Abstract
Boron (B) is one of the most problematic impurities to remove from metallurgical grade silicon in the production of more pure solar grade silicon (SoG-Si). In the present work, recent progresses in the application of reactive gases for B removal from molten silicon is reviewed. Moreover, in order to clarify the mechanisms and kinetics of gas-based B-refining, an experimental procedure using humidified Ar, N2, and H2 gases applied to boron-doped silicon melt is described. It is shown that the kinetics and extent of B removal is depending on the type of humidified gas. The thermodynamics and kinetics of B removal from molten silicon are studied to explain experimental observations. The mass transfer coefficients of B are calculated and possible mechanisms for B removal by the reactive gases are proposed:
It is shown that the lower equilibrium partial pressure of HBO gas at higher temperatures causes slower B removal rate.
Similar content being viewed by others
Introduction
Silicon is the main material used for the fabrication of solar cells and will likely continue to be a main material in photovoltaic industry for decades to come. The production of solar grade silicon (SoG-Si) through a metallurgical route is more energy efficient and environmentally benign than the traditional chemical route (Siemens process). Application of a gas refining technique could further improve the efficiency of a metallurgical refining process for low-boron SoG-Si. The removal of B from silicon by gas treatment has been studied by several researchers using plasma technique or gas blowing. The application of H2O gas in the reactive gas in plasma refining[1–6] and H2-H2O gas mixtures through non-plasma-aided gas blowing[7,8] has shown faster B removal compared to the application of pure H2,[8–10] O2,[9,11] and CO2[11] as reactive gases. The plasma refining experiments with H2-O2 mixtures[12,13] as the reactive gas have also indicated faster B removal from silicon than using H2, O2, CO2 gases. This has been suggested to be due to the removal of B from silicon through the formation of volatile H-B-O species at the silicon melt surface, which is more favorable than formation of volatile H-B and B-O species. It has been proposed that formation of HBO is more favorable than other species.[2,6] Tang et al.[14] has recently reported the changes in the Gibbs energy of formation of HBO (g) as:
The mechanism of HBO formation in the silicon refining by H2O containing gases is still not well-known. Khattak et al.[2] proposed that the removal of B at the melt surface occurs through reaction with the SiO and H2 gases as:
According to this study, the SiO gas in Reaction [2] is produced through the simultaneous oxidation of silicon with H2O to H2 and SiO gas. Altenberend[15] introduced an enrichment factor, which is the ratio of B over Si in the gas phase, and reported that this factor is proportional to the square root of the H2 concentration in the gas. The kinetics of B removal is dependent on both the refining method and also the reactive gas composition. Reaction rate constants in the range 2 × 10−5 to 1.4 × 10−4 m/s have been reported for B removal by different plasma techniques.[5] The largest rate constants for plasma aided B removal have been achieved by applying Ar-1.24 pctH2O and Ar-7.5 pctH2O[5] reactive gases, resulting in rate constants as 1.4 × 10−4 and 7.8 × 10−4 m/s, respectively. Lower rate constants have been reported from the non-plasma-aided blowing of H2-H2O gas mixtures (1.0 × 10−6 to 1.3 × 10−5 m/s).[7,8] The highest rate constant for such conditions has been reported for H2-3.2 pctH2O at 1723 K (1450 °C).[8]
In the present study, the gas-based removal of B from molten silicon is studied experimentally. The obtained results are discussed based on thermodynamic and kinetic considerations of the refining system. These are used further to explain the mechanism of B removal by humidified gases.
