Abstract
Background
IgA nephropathy (IgAN), the most common primary glomerulonephritis, often presents as advanced renal failure with end-stage renal disease at diagnosis. Tubulointerstitial injury and fibrosis on histology are the most important predictors of renal outcome. A non-invasive biomarker is required for assessment of progression in IgA nephropathy. We investigated the utility of blood profibrotic molecules, TGF-β1 and miRNA-21-5p (miR-21), to identify a non-invasive biomarker for renal fibrosis in IgAN.
Materials and methods
The study included 30 IgAN (mean age 31.5 ± 9 years) at the time of initial diagnosis, 25 age–sex-matched healthy controls and 10 Lupus nephritis patients as disease controls. Serum TGF-β1 was analyzed by enzyme-linked immunosorbent assay and plasma miR-21 by qRT-PCR, normalized with U6-snRNA. The levels were correlated with clinical features, laboratory parameters, histological Oxford MEST-C score and renal outcome.
Results
The serum TGF-β1 and plasma miR-21 were significantly higher in patients with IgAN than in healthy controls. TGF-β1 significantly correlated with serum creatinine, eGFR, Oxford T score and miR-21. High plasma miR-21 was significantly associated with T score and interstitial inflammation. On multivariate analysis, high levels of TGF-β1 and miR-21 correlated with lower eGFR and T score, respectively. On a follow-up period of 21.5 months, high miR-21 expression at diagnosis was associated (p = 0.02) with a poor renal outcome having a shorter time to doubling of serum creatinine.
Conclusion
High blood TGF-β1 and miR-21 expression at diagnosis of IgAN show significant correlation with renal function and degree of chronic tubulointerstitial injury on histology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunoglobulin A nephropathy (IgAN) is the most common primary glomerulonephritis in the world [1]. IgAN, characterized by the deposition of polymeric IgA in the mesangium, shows a variable clinical course [2, 3]. It is estimated that about 30–40% of affected individuals will progress to end-stage renal disease over a course of 20 years [4, 5]. At present, the major clinical tools for predicting prognosis of IgAN are baseline renal function, level of proteinuria, and histological grading. The working group of the International network and the Renal Pathology society had proposed a Oxford histological score based on the presence of mesangial hypercellularity (M), segmental glomerulosclerosis (S), and tubular atrophy/interstitial fibrosis [6, 7]. Among these, tubular atrophy and interstitial fibrosis is the most important predictor of progression and clinical outcome of IgAN, contributing to decline in renal function [8, 9]. Non-invasive biomarkers for early diagnosis of renal fibrosis can act as important predictors of prognosis in IgAN.
Transforming growth factor β (TGF-β), a cytokine with multiple biological properties, is involved in a pathophysiological process that leads to chronic kidney disease (CKD) and end-stage renal disease (ESRD) [10]. Activation of profibrotic pathways lead to elevated TGF-β1 levels in kidney [11].
MicroRNAs (miRNAs) are noncoding, single-stranded RNA molecules 20–25 nucleotides in length, regulating gene expression through post-transcriptional degradation of messenger RNA or translational inhibition of protein synthesis by binding to 3ʹ-UTR [12]. Several studies [13, 14] have shown upregulation of miR-21 in kidney fibroblasts cells upon TGF-β induction.
We investigated the utility of blood TGF-β1 and miR-21 in IgAN, aiming to explore the role of these molecules as non-invasive biomarkers of kidney fibrosis predicting the progression of IgAN.
Materials and methods
Patients
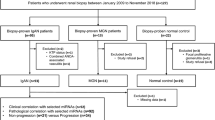
The study enrolled 65 participants including 30 patients of IgAN, 10 biopsy-proven Lupus Nephritis and 25 age and sex-matched persons as healthy controls and disease controls, respectively, from July 2017 to July 2019, at the Sanjay Gandhi Postgraduate Institute of Medical Sciences (Lucknow, India). The diagnosis of IgAN was made on renal biopsy after integration of light microscopy features and immunofluorescence, characterized by presence of dominant IgA deposits. Children (age < 18 years), elderly (age > 60 years), and patients with known systemic disease including diabetes mellitus, hypertension, heart disease or lung fibrosis were excluded. IgAN, secondary to liver disease, were also excluded.
