Abstract
Introduction
Baricitinib is an oral Janus kinase (JAK)1/JAK2 inhibitor approved to treat rheumatoid arthritis (RA). This study aimed to investigate patients’ characteristics, prescription patterns, effectiveness, and treatment persistence in patients receiving baricitinib in real-world practice in Spain.
Methods
This retrospective longitudinal cohort study conducted in five rheumatology units included adults with RA initiating baricitinib (Sep-2017–May-19) with at least a 6-month-follow-up. Demographic/clinical characteristics, prescription patterns, and changes in disease activity and pain level were collected until treatment discontinuation/end of follow-up. Treatment persistence was estimated by Kaplan–Meier methods.
Results
Data from 182 patients were included (mean (SD)): 83.5% women, 62.2 (12.3) years, body mass index 26.8 (5.1), disease duration 13.2 (10.8) years and Charlson Comorbidity Index score 2.4 (2.0). All patients had received at least one conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) before starting baricitinib and 78.0% at least one biologic disease-modifying anti-rheumatic drugs (bDMARD). Furthermore, 90.1% started with baricitinib 4 mg/day; 43.4% in monotherapy. One hundred and twelve (61.5%) of patients continued baricitinib at data collection time; mean persistence was 14.1 (0.5) months. Overall treatment persistence was 79.7/64.8/59.1% at 6/12/18 months. Seventy (38.5%) patients discontinued baricitinib during follow-up due to loss of efficacy (68.6%) or adverse events (18.6%). In those patients with available scores at the different observed cut-off points, remission or low disease activity was reported in 71.6 and 76.3% of patients at 6/12 months at any index: Disease Activity Score 28 joints using erythrocyte sedimentation rate (DAS28-ESR) (73.1 and 73.5%), Simplified Disease Activity Index (SDAI) (62.4 and 75.0%), and Clinical Disease Activity Index (CDAI) (66.7 and 78.1%). Good or moderate European League Against Rheumatism (EULAR)-response was noted in 80.0 and 78.2% of patients, respectively. Improvement from baseline in pain (Visual Analog Scale) was 2.5 cm and 3.0 cm at 6/12 months, respectively.
Conclusions
This Spanish cohort of patients treated with baricitinib had a long-standing and refractory disease. Nevertheless, high persistence and improvements in disease activity and pain were found at 6 and 12 months after treatment initiation, independently of the composite disease activity measure used, reinforcing the effectiveness of baricitinib in routine clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Baricitinib is an oral Janus kinase (JAK)1/JAK2 inhibitor approved to treat moderate-to-severe rheumatoid arthritis (RA). Despite a robust clinical development, it is important to complement these results with data in real-life conditions. |
The ORBIT-RA retrospective study describes patient’s characteristics and prescription patterns of baricitinib and to estimate its effectiveness and persistence in a cohort of real-world Spanish RA patients. |
Baricitinib was mostly used in RA patients with failure to conventional synthetic disease modifying anti-rheumatic drugs and biologics. In them, baricitinib proved to be effective not only to achieve remission or low activity of the disease. Also in the control of Patient Reported Outcomes, such as pain. |
In general, these results are consistent with those obtained in baricitinib clinical trials and previous observational studies, which reinforces baricitinib effectiveness for the treatment of RA. |
Introduction
Rheumatoid arthritis (RA) is a chronic, multisystemic, autoimmune inflammatory disease characterized by joint inflammation with pain and swelling as its main clinical manifestations (1). The inflammation causes progressive joint destruction and disability (1, 2). RA is associated with extra-articular manifestations such as nodules, interstitial lung disease, and fatigue, as well as specific comorbidities such as depression and cardiovascular disease, which leads to an increase in mortality (1,2,3,4). RA incidence varies, depending on the country, from 0.1 to 0.5 RA cases per 1000 inhabitants/year, with a prevalence of 0.2–1.1% in developed countries, and higher values in women (1,2,3,4,5). In Spain, the prevalence is 0.88% in women and 0.76% in men, peaking at middle age, especially over 60 years (51.3%), with an incidence of 11.3/100,000 cases in women and 5.2/100,000 cases in men (5, 6).
The 2019 EULAR (European League Against Rheumatism) treatment recommendations focus on early intervention and treat-to-target (T2T) strategies using old and new disease-modifying anti-rheumatic drugs (DMARDs) (7). The treatment target should be remission or low disease activity, considering factors such as comorbidities and safety issues (1,2,3, 7). Conventional synthetic DMARDs (csDMARDs), mainly methotrexate (MTX), with or without low dose of glucocorticoids (GCC), have long been the cornerstone of treatment (1,2,3, 7). In case of inadequate response to this first-line treatment, a switch to biologics (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) as second- or third-line treatments is recommended for patients with poor prognostic factors (1,2,3, 7, 8). This strategy has proven to be beneficial in various disease outcomes, but unmet needs and a great disease burden are still recognized (9). The main barriers to the implementation of an effective T2T strategy are well known and settle in the health system (costs, access to healthcare, delayed referral, and diagnosis with difficulty in accessing specialists), patients (adherence, fears about toxicity), and physician factors (knowledge and experience) (1,2,3, 7, 9, 10).
