Abstract
Cancer is a leading global cause of mortality, which underscores the imperative of early detection for improved patient outcomes. Biorecognition molecules, especially aptamers, have emerged as highly effective tools for early and accurate cancer cell identification. Aptamers, with superior versatility in synthesis and modification, offer enhanced binding specificity and stability compared with conventional antibodies. Hence, this article reviews diagnostic strategies employing aptamer-based biohybrid nano-biosensing technologies, focusing on their utility in detecting cancer biomarkers and abnormal cells. Recent developments include the synthesis of nano-aptamers using diverse nanomaterials, such as metallic nanoparticles, metal oxide nanoparticles, carbon-derived substances, and biohybrid nanostructures. The integration of these nanomaterials with aptamers significantly enhances sensitivity and specificity, promising innovative and efficient approaches for cancer diagnosis. This convergence of nanotechnology with aptamer research holds the potential to revolutionize cancer treatment through rapid, accurate, and non-invasive diagnostic methods.


Reproduced with permission from Kohlberger et al. (2022) [35], ©International Union of Biochemistry and Molecular Biology, 2024

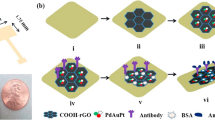
Reproduced with permission from Liu et al. (2023) [62], ©Elsevier, 2023

Reproduced with permission from Chen et al. (2023) [72], © Elsevier, 2023

Reproduced with permission from Borghei et al. (2016) [132], ©Elsevier, 2016

Reproduced with permission from Park and Ban (2023) [136], ©Springer Nature, 2023

Reproduced with permission from Jiang et al. (2018) [144], ©Elsevier, 2018

Reproduced with permission from Sun et al. (2017) [149], ©Springer, 2017

Reproduced with permission from Yang et al. (2014) [155], ©Elsevier, 2014

Reproduced with permission from Zhang et al. (2011) [162], ©Elsevier, 2011

Reproduced with permission from Zhang et al. (2023) [165], ©Elsevier, 2023

Reproduced with permission from Hao et al. (2019) [174], ©Springer, 2019

Reproduced with permission from Wang et al. (2020) [180], ©Elsevier, 2020
Similar content being viewed by others
References
Jeevanandam J, Sabbih G, Tan KX, Danquah MK. Oncological ligand-target binding systems and developmental approaches for cancer theranostics. Mol Biotechnol. 2021;63:167–83.
Kavitha R, Jothi DK, Saravanan K, Swain MP, Gonzáles JLA, Bhardwaj RJ, et al. Colony optimization-enabled CNN deep learning technique for accurate detection of cervical cancer. Biomed Res Int. 2023;2023:1742891.
Sudhakar A. History of cancer, ancient and modern treatment methods. J Cancer Sci Ther. 2009;1:1–4.
Blackley D, Behringer B, Zheng S. Cancer mortality rates in Appalachia: descriptive epidemiology and an approach to explaining differences in outcomes. J Commun Health. 2012;37:804–13.
Alberini A, Rheinberger CM, Ščasný M. All cancers are not created equal: how do survival prospects affect the willingness to pay to avoid cancer? J Benefit Cost Anal. 2023;14(1):93–113.
Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809.
Baghdadli A, Arcuri GG, Green CG, Gauthier LR, Gagnon P, Gagnon B. The fast cognitive evaluation (FaCE): a screening tool to detect cognitive impairment in patients with cancer. BMC Cancer. 2023;23:1–12.
Ren B, Liu X, Suriawinata AA. Pancreatic ductal adenocarcinoma and its precursor lesions: histopathology, cytopathology, and molecular pathology. Am J Pathol. 2019;189:9–21.
He L, Long LR, Antani S, Thoma GR. Histology image analysis for carcinoma detection and grading. Comput Methods Programs Biomed. 2012;107:538–56.
Varghese J, Saleema JS. Machine learning techniques for automated nuclear atypia detection in histopathology images: a review. In: Inventive computation and information technologies. Proceedings of ICICIT 2022; 2023. p. 717–40.
Rastogi A. Changing role of histopathology in the diagnosis and management of hepatocellular carcinoma. World J Gastroenterol. 2018;24:4000–13.
Kim T, Rao J. “SMART” cytology: the next generation cytology for precision diagnosis. Amsterdam: Elsevier; 2023.
Morrison WB, DeNicola DB. Advantages and disadvantages of cytology and histopathology for the diagnosis of cancer. Semin Vet Med Surg. 1993;8:222–7.
Siddiqui EA, Chaurasia V, Shandilya M. Detection and classification of lung cancer computed tomography images using a novel improved deep belief network with Gabor filters. Chemom Intell Lab Syst. 2023;235:104763.
Kuhl CK. Abbreviated magnetic resonance imaging (MRI) for breast cancer screening: rationale, concept, and transfer to clinical practice. Annu Rev Med. 2019;70:501–19.
Veenhuizen SGA, de Lange SV, Bakker MF, Pijnappel RM, Mann RM, Monninkhof EM, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278–86.
Hwang EJ, Nam JG, Lim WH, Park SJ, Jeong YS, Kang JH, et al. Deep learning for chest radiograph diagnosis in the emergency department. Radiology. 2019;293:573–80.
Zebari DA, Ibrahim DA, Zeebaree DQ, Haron H, Salih MS, Damaševičius R, et al. Systematic review of computing approaches for breast cancer detection based computer aided diagnosis using mammogram images. Appl Artif Intell. 2021;35:2157–203.
Ge J, Zhang Q, Zeng J, Gu Z, Gao M. Radiolabeling nanomaterials for multimodality imaging: new insights into nuclear medicine and cancer diagnosis. Biomaterials. 2020;228: 119553.
Ayana G, Park J, Jeong J-W, Choe S-W. A novel multistage transfer learning for ultrasound breast cancer image classification. Diagnostics. 2022;12:135.
Sivasubramanian M, Hsia Y, Lo L-W. Nanoparticle-facilitated functional and molecular imaging for the early detection of cancer. Front Mol Biosci. 2014;1:15.
Wu J, Hu S, Zhang L, Xin J, Sun C, Wang L, et al. Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis. Theranostics. 2020;10:4544.
