Abstract
Introduction
Alport syndrome (ALP) is a rare genetic condition characterized by progressive involvement of the basal membranes and renal dysfunction. The purpose of the study was to evaluate urinary (u) and serum (s) levels of tumor growth factor (TGF)-beta(β) and high mobility group box (HMGB)-1 in ALP patients with normal renal function, albuminuria and proteinuria.
Methods
A prospective, single-center study was performed with a follow-up period of 12 months, enrolling 11 pediatric ALP patients and 10 healthy subjects (HS). Normal values of serum creatinine, albuminuria and proteinuria, as well as unaltered estimated glomerular filtration rate (eGFR) were required at enrollment.
Results
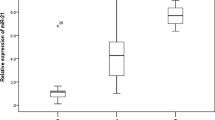
ALP patients had significantly higher levels of serum and urinary HMGB1 compared to HS. The same trend was observed for TGF-β1, with higher values in ALP patients than in HS. HMGB1 and TGF-β1 correlated with each other and with markers of renal function and damage. Urinary biomarkers did not correlate with eGFR, whereas sHMGB1 and sTGF-β1 were negatively related to filtration rate (r: − 0.66; p = 0.02, r: − 0.96; p < 0.0001, respectively). Using proteinuria as a dependent variable in a multiple regression model, only the association with sTGF-β1 (β = 0.91, p < 0.0001) remained significant.
Conclusions
High levels of HMGB1 and TGF-β1 characterized ALP patients with normal renal function, highlighting the subclinical pro-fibrotic and inflammatory mechanisms triggered before the onset of proteinuria. Further studies are needed to evaluate the role of HMGB1 and TGFβ-1 in ALP patients.
Graphic abstract





Similar content being viewed by others
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bruni V, Petrisano M, Tarsitano F, Falvo F, Parisi F, Cucinotta U et al (2019) “Focus on pediatric nephrology” Alport’s syndrome. J Biol Regul Homeost Agents 33:19–24
Chimenz R (2019) Introduction to the special issue: “Focus on pediatric nephrology.” J Biol Regul Homeost Agents 33:1
Nozu K, Minamikawa S, Yamada S, Oka M, Yanagita M, Morisada N et al (2017) Characterization of contiguous gene deletions in COL4A6 and COL4A5 in Alport syndrome-diffuse leiomyomatosis. J Hum Genet 62:733–735
Meloni I, Muscettola M, Raynaud M, Longo I, Bruttini M, Moizard MP et al (2002) FACL4, encoding fatty acid-CoA ligase 4, is mutated in nonspecific X-linked mental retardation. Nat Genet 30:436–440
Proesmans W, Van Dyck M (2004) Enalapril in children with Alport syndrome. Pediatr Nephrol 19:271–275
Webb NJ, Lam C, Shahinfar S (2011) Efficacy and safety of losartan in children with Alport syndrome—results from a subgroup analysis of prospective, randomized, placebo- or amlodipine-controlled trial. Nephrol Dial Transplant 26:2521–2526
Giani M, Mastrangelo A, Villa R, Turolo S, Marra G, Tirelli AS et al (2013) Alport syndrome: the effects of spironolactone on proteinuria and urinary TGF-β1. Pediatr Nephrol 28:1837–1842
Daina E, Cravedi P, Alpa M, Roccatello D, Gamba S, Perna A et al (2015) A multidrug, antiproteinuric approach to Alport syndrome: a ten-year cohort study. Nephron 130:13–20
Mallett A, Tang W, Clayton PA, Stevenson S, McDonald SP, Hawley CM et al (2014) End-stage kidney disease due to Alport syndrome: outcomes in 296 consecutive Australia and New Zealand Dialysis and Transplant Registry cases. Nephrol Dial Transplant 29:2277–2286
Shang S, Peng F, Wang T, Wu X, Li P, Li Q et al (2019) Genotype–phenotype correlation and prognostic impact in Chinese patients with Alport syndrome. Mol Genet Genom Med. 29:e741
Levey AS, Cattran D, Friedman A, Miller WG, Sedor J, Tuttle K et al (2009) Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 54:205–226
Devarajan P (2010) The use of targeted biomarkers for chronic kidney disease. Adv Chronic Kidney Dis 17:469–479
Schnaper HW (2014) Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol 29:193–202
Sayers R, Kalluri R, Rogers KD, Shiel CF, Meehan DT, Cosgrove D (1999) Role for transforming growth factor-beta1 in Alport renal disease progression. Kidney Int 56:1662–1673
Goumenos DS, Tsakas S, El Nahas AM, Alexandri S, Oldroyd S, Kalliakmani P et al (2002) Transforming growth factor-β1 in the kidney and urine of patients with glomerular disease and proteinuria. Nephrol Dial Transplant 17:2145–2152
den Haan JMM, Kraal G, Bevan MJ (2007) Cutting edge: lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol 178:5429–5433
Chirico V, Lacquaniti A, Salpietro V, Munafò C, Calabrò MP, Buemi M et al (2014) High-mobility group box 1 (HMGB1) in childhood: from bench to bedside. Eur J Pediatr 173:1123–1136
Arrigo T, Chirico V, Salpietro V, Munafò C, Ferraù V, Gitto E et al (2013) High-mobility group protein B1: a new biomarker of metabolic syndrome in obese children. Eur J Endocrinol 168:631–638