The Janus kinase inhibitors (JAKi) are a novel class of tsDMARD that modulate the effect of multiple cytokines proven to be pivotal in the pathogenesis of RA. Baricitinib is an oral small molecule that provides reversible inhibition of JAK1 and JAK2 with a half-life of 12.5 h (1,2,3, 7, 8, 11, 12). Baricitinib has shown efficacy in clinical trials involving all clinically relevant RA patient populations: MTX naïve, MTX inadequate responders (MTX-IR), and bDMARD-IR (12,13,14). Since September 2017, baricitinib has been reimbursed and marketed in Spain for the treatment of moderate-to-severe active RA in adult patients who have responded inadequately to, or who are intolerant to one or more csDMARDs, and can be prescribed as monotherapy or in combination with MTX (15).
When marketing a new drug, it is important to know its prescription pattern and to estimate its effectiveness in the real world. The existence of a large variability in the rheumatologists’ prescribing patterns (16) and the hurdles in the implementation of the T2T strategies (1,2,3, 9) create a need to understand drug efficacy in a real-world setting. Moreover, from the clinicians’ point of view, there are substantial differences in patient characteristics between RCTs, registries, and observational studies in RA (16). Clinical trials are the methodological gold standard for the evaluation of the efficacy and safety of new drugs, but they are inherently characterized by the lack of external validity as compared to observational studies (16, 17).
To date, limited evidence on baricitinib usage in the real world is available (18,19,20,21). Two observational, retrospective, and prospective studies of 56 and 446 RA patients treated with baricitinib were conducted in Italy (20, 21). An observational, retrospective study with 40 RA patients conducted in Spain, published by Rosas et al. (18), and a retrospective study in UK with 69 RA patients (19), concluded that baricitinib showed effectiveness and safety in real-world conditions.
The present study was designed to investigate patients’ characteristics, patterns of prescription, effectiveness, and drug persistence in a cohort of RA patients treated with baricitinib in a real-world setting in Spain.
Methods
Study Design
This is an observational, retrospective, longitudinal cohort study based on medical chart reviews, conducted in five tertiary Spanish public hospitals distributed along different geographic areas.
The study inclusion period was from September 1, 2017 (date of approval of baricitinib in Spain) to May 31, 2019. The data collection period started in December 2019, to guarantee a minimum follow-up of 6 months for each patient (Fig. 1). The investigators extracted data from each patient’s corresponding electronic clinical record form until treatment discontinuation or end of study follow-up (per patient data collection date). The study did not have an impact on any treatment decision during clinical practice due to the retrospective nature of the data collection. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the Ethics Committees of Virgen Macarena and Virgen Rocio University Hospitals in Seville (Spain). Informed consent was collected when required by each ethics committee.
Patient Selection Criteria
Patients eligible for inclusion in this study had a RA diagnosis according to the 2010 ACR/EULAR criteria, initiated baricitinib (according to the summary of product characteristics (SmPC)) during the inclusion period, were aged ≥ 18 years, and had a complete medical record at the participating site or at least since the RA diagnosis. Patients were excluded if they had previously participated in any baricitinib clinical trial. No other explicit exclusion criteria were defined.
Study Objectives
The primary objective was to describe the demographic and clinical characteristics and prescription patterns among RA patients treated with baricitinib in real clinical practice in Spain. The secondary objectives were aimed at estimating baricitinib treatment persistence, effectiveness using different validated index/scales, and impact of the treatment on the concomitant medication for RA.
Study Variables
The following variables were collected at each site:
Baseline Patient Demographics and Clinical Characteristics
Patients’ characteristics included age, gender, body mass index (BMI), smoking status, disease duration, working status, and Charlson Comorbidity Index (CCI). CCI includes age, myocardial infarction, congestive heart failure (CHF), peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), connective tissue disease, peptic ulcer disease, liver disease: mild/moderate or severe, diabetes mellitus: uncomplicated/end-organ damage, hemiplegia, moderate-to-severe chronic kidney disease (CKD), any solid tumor: without metastasis/metastatic solid tumor, leukemia, lymphoma, and acquired immunodeficiency syndrome (AIDS). Each condition receives 1, 2, 3, or 6 points, depending on its independent mortality risk. Total score for CCI, which is derived by the sum of all comorbid conditions, rates from 0 to 37, with higher values indicating a more severe condition and a worse prognosis.