Cengiz Ozalp V, Kavruk M, Dilek O, Tahir BA. Aptamers: molecular tools for medical diagnosis. Curr Top Med Chem. 2015;15:1125–37.
Odeh F, Nsairat H, Alshaer W, Ismail MA, Esawi E, Qaqish B, et al. Aptamers chemistry: chemical modifications and conjugation strategies. Molecules. 2019;25:3.
Ștefan G, Hosu O, De Wael K, Lobo-Castañón MJ, Cristea C. Aptamers in biomedicine: selection strategies and recent advances. Electrochim Acta. 2021;376: 137994.
Cheng X, Xia X, Ren D, Chen Q, Xu G, Wei F, et al. Programmable CRISPR-Cas12a and self-recruiting crRNA assisted dual biosensing platform for simultaneous detection of lung cancer biomarkers hOGG1 and FEN1. Anal Chim Acta. 2023;1240: 340748.
Stephens M. The emerging potential of aptamers as therapeutic agents in infection and inflammation. Pharmacol Ther. 2022;238: 108173.
Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol. 2022;20:262.
Khan S, Hossain MK. Classification and properties of nanoparticles. In: Rangappa SM, Parameswaranpillai J, Yashas Gowda TG, Siengchin S, Ozgur Seydibeyoglu M, editors. Nanoparticle-based polymer composites. Amsterdam: Elsevier; 2022. p. 15–54.
Kurup CP, Lim SA, Ahmed MU. Nanomaterials as signal amplification elements in aptamer-based electrochemiluminescent biosensors. Bioelectrochemistry. 2022;147: 108170.
Thevendran R, Citartan M. Assays to estimate the binding affinity of aptamers. Talanta. 2022;238: 122971.
Liu Z, Huang Q, Yan Y, Yao J, Zhong F, Xie S, Zhang M, Zhang H, Jin M, Shui L. A multi-unit integrated electrochemical biosensor array for synergistic signal enhancing carbohydrate antigen 125 detection. Sens Actuators B. 2023;393: 134224.
Khazaei M, Hosseini MS, Haghighi AM, Misaghi M. Nanosensors and their applications in early diagnosis of cancer. Sens Bio-Sens Res. 2023;41:100569.
McKeague M, DeRosa MC. Challenges and opportunities for small molecule aptamer development. J Nucleic Acids. 2012;2012:1–20.
Kohlberger M, Gadermaier G. SELEX: critical factors and optimization strategies for successful aptamer selection. Biotechnol Appl Biochem. 2022;69:1771–92.
Pundir M, De Rosa MC, Lobanova L, Abdulmawjood S, Chen X, Papagerakis S, et al. Structural properties and binding mechanism of DNA aptamers sensing saliva melatonin for diagnosis and monitoring of circadian clock and sleep disorders. Anal Chim Acta. 2023;1251: 340971.
Joseph DF, Nakamoto JA, Garcia Ruiz OA, Peñaranda K, Sanchez-Castro AE, Castillo PS, et al. DNA aptamers for the recognition of HMGB1 from Plasmodium falciparum. PLoS ONE. 2019;14: e0211756.
Kushwaha A, Takamura Y, Nishigaki K, Biyani M. Competitive non-SELEX for the selective and rapid enrichment of DNA aptamers and its use in electrochemical aptasensor. Sci Rep. 2019;9:1–11.
Stoltenburg R, Nikolaus N, Strehlitz B. Capture-SELEX: selection of DNA aptamers for aminoglycoside antibiotics. J Anal Methods Chem. 2012;2012:415697.
Wang H, Zhang L, Sun H, Xu S, Li K, Su X. Screening and application of inhibitory aptamers for DNA repair protein apurinic/apyrimidinic endonuclease 1. Int J Biol Macromol. 2023;242: 124918.
Yin S, Li Y, Hossain MN, Sun C, Kraatz H-B. Electrochemical detection of 25-hydroxyvitamin D3 using an oligonucleotide aptasensor. Sens Actuators B. 2021;340: 129945.
Mann D, Reinemann C, Stoltenburg R, Strehlitz B. In vitro selection of DNA aptamers binding ethanolamine. Biochem Biophys Res Commun. 2005;338:1928–34.
Zhu Q, Liu G, Kai M. DNA aptamers in the diagnosis and treatment of human diseases. Molecules. 2015;20:20979–97.
Lakhin A, Tarantul V, Gening L. Aptamers: problems, solutions and prospects. Acta Nat. 2013;5:34–43.
Shien Yeoh T, Yusof Hazrina H, Bukari BA, Tang T-H, Citartan M. Generation of an RNA aptamer against LipL32 of Leptospira isolated by tripartite-hybrid SELEX coupled with in-house Python-aided unbiased data sorting. Bioorg Med Chem. 2023;81: 117186.
Ospina-Villa JD, Dufour A, Weber C, Ramirez-Moreno E, Zamorano-Carrillo A, Guillen N, et al. Targeting the polyadenylation factor EhCFIm25 with RNA aptamers controls survival in Entamoeba histolytica. Sci Rep. 2018;8:1–11.
Sakyi SA, Aboagye SY, Otchere ID, Liao AM, Caltagirone TG, Yeboah-Manu D. RNA aptamer that specifically binds to mycolactone and serves as a diagnostic tool for diagnosis of Buruli ulcer. PLoS Negl Trop Dis. 2016;10: e0004950.
Weiss S, Proske D, Neumann M, Groschup MH, Kretzschmar HA, Famulok M, et al. RNA aptamers specifically interact with the prion protein PrP. J Virol. 1997;71:8790–7.
Germer K, Leonard M, Zhang X. RNA aptamers and their therapeutic and diagnostic applications. Int J Biochem Mol Biol. 2013;4:27.
Rozenblum GT, Lopez VG, Vitullo AD, Radrizzani M. Aptamers: current challenges and future prospects. Expert Opin Drug Discov. 2016;11:127–35.
Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16:181–202.
Chen S, Cao Z, Prettner K, Kuhn M, Yang J, Jiao L, et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023;9:465–72.
Liang H, Zhou X, Zhu Y, Li D, Jing D, Su X, et al. Association of outdoor air pollution, lifestyle, genetic factors with the risk of lung cancer: a prospective cohort study. Environ Res. 2023;218: 114996.
