Abstract
The catnip plant, Nepeta cataria L., produces bioactive iridoid monoterpenes known as nepetalactones. These monoterpenes follow a noncanonical biosynthetic route starting as isoprene monomers in the methylerythritol (MEP) pathway after condensation via geranyl pyrophosphate synthase (GPPS) and later cyclization through the iridoid pathway via nepetalactol-related short-chain dehydrogenase enzymes (NEPS). Nepetalactones exhibit insect repellant properties against disease-carrying vectors such as mosquitoes, bed bugs and ticks. Chemical elicitors such as salicylic acid have been used to induce biochemical changes in medicinal and aromatic plant species including catnip that can temporarily increase the yield and quality of secondary metabolites. This is the first study to correlate the effects of short-term salicylic acid application on the genes in the nepetalactone biosynthetic pathway. Both 0.5 mM SA and 1.0 mM SA resulted in upregulation of GPPS and NEPS1. However, within the 24-h time course of the study significant changes in nepetalactone yield were not observed. Correlation analysis revealed a significant relationship between the mRNA transcript abundance for GPPS and NEPS1 demonstrating a low positive co-expression strength for the two genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Catnip, Nepeta cataria, is an aromatic herb that belongs to the Lamiaceae family, the Nepetoidae subfamily and the tribe Menthae (Payandeh et al. 2015). It is widely known for eliciting a euphoric effect on cats and other feline animals due to its volatile secondary product, nepetalactone, contained in the essential oil of the plant (Reichert et al. 2016). Current research has also shown catnip essential oil to be an effective, natural insect repellant especially against urban pests such as mosquitoes, bed bugs, and ticks (Reichert et al. 2019; González et al. 2022; Patel et al. 2023).
The bioactive nepetalactones can be procured from catnip essential oil via steam distillation and hydro-distillation of the aerial plant material or through extraction by organic solvents such as methanol and ethanol (Adiguzel et al. 2009; Gomes et al. 2020). Common production practices to obtain catnip biomaterial include growing catnip from seed and procuring transplants from undomesticated populations (Reichert et al. 2016). Commercial production of catnip, however, has been limited since varieties tend to produce relatively low quantities of biomass and essential oil (Park et al. 2007). More recent breeding studies to improve catnip cultivation and make catnip an economically viable option for small farmers have led to cultivars with marked improvement in vegetative and morphological performance. Simon and Reichert (2020) developed a high performing catnip cultivar, ‘CR9,’ specifically bred to produce higher amounts of biomass and yield essential oil richer in (Z,E)-nepetalactone. Another catnip cultivar, ‘CR3,’ was purposefully bred to yield greater quantities of total essential oil while also accumulating both (E,Z)-nepetalactone and (Z,E)-nepetalactone (Simon et al. 2019). Subsequent catnip germplasm studies have been conducted to assess the effects of genotype, harvest time, and their interactions on biomass yield and terpene composition. Patel et al. (2022) demonstrated that patented cultivars such as ‘CR3’ and ‘CR9’ exhibit improved field performance and accumulate greater biomass when compared to the yields of underdeveloped genotypes.
Among the production practices used to improve the performance and quality of various medicinal plants, numerous short-term abiotic elicitation studies have been shown to influence essential oil and secondary metabolite concentration in addition to their related biosynthetic genes in Lamiaceae species. One study conducted with peppermint (Mentha x piperita) evaluated the influence of methyl jasmonate treatment on menthol production and gene expression of pulegone reductase (pr), menthofuran synthase (mfs), and limonene synthase (ls). The transcripts of these genes involved in the menthol biosynthesis pathway were upregulated within 4–12 h of methyl jasmonate application followed by downregulation after 24–48 h (Afkar et al. 2013). Majdi et al. (2017) executed a rapid elicitation study on thyme (Thymus vulgaris L.) with methyl jasmonate, salicylic acid, trans-cinnamic acid and ultraviolet (UV-C) treatments. Sampling was conducted 24 h after elicitor application which led to the upregulation of genes in the thymol/carvacrol biosynthetic pathway. Another report investigated the physiological responses, secondary metabolite production, and gene expression of Prunella vulgaris L. in response to exogenous methyl jasmonate and salicylic acid (Tang et al. 2022). After 24 h both treatments resulted in changes in secondary metabolites including phenolics and flavonoids, while also influencing the activity of antioxidant enzymes and the expression of genes in the phenylpropanoid metabolic pathway. Another study performed with lavender (Lavandula angustifolia) assessed the effects of 1.0 mM methyl jasmonate and 1.0 mM salicylic acid on the induction of two genes, encoding borneol synthase (BRS) and fenchol synthase (FES), involved in the monoterpene pathway. Though samples were harvested after 24, 48 and 72 h of treatment, the expression of BRS was only significantly increased after 72 h in response to methyl jasmonate which may be useful for advancements in metabolic engineering (Hosseini et al. 2023).
However, in catnip, the use of chemical elicitors to improve agronomic productivity is limited. In Iran, Ozhan et al. (2017) treated catnip (Nepeta cataria L.) with a combinatorial formulation of humic acid, citric acid, and chitosan. These biostimulants exhibited a positive effect on the vegetative characteristics and nepetalactone yield of the treated plants. Aničić et al. (2022) explored the effects of methyl jasmonate on iridoid biosynthesis and identified elevated expression of key biosynthetic genes in Nepeta rtanjensis and Nepeta nervosa.