Blood samples in IgAN at the time of biopsy and from both groups of controls were collected by using ethylene diaminetetraacetic acid (EDTA) and plain tubes. Cell-free plasma and serum was isolated from blood samples by centrifugation at 1500 r.p.m. for 10 min to prevent contamination by cellular nucleic acids and stored at − 80 °C.
The study was approved by the Institutional Ethics Committee. The clinical and biochemical parameters were obtained from the Hospital information system (HIS) and patient case files. The glomerular filtration rate (eGFR) was estimated using the modification of diet in kidney disease (MDRD) equation.
Renal biopsy assessment
Renal biopsy evaluation was performed by light microscopy and immunofluorescence. The renal biopsy for light microscopy was collected in 10% buffered formalin and routinely processed. The paraffin-embedded tissue was serially sectioned at 3 μm thickness and stained with hematoxylin and eosin, periodic acid-schiff, Masson’s trichrome, and sliver methenamine. Renal biopsy for immunofluorescence (IF) was received in normal saline and direct IF was performed for IgA, IgG, IgM, C3, C1q, kappa and lambda. The pathologic variables were scored according to the Oxford classification using the MEST-C scores [6, 7]. The mesangial hypercellularity score (M) was defined as M0 if the score was ≤ 0.5 and M1, if the score was > 0.5. Presence of endocapillary hypercellularity (E1) and segmental glomerulosclerosis (S1) was noted. Tubular atrophy and interstitial fibrosis (T) was semi-quantitatively scored according to the percentage of the cortical area involved: T0 for 0–25%, T1 for 26–50% and T2 for > 50% [6]. The presence of crescents were scored C0 for no cellular or fibrocellular crescents; C1 for cellular/fibrocellular crescents in < 25% of glomeruli; and C2 for crescents in ≥ 25% of glomeruli [7].
Determination of serum TGF-β1
Serum TGF-β1 level was estimated by using ELISA kits DY240 (R&D) according to the manufacture’s protocol. Briefly, ELISA plates were coated with mouse anti-human TGF-β1 capture antibodies at room temperature overnight. After blocking, diluted test serum was added, and TGF-β1 was detected with streptavidin–horseradish peroxidase (HRP). TGF-β1 levels were determined from OD450 values within the linear range of the standard curve via comparison to values obtained from dilutions of purified TGF-β1 in duplicates.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) for miRNA-21-5p
MiRNA from 200 μl of plasma was extracted using the mirVana PARIS Kit (Ambion, Austin, TX, USA) according to the manufacturer’s protocol. Total RNA was eluted in 50 μl of RNase-free water and stored at − 80 °C until analysis. The quantity and quality of RNA were assessed by NanoDrop 2000 thermo scientific spectrophotometer (NanoDrop Technologies). The concentration and purity of the miRNA were evaluated by using the ultraviolet spectrophotometer. Plasma samples with RNA concentration of < 10 ng/µl were not included in the analysis of miRNA.
Reverse transcription (ABI prism 7900 Sequence detection system, Applied Biosystem) was performed using the ΔTaqMan MicroRNA Reverse Transcription Kit (Thermofisher) with the specific TaqMan probe microRNA Assay (Thermofisher) for miR-21-5p (Assay ID397). U6-snRNA (Assay ID1973) was used as reference. Real-time PCR was performed with TaqMan Universal Master Mix II no UNG (Thermofisher) and miRNA specific primers (Thermofisher). For RT-QPCR, 10 μl 2 × miRcute miRNA Premix, 0.4 μl Primer (10 μM), 2 μl miRNA cDNA and 7.2 μl ddH2O were mixed to make a 20 μl reaction volume. Each sample was run in triplicate. Real-time quantitative polymerase chain reaction (RT-QPCR) was performed at 94 °C for 2min, followed by 40 cycles at 94 °C for 20s and 60 °C for 34s. U6-snRNA was used as an endogenous control for normalizing the miRNA expression in plasma. Expression levels of miRNA was calculated based on the comparative threshold cycle method (RQ = 2−ΔΔCt) [15].