Sundaram P, Kurniawan H, Byrne ME, Wower J. Therapeutic RNA aptamers in clinical trials. Eur J Pharm Sci. 2013;48:259–71.
Lawler M, Davies L, Oberst S, Oliver K, Eggermont A, Schmutz A, et al. European groundshot: addressing Europe’s cancer research challenges: a Lancet Oncology Commission. Lancet Oncol. 2023;24:e11-56.
Takita S, Nabok A, Lishchuk A, Mussa MH, Smith D. Enhanced performance electrochemical biosensor for detection of prostate cancer biomarker PCA3 using specific aptamer. Eng. 2023;4:367–79.
Chinnappan R, Ramadan Q, Zourob M. An integrated lab-on-a-chip platform for pre-concentration and detection of colorectal cancer exosomes using anti-CD63 aptamer as a recognition element. Biosens Bioelectron. 2023;220: 114856.
Sun D, Ma Y, Wu M, Chen Z, Zhang L, Lu J. Recent progress in aptamer-based microfluidics for the detection of circulating tumor cells and extracellular vesicles. J Pharm Anal. 2023;13:340–54.
Liu Z, Parveen N, Rehman U, Aziz A, Sheikh A, Abourehab MAS, et al. Unravelling the enigma of siRNA and aptamer mediated therapies against pancreatic cancer. Mol Cancer. 2023;22:1–22.
Bapat RS, Bhattad R, Dhabadgav R. Awareness, knowledge and practice of self-breast examination in young women. Indian J Gynecol Oncol. 2023;21:4.
Tan W, Zhao Z, Zhang L. Aptamer properties, functions, and applications. In: Zhang L, Tang X, Xi Z, Chattopadhyaya J, editors. Nucleic acids in medicinal chemistry and chemical biology: drug development and clinical applications. US: Wiley; 2023. p. 443–82.
Liu W, Wang Y, Zhang Y, Yu T, Ge J. Analysis of breast cancer biomarker HER2 based on single stranded DNA aptamer and enzyme signal amplification. Int J Electrochem Sci. 2023;18: 100056.
Liu M, Yang T, Chen Z, Wang Z, He N. Differentiating breast cancer molecular subtypes using a DNA aptamer selected against MCF-7 cells. Biomater Sci. 2018;6:3152–9.
Li W-M, Zhou L-L, Zheng M, Fang J. Selection of metastatic breast cancer cell-specific aptamers for the capture of CTCs with a metastatic phenotype by cell-SELEX. Mol Ther Nucleic Acids. 2018;12:707–17.
Drake BF, Khan S, Wang M, Hicks V, Nichols K, Taylor M, et al. Definitive treatment and risk of death among men diagnosed with metastatic prostate cancer at the Veterans Health Administration. Ann Epidemiol. 2023;79:24–31.
Jolly P, Formisano N, Estrela P. DNA aptamer-based detection of prostate cancer. Chem Pap. 2015;69:77–89.
Wang Y, Luo Y, Bing T, Chen Z, Lu M, Zhang N, et al. DNA aptamer evolved by cell-SELEX for recognition of prostate cancer. PLoS ONE. 2014;9: e100243.
Duan M, Long Y, Yang C, Wu X, Sun Y, Li J, et al. Selection and characterization of DNA aptamer for metastatic prostate cancer recognition and tissue imaging. Oncotarget. 2016;7:36436.
Campos-Fernández E, Barcelos LS, Souza AG, Goulart LR, Alonso-Goulart V. Post-SELEX optimization and characterization of a prostate cancer cell-specific aptamer for diagnosis. ACS Omega. 2020;5:3533–41.
Huang Z-X, Xie Q, Guo Q-P, Wang K-M, Meng X-X, Yuan B-Y, et al. DNA aptamer selected for specific recognition of prostate cancer cells and clinical tissues. Chin Chem Lett. 2017;28:1252–7.
Lobb RJ, Visan KS, Wu L-Y, Norris EL, Hastie ML, Everitt S, et al. An epithelial-to-mesenchymal transition induced extracellular vesicle prognostic signature in non-small cell lung cancer. Commun Biol. 2023;6:68.
Chen J, Xu J, Xiang J, Wan T, Deng H, Li D. A multivalent activatable aptamer probe with ultralow background signal and high sensitivity for diagnosis of lung adenocarcinoma. Talanta. 2023;253: 124056.
Petrella F, Rizzo S, Attili I, Passaro A, Zilli T, Martucci F, et al. an overview of treatment options. Curr Oncol. 2023;30:3160–75.
Wang H, Qin M, Liu R, Ding X, Chen ISY, Jiang Y. Characterization of a bifunctional synthetic RNA aptamer and a truncated form for ability to inhibit growth of non-small cell lung cancer. Sci Rep. 2019;9:1–12.
Zhao Z, Xu L, Shi X, Tan W, Fang X, Shangguan D. Recognition of subtype non-small cell lung cancer by DNA aptamers selected from living cells. Analyst. 2009;134:1808–14.
Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338–44.
Nuzzo S, Roscigno G, Affinito A, Ingenito F, Quintavalle C, Condorelli G. Potential and challenges of aptamers as specific carriers of therapeutic oligonucleotides for precision medicine in cancer. Cancers. 2019;11:1521.
O’Reilly M, Linehan A, Krstic A, Kolch W, Sheahan K, Winter DC, et al. Oncotherapeutic strategies in early onset colorectal cancer. Cancers. 2023;15:552.
Chen C, Zhou S, Cai Y, Tang F. Nucleic acid aptamer application in diagnosis and therapy of colorectal cancer based on cell-SELEX technology. NPJ Precis Oncol. 2017;1:1–7.
Li W, Wu C-C, Wang S, Zhou L, Qiao L, Ba W, Liu F, Zhan L, Chen H, Yu J-S, Fang J. Identification of the target protein of the metastatic colorectal cancer-specific aptamer W3 as a biomarker by aptamer-based target cells sorting and functional characterization. Biosens Bioelectron. 2022;213: 114451.
