \( p_{{{\text{O}}_{2} }} \) ≤ 10−20 atm with 5 wt% carbon for 2 h at 1400 °C resulted in two phases, i.e., a metallic iron phase and a non-metallic B–Dy–Nd phase. The targeted separation of neodymium from iron in the roasting step was successfully achieved. The iron phase neatly separated from the REEs phase without the need of distinctly carrying out an oxidation and a reduction step. The most favorable roasting conditions were 5 wt% carbon; no flux; no cooling by quenching, a roasting temperature of 1400 °C and a roasting time of 2 h. The beneficial effect of the optimized roasting was corroborated by leaching with the water-containing ionic liquid [Hbet][Tf2N]. The total process duration (roasting + leaching) was 17 times shorter compared to the optimum conditions of previous work in our group (2.3 h instead of 39 h) [17]. In conclusion, a proof-of-concept was given that the recycling of Nd–Fe–B permanent magnets can be made more sustainable by optimizing their pretreatment (roasting): in fact, in only one roasting step the iron is reduced, the formation of NdFeO3 is avoided, and, hence, the consequent leaching process is intensified.
References
Yang Y, Walton A, Sheridan R, Güth K, Gauß R, Gutfleisch O, Buchert M, Steenari B-M, Van Gerven T, Jones PT, Binnemans K (2017) REE recovery from end-of-life NdFeB permanent magnet scrap: a critical review. J Sustain Metall. https://doi.org/10.1007/s40831-016-0090-4
Diehl O, Schönfeldt M, Brouwer E, Dirks A, Rachut K, Gassmann J, Güth K, Buckow A, Gauß R, Stauber R, Gutfleisch O (2018) Towards an alloy recycling of Nd-Fe-B permanent magnets in a circular economy. J Sustain Metall 4:163–175. https://doi.org/10.1007/s40831-018-0171-7
Yoon H-S, Kim C-J, Chung KW, Kim S-D, Kumar JR (2015) Recovery process development for the rare earths from permanent magnet scraps leach liquors. J Braz Chem Soc 26:1143–1151. https://doi.org/10.5935/0103-5053.20150077
Abrahami ST, Xiao Y, Yang Y (2015) Rare-earth elements recovery from post-consumer hard-disc drives. Miner Process Extr Metall 124:106–115. https://doi.org/10.1179/1743285514Y.0000000084
Saeki T, Akahori T, Miyamoto Y, Kyoi M, Okamoto M, Okabe TH, Hiroshige Y, Nemoto T (2014) Environment-friendly recycling process for rare earth metals in end-of-life electric products. Rare Metal Technology 2014. Wiley, Hoboken, pp 103–106
Lee CH, Chen YJ, Liao CH, Popuri SR, Tsai SL, Hung CE (2013) Selective leaching process for neodymium recovery from scrap Nd-Fe-B magnet. Metall Mater Trans A. https://doi.org/10.1007/s11661-013-1924-3
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. https://doi.org/10.1016/j.jclepro.2012.12.037
Zakotnik M, Harris IR, Williams AJ (2009) Multiple recycling of NdFeB-type sintered magnets. J Alloys Compd 469:314–321. https://doi.org/10.1016/j.jallcom.2008.01.114
Vander Hoogerstraete T, Blanpain B, Van Gerven T, Binnemans K (2014) From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid. RSC Adv 4:64099–64111. https://doi.org/10.1039/C4RA13787F
Koyama K, Kitajima A, Tanaka M (2009) Selective leaching of rare-earth elements from Nd-Fe-B magnet. Kidorui (Rare Earths) 54:36–37
Yoon H-S, Kim C-J, Lee J-Y, Kim S-D, Kim J-S, Lee J-C (2003) Separation of neodymium from NdFeB permanent magnetic scrap. J Korean Inst Resour Recycl 12:57–63
Orefice M, Binnemans K, Vander Hoogerstraete T (2018) Metal coordination in the high-temperature leaching of roasted NdFeB magnets with the ionic liquid betainium bis(trifluoromethylsulfonyl)imide. RSC Adv 8:9299–9310. https://doi.org/10.1039/C8RA00198G
Jakobsson LK, Tranell G, Jung I (2016) Experimental investigation and thermodynamic for recycling of NdFeB magnet scrap. Metall Mater Trans B 48:60–72. https://doi.org/10.1007/s11663-016-0748-0
Firdaus M, Rhamdhani MA, Rankin WJ, Pownceby M, Webster NAS, D’Angelo AM, McGregor K (2018) High temperature oxidation of rare earth permanent magnets. Part 1—Microstructure evolution and general mechanism. Corros Sci 133:374–385. https://doi.org/10.1016/j.corsci.2018.01.040
Lee J, Kim W, Jeong J, Yoon I (1998) Extraction of neodymium from Nd-Fe-B magnet scraps by sulfuric acid (in Korean). J Korean Inst Met Mater 36:967–972
Piyawit W, Sawananusorn P, Srikhang L, Buahombura P, Akkarapattanagoon N, Patcharawit T, Khumkoa S (2018) Selective extraction and recovery of rare earth metals (REMs) from NdFeB magnet grinding sludge. In: Davis BR, Moats MS, Wang S (eds) Extraction 2018: proceedings of the first global conference on extractive metallurgy. TMS, Pittsburgh, PA, pp 2399–2407