Experimental Procedure
Electronic grade polycrystalline silicon (9N) was B-doped by melting silicon in a graphite crucible in an induction furnace under Ar flow. The melt was kept for 1 hour at 1873 K (1600 °C), and cast in a water cooled copper mould to minimize B segregation. The prepared master Si-B alloy was analysed by a high resolution inductively coupled plasma mass spectrometry (ICP-MS) and it showed B concentration as 430 ± 10 ppmw. Mixtures of this master alloy and the polycrystalline silicon were melted in graphite crucible with 75 mm inside diameter and 85 mm outside diameter for making B-containing silicon melts. Gas refining of the silicon melts was done in an induction furnace, the experimental setup is schematically shown in Figure 1. The furnace chamber was purged for oxygen through evacuation twice followed by argon (+99.999 pctAr) flushing. The heating and melting was then done under Ar flow, while the temperature at 10 mm above the crucible bottom was measured by a thermocouple type C located in an alumina insulating tube. Pure Ar, N2, and H2 gases (5 N) were moisturized by a humidifier (P10 series humidifier, Cellkraft AB, Stockholm, Sweden) and blown over a 400 g B-doped Si-melt with flow of 3 NL/min using a lance with 4 mm inner diameter from 40 mm above the melt surface. The accuracy of the humidifier was 1.5 pctRH (relative humidity), a typical RH as 40 pct is obtained for 3vol pct H2O in Ar at 313 K (40 °C). The partial pressure of oxygen in the outlet was continuously measured by an oxygen analyser (Rapidox 2100ZR Oxygen Analyser, Cambridge Sensotec Ltd., Cambridgeshire, UK), with a detection limit of 10−20 ppmw. There are ports and windows on the top of the furnace which make it possible to watch the melt surface and insert a quartz sampling tube. Watching the melt surface is beneficial to observe the formation of any condensed phases over the melt during the refining. Samples from the melt, each around 2 g, were taken throughout the refining. Additional experimental details are shown in Table I. The silicon melt was cast after refining in the water cooled copper mould. Three parallels from each taken sample with around 30 mg weight were analysed by ICP-MS to determine the concentration of B. In addition to the ICP-MS analysis, the resistivity of the final cast silicon was measured by a Jandel 4 point probe (Jandel Engineering, Ltd., Bedfordshire, UK) and the corresponding B concentrations then determined according to the ASTM F723-99 standard for conversion between resistivity and dopant density for B-doped silicon. It was found in the experiments that an oxide surface layer is formed over the silicon melt during refining. This surface layer was also analyzed by electron probe microanalysis (EPMA).
Results
The obtained results from the experimental work are described as follows.
Oxygen Partial Pressure
The oxygen partial pressure in the gas outlet during the heating and refining were measured. It was observed that the oxygen partial pressure is rapidly decreasing in the furnace during heating with a highest rate at around 1273 K (1000 °C) to very low partial pressures, i.e., 1 × 10−14 ppm. This decrease in oxygen partial pressure is due to the fast interaction of oxygen with the graphite crucible with increasing temperature and CO gas formation. The oxygen partial pressure is significantly reduced during heating with a short rise in p O2 at the start of melting and subsequent decrease during the refining step with the introduction of humidified gases. This shows that free oxygen in the gas phase during the refining process is at a very low level. The oxygen partial pressure is levelled off after some refining time. The observed oxygen partial pressures for all the experiments were below 1 × 10−17 ppm during the main gas blowing cycle. When humidified Ar and N2 were used, the oxygen partial pressures were mostly between 1 × 10−17 and 1 × 10−18 ppm, while when humidified H2 was used pO2 decreased to below 1 × 10−20 ppm; the detection limit of the oxygen gas analyzer.
Boron Removal from Silicon Melts
The measured chemical compositions of silicon samples presented in Table I show B removal from silicon for all the experiments. The measured B concentrations by the resistivity method are in relatively good agreement with the ICP-MS analysis. In order to evaluate the effectiveness of the refining conditions on the B removal, the degree of B removal was calculated as:
where C B,0 is the initial concentration of B in silicon and C B,t is the concentration of B at refining time t. It is worth mentioning that the silicon loss during the refining is not significant, a few weight percentages, i.e., 3 pct, and it does not affect the above definition for B removal. The results (Figure 2) show that for all experiments B is removed with an initial high rate stage followed with a second slower stage. For a given refining time, i.e., 60 minutes, the B removal degree by using H2-H2O gas mixtures is higher than that for Ar-H2O and N2-H2O gases. The rate and extent of B removal for a given steam concentration, 3 pctH2O, is highest when humidified H2 is used, and lowest for N2-3 pctH2O. The B removal rates by H2-H2O gases with higher moisture concentrations (5.9 pctH2O and 11 pct H2O) at 1773 K (1500 °C) are more or less the same as for a temperature of 1703 K (1430 °C) for 3 pctH2O at initial, independent of H2O content in the experiments in this study. Longer refining times, however, they show different behavior and the B removal kinetics is slower for 5.9 and 11 pctH2O concentrations. For a given gas phase composition as H2-3 pctH2O, the kinetics of B removal is slower at higher temperature as clearly seen through comparing the Experiments 3 and 6.