Follow-up
The follow-up data were collected from the hospital records and telephonic consultations. Time to doubling of serum creatinine from the initial level at the time of initial diagnosis was recorded. Follow-up samples could not be collected from many patients due to the emergence of COVID pandemic. The follow-up samples, available in occasional patients, were, therefore, not included in the study.
Statistical analysis
Continuous variables were compared using unpaired Student’s test or ANOVA (Analysis of Variance) and categorical data were analyzed using the Chi-square test. Correlation between different parameters was performed using Pearson and spearman’s correlation coefficient. Simple linear and multivariate regression analysis was used to study the interrelationship between histological scores, clinical parameters and profibrotic biomarkers. Kaplan–Meier plots with log rank test and Cox Proportional Hazards regression was used to evaluate time to doubling of serum creatinine on follow-up with various variables. IBM SPSS (Statistical Package of the Social Science), version 20, was used for statistical analysis and P value less than 0.05 was considered as significant.
Results
Table 1 mentions the baseline characteristics of IgAN, disease controls (lupus nephritis) and healthy controls. The mean age in IgAN was 31.5 ± 9 years (range 18–49) and M: F ratio was 1.5:1. Twenty-two patients had hypertension. The mean 24-h proteinuria was 4.5 ± 4gm/day (range 0.33–16.3). The mean s. creatinine was 2.80 ± 1.6 mg/dl and the mean eGFR was 39.8 ± 27.8 ml/min/1.73 m2 (range 8–92).
On the basis of Haas’s classification for IgAN, 5 (16.6%) were class I, 4 (13.3%) class II, 3 (10%) class III, 7 (23.3%) class IV and 10 (33.3%) class V. According to Oxford classification, most (25/30; 83.3%) of the renal biopsies showed mesangial hypercellularity (M1). E1, S1 and C2 were seen in 11/30 (36.6%), 14/30 (46.6%) and 2/30 (6.6%) renal biopsies, respectively. Mild to moderate interstitial inflammation was present in 25/30 (83.2%) biopsies. Tubular atrophy/interstitial fibrosis (T1/T2) was found in 63% (n = 19) renal biopsies.
Serum TGF-β1 and Plasma miR-21-5p
The mean levels of serum TGF-β1 (Table 1) in healthy controls, IgAN and LN were 54.8 ± 15.3 pg/ml, 104.8 ± 36.7 pg/ml, 185 ± 27.8 pg/ml, respectively. In comparison with healthy controls, serum TGF-β1 was significantly higher in IgAN (p = 0.001) and LN (p = 0.007).
The mean relative expression of miR-21 measured by qRT-PCR after normalization with U6-snRNA in healthy controls and IgAN were 2.1 ± 0.93 and 4.05 ± 3.1, respectively. The plasma miR-21-5p expression was significantly elevated in IgAN (p = 0.001, Table 1).
Correlation of TGF-β1 and miR-21-5p levels with clinico-pathological parameters
Patients with decreased eGFR had significantly increased TGF-β1 levels (≥ 60 ml/min/1.73m2, 87.2 ± 34.8 pg/ml: < 60 ml/min/1.73m2 119.7 ± 34.5 pg/ml) (p = 0.001).
IgAN with presence of interstitial fibrosis/tubular atrophy (IF/TA) (T0 vs T1: 75.8 ± 33.6 vs 119 ± 25.5 pg/ml, p = 0.002; T0 vs T2: 75.8 ± 33.6 vs 134 ± 13.2 pg/ml, p = 0.004) and interstitial inflammation (absence: 60.14 ± 16.2 vs presence: 114.2 ± 31.2 pg/ml, p = 0.001) showed significantly raised TGF-β1 levels (Table 2). This correlation was not found with other histological features (M1, E1, S1, C1) of the MEST-C scores.