The disease characteristics collected were time from RA diagnosis, previous and concomitant treatments, seropositivity status, presence of extra-articular manifestations, and presence of bone erosions. The Disease Activity Score 28 joints using erythrocyte sedimentation rate (DAS28-ESR), Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), EULAR response criteria, and pain by visual analogic scale (VAS) ranging from 0 (no pain) to 10 (maximum pain) cm were collected. Laboratory tests included acute phase reactants (C-reactive protein (CRP) in mg/l and erythro-sedimentation rate (ESR) in mm/h), hemoglobin (g/l), platelets (mm3 or 109/l), leukocytes (109/l or cells/l), triglycerides (mg/dl or mmol/l), total cholesterol (mg/dl or mmol/l), HDL (mg/dl or mmol/l), LDL (mg/dl or mmol/l), serum creatinine, creatine kinase (CK U/l), and glomerular filtration rate (GFR, ml/min).
Baricitinib Prescription Patterns
Date of baricitinib treatment initiation, posology and changes in dosing, and date and reasons of definitive treatment discontinuation (if applicable) were collected. History of previous csDMARDs or bDMARDs was recorded to assess previous treatment exposures. Concomitant treatment medications for RA (GCC, csDMARDs, and non-steroid anti-inflammatory drugs (NSAIDs) were also recorded after baricitinib initiation.
Baricitinib Effectiveness and Pain Assessment
Effectiveness assessments available at 6 and 12 months using DAS28-ESR, SDAI, CDAI, and EULAR response, and pain level based on VAS were obtained.
Persistence was summarized by mean treatment duration on baricitinib until discontinuation or end of follow-up, and by the percentage of patients who maintained baricitinib at 6, 12, and 18 months of follow-up.
Changes from baseline in DAS28-ESR, SDAI, and CDAI, and pain scores were calculated as the difference between baseline (baricitinib initiation) and follow-up scores (at 6 and 12 months). EULAR response criteria, evaluated by improvement in the DAS28-ESR from baseline, were described (good response, moderate response, and non-response) at 6 and 12 months.
Statistical Analysis
A descriptive statistical analysis of the patients’ demographic and clinical characteristics was performed. Summaries were estimated for patients with valid observations. Continuous variables were summarized using the mean, standard deviation (SD), median, and 25th and 75th percentiles (P25 and P75, respectively). Categorical variables were summarized using frequencies and percentages.
Changes from baseline in clinical scores (DAS28-ESR, SDAI, CDAI, and EULAR response criteria) and patient’s pain assessment were reported using 95% confidence intervals (CI) of the baseline follow-up difference and compared using paired t test; however, no inferential statistics should be considered as the testing hypothesis.
Baricitinib treatment persistence was estimated as the mean time to discontinuation using the Kaplan–Meier method, and treatment maintenance at 6, 12, and 18 months after baseline. Patients who had discontinued baricitinib at data extraction date due to loss of follow-up, lack of efficacy, adverse events, or other reasons, were considered for persistence analysis up to the date of discontinuation. For patients who had not discontinued baricitinib, data was censored at the end of the follow-up (administrative censoring). Deaths were not applied for censoring since that information was not available.
Additionally, analyses were conducted among different groups of patients to assess the persistence rates at 6 and 12 months according to: (1) the number of previous bDMARDs (0/ < 3/ ≥ 3), (2) seropositivity status (rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA): (ACPA+ and RF+/ACPA+ and RF−/ACPA− and RF+/ACPA− and RF−), (3) age (< 65 years/ ≥ 65 years), (4) gender, (5) CCI (≤ 2/ > 2), and (6) concomitant use of csDMARDs (baricitinib monotherapy (without csDMARDs)/baricitinib+ csDMARD).
Results
Demographic and Clinical Characteristics
One hundred and eighty-two patients were included in the study, mostly women (83.5%) with a mean (SD) age of 62.2 (12.3) years, and a mean time since RA diagnosis of 14.6 (10.8) years. Mean BMI was 26.8 (5.1) kg/m2 and 14.8% were current smokers. Most of the patients were retired (32.4%) (Table 1).
A significant proportion of patients presented poor prognosis variables: RF+ (79.1%), ACPA+ (80.2%), bone erosions (64.8%), extra-articular manifestations (31.9%), and a mean CCI of 2.4 (2.0). Laboratory tests prior to baricitinib treatment showed a mean CRP of 8.6 (15.8) mg/l and an ESR of 29.7 (24.0) mm/h. Results of the remaining laboratory tests are shown in Table 1.