Analysis of the Passive Surface Layer
As earlier mentioned, the formation of a passive oxide layer on the silicon melt surface was observed during the silicon refining in some experiments. Figure 3 shows a cross section of the passive surface layer formed over Si-B melt treated by H2-3 pctH2O gas. This layer covered a part of the surface after 1 h of refining time. This cross section shows a very good contact between the layer and silicon. This layer is SiO2 and it is porous with regard to the presented X-ray map of the elements. It is worth mentioning that for the majority of the experiments, except Exp. 2 and Exp. 6, this passive oxide layer was observed after around 30 minutes.
Discussion
Kinetics of B Removal
Assuming first order reaction kinetics for the removal of B from liquid silicon, the relationship between the B concentration and refining time t can be written as:
where A/V is the ratio of melt surface over the melt volume, and k B is the total mass transfer coefficient for B removal from the melt to the gas phase. The value of k B is the slope of a plotted line of the left side of Eq. [4] against (A/V)t as illustrated in Figure 4. For calculating k B we may consider the first 30 minutes of refining due to the negligible amount of passive surface layer formation within this refining time. This yields k B = 7×10−6, 6 × 10−6, 1.3 × 10−5, 1.4 × 10−5, 1.3 × 10−5, and 7 × 10−6 m/s for Experiments 1 to 6, respectively.
The removal of B from silicon occurs through a first order reaction as seen for Experiment 6 for the whole refining time due to no formation of the passive oxide layer. The slower B removal for other experiments after some refining time is due to the formation of this layer decreasing the effective A/V ratio. The SiO2 layer plays an important role in the refining kinetics; it is formed at lower temperatures and higher oxygen potential by a passive silicon oxidation scheme:
The oxide layer was observed for Exp. 3 after 1 hour refining. For Exp. 4 and Exp. 5, which were carried out at a higher temperature and higher H2O contents, it was observed after 30 minutes refining; i.e., the application of a higher steam concentration provided more favorable thermodynamic conditions for the formation of SiO2.[16] In Exp. 6 with 100 deg higher temperature compared to Exp. 3, no oxide layer was observed and it may be concluded that for a given H2O content in the gas, the passive layer is less stable at higher temperatures, which is in accord with thermodynamic calculations. Predicting that at higher temperatures, the stability of SiO gas is increased and may hence postpone the formation of the passive oxide layer.
Mechanism of B Removal
The removal of B by the humidified gases can potentially take place according to the following oxidation reaction:
Simultaneously, the oxidation of silicon to SiO gas is taking place in the system:
The B removal rate is increased with increasing H2O content in the refining gases[7,14] as long as the passive oxide layer is not covering the surface. In parallel, more SiO gas is produced with higher H2O contents in the refining gases due to maintaining larger driving force for the Reaction [7]. On the other hand, more SiO gas is produced at higher temperatures with regard to the changes of ΔG° with temperature for Reaction [7]. Hence, increasing the refining temperature has to increase the kinetics of B removal, if Reaction [2] is the controlling step of B removal. However, the results of this work and the previous works[8,15] show that the rate of B removal is decreased with increasing temperature and Reaction [2] cannot explain the effect of temperature. If we assume that Reaction [6] is the main reaction for B removal, and that H2 does not play a role in the removal kinetics, it is difficult to explain the difference in B removal rate with gas composition change in experiments 1, 2, and 3 where the steam concentration in the reactive gas phase is the same, while the carrier gas different (Ar, N2, H2, respectively). According to the present results and the previous work at NTNU,[8] the B removal rate is higher than any other type of gas when H2-H2O mixtures are used. Therefore, we may conclude that hydrogen plays a key role in the reaction mechanism as observed in literature as well.[5,12] We propose that there may be two different, potentially both contributing mechanisms to this result; the first mechanism is that hydrogen is dissolved in silicon and that B removal occurs through the interaction of the dissolved B and H species with H2O gas at the melt-gas interfacial area:
Hence the process requires considerable amount of hydrogen to provide enough dissolved atomic hydrogen close to the reaction interface for Reaction [9]. This mechanism may explain the faster B removal with increasing the H2/Ar ratio in Ar-H2-H2O gas mixtures.[8] When H2 gas is not purged into the system, i.e., Experiments 1 and 2, the B removal occurs through the dissociation of H2O at the melt surface into H and O elements and further B removal by Reaction [9]. Obviously, the rate of B removal in this mechanism will be slower than H2-H2O mixtures due to much lower dissolved hydrogen in the melt.