Liu Q, Jin C, Wang Y, Fang X, Zhang X, Chen Z, et al. Aptamer-conjugated nanomaterials for specific cancer cell recognition and targeted cancer therapy. NPG Asia Mater. 2014;6: e95.
Hung L-Y, Wang C-H, Che Y-J, Fu C-Y, Chang H-Y, Wang K, Lee G-B. Screening of aptamers specific to colorectal cancer cells and stem cells by utilizing on-chip Cell-SELEX. Sci Rep. 2015;5:1–12.
Özdemir Ç, Kar YD, Eroğlu N, Şen HS, Şenol Y, İbrahim E. The relationship of bone marrow fibrosis at diagnosis with prognosis and survival in childhood acute lymphoblastic leukemia. Eur J Res. 2023;9:375–83.
Wang K, She Q, Li M, Zhao H, Zhao W, Chen B, et al. Prognostic significance of frailty status in patients with primary lung cancer. BMC Geriatr. 2023;23:1–9.
Yazdian-Robati R, Arab A, Ramezani M, Abnous K, Taghdisi SM. Application of aptamers in treatment and diagnosis of leukemia. Int J Pharm. 2017;529:44–54.
Rus I, Tertis M, Pop A, Fizeşan I, Bogdan D, Matei E, et al. The use of a new selective AB3 aptamer for the hematologic tumor cells’ detection. Sens Actuators B. 2023;394: 134389.
Poturnayová A, Buríková M, Bízik J, Hianik T. DNA aptamers in the detection of leukemia cells by the thickness shear mode acoustics method. ChemPhysChem. 2019;20:545–54.
Yang M, Jiang G, Li W, Qiu K, Zhang M, Carter CM, et al. Developing aptamer probes for acute myelogenous leukemia detection and surface protein biomarker discovery. J Hematol Oncol. 2014;7:1–14.
Zeng Z, Parekh P, Li Z, Shi Z-Z, Tung C-H, Zu Y. Specific and sensitive tumor imaging using biostable oligonucleotide aptamer probes. Theranostics. 2014;4:945.
Yang C, Wang Y, Ge MH, Fu YJ, Hao R, Islam K, et al. Rapid identification of specific DNA aptamers precisely targeting CD33 positive leukemia cells through a paired cell-based approach. Biomater Sci. 2019;7:938–50.
Yu Y, Duan S, He J, Liang W, Su J, Zhu J, et al. Highly sensitive detection of leukemia cells based on aptamer and quantum dots. Oncol Rep. 2016;36:886–92.
Cabibbo G, Celsa C, Calvaruso V, Petta S, Cacciola I, Cannavò MR, et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J Hepatol. 2019;71:265–73.
Wu X, Chen J, Wu M, Zhao JX. Aptamers: active targeting ligands for cancer diagnosis and therapy. Theranostics. 2015;5:322.
Chehelgerdi M, Chehelgerdi M. The use of RNA-based treatments in the field of cancer immunotherapy. Mol Cancer. 2023;22:106.
Goh KW, Stephen A, Wu YS, Sim MS, Batumalaie K, Gopinath SC, et al. Molecular targets of aptamers in gastrointestinal cancers: cancer detection, therapeutic applications, and associated mechanisms. J Cancer. 2023;14:2491.
Li X, Zhang P, Dou L, Wang Y, Sun K, Zhang X, et al. Detection of circulating tumor cells in breast cancer patients by nanopore sensing with aptamer-mediated amplification. ACS Sens. 2020;5:2359–66.
Zahra Q, Khan QA, Luo Z. Advances in optical aptasensors for early detection and diagnosis of various cancer types. Front Oncol. 2021;11:632165.
Kivrak E, Ince-Yardimci A, Ilhan R, Kirmizibayrak PB, Yilmaz S, Kara P. Aptamer-based electrochemical biosensing strategy toward human non-small cell lung cancer using polyacrylonitrile/polypyrrole nanofibers. Anal Bioanal Chem. 2020;412:7851–60.
Li L, Wan J, Wen X, Guo Q, Jiang H, Wang J, et al. Identification of a new DNA aptamer by tissue-SELEX for cancer recognition and imaging. Anal Chem. 2021;93:7369–77.
Pu A, Raj G, John J, Mohan M, John F, George J. Aptamers: features, synthesis and applications. Chem Biodivers. 2023;20: e202301008.
Cerchia L, De Franciscis V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010;28:517–25.
Li Y, Tam WW, Yu Y, Zhuo Z, Xue Z, Tsang C, et al. The application of aptamer in biomarker discovery. Biomark Res. 2023;11:70.
Ruiz Ciancio D, Vargas MR, Thiel WH, Bruno MA, Giangrande PH, Mestre MB. Aptamers as diagnostic tools in cancer. Pharmaceuticals. 2018;11:86.
Chiang S-C, Han C-L, Yu K-H, Chen Y-J, Wu K-P. Prioritization of cancer marker candidates based on the immunohistochemistry staining images deposited in the human protein atlas. PLoS ONE. 2013;8: e81079.
Jiang L, Lin X, Chen F, Qin X, Yan Y, Ren L, et al. Current research status of tumor cell biomarker detection. Microsyst Nanoeng. 2023;9:123.
Barough MS, Jalili N, Shafiee S, Salehi M, Naseri N, Javidi MA, et al. Anti-MUC1 nanobody can synergize the Tamoxifen and Herceptin effects on breast cancer cells by inducing ER, PR and HER2 overexpression. Int Immunopharmacol. 2023;124: 110792.
Sola M, Menon AP, Moreno B, Meraviglia-Crivelli D, Soldevilla MM, Cartón-García F, et al. Aptamers against live targets: is in vivo SELEX finally coming to the edge? Mol Ther Nucleic Acids. 2020;21:192–204.
Yan AC, Levy M. Aptamer-mediated delivery and cell-targeting aptamers: room for improvement. Nucleic Acid Ther. 2018;28:194–9.
DeRosa MC, Lin A, Mallikaratchy P, McConnell EM, McKeague M, Patel R, et al. In vitro selection of aptamers and their applications. Nat Rev Methods Primers. 2023;3:54.
Schüling T, Eilers A, Scheper T, Walter J. Aptamer-based lateral flow assays. AIMS Bioeng. 2018;5:78–102.