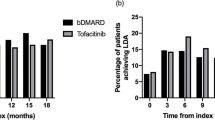
Most patients had received ≥ 3 prior csDMARDs (52.2%). The overall number of patients who had previously been treated with one, two, or three or more bDMARDs was 24.2, 17.0, and 36.8%, respectively (Table 2 and Fig. 2). Furthermore, 3.8% of patients had previously been treated with another JAKi (tofacitinib), 76.4% with GCC and 33.0% with NSAIDs.
The most common treatments received immediately prior to baricitinib were csDMARDs (69.8%), bDMARDs (61.5%), GCC (54.9%), and NSAIDs (20.9%). MTX (63.0%) and leflunomide (33.1%) were the most frequently used csDMARD. Among patients receiving bDMARDs, 44.7% were treated with a TNF inhibitor (TNFi) and 55.3% with a non-TNF biologic (mainly abatacept, 56.4%).
Patients included in the study had moderate (63.4, 56.3, and 54.1%) and high (21.8, 34.1, and 38.5%) disease activity at baseline according to DAS28-ESR, CDAI, and SDAI, respectively. Mean pain assessment at baseline was 6.6 (2.0) cm (Table 1).
Baricitinib Prescription Patterns
Most patients included in the study started treatment with baricitinib at 4 mg/day (90.1%), and 43.4% received baricitinib as monotherapy (Table 3). Eighteen patients (9.9%) changed the starting dose during follow-up; of the total number of changes (n = 20), 14 (70.0%) were dose reductions to 2 mg/day due to remission (64.3%) and adverse events (14.3%). The mean time until change in posology was 272 (200) days, and 326 (195) days until decreasing the dose from 4 to 2 mg daily. Six (30.0%) dose increases from 2 to 4 mg daily were observed. The mean time until increasing the dose from 2 to 4 mg daily was 92 (40) days. Baricitinib treatment was discontinued during follow-up in 70 patients (38.5%). The main reasons for discontinuation were lack of efficacy (68.6%; (n = 48/182); 60.4% primary, 39.6% secondary) and adverse reactions (18.6%; (n = 13/182). The main patient sociodemographic and clinical characteristics of the patients who discontinued treatment due to lack of efficacy are provided in Table 1S. They were different in weight, RF, ACPA positivity, and erosions. The median [P25; P75] time of baricitinib treatment during the study period was 373 days (197; 569) (Table 3).
A total of 150 patients (82.4%) received concomitant treatments together with baricitinib (in any time of baricitinib treatment): DMARDs (56.6%), systemic corticosteroids (54.9%) and NSAIDs (20.3%). From the total number of concomitant GCC treatments (n = 112), 23.2% (n = 26) changed after baricitinib initiation to reduce or stop in 96.2% of the cases. Median time until reduction or withdrawal of GCC was 116 and 111 days, respectively. With respect to NSAIDs administered during baricitinib treatment, 73.0% were occasionally taken.
Baricitinib Effectiveness
At baseline, the number of tender joint counts (TJC) and swollen joint counts (SJC) was 6.7 (5.8) and 4.4 (3.7), respectively (Table 1). Patients treated with baricitinib showed a reduction (mean (95%CI)) of 4.4 (− 5.5; − 3.3) and 5.2 (− 6.5; − 4.0 to 4) for TJC; and 2.8 (− 3.6; − 2.0) and 4.0 (− 4.9; − 3.1) for SJC at 6 and 12 months, respectively. Pain assessment (VAS 10 cm) was 6.6 (2.0) at baseline (Table 1) and showed a reduction of 2.5 (− 3.0; − 2.0) and 3.0 (− 3.6; − 2.5) cm at 6 and 12 months, respectively. These changes were statistically significant (p value < 0.0001). Figure 3 and Table 4 show the evolution progress and changes in the score of the different indexes during the follow-up period.
The activity indexes measured at baseline (DAS28-ESR, CDAI, and SDAI) were 4.4 (1.1), 23.6 (10.5), and 25.0 (10.4), respectively (Table 1). In those patients with available scores at the different observed cut-off points, reductions at 6 and 12 months were (mean (95% CI)) 1.7 (− 2.0; − 1.4) and 1.7 (− 2.1; − 1.2) for DAS28-ESR; 13.0 (− 15.3; − 10.6) and 13.7 (− 16.7; − 10.7) for CDAI; and 13.4 (− 15.8; − 11.0) and 14.4 (− 17.4; − 11.3) for SDAI (Table 4, Fig. 3). The changes from baseline at 6 and 12 months were statistically significant for all indexes (p value < 0.0001). The percentage of patients who presented remission or low disease activity according to DAS28-ESR, CDAI, and SDAI was 73.1% (n = 57/78), 66.7% (n = 62/93), and 62.4% (n = 58/93) at 6 months; and 73.5% (n = 36/49), 78.1% (n = 50/64), and 75.0% (n = 48/64) at 12 months, respectively. Based on previous data, remission or low disease activity was reported in 71.6% (n = 83/116) and 76.3% (n = 58/76) of patients at 6/12 months at any index.