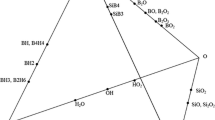
In order to study the hydrogen solubility in silicon, the binary Si-H system was studied using the developed database for SoG-Si at SINTEF/NTNU[17] and applying the FactSage thermodynamic software. The Si-rich part of the phase diagram at elevated temperatures is shown in Figure 5 and it shows that at the applied refining temperatures there are significant H solubility in silicon. For instance at 1703 K (1430 °C) around 25 ppma hydrogen exist in the melt at 1 atm pressure. It is worth mentioning that higher solubility of H in silicon as 60 ppma has been measured by experimental study by Ikari et al.[18] for the temperature range of 1703 K to 1773 K (1430 °C to 1500 °C). Hence, there is always considerable amount of dissolved hydrogen in the melt surface during the B elimination to very low levels. The high solubility of H2 in silicon was also confirmed in the present study. As shown in Figure 6, the formation of many pores in the solidified silicon under H2 atmosphere indicates the presence of dissolved hydrogen in the melt. The solubility of hydrogen in solid silicon is much less than liquid silicon (Figure 5) and it causes the formation of H2 bubbles during solidification.
In the present study, a graphite crucible was used to contain the silicon which results in carbon saturation of the melt. The high activity of carbon in the melt, in addition to dissolved hydrogen, ensures a low solubility of oxygen. In addition, the low partial pressure of oxygen in the gas (i.e., 10−18 ppm) makes the availability of oxygen limited. As we did not observe significant effect of increasing the H2O content in the H2-H2O gas mixtures (above 3 vol pct) on B removal kinetics at initial stages, we do not consider dissolved oxygen a major contributor in the B removal reaction mechanism. More experimental work may clarify more details about the role of dissolved oxygen in the reaction mechanism.
In addition to the role of dissolved hydrogen on the kinetics of the B removal, other researchers[15] have suggested that the presence of H2 suppresses the formation of condensed SiO2 by oxidation of SiO in the gas boundary layer, hence enhancing the diffusion rate of HBO products. Given that the p O2 in the H2 containing gases used in the present study was below the detection limit, allowing less oxygen in the gas phase as compared to N2 and Ar carried gases, this is also a possible contributing factor to the increased removal rate for H2 carried gases.
The thermodynamic equilibrium in the system for refining of silicon melt containing 30 ppmw B by Ar-3 pctH2O, N2-3 pctH2O, H2-3 pctH2O gases was studied through calculations using the FactSage thermodynamic software, with the recently developed data base for solar grade silicon at SINTEF/NTNU. The results for the melt treatment at different temperatures for H2-3 pctH2O are shown in Figure 7. It is seen that among the B-containing species the partial pressure of HBO gas is the highest in the system and B removal through the formation of HBO is the most possible reaction. The thermodynamics calculations show very low oxygen partial pressures, which is in agreement with the measured low oxygen partial pressures by the oxygen analyzer in this work. The thermodynamic calculations indicated the existence of solid SiO2 in the system, although significant amount of SiO gas co-exists. We may propose that the reason of not observing a passive oxide layer (SiO2) for some experiments is due to the lack of solid SiO2 nucleation on the silicon surface. This explanation may be supported by the observation of an oxide layer after some refining time, i.e., 30 minutes, and not from the start of gas blowing. Relatively fast oxide layer growth after nucleation may indicate thermodynamic stability of SiO2 in the system. The higher stability of SiO gas at higher temperatures may reduce the possibility of SiO2 layer formation, which is clearly seen through comparing Experiments 3 and 6. We may propose that when N2 gas is used, i.e., Experiment 2, it affects the interfacial energies between the liquid silicon and gas so that the nucleation of solid SiO2 becomes more difficult and this postpones or even stops the formation of solid SiO2 on the surface.
The slower B removal rate at higher temperatures can now be explained considering the thermodynamic equilibrium. Figure 7 shows that the equilibrium partial pressure of the HBO gas is decreased with increasing temperature. According to kinetic principles, this means smaller driving force for the chemical Reaction [9] at the melt surface and therefore a lower B removal rate. This is important from practical point of view in choosing the process temperature.
