Abstract
The removal of boron in pure silicon by gas mixtures has been examined in the laboratory. Water-vapor-saturated hydrogen was used to remove boron doped in electronic-grade silicon in a vacuum frequency furnace. Boron concentrations in silicon were reduced from 52 ppm initially to 0.7 ppm and 3.4 ppm at 1450°C and 1500°C, respectively, after blowing a H2-3.2%H2O gas mixture for 180 min. The experimental results indicate that the boron removal as a function of gas-blowing time follows the law of exponential decay. After 99% of the boron is removed, approximately 90% of the silicon can be recovered. In order to better understand the gaseous refining mechanism, the quantum chemical coupled cluster with single and double excitations and a perturbative treatment of triple excitations method was used to accurately predict the enthalpy and entropy of formation of the HBO molecule. A simple refining model was then used to describe the boron refining process. This model can be used to optimize the refining efficiency.
Similar content being viewed by others
Introduction
The removal of boron in metallurgical grade silicon (MG-Si) is one of the most crucial tasks for upgrading to the photovoltaic silicon feedstock. Several processes have been reported for this purpose. Molten flux treatment1–4 and plasma5–11 are two of the most common methods for the removal of boron in silicon. Although the molten flux treatment can effectively remove boron, it will inevitably contaminate the silicon feedstock. Consequently, additional refining step(s), e.g., acid leaching and directional solidification, is(are) often necessary in order to remove the contaminated impurities from the flux-treated silicon. Since the 1990s, steam-added plasma treatment has been extensively studied in refining silicon feedstock. Suzuki et al.5 reported the method of Ar-H2O plasma for the removal of boron from MG-Si. 98.9% of the boron in MG-Si can be removed using an Ar-1.24 vol.% H2O plasma gas after a 25-min treatment. Ikeda and Maeda6 tested the Ar-H2O plasma gases with different water vapor pressures. They concluded that the highest water vapor pressure, 1.24 vol.% H2O, has the highest apparent rate constant for boron removal. Similar experiments were carried out by Nakamura et al.7 and Yuge et al.8 Recently, Wu et al.10 reported their experimental results for the removal of boron by an Ar-H2O-O2 mixture. Boron was reduced from 18 ppm to 2 ppm after the first 10 min. However, they found no further removal of boron after treatment for longer times. Lee et al.9 tested the method of refining boron by a steam plasma method utilizing the electromagnetic casting process. After a 3-min treatment by the Ar-H2O-H2 plasma gas, more than 60% of the boron could be removed from silicon melts.
Simple humidified hydrogen treatment can also remove boron in pure silicon rather efficiently.12,13 Theuerer13 reported the method of boron removal by humidified hydrogen about 60 years ago. He found that the efficiency of boron removal is proportional to the partial pressure of water vapor by
where B is the boron concentration after treatment, B 0 is the initial boron concentration, \( P_{{{\text{H}}_{ 2} {\text{O}}}} \) is the partial pressure of water vapor in the gas phase, t is the time of treatment in min, and k is the rate constant. Below we will show that this relation is also applicable for the steam-added plasma gas mixtures.5–10
In order to define the boundary conditions of boron removal by humidified hydrogen, the thermochemical properties of the boron-containing gaseous species are the essential aspects. The JANAF Thermochemical Tables14 are frequently used to evaluate the refining efficiency in the literature. However, as indicated by both experiments and quantum chemistry calculations,15–18 the recommended value of the enthalpy of formation for the HBO molecule is questionable. As a consequence, the tabulated properties of HBO in the JANAF Thermochemical Tables14 may not be accurate enough for the analysis of current refining processes. A new assessment is necessary to correctly describe the refining chemistries.
In the present study, the removal of boron in silicon by an H2-H2O mixture was first studied experimentally. The enthalpy and entropy of formation of the HBO molecule have been calculated using the ab initio coupled cluster with single and double excitations and a perturbative treatment of triple excitations [CCSD(T)] technique. Applying these new values for the thermochemical properties of HBO, the experimental results can be reproduced within their uncertainties. The apparent rate constants for boron removal at 1450°C and 1550°C are also evaluated. A simple dynamic model is proposed for the further optimization of the refining process.