At 6 months, 80.0% (n = 75) of patients showed moderate or good response according to EULAR criteria. A similar response was observed from 6 to 12 months of follow-up in patients who continue in treatment (78.2%) (n = 46).
A mean (95% CI) reduction at 6 and 12 months compared to baseline in levels of CRP (− 4.7 (− 7.7; − 1.8) (n = 115) and − 4.9 (− 9.0; − 0.9) (n = 85) mg/l) and ESR (− 4.0 (− 8.1; 0.1) (n = 113) and − 6.3 (− 11.9; − 0.7) (n = 65) mm/h) was observed. Changes in laboratory values showed a reduction in hemoglobin (− 0.4 [− 1.0; 0.1] g/l) and leukocytes (− 0.4 [− 2.1; 0.9] 109/l); and a small increase in platelets 42 [− 10.0; 83.0] 109/l, total cholesterol 14 [− 3.0; 35.0] mg/dl, creatinine 0.1 [0.0; 0.1], and CK 44 [− 33.0; 62.0] (median [P25; P75] values) when considering baseline values and last available record in the medical chart after baricitinib initiation (Table 5).
Baricitinib Persistence
At the time of data collection, 61.5% of patients were on treatment with baricitinib, and the mean time on treatment was 14.1 (0.5) months. Baricitinib showed a persistence at 6, 12, and 18 months of 79.7, 64.8, and 59.1%, respectively. The persistence varied among the different subpopulations studied, ranging from 90.0, 81.9, and 72.6% in patients with no previous bDMARDs to 80.8, 65.5, and 58.0% in patients who failed < 3 previous bDMARDs and to 71.9, 53.4, and 51.3% in patients who failed ≥ 3 bDMARDs at 6, 12, and 18 months, respectively (Fig. 4). Regarding seropositive status, ACPA+ RF+ subpopulation showed the best persistence with values of 82.2, 71.0, and 64.5% at 6, 12, and 18 months, respectively; in the ACPA- RF- subgroup persistence values were 70.4, 45.4, and 45.4% at 6, 12, and 18 months, respectively.
There were no relevant differences in persistence of treatment according to age or gender. However, higher values were found in patients who had less than 2 CCI in comparison with those who had ≥ 2, and in those who were on baricitinib in combination with csDMARDs, as opposed to those in monotherapy (Fig. 4). Additional information on the baseline patient sociodemographic and clinical characteristics of these two subgroups according to treatment (combination and monotherapy) is provided in Table 2S. Patients in monotherapy seemed to be older, had longer disease, high levels of CRP, and a lower percentage of smokers, although no formal statistical tests for comparison were made.
Discussion
The ORBIT-RA study is an observational study conducted in Spain to describe the use of baricitinib in real-world conditions. This study showed that baricitinib provides remission or low disease activity and pain relief, even in those who had previously failed several csDMARDs and bDMARDs, thus reinforcing its effectiveness in real clinical practice.
Patients included in the ORBIT-RA study were predominantly older females with moderate to highly active RA before starting baricitinib treatment. Clinical and sociodemographic characteristics of these patients were reasonably aligned with those of previous observational studies conducted in Spain, Italy, and the UK (18,19,20,21) in terms of gender, age, years of disease, seropositivity status, comorbidities, and disease activity scores (DAS28-ESR, SDAI and CDAI). Our study showed baseline moderate levels of disease activity scores (DAS28ESR 4.3 ± 1.1, SDAI 10.9 ± 10.3 and CDAI 23 ± 10 like other observational studies (18,19,20,21) and lower than those of the clinical trials with baricitinib. In the RA-BEAM study, the DAS28-ESR was 6.4 ± 1.0 and SDAI 40 ± 13 (14). This differences in activity are basically due to that the patients in clinical practice have different clinical characteristics than those included in clinical trials (16, 17), and because in clinical practice the treat to target strategy is applied, and that prevents patients from having high levels of the disease. Compared to the ORBIT-RA study, the sample from UK and Italian prospective studies included younger patients, with less time since diagnosis and lower percentages of seropositivity (19, 20).