Kovacevic KD, Gilbert JC, Jilma B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv Drug Deliv Rev. 2018;134:36–50.
Shweta, Sood S, Sharma A, Chadha S, Guleria V. Nanotechnology: a cutting-edge technology in vegetable production. J Hortic Sci Biotechnol. 2021;96:682–95.
Baig N. Two-dimensional nanomaterials: a critical review of recent progress, properties, applications, and future directions. Composites A. 2022;165:107362.
Kumar Kulabhusan P, Hussain B, Yüce M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics. 2020;12:646.
Bousiakou L, Al-Dosary O, Economou A, Subjakova V, Hianik T. Current trends in the use of semiconducting materials for electrochemical aptasensing. Chemosensors. 2023;11:438.
Jo H, Ban C. Aptamer-nanoparticle complexes as powerful diagnostic and therapeutic tools. Exp Mol Med. 2016;48:e230–330.
Zhang Y, Zhou N. Electrochemical biosensors based on micro-fabricated devices for point-of-care testing: a review. Electroanalysis. 2022;34:168–83.
Police Patil AV, Chuang Y-S, Li C, Wu C-C. Recent advances in electrochemical immunosensors with nanomaterial assistance for signal amplification. Biosensors. 2023;13:125.
Reinemann C, Strehlitz B. Aptamer-modified nanoparticles and their use in cancer diagnostics and treatment. Swiss Med Wkly. 2014;144: w13908.
Huang Z, Qiu L, Zhang T, Tan W. Integrating DNA nanotechnology with aptamers for biological and biomedical applications. Matter. 2021;4:461–89.
Choi Y-E, Kwak J-W, Park JW. Nanotechnology for early cancer detection. Sensors. 2010;10:428–55.
Aslan Y, Atabay M, Chowdhury HK, Göktürk I, Saylan Y, Inci F. Aptamer-based point-of-care devices: emerging technologies and integration of computational methods. Biosensors. 2023;13:569.
Fu Z, Xiang J. Aptamer-functionalized nanoparticles in targeted delivery and cancer therapy. Int J Mol Sci. 2020;21:9123.
Loiseau A, Zhang L, Hu D, Salmain M, Mazouzi Y, Flack R, et al. Core–shell gold/silver nanoparticles for localized surface plasmon resonance-based naked-eye toxin biosensing. ACS Appl Mater Interfaces. 2019;11:46462–71.
Catanzaro L, Scardaci V, Scuderi M, Condorelli M, D’Urso L, Compagnini G. Surface plasmon resonance of gold nanoparticle aggregates induced by halide ions. Mater Chem Phys. 2023;308: 128245.
Weremfo A, Fong STC, Khan A, Hibbert DB, Zhao C. Electrochemically roughened nanoporous platinum electrodes for non-enzymatic glucose sensors. Electrochim Acta. 2017;231:20–6.
Menichetti A, Mavridi-Printezi A, Mordini D, Montalti M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J Funct Biomater. 2023;14:244.
Ruiz-Fresneda MA, Schaefer S, Hübner R, Fahmy K, Merroun ML. Exploring antibacterial activity and bacterial-mediated allotropic transition of differentially coated selenium nanoparticles. ACS Appl Mater Interfaces. 2023;15:29958–70.
Fu Y, Ganeev RA, Krishnendu P, Zhou C, Rao KS, Guo C. Size-dependent off-resonant nonlinear optical properties of gold nanoparticles and demonstration of efficient optical limiting. Opt Mater Express. 2019;9:976–91.
Chun L, Kim S-E, Cho M, Choe W-S, Nam J, Lee DW, et al. Electrochemical detection of HER2 using single stranded DNA aptamer modified gold nanoparticles electrode. Sens Actuators B. 2013;186:446–50.
Zhu Y, Chandra P, Shim Y-B. Ultrasensitive and selective electrochemical diagnosis of breast cancer based on a hydrazine-Au nanoparticle-aptamer bioconjugate. Anal Chem. 2013;85:1058–64.
Borghei Y-S, Hosseini M, Dadmehr M, Hosseinkhani S, Ganjali MR, Sheikhnejad R. Visual detection of cancer cells by colorimetric aptasensor based on aggregation of gold nanoparticles induced by DNA hybridization. Anal Chim Acta. 2016;904:92–7.
Li C-H, Kuo T-R, Su H-J, Lai W-Y, Yang P-C, Chen J-S, et al. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci Rep. 2015;5:1–11.
Yaiwong P, Anuthum S, Sangthong P, Jakmunee J, Bamrungsap S, Ounnunkad K. A new portable toluidine blue/aptamer complex-on-polyethyleneimine-coated gold nanoparticles-based sensor for label-free electrochemical detection of alpha-fetoprotein. Front Bioeng Biotechnol. 2023;11:1182880.
Chen LQ, Xiao SJ, Peng L, Wu T, Ling J, Li YF, et al. Aptamer-based silver nanoparticles used for intracellular protein imaging and single nanoparticle spectral analysis. J Phys Chem B. 2010;114:3655–9.
Park J, Ban C. Development of a one-shot dual aptamer-based fluorescence nanosensor for rapid, sensitive, and label-free detection of periostin. Sci Rep. 2023;13:10224.
Zhou L, Wang W, Chen Y, Fan J, Tong C, Liu B. Aptamer-tagged silver nanoclusters for cell image and Mucin1 detection in vitro. Talanta. 2019;205: 120075.
Yin J, He X, Wang K, Xu F, Shangguan J, He D, et al. Label-free and turn-on aptamer strategy for cancer cells detection based on a DNA-silver nanocluster fluorescence upon recognition-induced hybridization. Anal Chem. 2013;85:12011–9.
Shan W, Pan Y, Fang H, Guo M, Nie Z, Huang Y, et al. An aptamer-based quartz crystal microbalance biosensor for sensitive and selective detection of leukemia cells using silver-enhanced gold nanoparticle label. Talanta. 2014;126:130–5.
Idris AO, Akanji SP, Orimolade BO, Olorundare FOG, Azizi S, Mamba B, et al. Using nanomaterials as excellent immobilisation layer for biosensor design. Biosensors. 2023;13:192.