Given the variety of abiotic elicitors utilized to enhance the medicinal and aromatic value of plants, one of the most commonly used exogenous compounds is salicylic acid. Well known for its ability to induce systemic acquired resistance in plants against pathogens, it is the primary signal responsible for regulating plant defense gene expression (Guru et al. 2022). Various modes of application such as soaking seeds, cultivation via hydroponics, and foliar spraying, have illustrated its protective role as a primary signaling hormone for plant immunity (Liu et al. 2022). The application of salicylic acid has been shown to both mitigate environmental stressors on medicinal plants while also increasing their bioactive constituents (Ahmad et al. 2018; Pacheco & Gorni 2021). Foliar application of salicylic acid on two catnip chemotypes (Nepeta cataria L. and Nepeta cataria var. citriodora) improved essential oil accumulation and plant growth under water stress conditions (Said-Al Ahl et al. 2016). But to the best of our knowledge, there are no short-term studies showing the effect of this elicitor on the nepetalactone-rich methanol extracts of catnip as well as the expression of genes involved in the nepetalactone biosynthetic pathway. As a result, we sought to determine the short-term effects of salicylic acid application onto catnip plants grown hydroponically by evaluating their fresh and dry masses, nepetalactone yield, and relative mRNA expression of genes involved in the nepetalactone biosynthetic pathway after a 24-h elicitation period. We also attempted to establish a relationship between the expression patterns of nepetalactone biosynthetic genes and the concentration of nepetalactone in the catnip leaves at the vegetative developmental stage after salicylic acid treatment.
2 Material and methods
2.1 Plant materials and growth conditions
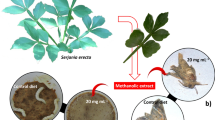
In the current experiment, Rutgers-bred ‘CR9’ catnip (Nepeta cataria L.) was used as the source of plant material. This study was conducted in the New Jersey Agricultural Experiment Station (NJAES) Research Greenhouse in climate-controlled conditions in New Brunswick, New Jersey. The ambient temperature ranged from 20 to 23 °C and the relative humidity averaged 57.8%. ‘CR9’ catnip seeds were planted in commercial soil contained within 128-plug polypropylene flats and subjected to a 16/8 h light/dark photoperiod. The seeds were allowed to germinate under a greenhouse mist chamber for approximately one week. Additional seedlings were removed from the flats so only 1 seedling remained per plug. Flats were transferred to greenhouse bench space and grew until seedlings reached 4 pairs of leaves at approximately 7 weeks of age (Fig. 1). Prior to transferring the seedlings to the hydroponic system, the soil was manually removed from the roots and the roots were thoroughly rinsed with water.
2.2 Hydroponic conditions
To cultivate the seedlings hydroponically, the Viagrow VCLN24 Clone Machine, 24-site Hydroponic Systems (Viagrow, East Point, GA, USA) were used. Each contained 2 gallons (7.57 L) of ½ strength Hoagland’s nutrient solution (PhytoTech Labs, Lenexa, KS, USA). Three clone machines were used to complete each replication of the experiment. The nutrient solution was set to a pH between 5.8 and 6.0 using “pH Down” and “pH Up” solutions (Viagrow, East Point, GA, USA). After 8 weeks of growth (Maboko and Plooy 2014), catnip seedlings of similar height and width (approximately 20 cm tall and 6.5 cm wide) were transferred to the Viagrow Clone 24 machines. Each seedling was placed in a neoprene collar and positioned with the shoots above the clone machine and the roots below in the ½ strength Hoagland’s nutrient medium (Fig. 2). Twelve plants were added to each clone machine. The seedlings were allowed to acclimatize to the hydroponic system for 1 week prior to treatment with salicylic acid to allow satisfactory development of the root system and adaptation to the hydroponic system.
2.3 Plant elicitation
After the 1-week acclimatization period, salicylic acid was added to each vessel of nutrient solution. The salicylic acid (SA) concentrations used were 0.0 mM (control), 0.5 mM, and 1.0 mM. After adding the appropriate concentration of SA to each box, the pH was re-adjusted to the working range. Three time-points were used for each concentration at 4-h, 12-h and 24-h. For each time-point, a total of 12 plants (three biological replicates; four plants per treatment concentration) were manually collected. For each replication and individual concentration, approximately 100 mg of young leaves were collected in a 1.5 mL Eppendorf tube (Eppendorf, Hamburg, Germany) and flash frozen in liquid nitrogen for RNA extraction. Upon manually collecting each plant, the shoots were separated from the roots in order to assess the fresh mass of each. Fresh masses of shoots and roots were measured immediately after plant collection. Following fresh biomass measurements, the plants were transferred to brown paper bags and oven-dried at 40 °C for 4 days until no further moisture loss was observed under these conditions. Samples subsequently used for methanol extraction and total RNA extraction were obtained from bulked leaves of all plants in each replication.
2.4 RNA Extraction and relative gene expression
To understand the molecular mechanisms influencing nepetalactone production in response to SA elicitation, seven of the key genes involved in nepetalactone biosynthesis were selected for quantitation by Real-time RT qPCR analysis. The catnip beta-6 tubulin (TUB6) gene was used as a reference housekeeping gene in this study. The primers for each selected gene were designed based on the Nepeta cataria L. sequencing data from the Mint Evolutionary Genomics Consortium (Boachon et al. 2018). All primer pairs were designed using the Primer Express software (Applied Biosystems, Thermo Fisher, Foster City, CA, USA). The primers are listed in Table 1. The primer sets for each gene were first validated to have similar amplification efficiency as the TUB6 gene. The total RNA of each replicate was isolated using the Spectrum™ Plant Total RNA Kit (MilliporeSigma, Burlington, MA, USA) following the instructions of the manufacturer. The concentrations were measured using a NanoDrop spectrophotometer (Thermo Fisher, Waltham, MA, USA). Forceps were cleaned with 70% ethanol and RNAse Away (Thermo Fisher, Waltham, MA, USA) in between each sample collection. Reverse transcription (RT) reaction was carried out with the High Fidelity cDNA Synthesis Kit (Applied Biosystems, Thermo Fisher, Foster City, CA, USA) using approximately 2 µg RNA. The RT products were used in qPCR reactions with the 2 × SYBR Green Master Mix from Applied Biosystems (Thermo Fisher, Foster City, CA, USA). The RT-qPCR assays for GPPS, GES, G8H, HGO, NEPS1, NEPS2 and Tubulin6 were run on the default setting at 95 °C for 3 min for initial denaturation and 40 cycles at 95 °C for 30 s followed by 60 °C for 30 s. The assays for ISY were ran on similar settings except the cycle number was increased from 40 to 60. The fold-change of gene expression was calculated by the 2^-ΔΔCt method. The RT-qPCR analysis was repeated three times per SA concentration and the gene expression values were averaged (Livak and Schmittgen 2001).