Regarding prescription patterns, we found that the use of baricitinib in the ORBIT-RA study followed recommendations in the SmPC. Most patients were bDMARD-IR (78%), which is quite different compared to Rosas et al.’s registry-based study in which 54% of patients were naïve to bDMARDs (18), supporting the great variability observed in prescription patterns in routine clinical practice (16, 17, 19). Treatment with baricitinib was started at 4 mg/day dose in 90.1% of patients since 2 mg daily is only indicated in patients older than 75 years, with a previous history of chronic or recurrent infections or renal impairment: explaining the use of this starting dose in only 18 patients of this cohort.
Unlike other JAKi, baricitinib has the advantage of permitting dose reductions from 4 to 2 mg/day in case of sustained control of the disease and these changes in dose have been described for the first time in our study. A total of 13 dose reductions from 4 to 2 mg/day were registered, nine of which were due to remission or low disease activity. In contrast, six dose increases were recorded, none of them associated with lack of effectiveness. In line with these results, a longer follow-up time would be needed to understand if the sustained control of the disease translates into a stable decrement in the dose of baricitinib.
Adverse events were the reason for two dose reductions from 4 to 2 mg/day, similar to what has previously been described in Rosas (18) and Fitton (19) studies. From the total number of patients included in the study, 13 discontinued baricitinib due to safety (7%), which is a slightly lower value than the ones reported in Rosas (10%), Guidelli (13%), and Fitton (13%) studies (18,19,20).
Concerning concomitant GCC treatments, 23.2% were changed after baricitinib initiation; 96.2% of which reduced or stopped as early as 116 days and 111 days (median), respectively. These data are consistent with results found in the Italian cohort (20), where the number of patients treated with GCC, as well as the dose, was halved per year, a result that may be explained by the remission or low disease activity achieved after starting baricitinib or the pain relief effect.
The patients included in the study with available activity measures (any index) showed an increase or maintenance in the percentages of remission or low activity at 6 and 12 months. This result is in agreement with that reported by Fitton et al. (19) at 6 months for DAS28 in the UK, but based on a longer follow-up period and analyzed using different disease activity indexes. Collectively, these data reveal that baricitinib is an effective treatment for managing RA signs and symptoms in real clinical practice.
Of interest, the results regarding pain assessment are consistent with those observed in other studies on the clinical benefit of baricitinib in pain control (19), already supported by the results of RA-BEAM (14), where a greater proportion of baricitinib-treated patients vs. adalimumab achieved pain relief as early as week 2. The importance of this finding is subscribed by the proportion of RA patients who rate pain as one of their top three priorities based on prior evidence (68–88%) (22). The QUEST-RA (23) and Studenic et al. (24) studies showed pain to be the major determinant of patient global assessment scores, explaining 75.6% of score variability. In addition, the American College of Rheumatology Pain Management Task Force also reinforces this evidence stating that “pain is probably the most important patient-reported outcome in rheumatology” (25). Results from the ORBIT-RA study showed that not only can remission be achieved with baricitinib, but also other patient-reported outcomes.
Baricitinib persistence was especially remarkable in our study, with higher values in patients not previously exposed to bDMARDs and lower ones in the bDMARDs-IR population, consistent with data obtained by Guidelli and Fitton (19, 20). Persistence with baricitinib was found to be associated with seropositivity, a connection that has already been described for abatacept and rituximab (26, 27), but not for tofacitinib (28), so further research is needed to confirm this relationship. Better persistence was also related to lower CCI scores and the use of baricitinib in combination, as it has been described for several bDMARDs (29,30,31,32,33,34). No impact on persistence was detected for variables such as age and gender. However, additional investigations will be necessary to determine whether all of these factors can be considered influential in treatment persistence or not.
Compared to previous observational studies available in the literature, some aspects of the design of the ORBIT-RA study can be highlighted, such as the large sample size, the long observational period (18 months, with a minimum follow-up per patient of 6 months), and the use of multiple disease activity scores to assess effectiveness. The main study limitations are related to its retrospective design and the availability of data in patients’ medical records to meet the study objectives, mainly for disease activity scores. Additionally, as routine clinical practice differs geographically, the generalizability of the results is also a limitation, as well as the lack of a comparator group to determine how the different assessed variables compares to other treatments. Finally, since the analysis of baricitinib persistence by subgroup was descriptive, any conclusion about the differences observed should be made with caution, and further research would be needed to confirm it.
Conclusions
Patients with RA who received baricitinib treatment showed a long-standing and refractory disease profile. Results from the ORBIT-RA study reinforced evidence that baricitinib provides remission or low disease activity at 6 months and maintains the response at 12 months in a high percentage of patients, even in those who have failed previously to several csDMARDs and bDMARDs. The major improvements in disease activity suggest the good effectiveness and pain relief of baricitinib treatment in real clinical practice for patients with moderate to highly active RA in Spain.