Kalambate P, Thirabowonkitphithan P, Kaewarsa P, Permpoka K, Radwan A, Shakoor R, et al. Progress, challenges, and opportunities of two-dimensional layered materials based electrochemical sensors and biosensors. Mater Today Chem. 2022;26: 101235.
Gao P, Wei R, Chen Y, Li X, Pan W, Li N, et al. Pt nanozyme-bridged covalent organic framework-aptamer nanoplatform for tumor targeted self-strengthening photocatalytic therapy. Biomaterials. 2023;297: 122109.
Bharti A, Rana S, Dahiya D, Agnihotri N, Prabhakar N. An electrochemical aptasensor for analysis of MUC1 using gold platinum bimetallic nanoparticles deposited carboxylated graphene oxide. Anal Chim Acta. 2020;1097:186–95.
Jiang Y, Sun D, Liang Z, Chen L, Zhang Y, Chen Z. Label-free and competitive aptamer cytosensor based on layer-by-layer assembly of DNA-platinum nanoparticles for ultrasensitive determination of tumor cells. Sens Actuators B. 2018;262:35–43.
Fu L, Zheng Y, Li X, Liu X, Lin C-T, Karimi-Maleh H. Strategies and applications of graphene and its derivatives-based electrochemical sensors in cancer diagnosis. Molecules. 2023;28:6719.
MubarakAli D, Kim H, Venkatesh PS, Kim J-W, Lee S-Y. A systemic review on the synthesis, characterization, and applications of palladium nanoparticles in biomedicine. Appl Biochem Biotechnol. 2023;195:3699–718.
Bi L, Teng Y, Baghayeri M, Bao J. Employing Pd nanoparticles decorated on halloysite nanotube/carbon composite for electrochemical aptasensing of HER2 in breast cancer patients. Environ Res. 2023;237: 117030.
Tabrizi MA, Shamsipur M, Saber R, Sarkar S. Flow injection amperometric sandwich-type aptasensor for the determination of human leukemic lymphoblast cancer cells using mwcnts-pdnano/ptca/aptamer as labeled aptamer for the signal amplification. Anal Chim Acta. 2017;985:61–8.
Sun D, Lu J, Wang X, Zhang Y, Chen Z. Voltammetric aptamer based detection of HepG2 tumor cells by using an indium tin oxide electrode array and multifunctional nanoprobes. Microchim Acta. 2017;184:3487–96.
Ou D, Sun D, Lin X, Liang Z, Zhong Y, Chen Z. A dual-aptamer-based biosensor for specific detection of breast cancer biomarker HER2 via flower-like nanozymes and DNA nanostructures. J Mater Chem B. 2019;7:3661–9.
Anandhi P, Rajeshkumar S. Copper nanoparticles in wound healing: a review. J Surv Fish Sci. 2023;10:49–63.
Baranwal J, Barse B, Di Petrillo A, Gatto G, Pilia L, Kumar A. Nanoparticles in cancer diagnosis and treatment. Materials. 2023;16:5354.
Gedi V, Kim Y-P. Detection and characterization of cancer cells and pathogenic bacteria using aptamer-based nano-conjugates. Sensors. 2014;14:18302–7.
Zhang Y, Xu Y, Li N, Qi N, Peng L, Yang M, et al. An ultrasensitive dual-signal ratio electrochemical aptamer biosensor for the detection of HER2. Colloids Surf B. 2023;222: 113118.
Yang X-H, Sun S, Liu P, Wang K-M, Wang Q, Liu J-B, Huang J, He L-L. A novel fluorescent detection for PDGF-BB based on dsDNA-templated copper nanoparticles. Chin Chem Lett. 2014;25:9–14.
Zhu Y, Wang H, Wang L, Zhu J, Jiang W. Cascade signal amplification based on copper nanoparticle-reported rolling circle amplification for ultrasensitive electrochemical detection of the prostate cancer biomarker. ACS Appl Mater Interfaces. 2016;8:2573–81.
Maiyo F, Singh M. Selenium nanoparticles: potential in cancer gene and drug delivery. Nanomedicine. 2017;12:1075–89.
Tan HW, Mo H-Y, Lau AT, Xu Y-M. Selenium species: current status and potentials in cancer prevention and therapy. Int J Mol Sci. 2019;20:75.
Jalalian SH, Ramezani M, Abnous K, Taghdisi SM. Targeted co-delivery of epirubicin and NAS-24 aptamer to cancer cells using selenium nanoparticles for enhancing tumor response in vitro and in vivo. Cancer Lett. 2018;416:87–93.
Liu ML, Zou HY, Li CM, Li RS, Huang CZ. Aptamer-modified selenium nanoparticles for dark-field microscopy imaging of nucleolin. Chem Commun. 2017;53:13047–50.
Meng F-L, Wang W, Jia W-D. Diagnostic and prognostic significance of serum miR-24-3p in HBV-related hepatocellular carcinoma. Med Oncol. 2014;31:1–6.
Zhang X, Li S, Jin X, Li X. Aptamer based photoelectrochemical cytosensor with layer-by-layer assembly of CdSe semiconductor nanoparticles as photoelectrochemically active species. Biosens Bioelectron. 2011;26:3674–8.
Xu Y, Zhang Y, Li N, Yang S, Chen J, Hou J, et al. An ultrasensitive ratiometric electrochemical aptasensor based on metal-organic frameworks and nanoflower-like Bi2CuO4 for human epidermal growth factor receptor 2 detection. Bioelectrochemistry. 2023;154: 108542.
Wu C-W, Harroun SG, Lien C-W, Chang H-T, Unnikrishnan B, Lai IP-J, et al. Self-templated formation of aptamer-functionalized copper oxide nanorods with intrinsic peroxidase catalytic activity for protein and tumor cell detection. Sens Actuators B. 2016;227:100–7.
Zhang Y, Yu Y, Kang K, Wang X, Zhu N, Wang X, et al. Nano-magnetic aptamer sensor incorporating AND logic recognition-launched hybridization chain reaction for organ origin identification of circulating tumor cells. Nano Today. 2023;49: 101817.
Yu MK, Kim D, Lee IH, So JS, Jeong YY, Jon S. Image-guided prostate cancer therapy using aptamer-functionalized thermally cross-linked superparamagnetic iron oxide nanoparticles. Small. 2011;7:2241–9.