2.5 Methanol extraction for LC/MS analysis
The dried leaf material was ground to a fine powder and then extracted. Approximately, 100 mg of ground plant powder was extracted with 10 mL of methanol and sonicated for 10 min in a Branson M5800 Ultrasonic Cleaner (Branson Ultrasonics, Brookfield, CT, USA). The samples were then allowed to stand at room temperature overnight. The supernatant was diluted 20 times by adding 50 μL of the extract to 950 μL of methanol and vortexed at full speed to homogenize the samples. The diluted extract was then centrifuged at 10,000×g for 5 min using the accuSpin Micro17 centrifuge (Fisher Scientific, Hampton, NH, USA). An aliquot of 750 μL was transferred to an HPLC vial and stored at −20 °C until further analysis.
2.6 Ultra-high performance liquid chromatography—quadrupole time-of-flight—mass spectrometry (UHPLC-QTOF/MS) analysis
An Agilent 1290 Infinity II LC in tandem with an Agilent 5456 QTOF were used for the quantitative analysis. A Waters Acquity UPLC BEH C18 1.7 µm (2.1 × 50 mm) column equipped with an Acquity BEH C18 1.7 µm (2.1 × 5 mm) pre-column provided the chromatographic separation. Water (0.1% formic acid) and acetonitrile (0.1% formic acid) were applied as the mobile phase. The gradient of the two was as follows: from 0–0.2 min 20–30% B, 0.2–3.5 min 30–45% B, 3.5–4.5 min isocratic 45% B, 4.5–4.6 min 45–100% B, 4.6–7 min isocratic 100% B, with a post-time of 1.2 min, flow rate of 0.4 ml/min, and injection volume of 1 µl. The column was heated to 30 °C. For the mass spectrometer, the sample was sent to waste from 0–0.3 min as well as 4.6–7 min. Mass spectra were acquired in positive mode, the range was set to 100–1200 m/z with an acquisition rate of 6 spectra/s. A gas temperature of 300 °C (12 L/min) and a sheath gas temperature of 325 °C (12 L/min), along with a nebulizer pressure of 25 PSI were produced by the AJS ESI. The capillary voltage was 4000 V compared to the nozzle voltage of 2000 V, with the Fragmentor set to 130 V. Reference masses of 121.050873 and 922.009798 m/z were used for the instrument. All data was collected in centroid form. A combination of MassHunter Qualitative Analysis and MassHunter Quantitative Analysis were used for quantitative measurements, with the former used to verify the existence of borderline peaks.
2.7 Data analysis
All experiments were conducted with 4 plants per experimental unit in 3 sets of biological replicates for a total of 36 plants per replication. The hydroponic experiments were set up as a 3 × 3 factorial scheme arranged in a completely randomized design. The two-way analysis of variance (ANOVA) for all the data was performed and the means were compared via Dunnett’s test or Tukey’s test at p < 0.05. The results are presented as bar graphs with standard deviation. The statistical analyses were performed using GraphPad Prism version 6.04 for Windows, GraphPad Software (www.graphpad.com).
3 Results
3.1 Nepetalactone yield
In this study, nepetalactones were the primary analyte of interest in the methanolic catnip extracts (Fig. 3). The short-term salicylic acid treatments did not lead to significant changes in the nepetalactone chemistry within the 24-h period of the study conducted in hydroponic conditions. The average (Z,E)-nepetalactone concentration averaged 1.23% dry mass (DM) across the control and SA-treated samples for all timepoints (Fig. 4a). The average (E,Z)-nepetalactone concentration averaged 0.15% DM for all samples (Fig. 4b). The averages for the total nepetalactone concentration varied from 2.04% DM in the 0.5 mM SA treated samples collected after 12 h, to 2.74% DM in the control samples collected after 4 h (Fig. 4c), not being different from each other. We also quantified the presence of a hydrolysis byproduct of nepetalactone: nepetalic acid. The average yield of nepetalic acid ranged from 0% DM in the control samples collected at the 24-h timepoint, to 0.04% DM in the 1.0 mM SA treated samples collected after 12 h, but there were no significant differences among the treatments (Fig. 4d).