Change history
02 June 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40744-022-00463-8
References
Scott IC, Machin A, Mallen CD, Hider SL. The extra-articular impacts of rheumatoid arthritis: moving towards holistic care [Internet]. BMC Rheumatol. 2018;2:1–15.
Carmona L, González-Álvaro I, Balsa A, Belmonte MA, Tena X, Sanmartí R. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis [Internet]. 2003;62(9):897–900.
Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of rheumatology criteria: a systematic review. Semin Arthritis Rheum [Internet]. 2006;36(3):182–8.
Se G. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am [Internet]. 2001;27(2):269–81.
Seoane-Mato D, Sánchez-Piedra C, Silva-Fernández L, Sivera F, Blanco FJ, Pérez Ruiz F, et al. Prevalence of rheumatic diseases in adult population in Spain (EPISER 2016 study): aims and methodology. Reumatol Clin. 2019;15(2):90–6.
Carbonell J, Cobo T, Balsa A, Descalzo MÁ, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatol [Internet]. 2008;47(7):1088–92.
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis [Internet]. 2020;79(6):S685–99.
Taylor PC. Clinical efficacy of launched JAK inhibitors in rheumatoid arthritis. Rheumatol (UK) [Internet]. 2019;58(Suppl 1):17–26.
Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36(5):685–95.
Silvagni E, Sakellariou G, Bortoluzzi A, Giollo A, Ughi N, Vultaggio L, et al. One year in review 2021: novelties in the treatment of rheumatoid arthritis [Internet]. Clin Exp Rheumatol. 2021;39:705–20.
Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69(3):506–17.
Dougados M, Van Der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76(1):88–95.
Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis [Internet]. J Mineralstoffwechsel N Engl J Med. 2016;23:60–1.
Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, del Carmen ML, Reyes Gonzaga J, et al. Baricitinib versus Placebo or adalimumab in rheumatoid arthritis. N Engl J Med [Internet]. 2017;376(7):652–62.
(EMEA). ETEAftEoMP. Olumiant®. Summary of Product Characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf.
Dhaon P, Das SK, Srivastava R, Dhakad U. Performances of Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI) appear to be better than the gold standard Disease Assessment Score (DAS-28-CRP) to assess rheumatoid arthritis patients. Int J Rheum Dis [Internet]. 2018;21(11):1933–9.
Takanashi S, Kaneko Y, Takeuchi T. CDAI and DAS28 in the management of rheumatoid arthritis in clinical practice [Internet]. Ann Rheum Dis. 2020;79:671–4.
Rosas J, Senabre-Gallego JM, Santos-Soler G, Bernal JA, Pons Bas A, Aire-Mb Grupo. Efficacy and safety of baricitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic DMARDs and/or biological DMARDs: data from a local registry. Reumatol Clin (Engl Ed). 2020. https://doi.org/10.1016/j.reuma.2020.04.011. (English, Spanish).
Fitton J, Melville AR, Emery P, Nam JL, Buch MH. Real-world single centre use of JAK inhibitors across the rheumatoid arthritis pathway. Rheumatol [Internet]. 2021;60(9):4048–54.
Guidelli GM, Viapiana O, Luciano N, De Santis M, Boffini N, Quartuccio L, et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: a real-life multicentre study. Clin Exp Rheumatol [Internet]. 2021;39(4):868–73.
Perrone V, Losi S, Rogai V, Antonelli S, Fakhouri W, Giovannitti M, et al. Real-world analysis of therapeutic patterns in patients affected by rheumatoid arthritis in Italy: a focus on baricitinib. Rheumatol Ther [Internet]. 2020;7(3):657–65.
Ten Klooster PM, Veehof MM, Taal E, Van Riel PLCM, Van De Laar MAFJ. Changes in priorities for improvement in patients with rheumatoid arthritis during 1 year of anti-tumour necrosis factor treatment. Ann Rheum Dis [Internet]. 2007;66(11):1485–90.
Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res [Internet]. 2012;64(2):206–14.
Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum [Internet]. 2012;64(9):2814–23.
American College of Rheumatology Pain Management Task Force. Report of the American College of Rheumatology Pain Management Task Force. Arthritis Care Res (Hoboken). 2010;62(5):590–9. https://doi.org/10.1002/acr.20005.
Alten R, Mariette X, Lorenz HM, Nüßlein H, Galeazzi M, Navarro F, et al. Predictors of abatacept retention over 2 years in patients with rheumatoid arthritis: results from the real-world ACTION study. Clin Rheumatol [Internet]. 2019;38(5):1413–24.
