Abstract
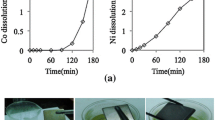
Neodymium-iron-boron (Nd-Fe-B) magnets were most widely applied to permanent magnetic products in the world due to their high magnetic force. The increasing growth of scrap Nd-Fe-B magnets resulted in disposal problems and the reduction of neodymium (Nd) valuable resources. In this study, we developed a simple hydrometallurgical precipitation process with pH adjustment to separate and recover Nd 100 pct recovery from scrap Nd-Fe-B magnets. Several physical and chemical methods such as demagnetization, grinding, screening, and leaching processes were also adopted to investigate the recovery of Nd and other metals from scrap Nd-Fe-B magnets. The leaching process was carried out with four leaching reagents such as NaOH, HCl, HNO3, and H2SO4. Batch studies were also conducted to optimize the leaching operating conditions with respect to leaching time, concentration of leaching reagent, temperature, and solid/liquid ratio for both HCl and H2SO4 leaching reagents. Nd was successfully separated and recovered with 75.41 wt pct from optimized H2SO4 leaching solution through precipitation. Further, the purity and weight percentage of the obtained Nd product was analyzed using scanning electron microscopy–energy-dispersive spectroscopy (SEM-EDS) analysis. An X-ray diffraction (XRD) study confirmed the obtained product of Nd was in the form of NdOOH and Nd(OH)3.








Similar content being viewed by others
Notes
PERKINELMER is a trademark of PerkinElmer, Inc., Waltham, MA.
SIEMENS is a trademark of Bruker, Billerica, MA.
References
W.T. Benecki, T.K. Clagett, and S.R. Trout: Permanent Magnets, 2010, http://www.waltbenecki.com/presentations.html, accessed July 18, 2011.
M. Katter: ElektronikPraxis Nr., 2010, pp. 14–26.
“Study on Rare Earths and Their Recycling,” Final Report for The Greens/EFA Group in the European Parliament, 2011, http://reinhardbuetikofer.eu/wp-content/uploads/2011/01/Rare-earths-study_Oeko-Institut_Jan-2011.pdf, accessed Jan. 18, 2012.
China Rare Earth, http://www.cre.net, accessed Aug. 21, 2011.
Disposal of Magnets, http://www.mceproducts.com/knowledge-base/article/article-dtl.asp?id=11, accessed Aug. 28, 2011.
Corrosion Source, http://www.corrosionsource.com/handbook/periodic/60.htm, accessed July 28, 2012.
M. Zakotnik, I.R. Harris, and A.J. Williams: J. Alloys Compd., 2009, vol. 469, pp. 314–21.
T. Kawasaki, M. Itoh, and K. Machida: Mater. Trans., 2003, vol. 44, pp. 1682–85.
S. Zhihong: Patent No. CN 200710156061 A, 2008, accessed July 10, 2012.
Department of Energy News: Process Recovers Valuable Neodymium from Magnetic Waste, U.S. 2001, http://www.eurekalert.org/features/doe/2001-07/dl-nlf060502.php, accessed July 12, 2012.
T. Hiroko: “Japan Recycles Minerals from Used Electronics,” NYTimes.com, Oct. 4, 2010, http://www.nytimes.com/2010/10/05/business/global/05recycle.html?pagewanted=all, accessed June 24, 2012.
Z. Xuanxu, Y. Danghua, and G. Lianping: J. Copper Eng., 2010, vol. 1, pp. 1009–3842.
W. Yijun, L. Yuhui, G. Junxun, W. Suling, and W. Guoqing: J. Hydrometall., 2006, vol. 25, pp. 4–197.
T. Jie, W. Chengfu, Z. Daowen, L. Hong, and T. Guihua: J. Rare Met. Cemented Carbides, 2009, abstract.
W. Wei and Z. Honglin: Chin. J. Rare Met., 2010, http://en.cnki.com.cn/Article_en/CJFDTOTALZXJS201001016.htm, accessed July 28, 2012.
Taiwan Environmental Protection Agency [TEPA]: http://www.niea.gov.tw/analysis/method/ListMethod.asp?methodtype=REFUSE (Chinese), accessed Apr. 18, 2011.
Chemical Reactions of the Elements, https://www.webelements.com/neodymium/chemistry.html, accessed July 15, 2011.
Treatise on Analytical Chemistry, I.M. Kolthoff and P.J. Elving, eds., Interscience, New York, NY, 1963, vol. 8, pp. 1–146.
S.R. Rao: Resource Recovery and Recycling from Metallurgical Wastes, Waste Management Series 7, Elsevier, Amsterdam, 2011, p. 256.
W. Xinchao, C. Roger, J. Viadero, and M. Karen: Environ. Eng. Sci., 2005, vol. 22, pp. 745–55.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, CH., Chen, YJ., Liao, CH. et al. Selective Leaching Process for Neodymium Recovery from Scrap Nd-Fe-B Magnet. Metall Mater Trans A 44, 5825–5833 (2013). https://doi.org/10.1007/s11661-013-1924-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11661-013-1924-3