Percentage dry mass of (Z,E)-nepetalactone (a), (E,Z)-nepetalactone (b), total nepetalactone (c), and nepetalic acid (d) found in hydroponically grown control ‘CR9’ plants, ‘CR9’ plants treated with 0.5 mM salicylic acid, and ‘CR9’ plants treated with 1.0 mM salicylic acid collected after 4, 12 and 24 h. Each data point is the average for each of the three biological replicates with four technical replicates against each biological replicate. Error bars represent standard deviation
3.2 Biomass
Measurements for fresh shoot mass, fresh root mass, dry shoot mass, and dry root mass were collected for all samples in the study. Regarding the fresh plant biomass, no significant differences were observed between the control plants and the plants treated with SA for both fresh shoot mass and fresh root mass. The value for fresh shoot mass averaged 1.21 g for both control and SA-treated samples across all timepoints (data not shown). While the average fresh root mass varied from 0.61 g for the roots treated with 1.0 mM SA collected after 24 h, to 1.05 g for untreated control roots collected after 12 h. Comparatively the short-term SA treatments did not lead to significant changes in dry plant biomass either. The average dry shoot mass across all samples and timepoints was 0.17 g. While the average dry root mass varied from 0.05 g in the 1.0 mM SA treated samples collected after 12 h, to 0.07 g in the control samples collected after 24 h.
3.3 Relative mRNA expression
To assess the short-term elicitation response of SA on catnip at a transcriptional level, seven of the key genes involved in the nepetalactone biosynthetic pathway were assayed via Real-time RT-qPCR: GPPS (geranyl pyrophosphate synthase), GES (geraniol synthase), G8H (geraniol 8-hydroxylase), HGO (hydroxy-geraniol oxidoreductase), ISY (iridoid synthase), NEPS1 (nepetalactol-related short-chain dehydrogenase enzyme 1), and NEPS2 (nepetalactol-related short-chain dehydrogenase enzyme 2). A two-way ANOVA was conducted that examined the effects of time and concentration of SA on the relative transcript abundance of these genes. For GES, G8H, HGO, ISY, and NEPS2, there were no significant interaction effects or main effects of time and SA concentration (Fig. 5a–e).
Real-time RT qPCR analyses of the expression of nepetalactone biosynthesis genes under the exogenous application of salicylic acid: GES (a), G8H (b), HGO (c), ISY (d), and NEPS2 (e). Expression was normalized to that of tubulin and the values in control plants were set to 1. Each data point is the average for each of the three biological replicates with four technical replicates against each biological replicate. Error bars indicate standard deviation
For the relative expression of GPPS and NEPS1, the two-way ANOVA revealed significant changes related to SA application. For GPPS mRNA expression, although there was no significant interaction between the concentration of SA and time (p = 0.5261) nor a significant main effect of time (p = 0.0608), the main effect of SA concentration was significant (p = 0.0011). The transcript abundance for GPPS across all time points averaged a 1.92-fold change at the 0.5 mM SA concentration and exhibited a 1.77-fold change at the 1.0 mM SA concentration. Dunnett’s Test for multiple comparisons found that the mean value of mRNA expression was significantly different between the control plants and the plants treated with 0.5 mM SA (p = 0.0010). The mean value was also significantly different between the control plants and the plants treated with 1.0 mM SA (p = 0.0048) (Fig. 6a,b).
(a) Real-time RT qPCR analysis of GPPS expression under the exogenous application of salicylic acid. (b) Real-time RT qPCR analysis of the main effect of salicylic acid concentration on the relative mRNA expression of GPPS. (c) Real-time RT qPCR analysis of NEPS1 expression under the exogenous application of salicylic acid. (d) Real-time RT qPCR analysis of the main effect of salicylic acid concentration on the relative mRNA expression of NEPS1. Expression was normalized to that of tubulin and the values in control plants were set to 1. Each data point is the average for each of the three biological replicates with four technical replicates against each biological replicate. Error bars indicate standard deviation. Asterisks (**) indicate significant differences (p < 0.01); asterisk (*) indicates statistically significant differences (p < 0.5). Letters in Fig. 6a and 6c indicate significant interactions for the main effect of time. Timepoints followed by the same letter do not differ according to Tukey’s Test (p ≤ 0.05). The comparisons between salicylic acid concentrations and the control values were assessed according to Dunnett’s Test (p ≤ 0.05)
Regarding the NEPS1 gene expression, similarly, there was no significant interaction between the concentration of SA and time (p = 0.2461). But the main effects of SA concentration (p = 0.0182) and time (p = 0.0168) did result in significant sources of variation for NEPS1 transcript abundance. Significant upregulation was not observed at the 4- or 12-h timepoints, but overexpression was observed at the 24-h timepoint (Fig. 6c).
The transcript abundance for NEPS1 across all time points averaged a 1.93-fold change at the 0.5 mM SA concentration and 1.90-fold change at the 1.0 mM SA concentration (Fig. 6d). Dunnett’s Test found that the mean value of NEPS1 mRNA expression was significantly different between the control plants and the plants treated with 0.5 mM SA (p = 0.0217). Significant differences were also found between the mean values of the control plants and the plants treated with 1.0 mM SA (p = 0.0275). According to Tukey’s Test, the mean transcript abundance for samples collected 4 h after treatment was not significantly different compared to the mean values of samples collected 12 h after treatment (p = 0.9728). However, the mean transcript abundances for samples collected after 4 h differed significantly from the mean values of samples collected after 24 h (p = 0.0252). Significant variation was also observed between the mean values collected 12 h and 24 h after SA treatment (p = 0.0398).
3.4 Correlation between nepetalactone chemistry and mRNA expression
To assess how the expression of nepetalactone biosynthetic genes are associated with the production of nepetalactone in response to SA elicitation, a Pearson correlation matrix was established to define any existing relationships between the variables (Fig. 7). We found a significant relationship between the transcript abundance of GES and G8H (p = 0.0006). There is a moderately positive correlation between their mRNA expression at a correlation strength of 0.62. Another significant relationship was found between the expression of GES and NEPS2 (p = 0.0005). These genes also displayed a moderately positive correlation of 0.62. We also found a significant relationship between GES and ISY (p = 0.0071) with a moderately positive correlation of 0.51. NEPS2 demonstrated a significant relationship in transcript abundance with G8H. The co-expression strength between the two genes is a low positive correlation of 0.46 (p = 0.0152). The transcript abundance of NEPS1 showed a significant relationship to the mRNA expression of GPPS (p = 0.020) though there was a low positive correlation of 0.45. The mRNA expression of the HGO gene demonstrated a significant relationship (p = 0.008) to the yield of nepetalic acid which led to a moderately negative correlation of −0.50. The two main isomers of nepetalactone, (Z,E)-nepetalactone and (E,Z)-nepetalactone, exhibited a significant relationship (p = 0.032) with a low negative correlation strength of −0.413. The (Z,E)-nepetalactone concentration was almost perfectly correlated to the total nepetalactone concentration at 0.976 (p = 5.730 e−018).