Experimental
Electronic grade silicon (EG-Si) was first doped with analytical reagent pure boron powder to about 150 ppmw in a high-purity crucible. For each measurement, 210 g B-doped Si (a mixture of 70 g 150 ppmw B and 140 g EG-Si) was used. The silicon materials were then put into a high-purity graphite crucible with an inner diameter of 70 mm and height of 150 mm. A PtRh-Pt thermocouple was placed into the bottom of the crucible by a graphite tube fixed on the wall of the crucible. After the crucible was positioned into the furnace, the chamber was closed to obtain vacuum. The system was then filled with hydrogen to an arbitrary pressure.
Hydrogen gas was first passed through a humidifier and then blown 50 mm over the melt through a 5 mm inner diameter quartz tube. The water temperature in the humidifier can be controlled within ±0.1°C. Hydrogen gas was saturated by water vapor at 25 ± 0.5°C. The steam content was fixed at 3.17 at.%. The gas inlet tube was heated to around 90°C in order to prevent water condensation. The purging gas rate was controlled at 3 L/min. Temperature profiles of the samples were carefully controlled by adjusting the induction power of the furnace.
During the gas blowing, the silicon melt was sampled by a quartz tube every 30 min. The duration of each experiment was 180 min. The boron contents of the samples were measured using a resistivity meter (Model RM2; Jandel, Leighton Buzzard, UK). For each sample, at least 8 points were measured for the resistivity. The boron contents can then be determined by the standard method. One set of samples was also analyzed by inductively coupled plasma (ICP)-mass spectroscopy (MS).
Results
Figure 1 shows the typical results of boron contents during the H2-H2O gas blowing at 1450°C and 1500°C, respectively. Si recovery rates in the present experiments were 89% at 1450°C and 91% at 1500°C. After a 3-h treatment, the boron removal rates were 99% at 1450°C and 93% at 1500°C. It is clear that the lower temperature favors boron removal.
A comparison of the boron contents measured by a resistivity meter with those by ICP-MS is shown in Fig. 2. In general, the boron contents determined by the resistivity meter are approximately 15–20% lower than those by ICP-MS. Nonetheless, the general agreement between the two sets of measurements is rather good. We will use the data of resistivity measurement for further analysis of the experimental results.
Quantum Chemistry Calculations
In order to calculate the enthalpy and entropy of formation, we employed ab initio electronic structure calculations. The most accurate quantum chemistry method that is practically applicable to a wide range of chemical problems is CCSD(T). We performed CCSD(T) calculations using the Gaussian 09 package.18 First, geometry optimizations were performed for the reference molecules H2 and O2 and for the target molecule HBO with the aug-cc-pVTZ basis set. To improve on the electronic energies, CCSD(T) calculations employing a larger basis set, aug-cc-pV5Z, were run using the geometries optimized in the previous step. The reference state of boron at 298 K is B(s). However, it is practically impossible to run the CCSD(T) calculations on solid boron. Therefore, CCSD(T) calculations are performed on the gas phase B atom. A literature value of the standard enthalpy of formation of B(g) is used to define the B reference energy by subtracting it from the calculated CCSD(T) energy for B(g). The most accurate estimate appears to be the theoretical value by Karton and Martin,19 570.3 kJ/mol, which differs from the tabulated value in the JANAF table, 560.0 kJ/mol. We used the former value. To calculate contributions from atomic motion, vibrational frequencies were calculated using the harmonic approximation, and rotations were included by treating the molecules as classical rigid rotors.