Chimenz R, Lacquaniti A, Colavita L, Chirico V, Fede C, Buemi M et al (2016) High mobility group box 1 and tumor growth factor β: useful biomarkers in pediatric patients receiving peritoneal dialysis. Ren Fail 38:1370–1376
Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev 220:35–46
Wu H, Ma J, Wang P (2010) HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol 21:1878–1890
Rademacher ER, Sinaiko AR (2009) Albuminuria in children. Curr Opin Nephrol Hypertens 18:246–251
Abulaban KM, Song H, Zhang X et al (2016) Predicting decline of kidney function in lupus nephritis using urine biomarkers. Lupus 25:1012–1018
Zickert A, Palmblad K, Sundelin B, Chavan S, Tracey KJ, Bruchfeld A et al (2012) Renal expression and serum levels of high mobility group box 1 protein in lupus nephritis. Arthritis Res Ther 14:R36
de Souza AW, Abdulahad WH, Sosicka P, Bijzet J, Limburg PC, Stegeman CA et al (2014) Are urinary levels of high mobility group box 1 markers of active nephritis in anti-neutrophil cytoplasmic antibody-associated vasculitis? Clin Exp Immunol 178:270–278
Woroniecki RP, Shatat IF, Supe K, Du Z, Kaskel FJ (2008) Urinary cytokines and steroid responsiveness in idiopathic nephrotic syndrome of childhood. Am J Nephrol 28:83–90
Zieg J, Blahova K, Seeman T, Bronsky J, Dvorakova H, Pechova M et al (2011) Urinary transforming growth factor-beta1 in children with obstructive uropathy. Nephrology 16:595–598
Wong MG, Perkovic V, Woodward M, Chalmers J, Li Q, Hillis GS et al (2013) Circulating bone morphogenetic protein-7 and transforming growth factor-beta1 are better predictors of renal end points in patients with type 2 diabetes mellitus. Kidney Int 83:278–284
Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addicks K et al (2003) Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int 63:438–446
Gross O, Perin L, Deltas C (2014) Alport syndrome from bench to bedside: the potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol Dial Transplant 29:iv124–iv130
Chen W, Tang D, Dai Y, Diao H (2019) Establishment of microRNA, transcript and protein regulatory networks in Alport syndrome induced pluripotent stem cells. Mol Med Rep 19:238–250
Tang SCW, Yiu WH (2020) Innate immunity in diabetic kidney disease. Nat Rev Nephrol 16:206–222
Mudaliar H, Pollock C, Komala MG, Chadban S, Wu HL, Panchapakesan U et al (2013) The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. Am J Physiol Ren Physiol 305:F143–F154
Tian S, Zhang L, Tang J, Guo X, Dong K, Chen SY (2015) HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury. Am J Physiol Ren Physiol 308:F69–F75
Chirico V, Lacquaniti A, Leonardi S, Grasso L, Rotolo N, Romano C et al (2015) Acute pulmonary exacerbation and lung function decline in patients with cystic fibrosis: high-mobility group box 1 (HMGB1) between inflammation and infection. Clin Microbiol Infect 21:368.e1–9
Rabadi MM, Ghaly T, Goligorksy MS, Ratliff BB (2012) HMGB1 in renal ischemic injury. Am J Physiol Renal Physiol 303:F873–F885
Abdulahad DA, Westra J, Bijzet J, Dolff S, van Dijk MC, Limburg PC et al (2012) Urine levels of HMGB1 in systemic lupus erythematosus patients with and without renal manifestations. Arthritis Res Ther 14:R184
Hayashida T, Wu MH, Pierce A, Poncelet AC, Varga J, Schnaper HW (2007) MAP-kinase activity necessary for TGFbeta1-stimulated mesangial cell type I collagen expression requires adhesion dependent phosphorylation of FAK tyrosine 397. J Cell Sci 120:4230–4240
Ortega-Velazquez R, Gonzalez-Rubio M, Ruiz-Torres MP, Diez-Marques ML, Iglesias MC, Rodriguez-Puyol M et al (2004) Collagen I upregulates extracellular matrix gene expression and secretion of TGF-beta 1 by cultured human mesangial cells. Am J Physiol Cell Physiol 286:C1335–C1343
Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH et al (2007) Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170:110–125
Bruchfeld A, Qureshi AR, Lindholm B, Barany P, Yang L, Stenvinkel P, Tracey KJ (2008) High mobility group box protein-1 correlates with renal function in chronic kidney disease (CKD). Mol Med 14:109–115
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial disclosure
The authors have no financial relationships relevant to this article to disclose.
Ethical approval
The study was approved by the Local Ethics Committee for Medical Research and carried out in accordance with the Helsinki Declaration.
Consent to participate
Both parents of each patient provided informed consent permitting data sampling and analysis at the time of initiation of the study.
Consent for publication
Both parents of each patient provided consent fort the publication of data at the time of initiation of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chimenz, R., Chirico, V., Basile, P. et al. HMGB-1 and TGFβ-1 highlight immuno-inflammatory and fibrotic processes before proteinuria onset in pediatric patients with Alport syndrome. J Nephrol 34, 1915–1924 (2021). https://doi.org/10.1007/s40620-021-01015-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-021-01015-z