Dupont D, Binnemans K (2015) Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]. Green Chem 17:2150–2163. https://doi.org/10.1039/C5GC00155B
Tanaka M, Oki T, Koyama K, Narita H, Oishi T (2013) Recycling of rare earths from scrap, 1st edn. Elsevier, Amsterdam
Parida SC, Dash S, Singh Z, Prasad R, Jacob KT, Venugopal V (2002) Thermodynamic studies on NdFeO3(s). J Solid State Chem 164:34–41. https://doi.org/10.1006/jssc.2001.9445
Maroufi S, Khayyam Nekouei R, Sahajwalla V (2017) Thermal isolation of rare earth oxides from Nd-Fe-B magnets using carbon from waste tyres. ACS Sustain Chem Eng 5:6201–6208. https://doi.org/10.1021/acssuschemeng.7b01133
Bian Y-Y, Guo S-Q, Xu Y-L, Tang K, Lu X-G, Ding W-Z (2015) Recovery of rare earth elements from permanent magnet scraps by pyrometallurgical process. Rare Met. https://doi.org/10.1007/s12598-015-0554-x
Yoon H-S, Kim C-J, Chung K, Jeon S, Park I, Yoo K, Jha M (2015) The effect of grinding and roasting conditions on the selective leaching of Nd and Dy from NdFeB magnet scraps. Metals (Basel) 5:1306–1314. https://doi.org/10.3390/met5031306
Dupont D, Binnemans K (2015) Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem 17:856–868. https://doi.org/10.1039/C4GC02107J
Davris P, Balomenos E, Panias D, Paspaliaris I (2016) Selective leaching of rare earth elements from bauxite residue (red mud), using a functionalized hydrophobic ionic liquid. Hydrometallurgy 164:125–135. https://doi.org/10.1016/j.hydromet.2016.06.012
Davris P, Marinos D, Balomenos E, Alexandri A, Gregou M, Panias D, Paspaliaris I (2018) Leaching of rare earth elements from ‘Rödberg’ ore of Fen carbonatite complex deposit, using the ionic liquid [Hbet][Tf2N]. Hydrometallurgy 175:20–27. https://doi.org/10.1016/J.HYDROMET.2017.10.031
Nockemann P, Thijs B, Pittois S, Thoen J, Glorieux C, Van Hecke K, Van Meervelt L, Kirchner B, Binnemans K (2006) Task-specific ionic liquid for solubilizing metal oxides. J Phys Chem B 110:20978–20992
Chipman J (1972) Thermodynamics and phase diagram of the Fe-C system. Metall Trans 3:55–64. https://doi.org/10.1007/BF02680585
Nakamoto M, Kubo K, Katayama Y, Tanaka T, Yamamoto T (2011) Extraction of rare earth elements as oxides from a neodymium magnetic sludge. Metall Mater Trans B 43B:468–476. https://doi.org/10.1007/s11663-011-9618-y
Kimizuka N, Yamamoto A, Ohashi H, Sugihara T, Sekine T (1983) The stability of the phases in the Ln2O3-FeO-Fe2O3 systems which are stable at elevated temperatures (Ln: lanthanide elements and Y). J Solid State Chem 49:65–76
Lyman JW, Palmer GM (1993) Recycling of neodymium iron boron magnet scrap, Report for the US Bureau of Mines
Firdaus M, Rhamdhani MA, Durandet Y, Rankin WJ, McGregor K (2016) Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste. J Sustain Metall 2:276–295. https://doi.org/10.1007/s40831-016-0045-9
Borra CR, Blanpain B, Pontikes Y, Binnemans K, Van Gerven T (2016) Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J Sustain Metall 2:28–37. https://doi.org/10.1007/s40831-015-0026-4
Onghena B, Binnemans K (2015) Recovery of Scandium(III) from aqueous solutions by solvent extraction with the functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide. Ind Eng Chem Res 54:1887–1898. https://doi.org/10.1021/ie504765v
Anastas PT, Warner JC (1998) Green chemistry: theory and practice, 1st edn. Oxford University Press, New York City
Acknowledgements
The research leading to these results received funding from the European Commission’s Horizon 2020 Programme ([H2020/2014-2019]) under Grant Agreement no. 674973 (MSCA-ETN DEMETER) and from the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme: Grant Agreement 694078—Solvometallurgy for critical metals (SOLCRIMET). This publication reflects only the authors’ view, exempting the Commission from any liability. The authors want to thank Magneti Ljubljana d.d. (Slovenia) for providing the Nd–Fe‒B permanent magnets, Tony Debecker and Kevin Wierinckx for crushing the magnets, and Dr. Bieke Onghena and Dr. Peter Tom Jones for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
The contributing editor for this article was Markus Reuter.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Martina Orefice and Amy Van den Bulck—Joint first authorship.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orefice, M., Van den Bulck, A., Blanpain, B. et al. Selective Roasting of Nd–Fe‒B Permanent Magnets as a Pretreatment Step for Intensified Leaching with an Ionic Liquid. J. Sustain. Metall. 6, 91–102 (2020). https://doi.org/10.1007/s40831-019-00259-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-019-00259-1