The calculations as outlined above give an enthalpy of formation at 298 K of HBO of −229.4 kJ/mol and entropy of formation of 202.9 J/K mol. The values in the JANAF table are −198.3 kJ/mol and 202.6 J/K mol, respectively. There is a difference of 31 kJ/mol in the enthalpies, but no difference is found between the entropies. There are other indications that the tabulated enthalpy of formation is erroneous. Previously, the standard enthalpy of formation has been estimated to be −251 kJ/mol (−60 kcal/mol) from multi-reference configuration interaction electronic structure calculations.17 In a later study employing the G2 method,20 devised for calculating accurate yet computationally affordable thermochemistry, the result was −234 kJ/mol (−55.9 kcal/mol). These calculated results together with the experiments described below serve to strongly suggest the need for a revision of the HBO enthalpy of formation in the JANAF table.
Discussion
Both Figs. 1 and 2 indicate that the concentrations of boron in liquid silicon decrease exponentially. As the reduction rate is faster at lower temperatures, it implies that the kinetics of boron removal by the H2-H2O gas mixture is controlled by chemical reactions occurring in the gas–liquid interface. Equation 1 obtained by Theuerer13 also suggested that boron removal involves heterogeneous reactions occurring at the surface of the molten silicon. The process involves the oxidation of both silicon and boron, and the evaporation of the oxidation products. It was observed that the chamber of the furnace was covered with a thin, white SiO2 layer after the experiment. This can be explained by initial oxidation of liquid Si by the water vapor to form gaseous SiO. Subsequently, SiO either condenses to SiO2 + Si or further reacts with steam and oxidizes to SiO2. A chemical analysis of boron in the deposits was not carried out in this work. However, Theuerer13 found the presence of a borate in the wall deposits.
Since the reaction occurs at the surface of the silicon melt, the reaction rate should be related to the ratio of area to volume by
This means that the larger the A/V ratio, the faster the reduction of boron concentration in silicon. However, many experimental results in the literature lack this value. The apparent mass transfer coefficient is then introduced to define the rate of boron reduction
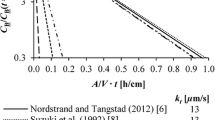
A comparison of the apparent mass transfer coefficients between the current work and those of steam-added plasma melting processes5–8 is shown in Fig. 3. It is obvious that Eq. 3 applies to both the experimental results of H2-H2O mixture and steam-added plasma. The boron apparent mass transfer coefficients for the steam-added plasma method vary about two orders of magnitude. The current experimental results are in the middle range. Nordstrand and Tangstad12 estimated the apparent mass transfer coefficient of B in gas–liquid reactions. They found that they are in the same range of the plasma refining process, at approximately 1.5 x 10−5 m/s. Compared with the apparent mass transfer coefficients of slagging treatment, on the order of 1 × 10−6 m/s, the gas refining process is expected to be more efficient.
A dynamic model has been developed in order to evaluate the possibilities to enhance the refining process. To avoid the complexity of kinetic modeling, the so-called effective reaction zone (ERZ)21 has been applied in the dynamic model. The basic assumptions of the ERZ model are: The kinetic limitations for the current refining process can be approximated by introducing an ERZ inside the vessel. Reactions occurring in the ERZ are all in equilibrium. The size and recirculation rates of the ERZ can be adjusted according to the experimental results. This simple dynamic model has been implemented in a MS Excel spreadsheet (Microsoft Corporation, Redmond, WA) using the ChemSheet22 add-in program (GTT-Technologies, Herzogenrath, Germany). Thermodynamic descriptions of the liquid metal phase were taken from our previous study.23 Properties of gaseous species in the Si-B-H-C-O system, except for HBO, were taken from the JANAF Thermochemical Tables.
Figure 4 shows the simulation results of the dynamic model. The initial parameters are the same as the initial experimental conditions: 52 ppm B in Si, 210 g Si, 3Nl/min gas blowing rate, H2-3.2%H2O mixture, and total blowing time is 180 min. Only two parameters were introduced to describe the ERZ: the ratio of gas that directly reacts with Si melt and the circulation rate for the ERZ and the rest of the melt. It is also assumed that the mixture between the ERZ and the rest of the mixture is perfect. It is clear that our newly calculated enthalpy of formation of HBO can reproduce the experimental results, whereas the JANAF data underestimate the refining efficiency significantly.