Wang AZ, Bagalkot V, Vasilliou CC, Gu F, Alexis F, Zhang L, et al. Superparamagnetic iron oxide nanoparticle-aptamer bioconjugates for combined prostate cancer imaging and therapy. ChemMedChem. 2008;3:1311–5.
Erkmen C, Tığ GA, Uslu B. Nanomaterial-based sandwich-type electrochemical aptasensor platform for sensitive voltammetric determination of leptin. Microchim Acta. 2022;189:396.
Erkmen C, Tig GA, Bengi U. First label-free impedimetric aptasensor based on Au NPs/TiO2 NPs for the determination of leptin. Sens Actuators B. 2022;358: 131420.
Pla L, Sancenon F, Martinez-Bisbal MC, Banuls C, Estan N, Botello-Marabotto M, et al. A new 8-oxo-7, 8–2′ deoxyguanosine nanoporous anodic alumina aptasensor for colorectal cancer diagnosis in blood and urine. Nanoscale. 2021;13:8648–57.
Sargazi S, Simge E, Mobashar A, Gelen SS, Rahdar A, Ebrahimi N, et al. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: a review. Chem Biol Interact. 2022;361: 109964.
Pasinszki T, Krebsz M, Tung TT, Losic D. Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors. 2017;17:1919.
Chen Q, Zhang Z, Xie L, Huang C, Lin X, Tang W, et al. A one-step aptasensor for ultrasensitive detection of lung cancer marker homocysteine based on multifunctional carbon nanotubes by square-wave voltammetry. Bioelectrochemistry. 2023;153: 108464.
Hao Z, Pan Y, Huang C, Wang Z, Zhao X. Sensitive detection of lung cancer biomarkers using an aptameric graphene-based nanosensor with enhanced stability. Biomed Microdevices. 2019;21:1–9.
Gedi V, Song CK, Kim GB, Lee JO, Oh E, Shin BS, et al. Sensitive on-chip detection of cancer antigen 125 using a DNA aptamer/carbon nanotube network platform. Sens Actuators B. 2018;256:89–97.
Li C, Meng Y, Wang S, Qian M, Wang J, Lu W, et al. Mesoporous carbon nanospheres featured fluorescent aptasensor for multiple diagnosis of cancer in vitro and in vivo. ACS Nano. 2015;9:12096–103.
Mohammadpour Z, Kamankesh M, Walsh T, Ghorbanzadeh S, Hamdi D, Akbari M, et al. A review on polymeric nanocomposites for the electrochemical sensing of breast cancer biomarkers. Microchem J. 2023;195:109528.
Saylan Y, Akgönüllü S, Yavuz H, Ünal S, Denizli A. Molecularly imprinted polymer based sensors for medical applications. Sensors. 2019;19:1279.
Foroozandeh A, Abdouss M, SalarAmoli H, Pourmadadi M, Yazdian F. An electrochemical aptasensor based on g-C3N4/Fe3O4/PANI nanocomposite applying cancer antigen_125 biomarkers detection. Process Biochem. 2023;127:82–91.
Wang D-E, Gao X, You S, Chen M, Ren L, Sun W, et al. Aptamer-functionalized polydiacetylene liposomes act as a fluorescent sensor for sensitive detection of MUC1 and targeted imaging of cancer cells. Sens Actuators B. 2020;309: 127778.
Lyu Y, Cui D, Huang J, Fan W, Miao Y, Pu K. Near-infrared afterglow semiconducting nano-polycomplexes for the multiplex differentiation of cancer exosomes. Angew Chem. 2019;131:5037–41.
Liu Y, Dykstra G. Recent progress on electrochemical (bio) sensors based on aptamer-molecularly imprinted polymer dual recognition. Sens Actuators Rep. 2022;4:100112.
Zhang C-H, Wang H, Liu J-W, Sheng Y-Y, Chen J, Zhang P, et al. Amplified split aptamer sensor delivered using block copolymer nanoparticles for small molecule imaging in living cells. ACS Sens. 2018;3:2526–31.
Hao N, Jiang L, Qian J, Wang K. Ultrasensitive electrochemical ochratoxin A aptasensor based on CdTe quantum dots functionalized graphene/Au nanocomposites and magnetic separation. J Electroanal Chem. 2016;781:332–8.
Patil PO, Pandey GR, Patil AG, Borse VB, Deshmukh PK, Patil DR, et al. Graphene-based nanocomposites for sensitivity enhancement of surface plasmon resonance sensor for biological and chemical sensing: a review. Biosens Bioelectron. 2019;139: 111324.
Shan H, Li X, Liu L, Song D, Wang Z. Recent advances in nanocomposite-based electrochemical aptasensors for the detection of toxins. J Mater Chem B. 2020;8:5808–25.
Chen Y, Chen J, Guo D, Yang P, Chen S, Zhao C, Xu C, Zhang Q, Lin C, Zhong S. Tryptophan metabolites as biomarkers for esophageal cancer susceptibility, metastasis, and prognosis. Front Oncol. 2022;12: 800291.
Khoshsafar H, Bagheri H, Hashemi P, Bordbar MM, Madrakian T, Afkhami A. Combination of an aptamer-based immunochromatography assay with nanocomposite-modified screen-printed electrodes for discrimination and simultaneous determination of tryptophan enantiomers. Talanta. 2023;253: 124090.
Wei B, Mao K, Liu N, Zhang M, Yang Z. Graphene nanocomposites modified electrochemical aptamer sensor for rapid and highly sensitive detection of prostate specific antigen. Biosens Bioelectron. 2018;121:41–6.
Yazdanparast S, Benvidi A, Banaei M, Nikukar H, Tezerjani MD, Azimzadeh M. Dual-aptamer based electrochemical sandwich biosensor for MCF-7 human breast cancer cells using silver nanoparticle labels and a poly (glutamic acid)/MWNT nanocomposite. Microchim Acta. 2018;185:1–10.
Naaz S, Sachdev S, Husain R, Pandey V, Ansari MI. Nanomaterials and nanocomposites: significant uses in plant performance, production, and toxicity response. In: Nanomaterials and nanocomposites exposures to plants: response, interaction, phytotoxicity and defense mechanisms. Springer; 2023. p. 1–18.