MiR-21-5p was found to be overexpressed in plasma of patients with moderate and severe fibrosis IF/TA (T1/T2) (T1: median relative expression [RQ] = 4.37 [1–9.8]) or severe (T2): median RQ = 7.68 [6.2–9.5]), in comparison to the patients with no fibrosis T0 (median RQ = 1.46 [0.23–7]). Presence of interstitial inflammation (absence RQ = 1.03[0.23–1.73] vs presence RQ = 4.29 [3.1–9.8]; p = 0.01) and endocapillary hypercellularity (E0 RQ = 2.9[1.6–4.3] vs E1 RQ = 5.06[3.37–9.8]; p = 0.049) also showed significant overexpression of plasma miR-21. This correlation was not found with M1, S1 and C1 histology scores.
Univariate linear regression showed significant positive correlation of serum TGF-β1 with plasma expression of miR-21-5p (spearman’s rho r = 0.615, p = 0.0001). High serum creatinine (r = 0.533, p = 0.002), eGFR (r = − 0.684, p = 0.0001), Haas’s class (r = 0.504, p = 0.007), degree of IF/TA (r = 0.626, p = 0.0001), interstitial inflammation (r = 0.541, p = 0.002) and acute tubular injury (r = 0.389, p = 0.034) were also found to be significantly correlated with higher levels of serum TGF-β1 levels. High plasma miR-21-5p correlated with IF/TA (spearman’s rho r = 0.768, p = 0.0001) and interstitial inflammation (spearman’s rho r = 0.417, p = 0.02) (Fig 1). Plasma level of miR-21 showed no significant association with demographic and clinical parameters as age, sex, hypertension, serum creatinine, eGFR and proteinuria.
A IgAN patients with presence of varying degree of fibrosis (IF/TA) (p = 0.041) shows shorter time to doubling of serum creatinine during median follow-up of 21.5 months. B Patients were divided into high and low plasma miR-21 levels as compared with its median level (3.03, IQR 0.23–10). High plasma miR-21 expression show shorter time to doubling of serum creatinine (p = 0.02)
To determine the independent contribution of each factor to TGF-β1 and miR-21-5p, multivariate models were constructed. Backward elimination regression method showed that high TGF-β1 significantly correlated with low eGFR (β = − 0.495, p = 0.001) and high plasma miR-21 (β = 0.360, p = 0.01). Plasma miR-21 significantly correlated with IF/TA (β = 0.728, p = 0.0001) on histology.
The median follow-up period was 21.5 months (range 4–30 months). Survival analysis was done to evaluate the impact of the clinico-pathological parameters on time to doubling of serum creatinine. Eleven (36.7%) patients of IgAN showed doubling of serum creatinine with median follow-up of 14.7 months (4–30 months). Patients were divided into high and low plasma miR-21 levels as compared with its median level (3.03, IQR 0.23–10). Shorter time to doubling of serum creatinine was seen in presence of interstitial fibrosis/tubular atrophy (IF/TA) (p = 0.041), interstitial inflammation (p = 0.038) and high plasma miR-21-5p expression (p = 0.02) at the time of initial diagnosis of IgAN (Fig 2). On univariate Cox regression analysis, relative expression of plasma miR-21 (Hazard ratio (HR): 0.133 confidence interval (CI) 95% = 0.017–1.068; p = 0.048) was more likely to have shorter time to doubling of serum creatinine. This association was not seen with TGF-β1 (p = 0.170). Multivariate cox regression analysis was not done due to small number of events.
Discussion
One-third of IgAN progresses to end-stage renal disease [5]. Tubular atrophy and interstitial fibrosis have significant influence on renal outcome [16]; however, this requires renal biopsy, an invasive procedure which cannot be done repeatedly. Serum creatinine is not a reliable early marker of disease progression. Circulating miRNAs, and profibrotic cytokines like TGF-β1 have shown to be non-invasive markers for progression of disease in kidney diseases including IgAN [17,18,19].