4 Discussion
As the global population at risk of contracting mosquito-borne diseases increases in response to changing climate and anthropogenic activity, solutions to effectively repel arbovirus vectors are necessary (Kraemer et al. 2019). Numerous studies have cited catnip essential oil and its bioactive nepetalactone isomers to offer both spatial and contact repellency against mosquito species (Bernier et al. 2005; Peterson and Coats 2011; Reichert et al. 2019). Chemical elicitors, such as salicylic acid, have been employed in various cultivation practices to enhance the synthesis of secondary metabolites produced in medicinal plants like catnip (Nepeta cataria L.) (Singh and Dwivedi 2018). This work is the first study to attempt to establish a correlation between the expression of nepetalactone biosynthetic genes and the production of nepetalactone in response to exogenous application of salicylic acid in hydroponically grown plants. Though salicylic acid has previously been tested on catnip (Said-Al Ahl et al. 2016), this is the first time that the effects of salicylic acid have ever been reported on the genes of the nepetalactone pathway. Furthermore, given the 24-h time course, this rapid study allowed us to assess the immediate transcriptional response of these genes as it relates to the whole plant physiology and chemical makeup.
In this study, we observed there were no significant differences in biomass after the salicylic acid treatments. Additionally, the salicylic acid did not result in significant changes in nepetalactone concentration for either the (Z,E)-nepetalactone or (E,Z)-nepetalactone isomer. Also, the nepetalic acid concentration and total nepetalactone yield didn’t reflect significant changes after salicylic acid application. It’s possible that the 24-h time course for the study is not enough time to observe significant differences in the accumulation of these metabolites, especially since nepetalactones were produced in relatively high quantities (up to 2.8% DM) within the catnip methanolic extracts of the aerial biomass for seedlings. Similar work with basil (Ocimum basilicum L.) demonstrated that although methyl salicylate treatment for 24 h did not significantly change methylchavicol concentrations in basil leaves, a 70% increase in methylchavicol production was observed after extending the elicitation period to 48 h (Deschamps et al. 2008).
Several of the genes involved in the nepetalactone biosynthesis pathway were assessed for their relative transcript abundance in response to elicitation with salicylic acid. No significant interactions were observed for GES, G8H, HGO, and NEPS2 regarding SA treatment. Data from previous studies suggests that geraniol synthase (GES) is a major gatekeeping step in the iridoid biosynthetic pathway as it diverts metabolic flux away from canonical monoterpene biosynthesis by converting geranyl pyrophosphate (GPP) to geraniol (Boachon et al. 2018). After the hydrolysis of GPP, hydroxylation and oxidation occur via geraniol 8-hydroxylase (G8H) and 8-hydroxygeraniol oxidoreductase (HGO) respectively which then yield a precursor compound, 8-oxogeranial (Lichman et al. 2020; Miettinen et al. 2014). Since these are enzymes involved in the intermediate steps, it’s possible that SA does not exert a direct effect on the regulation of their transcript abundance or secondary metabolite products. Conversely, a study involving Catharanthus roseus, demonstrated significant upregulation of geraniol-10 hydroxylase (G10h) following foliar application of salicylic acid (Soltani et al. 2020). Experimental trials with Nepeta nervosa showed the application of 250 µM methyl jasmonate in vitro upregulated the expression of NEPS, but did not increase iridoid accumulation in the leaves (Aničić et al. 2022). Future work may elucidate how the induction of certain pathway genes corresponds to the accumulation of bioactive catnip metabolites.
The first committed step of the iridoid pathway begins with the reductive cyclization of 8-oxogeranial into nepetalactol which is catalyzed by iridoid synthase (ISY) (Lichman et al. 2020). In recent findings, several enzymes have been found to act in conjunction with ISY to direct the formation of nepetalactone stereoisomer formation. In addition to characterizing the iridoid synthase enzyme, Lichman et al. (2019) identified three nepetalactol-related short-chain reductase/dehydrogenases (NEPS1, NEPS2, and NEPS3). NEPS1 is a dehydrogenase responsible for catalyzing the formation of (Z,E)-nepetalactone or (Z,Z)-nepetalactone from the analogous nepetalactol isomer. While both NEPS2 and NEPS3 have been associated with cyclase functionality in the formation of the nepetalactol isomers from the reactive ISY product (Lichman et al. 2019, 2020). This work aimed to assess how select NEPS genes respond to salicylic acid elicitation in the ‘CR9’ catnip cultivar which was selectively bred to contain high (Z,E)-nepetalactone within its essential oil (Reichert et al. 2016). Subsequent analysis of the biochemical pathway has uncovered additional genes, namely major latex protein-like genes (MLPL) in addition to NEPS4 and NEPS5 that comprise syntenic gene clusters within the Nepeta genome and are responsible for the formation of (Z,E)-nepetalactone. Since the expression of these genes largely correspond to the in planta chemistry for catnip (Lichman et al. 2020), these genes should also be assayed in future RT-qPCR studies. This approach may indicate how the upregulation patterns of the NEPS and MLPL genes or ratios of their transcript abundances could lead to higher production of individual nepetalactone isomers.