Courvoisier DS, Chatzidionysiou K, Mongin D, Lauper K, Mariette X, Morel J, et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: Results from a collaboration of 16 registries. Rheumatol (UK) [Internet]. 2021;60(2):820–8.
Harrold L, Shan Y, Rebello S, Guo L, Connolly S, Zhuo J, Kelly S, Lehman T. Association between baseline anti-citrullinated protein antibody status and response to abatacept or non-TNF inhibitor therapy in patients with RA: results from a US National Observational Study [abstract]. Arthritis Rheumatol. 2019;71(suppl 10):8. https://acrabstracts.org/abstract/association-between-baseline-anti-citrullinated-protein-antibody-status-and-response-to-abatacept-or-non-tnf-inhibitor-therapy-in-patients-with-ra-results-from-a-us-national-observational-study/. Accessed 19 Dec 2021.
Gabay C, Riek M, Scherer A, Finckh A. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: Data from the Swiss Clinical Quality Management Registry. Rheumatol (UK) [Internet]. 2015;54(9):1664–72.
Soliman MM, Ashcroft DM, Watson KD, Lunt M, Symmons DPM, Hyrich KL. Impact of concomitant use of DMARDs on the persistence with anti-TNF therapies in patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis [Internet]. 2011;70(4):583–9.
Heiberg MS, Koldingsnes W, Mikkelsen K, Rødevand E, Kaufmann C, Mowinckel P, et al. The comparative one-year performance of anti-turmor necrosis factor α drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Care Res [Internet]. 2008;59(2):234–40.
Favalli EG, Pregnolato F, Biggioggero M, Becciolini A, Penatti AE, Marchesoni A, et al. Twelve-year retention rate of first-line tumor necrosis factor inhibitors in rheumatoid arthritis: real-life data from a local registry. Arthritis Care Res [Internet]. 2016;68(4):432–9.
Zink A, Listing J, Kary S, Ramlau P, Stoyanova-Scholz M, Babinsky K, et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis [Internet]. 2005;64(9):1274–9.
Kristensen LE, Saxne T, Nilsson JÅ, Geborek P. Impact of concomitant DMARD therapy on adherence to treatment with etanercept and infliximab in rheumatoid arthritis. Results from a six-year observational study in southern Sweden. Arthritis Res Ther [Internet]. 2006;8:6.
Acknowledgements
The authors would like to thank the patients, investigators, and institutions for their participation in this study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Eli Lilly and Company, Indianapolis, IN, USA.
Medical Writing, Editorial, and Other Assistance
Assistance in the preparation of this article was provided by IQVIA and funded by Eli Lilly and Company, Indianapolis, IN, USA. The authors would like to acknowledge Jennifer Redondo (trainee at Lilly during the development of the article) for her contributions (data quality check-review and medical writing assistance).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for their contribution this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
All authors contributed to the study conception, design, and data analysis. All authors have reviewed previous versions of the manuscript and have read and approved the final one.
Prior Presentation
These data were previously accepted as an abstract for the congress of the Spanish Society of Rheumatology 2020 (SER2020) and presented as a poster in the congress of the Argentine Society of Rheumatology 2020 (SAR 2020).
Disclosures
Mercedes Núnez and Silvia Díaz are employees of Eli Lilly and Company (Spain) and hold Eli Lilly and Company shares. José Inciarte-Mundo was an employee of Eli Lilly and Company (Spain) at the time the study was conducted. Blanca Hernández-Cruz, José Rosas, César Díaz-Torné, Joaquín Belzunegui, Rosario García-Vicuña, Ana Pons, Ana M Millán, Sicylle Jeria-Navarro, Jesús A Valero, Noelia García-Castañeda, Cristina Valero, Irene Llorente and Alberto Calvo have received an economic compensation for their participation in the study through data collection, analysis, and interpretation tasks.
Compliance with Ethics Guidelines
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the Ethics Committees of Virgen de la Macarena and Virgen del Rocio University Hospitals in Seville (Spain). Informed consent was collected when required by each ethics committee.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised: As the authors noticed some of the values in Table 1 and Table 2 of this article, the data in the column 2 and row 67 headed “Baseline patient sociodemographic and clinical characteristics” and the data in the column 3 and row 12 headed “Number of RA treatments received prior to baricitinib treatment” were incorrectly published.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hernández-Cruz, B., Rosas, J., Díaz-Torné, C. et al. Real-World Treatment Patterns and Clinical Outcomes of Baricitinib in Rheumatoid Arthritis Patients in Spain: Results of a Multicenter, Observational Study in Routine Clinical Practice (The ORBIT-RA Study). Rheumatol Ther 9, 589–608 (2022). https://doi.org/10.1007/s40744-021-00423-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-021-00423-8