The hallmark of progressive CKD is excessive extracellular matrix (ECM) production, leading to renal fibrosis. TGF-β superfamily members have been implicated to play pathogenetic role in development of CKD [20]. TGF-β1 is a multifunctional cytokine that is well recognized to regulate a broad spectrum of cellular processes such as growth, differentiation, apoptosis, wound repair, and the pathogenesis of fibrosis [21]. TGF-β1 has been considered a potent profibrotic mediator in this process [22, 23].
Studies on IgAN suggest a predictive utility of TGF-β1 and miR-21 expression analysis in tissue and urine [24,25,26]. However, the value of plasma miR-21 levels as a prognostic factor for renal fibrosis in IgAN has not been reported. Therefore, we investigated the predictive role of TGF-β1and miR-21 in blood in IgAN and their association with disease severity and renal outcome.
Wu et al. [27] reported that the TGF-β1/Smad signaling pathway plays an important role in glomerular sclerosis, renal tubular injury and interstitial fibrosis in children with IgAN. Similarly, our study also shows significant correlation of TGF-β1 with renal acute tubular injury, degree of interstitial fibrosis and interstitial inflammation. Further, study done on unilateral ureteral obstruction (UUO) model suggests that miR-21 is upregulated after in-vitro stimulation of human renal tubular epithelial cells by TGF-β1 [28]. We also found significant positive correlation between TGF-β1 and miR-21-5p (r = 0.615, p = 0.0001).
Several miRNAs are found to be associated with kidney disease progression and fibrosis [19, 29, 30]. Circulating miRNA-1825 from plasma and in urinary exosomes of CKD patients correlated with reduced eGFR [31]. MiR-21-5p has been shown to be involved in the pathogenesis of diabetic kidney disease [32], CKD, CKD with cardiovascular disease and renal fibrosis [33].
MiR-21-5p was overexpressed in renal tissue of CKD [34], IgAN [35], renal fibrosis [36] and in diabetic nephropathy [37]. Its expression in renal tissue of CKD patients was also found to significantly correlate with eGFR, fibrosis, proteinuria and glomerulosclerosis [34]. High miR-21-5p renal tissue expression in diabetic nephropathy is associated with poor renal survival [36].
Marie-Flore Hennino et al. showed high expression of tissue miR-21-5p in patients of IgAN displaying tubular atrophy, interstitial fibrosis (miR-21-5p T0 RQ median = 1.23, T1 RQ = 3.01, T2 RQ = 3.90) and glomerulosclerosis (S0 RQ median = 1.31, S1 RQ = 2.65) [35]. We also found that circulating plasma miR-21-5p correlate with degree of interstitial fibrosis and tubular atrophy (r = 0.768, p = 0.0001) in IgAN. There was no association with degree of glomerulosclerosis and presence of crescents. Xu BY et al. in 2020, reported upregulated level of miRNA-21 in 48.6% (18/37) of IgAN patients in peripheral blood mononuclear cells (PBMCs) [38].
There were no studies done on IgAN patients to show expression of miRNA-21-5p in plasma. We showed that plasma miRNA-21-5p levels in IgAN correlate with presence of acute tubular injury, tubular atrophy and interstitial inflammation. Our findings suggest that recruitment of inflammatory cells due to renal interstitial inflammation and tubular injury in IgAN releases cytokines like TGF-β, which causes upregulation of miR-21 and accumulation of extracellular matrix leading to renal fibrosis. The limitation of our study is the small number of patients with a limited power to exclude smaller differences between the groups. Secondly, follow-up samples could were not collected due to emergence of COVID pandemic.
Lv et al. showed 1.3-fold change of miR-21-5p in patients of UUO with renal fibrosis as compared to controls without renal fibrosis. They showed a correlation of miR-21-5p with eGFR and interstitial fibrosis (r = 0.35, p = 0.04) [39]. In lupus nephritis, it has been shown that miR-21 expression in urinary exosome was 6.6 fold upregulated and cut off value of > 4.15 correlated with severe renal fibrosis (81% sensitivity and 72% specificity) [40].