Conclusion
The removal of boron by humidified hydrogen has been examined on the laboratory scale. Boron concentrations in liquid silicon were reduced from 52 ppm initially to 0.7 ppm and 3.4 ppm, after gas blowing for 180 min at 1450°C and 1500°C, respectively. The experimental results indicate that the boron removal as a function of treatment time follows the law of exponential decay. As the rate increases with decreasing temperature, it indicates that the process is controlled by chemical reactions occurring in the gas–liquid interface. After 99% of the boron is removed, approximately 90% of the Si can be recovered.
In order to better understand the gaseous refining mechanism, the ab initio CCSD(T) technique was used to predict the enthalpy and entropy of formation of the HBO molecule. A simple refining model was thereafter used to describe the boron refining process. This model can be used to optimize the refining efficiency in the future.
References
J. Dietl, Electrochem. Soc. 127, C368 (1980).
K. Suzuki, T. Sugiyama, K. Takano, and N. Sano, J. Jpn. Inst. Met. 54, 168 (1990).
T. Weiss and K. Schwerdtfeger, Metall. Mater. Trans. B 25, 497 (1994).
L.A.V. Teixeira and K. Morita, ISIJ Int. 49, 783 (2009).
K. Suzuki, T. Kumagai, and N. Sano, ISIJ Int. 32, 630 (1992).
T. Ikeda and M. Maeda, Mater. Trans. JIM 37, 983 (1996).
N. Nakamura, H. Baba, Y. Sakaguchi, and Y. Kato, Mater. Trans. JIM 45, 858 (2004).
N. Yuge, M. Abe, K. Hanazawa, H. Baba, N. Nakamura, Y. Kato, Y. Sakaguchi, S. Hiwasa, and F. Aratani, Prog. Photovolt. Res. Appl. 9, 203 (2001).
B.P. Lee, H.M. Lee, D.H. Park, J.S. Shin, T.U. Yu, and B.M. Moon, Sol. Energy Mater. Sol. Cells 95, 56 (2011).
J.J. Wu, W.H. Ma, B. Yang, Y.N. Dai, and K. Morita, Trans. Nonferr. Met. Soc. China 19, 463 (2009).
S. Tsao and S.S. Lian, Materials Science Forum (Proc. Pricm 5: The Fifth Pacific Rim International Conference on Advanced Materials and Processing) 475–479 (2005), pp. 2595–2598.
E.F. Nordstrand and M. Tangstad, Metall. Mater. Trans. B 43, (2012) (published online 01 May 2012).
H.C. Theuerer, Trans. Am. Inst. Min. Metall. Eng. 206, 1316 (1956).
M.W. Chase, NIST-JANAF Thermochemical Tables (Woodbury, NY: NIST, 1998).
N.L. Garland, C.T. Stanton, and H.H. Nelson, J. Chem. Phys. 95, 2511 (1991).
A.I. Boldyrev and J. Simons, J. Chem. Phys. 110, 3765 (1999).
M. Page, J. Chem. Phys. 93, 3639 (1989).
A. Karton and J.M.L. Martin, J. Phys. Chem. A 111, 5936 (2007).
M.J. Frisch, et al., Gaussian 09, Revision B.1 (Wallingford, CT: Gaussian, Inc., 2009).
X. Duan, D.P. Linder, M. Page, and M.R. Soto, J. Mol. Struct: Theochem. 465, 231 (1999).
M.A. van Ende, Y.M. Kim, M.K. Cho, J. Choi, and I.H. Jung, Mater. Trans. 42, 477 (2011).
GTT-Technologies, http://www.chemsheet.com/ (2012)
K. Tang, E.J. Øvrelid, G. Tranell, and M. Tangstad, Mater. Trans. JIM 50, 1978 (2009).
Acknowledgements
The authors acknowledge the financial support provided by the Research Council of Norway (NFR).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Tang, K., Andersson, S., Nordstrand, E. et al. Removal of Boron in Silicon by H2-H2O Gas Mixtures. JOM 64, 952–956 (2012). https://doi.org/10.1007/s11837-012-0368-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-012-0368-3