In the present work, GPPS and NEPS1, were upregulated by salicylic acid at both the 0.5 mM and 1.0 mM concentrations. Nepetalactone biosynthesis in Nepeta species begins with geranyl pyrophosphate (GPP) catalyzed by the GPPS enzyme (Palmer et al. 2022). As one of the earliest enzymes involved in the terpene pathway, we theorized that GPPS would respond to stress induced by salicylic acid. Given their up-regulation and the role salicylic acid plays in plant defense response, GPPS and NEPS may also be responsible for mitigating initial plant defense responses in catnip in addition to their traditional roles in monoterpene biosynthesis. According to Tounekti et al. (2013), salicylic acid generally upregulates the terpenoid biosynthetic pathway during the transcription. Another study observed that salicylic acid dramatically induced the expression of two terpene synthase unigenes in Santalum album (Zhang et al. 2019). Work with Artemisia annua L. found that salicylic acid both upregulated several genes in the artmesinin biosynthetic pathway and led to the downstream increase in artemisinin concentration post-treatment (Pu et al. 2009). Also, salicylic acid treatment on Salvia miltiorrhiza hairy roots was shown to enhance diterpene production and increase the expression of relevant pathway genes such as geranyl pyrophosphate synthase (GPPS).
GPPS is one of the first genes in the monoterpene pathway so its overexpression may contribute to higher production of nepetalactone in addition to higher production of other aromatic terpene compounds that are utilized for plant-pathogen defense. Since GPPS is involved in the early stages of the monoterpene pathway, its products can be used as precursor molecules for different terpenes including geraniol, linalool and cineole (Zebec et al. 2016). Because the expression of GPPS was positively correlated with expression of NEPS1, this may suggest overexpression of these genes can be used as targets to direct the flux of precursor molecules towards the production of nepetalactone via metabolic engineering (Hao et al. 2015).
The present study provides evidence that GPPS and NEPS1 are the first steps in the nepetalactone biosynthetic pathway to respond to salicylic acid application. Longer term studies should evaluate if other genes, including MLPL and additional NEPS, respond similarly and explore the duration of treatment needed to reflect significant changes in nepetalactone concentration.
5 Conclusion
Since catnip produces nepetalactone as a plant defense mechanism, we sought to uncover existing relationships between the nepetalactone biosynthetic pathway and salicylic acid, a well-known phytohormone involved in plant immunity. Though short-term application of salicylic acid did not significantly contribute to changes in biomass, nepetalactone or nepetalic acid concentration, we did observe increases in mRNA expression for both GPPS and NEPS1. These genes are involved in the biosynthetic pathway that leads to the production of nepetalactone, the primary component of catnip essential oil and the bioactive molecule responsible for insect repellency. Future work involving salicylic acid as well as other abiotic elicitors such as methyl jasmonate, chitosan or humic acid for longer time periods may elicit stronger effects on the secondary metabolite yield in catnip. Evaluating the responses of additional pathway genes may also lead to promising findings that help us to better understand the biochemical control of plant natural products during the transcription. This work has important implications for genetic and metabolic engineering of catnip and other aromatic plant species.
Data availability
The raw data that supports the findings of this study are available from the corresponding author, upon reasonable request.
References
Adiguzel A, Ozer H, Sokmen M, Gulluce M, Sokmen A, Kilic H, Sahin F, Baris O (2009) Antimicrobial and antioxidant activity of the essential oil and methanol extract of Nepeta cataria. Polish J Microbiol 58(1):69–76
Afkar S, Karimzadeh G, Jalali Javaran M, Sharifi M, Behmanesh M (2013) Influence of methyl jasmonate on menthol production and gene expression in peppermint (Mentha x piperita L.). J Med Plants Byprod 2(1):75–82
Ahmad B, Jaleel H, Sadiq Y, Khan MAM, Shabbir A (2018) Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul 86(2):273–286. https://doi.org/10.1007/s10725-018-0427-z
Aničić N, Filipović B, Dmitrović S, Nestorović Živković J, Skorić M, Gašić U, Milutinović M, Matekalo D, Petrović L, Božunović J, Banjanac T, Šiler B, Todorović M, Lukić T, Mišić D (2022) Elicitation effects of methyl jasmonate on iridoid biosynthesis in leaves of Nepeta rtanjensis and Nepeta nervosa. Book of Abstracts: 4th International Conference on Plant Biology and 23rd SPPS Meeting; 2022 Oct 6–8; Belgrade, Serbia, 122
Bernier UR, Furman KD, Kline DL, Allan SA, Barnard DR (2005) Comparison of contact and spatial repellency of catnip oil and N, N-Diethyl-3-methylbenzamide (deet) against mosquitoes. J Med Entomol 42(3):306–311. https://doi.org/10.1093/jmedent/42.3.306