Kaplan–Meier analysis revealed that patients with severe tubular atrophy/interstitial fibrosis and high plasma miR-21 show shorter time to doubling of serum creatinine, suggesting its role on in progression of IgA nephropathy. These data also show that the plasma miR-21 parallel with the severity of tubulointerstitial damage in IgAN. This suggests potential value of plasma miR-21 as diagnostic non-invasive biomarker. Therefore, administration of antagonist molecules of miR-21 can successfully prevents the development of renal fibrosis in IgA nephropathy patients.
In conclusion, our findings suggest that both TGF-β1 and miRNA-21-5p have a role on the profibrotic pathways in IgAN. The expression of circulating miRNA-21-5p has a predictive role in determining poor renal outcome. Therefore, further larger studies are required to confirm it utility as a non-invasive profibrotic biomarker for the prediction of IgAN progression.
Data availability
The datasets used/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IgAN:
-
IgA nephropathy
- LN:
-
Lupus nephritis
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- TGF-β1:
-
Transforming growth factor-β1
- UUO:
-
Unilateral ureteral obstruction
- RQ:
-
Relative expression
References
Rantala I, Mustonen J, Hurme M, Syrjänen J, Helin H (2001) Pathogenetic aspects of IgA nephropathy. Nephron 88(3):193–198
van der Boog PJ, van Kooten C, de Fijter JW, Daha MR (2005) Role of macromolecular IgA in IgA nephropathy. Kidney Int 67(3):813–821
Lim CS, Yoon HJ, Kim YS et al (2003) Clinicopathological correlation of intrarenal cytokines and chemokines in IgA nephropathy. Nephrology (Carlton) 8(1):21–27
Donadio JV, Grande JP (2002) IgA nephropathy. N Engl J Med 347(10):738–748
Le W, Liang S, Hu Y et al (2012) Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27(4):1479–1485
Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT et al (2009) The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 76(5):546–556
Trimarchi H, Barratt J, Cattran DC et al (2017) Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int 91(5):1014–1021
Worawichawong S, Worawichawong S, Radinahamed P et al (2016) Urine epidermal growth factor, monocyte chemoattractant protein-1 or their ratio as biomarkers for interstitial fibrosis and tubular atrophy in primary glomerulonephritis. Kidney Blood Press Res 41(6):997–1007
Coppo R, Troyanov S, Bellur S et al (2014) Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86(4):828–836
Meng XM, Chung AC, Lan HY (2013) Role of the TGF-β/BMP-7/Smad pathways in renal diseases. Clin Sci (Lond) 124(4):243–254
Wang W, Koka V, Lan HY (2005) Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 10(1):48–56
Esteller M (2011) Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874
Szeto CC, Li PK (2014) MicroRNAs in IgA nephropathy. Nat Rev Nephrol 10(5):249–256
Glowacki F, Savary G, Gnemmi V et al (2013) Increased circulating miR-21 levels are associated with kidney fibrosis. PLoS One 8(2):e58014
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
Humphreys BD (2018) Mechanisms of renal fibrosis. Annu Rev Physiol 80:309–326
Zhang W, Zhou X, Zhang H, Yao Q, Liu Y, Dong Z (2016) Extracellular vesicles in diagnosis and therapy of kidney diseases. Am J Physiol Renal Physiol 311(5):F844–F851
Leleu D, Levionnois E, Laurent P et al (2020) Elevated circulatory levels of microparticles are associated to lung fibrosis and vasculopathy during systemic sclerosis. Front Immunol 11:532177
Saejong S, Townamchai N, Somparn P et al (2020) MicroRNA-21 in plasma exosome, but not from whole plasma, as a biomarker for the severe interstitial fibrosis and tubular atrophy (IF/TA) in post-renal transplantation [published online ahead of print, 2020 Jun 21]. Asian Pac J Allergy Immunol. https://doi.org/10.12932/AP-101019-0656
Zeisberg M, Hanai J, Sugimoto H et al (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9(7):964–968