In order to study the importance of the mass transport in the melt and the gas phases, more experimental work with changing the process conditions, i.e., gas velocity, gas composition, and so forth are required.
Conclusions
The removal of B from molten silicon by humidified Ar, N2, and H2 gases was studied. It was found that H2-H2O gas mixtures were most effective for B removal compared to the other gas mixtures investigated. Furthermore, the kinetics of B removal was faster at lower temperatures. A mass transfer coefficient for B removal by H2-3 pctH2O as k B = 1.3 × 10−5 m/s at 1703 K (1430 °C) was obtained. Based on the experimental observations and thermodynamics of the system, it was proposed that B removal from silicon is taking place through simultaneous hydrogen dissociation into the melt and boron conversion into HBO gas by the dissolved hydrogen and H2O gas at the melt-gas interface. The presence of H2 in the refining gas may also suppress the SiO oxidation in the gas boundary layer, avoiding condensed species and hence enhancing the diffusion of HBO product with a subsequent increase in the B removal rate. The rate of B removal from molten silicon is decreased with increasing temperature due to the smaller equilibrium partial pressure of HBO gas and lowering the driving force of the chemical reaction of B removal at the melt-gas interfacial area.
References
T. Ikeda, M. Maeda, Mater. Trans. 37, 983–987 (1996)
Khattak C.P., Joyce D.B., and Schmid F.: Report NREL/SR-520-27593, National Renewable Energy Laboratory, 1999
C.P. Khattak, D.B. Joyce, F. Schmid, Sol. Energy Mater. Sol. Cells 74, 77–89 (2002)
N. Yuge, M. Abe, K. Hanazawa, H. Baba, N. Nakamura, Y. Kato, Y. Sakaguchi, S. Hiwasa, F. Aratani, Prog. Photovolt. Res. Appl. 9, 203–209 (2001)
N. Nakamura, H. Baba, Y. Sakaguchi, Y. Kato, Mater. Trans. 45(3), 858–864 (2004)
J.J. Wu, W.H. Ma, Y.N. Dai, K. Morita, Trans. Nonferr. Met. Soc. China 19, 463–467 (2009)
H.C. Theuerer, J. Metals 8, 1316–1339 (1956)
E. Nordstrand, M. Tangstad, Metall. Mater. Trans. B 43B, 814–822 (2012)
S. Tsao, S.S. Lian, Mater. Sci. Forum 475–479, 2595–2598 (2005)
S. Rousseau, M. Benmansour, D. Morvan, J. Amouroux, Sol. Energy Mater. Sol. Cells 91, 1906–1915 (2007)
K. Suzuki, K. Sakaguchi, T. Nakagiri, N. Sano, J. Jpn. Inst. Met. 54, 161–167 (1990)
C. Alemany, C. Trassy, B. Pateyron, K.-I. Li, Y. Delannoy, Sol. Energy Mater. Sol. Cells 72, 41–48 (2002)
E. Fourmond, C. Ndzogha, D. Pelletier, Y. Delannoy, C. Trassy, Y. Caratini, Y. Baluais, and R. Einhaus: 19th European Photovoltaic Solar Energy Conference, Paris, France, 7–11 June, 2004
K. Tang, S. Andersson, E. Nordstrand, M. Tangstad, JOM 64(8), 952–956 (2012)
J. Altenberend: Ph.D. Thesis, Universite de granoble, 2012
C. Wagner, J. Appl. Phys. 29, 1295–1297 (1958)
K. Tang, E.J. Øvrelid, G. Tranell, and M. Tangstad: 12th International Ferroalloys Congress, June 6–9, Helsinki, Finland, 2010, pp. 619–29
A. Ikari, H. Sasaki, E. Tokizaki, K. Terashima, S. Kimura, Jpn. J. Appl. Phys. 34(8), L1017–L1019 (1995)
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript submitted October 4, 2013.
Rights and permissions
About this article
Cite this article
Safarian, J., Tang, K., Hildal, K. et al. Boron Removal from Silicon by Humidified Gases. Metallurgical and Materials Transactions E 1, 41–47 (2014). https://doi.org/10.1007/s40553-014-0007-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40553-014-0007-8