Boachon B, Buell CR, Crisovan E, Dudareva N, Garcia N, Godden G, Henry L, Kamileen MO, Kates HR, Kilgore MB, Lichman BR, Mavrodiev EV, Newton L, Rodriguez-Lopez C, O’Connor SE, Soltis D, Soltis P, Vaillancourt B, Wiegert-Rininger K, Zhao D (2018) Phylogenomic mining of the mints reveals multiple mechanisms contributing to the evolution of chemical diversity in Lamiaceae. Mol Plant 11(8):1084–1096. https://doi.org/10.1016/j.molp.2018.06.002
Deschamps C, Raskin I, Simon JE (2008) Regulation of essential oil accumulation in basil (Ocimum basilicum L.) in response to elicitation. Intl J Plant Sci 169(8):981–986. https://doi.org/10.1086/590454
Gomes EN, Allen K, Jaworski K, Zorde M, Lockhart A, Besancon T, Brown T, Reichert W, Wu Q, Simon JE (2020) Catnip (Nepeta cataria L.): recent advances in botany, horticulture and production. In: Máthé Á (ed) Medicinal and aromatic plants of North America. Springer International Publishing, Cham, pp 247–284. https://doi.org/10.1007/978-3-030-44930-8_11
González J, Lockhart A, Wu Q, Simon JE, Toledo A (2022) Repellency of novel catnip (Nepeta cataria) cultivar extracts against Ixodes scapularis and Haemaphysalis longicornis (Acari: Ixodida: Ixodidae). Ticks Tick-Borne Dis 13(6):102046. https://doi.org/10.1016/j.ttbdis.2022.102046
Guru A, Dwivedi P, Kaur P, Pandey DK (2022) Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S Afr J Botany 149:1029–1043. https://doi.org/10.1016/j.sajb.2021.10.014
Hao X, Shi M, Cui L, Xu C, Zhang Y, Kai G (2015) Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotech Appl Biochem 62(1):24–31. https://doi.org/10.1002/bab.1236
Hosseini S, Maroufi A, Hassani SH (2023) Relative expression analysis of the monoterpene biosynthesis genes in Lavender (Lavandula angustifolia) in response to salicylic acid and methyl jasmonate. J Plant Res (iranian J Biol) 36(1):29–45
Kraemer MUG, Reiner RC, Brady OJ, Messina JP, Gilbert M, Pigott DM, Yi D, Johnson K, Earl L, Marczak LB, Shirude S (2019) Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiology. https://doi.org/10.1038/s41564-019-0376-y
Lichman BR, Kamileen MO, Titchiner GR, Saalbach G, Stevenson CEM, Lawson DM, O’Connor SE (2019) Uncoupled activation and cyclization in catmint reductive terpenoid biosynthesis. Nat Chem Biol 15(1):Article 1. https://doi.org/10.1038/s41589-018-0185-2
Lichman BR, Godden GT, Hamilton JP, Palmer L, Kamileen MO, Zhao D, Vaillancourt B, Wood JC, Sun M, Kinser TJ, Henry LK, Rodriguez-Lopez C, Dudareva N, Soltis DE, Soltis PS, Buell CR, O’Connor SE (2020) The evolutionary origins of the cat attractant nepetalactone in catnip. Sci Adv 6(20):eaba0721. https://doi.org/10.1126/sciadv.aba0721
Liu J, Qiu G, Liu C, Li H, Chen X, Fu Q, Lin Y, Guo B (2022) Salicylic acid, a multifaceted hormone, combats abiotic stresses in plants. Life 12(6):886. https://doi.org/10.3390/life12060886
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Maboko MM, Plooy CPD (2014) Yield of two hydroponically grown tomato cultivars as affected by transplanting stage or direct seeding. HortScience 49(4):438–440. https://doi.org/10.21273/HORTSCI.49.4.438
Majdi M, Malekzadeh-Mashhady A, Maroufi A, Crocoll C (2017) Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme (Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors. Plant Phys Biochem 115:152–162. https://doi.org/10.1016/j.plaphy.2017.03.016
Miettinen K, Dong L, Navrot N, Schneider T, Burlat V, Pollier J, Woittiez L, van der Krol S, Lugan R, Ilc T, Verpoorte R, Oksman-Caldentey K-M, Martinoia E, Bouwmeester H, Goossens A, Memelink J, Werck-Reichhart D (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Comms 5:3606. https://doi.org/10.1038/ncomms4606
Ozhan N, Goldani M, Naghdi Badi HA, Mehrafarin A, Parsa M (2017) Changes in nepetalactone content and biochemical traits of catnip (Nepeta cataria L.) in response to induction of biostimulants compounds. J Med Plants 16(64):32–44
Pacheco AC, Gorni PH (2021) Elicitation with salicylic acid as a tool for enhance bioactive compounds in plants. In: Hayat S, Siddiqui H, Damalas CA (eds) Salicylic Acid—A Versatile Plant Growth Regulator. Springer International Publishing, Cham, pp 1–15. https://doi.org/10.1007/978-3-030-79229-9_1
Palmer L, Chuang L, Siegmund M, Kunert M, Yamamoto K, Sonawan P, O’Connor SE (2022) In vivo\characterization of key iridoid biosynthesis pathway genes in catnip (Nepeta cataria). Planta, 256(5): 99. https://doi.org/10.1007/s00425-022-04012-z
Park C-H, Tannous P, Juliani H, Wu Q, Sciarappa W, Vanvranken R, Dalponte D, Simon J (2007) Catnip as a source of essential oils. In: Janick J, Whipkey A (eds) Issues in new crops and new uses. ASHS Press, Alexandria, VA, pp 311–315
Patel H, Gomes EN, Yuan B, Lyu W, Wu Q, Simon JE (2022) Investigation of volatile iridoid terpenes in Nepeta cataria L. (catnip) genotypes. Molecules 27(20):Article 20. https://doi.org/10.3390/molecules27207057
Patel HK, Gomes EN, Wu Q, Patel N, Kobayashi DY, Wang C, Simon JE (2023) Volatile metabolites from new cultivars of catnip and oregano as potential antibacterial and insect repellent agents. Front Plant Sci 14:1124305. https://doi.org/10.3389/fpls.2023.1124305
Payandeh M, Bordbar F, Mirtadzadini M, Khaniki GRB (2015) New chromosome counts for Nepeta Lamiaceae from flora of Iran. Biyolojik Çeşitlilik ve Koruma 8(2):Article 2