Sureshbabu A, Muhsin SA, Choi ME (2016) TGF-β signaling in the kidney: profibrotic and protective effects. Am J Physiol Renal Physiol 310(7):F596–F606
Ding Y, Choi ME (2014) Regulation of autophagy by TGF-β: emerging role in kidney fibrosis. Semin Nephrol 34(1):62–71
Lee SY, Kim SI, Choi ME (2015) Therapeutic targets for treating fibrotic kidney diseases. Transl Res 165(4):512–530
Wang G, Kwan BC, Lai FM et al (2010) Intrarenal expression of microRNAs in patients with IgA nephropathy. Lab Invest 90(1):98–103
Szeto CC, Ng JK, Fung WW et al (2020) Kidney microRNA-21 expression and kidney function in IgA nephropathy. Kidney Med 3(1):76-82.e1
Liang S, Cai GY, Duan ZY et al (2017) Urinary sediment miRNAs reflect tubulointerstitial damage and therapeutic response in IgA nephropathy. BMC Nephrol 18(1):63
Wu W, Jiang XY, Zhang QL, Mo Y, Sun LZ, Chen SM (2009) Expression and significance of TGF-beta1/Smad signaling pathway in children with IgA nephropathy. World J Pediatr 5(3):211–215
Zarjou A, Yang S, Abraham E, Agarwal A, Liu G (2011) Identification of a microRNA signature in renal fibrosis: role of miR-21. Am J Physiol Renal Physiol 301(4):F793–F801
Tangtanatakul P, Klinchanhom S, Sodsai P et al (2019) Down-regulation of let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pac J Allergy Immunol 37(4):189–197
Min QH, Chen XM, Zou YQ et al (2018) Differential expression of urinary exosomal microRNAs in IgA nephropathy. J Clin Lab Anal 32(2):e22226
Muralidharan J, Ramezani A, Hubal M et al (2017) Extracellular microRNA signature in chronic kidney disease. Am J Physiol Renal Physiol 312(6):F982–F991
Zang J, Maxwell AP, Simpson DA, McKay GJ (2019) Differential expression of urinary exosomal microRNAs miR-21-5p and miR-30b-5p in individuals with diabetic kidney disease. Sci Rep 9(1):10900
Peters LJF, Floege J, Biessen EAL, Jankowski J, van der Vorst EPC (2020) MicroRNAs in chronic kidney disease: four candidates for clinical application. Int J Mol Sci 21(18):6547
Yu Y, Bai F, Qin N et al (2018) Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron 139(3):269–282
Hennino MF, Buob D, Van der Hauwaert C et al (2016) miR-21-5p renal expression is associated with fibrosis and renal survival in patients with IgA nephropathy. Sci Rep 6:27209
Lv LL, Cao YH, Ni HF et al (2013) MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305(8):F1220–F1227
McClelland AD, Herman-Edelstein M, Komers R et al (2015) miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129(12):1237–1249
Xu BY, Meng SJ, Shi SF et al (2020) MicroRNA-21-5p participates in IgA nephropathy by driving T helper cell polarization. J Nephrol 33(3):551–560
Lv CY, Ding WJ, Wang YL et al (2018) A PEG-based method for the isolation of urinary exosomes and its application in renal fibrosis diagnostics using cargo miR-29c and miR-21 analysis. Int Urol Nephrol 50(5):973–982
Solé C, Moliné T, Vidal M, Ordi-Ros J, Cortés-Hernández J (2019) An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cells 8(8):773
Acknowledgements
The author is thankful to Indian Council of Medical Research for providing fellowship (2017-3612/CMB-BMS).
Author information
Authors and Affiliations
Contributions
NB, VA, SK, VA, and NP participated in study conception and design. NB and VA were involved in data acquisition, interpretation and writing the manuscript. VA performed critical revision of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Ethical approval and consent to participate
The study was approved by the Institutional Ethical Review Committee of Sanjay Gandhi Postgraduate Institute of Medical Sciences in Lucknow, India (IEC code: 2017-129-PhD-99). Informed consent was obtained from all participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bharti, N., Agrawal, V., Kamthan, S. et al. Blood TGF-β1 and miRNA-21-5p levels predict renal fibrosis and outcome in IgA nephropathy. Int Urol Nephrol 55, 1557–1564 (2023). https://doi.org/10.1007/s11255-023-03464-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-023-03464-w