Peterson CJ, Coats JR (2011) Catnip essential oil and its nepetalactone isomers as repellents for mosquitoes. In: Gretchen EP, Joel RC (eds) Recent Developments in Invertebrate Repellents, vol 1090. American Chemical Society, Washington, pp 59–65. https://doi.org/10.1021/bk-2011-1090.ch004
Pu G-B, Ma D-M, Chen J-L, Ma L-Q, Wang H, Li G-F, Ye H-C, Liu B-Y (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep 28(7):1127–1135. https://doi.org/10.1007/s00299-009-0713-3
Reichert W, Park HC, Juliani HR, Simon JE (2016) ‘CR9’: A new highly aromatic catnip Nepeta cataria L. cultivar rich in Z E-nepetalactone. HortScience 51(5):588–591. https://doi.org/10.21273/HORTSCI.51.5.588
Reichert W, Ejercito J, Guda T, Dong X, Wu Q, Ray A, Simon JE (2019) Repellency assessment of Nepeta cataria essential oils and isolated nepetalactones on Aedes aegypti. Sci Rep 9:1524. https://doi.org/10.1038/s41598-018-36814-1
Said-Al Ahl H, Sabra AS, Hegazy MH (2016) Salicylic acid improves growth and essential oil accumulation in two Nepeta cataria chemotypes under water stress conditions. Ital J Agrometeorol 21:25–36. https://doi.org/10.19199/2016.1.2038-5625.025
Simon JE, Reichert W (2020) Catnip cultivar ‘CR9’ (United States Patent US10602702B2). https://patents.google.com/patent/US10602702B2/en
Simon JE, Reichert W, Wu Q (2019) Catnip cultivar ‘CR3’ (United States Patent US10512231B2). https://patents.google.com/patent/US10512231B2/en
Singh A, Dwivedi P (2018) Methyl-jasmonate and salicylic acid as potent elicitors for secondary metabolite production in medicinal plants: a review. J Pharmacog Phytochem 7(1):750–757
Soltani N, Nazarian-Firouzabadi F, Shafeinia A, Sadr AS, Shirali M (2020) The expression of Terpenoid Indole Alkaloid (TIAs) pathway genes in Catharanthus roseus in response to salicylic acid treatment. Mol Biol Rep 47(9):7009–7016. https://doi.org/10.1007/s11033-020-05759-y
Tang H, Hu J, Zhao M, Cao L, Chen Y (2022) Comparative study of the physiological responses, secondary metabolites, and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiologiae Plantarum 45(2):20. https://doi.org/10.1007/s11738-022-03498-0
Tounekti T, Hernández I, Munné-Bosch S (2013) Salicylic acid biosynthesis and role in modulating terpenoid and flavonoid metabolism in plant responses to abiotic stress. In: Hayat S, Ahmad A, Alyemeni MN (eds) Salicylic acid: plant growth and development. Springer, Cham, pp 141–162. https://doi.org/10.1007/978-94-007-6428-6_8
Zebec Z, Wilkes J, Jervis AJ, Scrutton NS, Takano E, Breitling R (2016) Towards synthesis of monoterpenes and derivatives using synthetic biology. Curr Opin Chem Biol 34:37–43. https://doi.org/10.1016/j.cbpa.2016.06.002
Zhang X, Niu M, Teixeira da Silva JA, Zhang Y, Yuan Y, Jia Y, Xiao Y, Li Y, Fang L, Zeng S, Ma G (2019) Identification and functional characterization of three new terpene synthase genes involved in chemical defense and abiotic stresses in Santalum album. BMC Plant Biol 19(1):115. https://doi.org/10.1186/s12870-019-1720-3
Acknowledgements
We acknowledge the New Jersey Agricultural Experiment Station and Hatch Project New Jersey#12170 for partial funding of this work as well as partial funding from the Office of Research TechAdvance initiative. The senior author conducted this work while being a Rutgers University Pipeline—Initiative for Maximizing Student Development (RUP-IMSD) Fellow. We are also thankful to the members of the New Use Agriculture and Natural Plant Products Program (NUANPP) at Rutgers University for their help with the greenhouse experiment set up and data collection, and the staff at the Rutgers greenhouse including Adam Morgan and Amy Abate for their assistance and support. We would also like to acknowledge the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES) (DOC_PLENO/proc. proc. n° 88881.129327/2016-01) for providing a Graduate Student Fellowship to Erik Nunes Gomes. This work was conducted as a part of the first author’s dissertation studies.
Funding
Funding was provided by The State University of New Jersey (Grant Nos: Hatch Project NJ#12170, and TechAdvance).
Author information
Authors and Affiliations
Contributions
Kirsten Allen: Conceptualization, Methodology, Formal Analysis, Investigation, Data Curation, Writing—original draft, Writing—reviewing and editing, Visualization. Erik Nunes Gomes: Conceptualization, Methodology, Formal Analysis, Data Curation, Writing—reviewing and editing, Visualization. Anthony Lockhart: Methodology, Validation, Writing—reviewing and editing. Qingli Wu: Resources, Writing—reviewing and editing, Supervision. Rong Di: Conceptualization, Validation, Resources, Writing—reviewing and editing, Supervision. James E. Simon: Conceptualization, Validation, Resources, Writing—reviewing and editing, Supervision, Project Administration, Funding Acquisition.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. We declare that this manuscript is original, has not been published and is not under consideration for publication elsewhere. The authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Allen, K.A., Gomes, E.N., Lockhart, A. et al. Short-term salicylic acid application induces the expression of GPPS and NEPS1 in catnip. Theor. Exp. Plant Physiol. 36, 299–312 (2024). https://doi.org/10.1007/s40626-024-00322-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-024-00322-7