Sharma A, Anjana RH, Goswami S. A comprehensive review on the heavy metal removal for water remediation by the application of lignocellulosic biomass-derived nanocellulose. J Polym Environ. 2022;30:1–18.
Mo T, Liu X, Luo Y, Zhong L, Zhang Z, Li T, et al. Aptamer-based biosensors and application in tumor theranostics. Cancer Sci. 2022;113:7–16.
Mahmoudpour M, Ding S, Lyu Z, Ebrahimi G, Du D, Dolatabadi JEN, et al. Aptamer functionalized nanomaterials for biomedical applications: recent advances and new horizons. Nano Today. 2021;39: 101177.
de Valega Negrao CvZ, Cerize NNP, Justo-Junior ADS, Liszbinski RB, Meneguetti GP, Araujo L, et al. Iron oxide nanoparticles coated with biodegradable block-copolymer PDMAEMA-b-PMPC and functionalized with aptamer for HER2 breast cancer cell identification. bioRxiv. 2023:2023-06.
Mazloum-Ardakani M, Barazesh B, Moshtaghiun SM. A distinguished cancer-screening package containing a DNA sensor and an aptasensor for early and certain detection of acute lymphoblastic leukemia. Clin Chim Acta. 2019;497:41–7.
Mazloum-Ardakani M, Barazesh B, Moshtaghioun SM. An aptasensor based on electrosynthesized conducting polymers, Cu2O-carbon dots and biosynthesized gold nanoparticles, for monitoring carcinoembryonic antigen. J Nanostruct. 2019;9:659–68.
Davodabadi F, Mirinejad S, Fathi-Karkan S, Majidpour M, Ajalli N, Sheervalilou R, et al. Aptamer-functionalized quantum dots as theranostic nanotools against cancer and bacterial infections: a comprehensive overview of recent trends. Biotechnol Prog. 2023;39: e3366.
Kumar V, Kukkar D, Hashemi B, Kim KH, Deep A. Advanced functional structure-based sensing and imaging strategies for cancer detection: possibilities, opportunities, challenges, and prospects. Adv Funct Mater. 2019;29:1807859.
Hejji L, Azzouz A, Kukkar D, Kim K-H. Recent advancements in nanomaterials-based aptasensors for the detection of emerging contaminants in foodstuffs. TrAC Trends Anal Chem. 2023;166:117194.
Klinghammer S, Voitsekhivska T, Licciardello N, Kim K, Baek C-K, Cho H, et al. Nanosensor-based real-time monitoring of stress biomarkers in human saliva using a portable measurement system. ACS Sens. 2020;5:4081–91.
Sharifi S, Vahed SZ, Ahmadian E, Dizaj SM, Eftekhari A, Khalilov R, et al. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens Bioelectron. 2020;150: 111933.
Purohit B, Vernekar PR, Shetti NP, Chandra P. Biosensor nanoengineering: design, operation, and implementation for biomolecular analysis. Sens Int. 2020;1: 100040.
Muhammad N, Hoo LT, Ahmad AN, Mohamad A, Abdullah Lim S. Accuracy of biosensors as rapid diagnostic and biochemical monitoring tools for non-communicable diseases management. In: Joshi SN, Chandra P, editors. Advanced micro-and nano-manufacturing technologies: applications in biochemical and biomedical engineering. Singapore: Springer; 2022. p. 57–75.
Jiang Y, Cui S, Xia T, Sun T, Tan H, Yu F, et al. Real-time monitoring of heavy metals in healthcare via twistable and washable smartsensors. Anal Chem. 2020;92:14536–41.
Timilsina SS, Jolly P, Durr N, Yafia M, Ingber DE. Enabling multiplexed electrochemical detection of biomarkers with high sensitivity in complex biological samples. Acc Chem Res. 2021;54:3529–39.
Röthlisberger P, Gasse C, Hollenstein M. Nucleic acid aptamers: emerging applications in medical imaging, nanotechnology, neurosciences, and drug delivery. Int J Mol Sci. 2017;18:2430.
Rusconi CP, Roberts JD, Pitoc GA, Nimjee SM, White RR, Quick G Jr, et al. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat Biotechnol. 2004;22:1423–8.
Bompiani K, Monroe D, Church F, Sullenger B. A high affinity, antidote-controllable prothrombin and thrombin-binding RNA aptamer inhibits thrombin generation and thrombin activity. J Thromb Haemost. 2012;10:870–80.
Ausländer D, Wieland M, Ausländer S, Tigges M, Fussenegger M. Rational design of a small molecule-responsive intramer controlling transgene expression in mammalian cells. Nucleic Acids Res. 2011;39: e155.
Göringer HU. Parasite-specific aptamers as biosynthetic reagents and potential pharmaceuticals. Trends Parasitol. 2012;28:106–13.
Lakhin AV, Kazakov AA, Makarova AV, Pavlov YI, Efremova AS, Shram SI, et al. Isolation and characterization of high affinity aptamers against DNA polymerase iota. Nucleic Acid Ther. 2012;22:49–57.
Eulberg D, Buchner K, Maasch C, Klussmann S. Development of an automated in vitro selection protocol to obtain RNA-based aptamers: identification of a biostable substance P antagonist. Nucleic Acids Res. 2005;33: e45.
NajafiKhoshnoo S, Kim T, Tavares-Negrete JA, Pei X, Das P, Lee SW, et al. A 3D nanomaterials-printed wearable, battery-free, biocompatible, flexible, and wireless pH sensor system for real-time health monitoring. Adv Mater Technol. 2023;8:2201655.
Bilibana MP, Citartan M, Fuku X, Jijana AN, Mathumba P, Iwuoha E. Aptamers functionalized hybrid nanomaterials for algal toxins detection and decontamination in aquatic system: current progress, opportunities, and challenges. Ecotoxicol Environ Saf. 2022;232: 113249.
Yuhan J, Zhu L, Zhu L, Huang K, He X, Xu W. Cell-specific aptamers as potential drugs in therapeutic applications: a review of current progress. J Control Release. 2022;346:405–20.
Langlois NI, Ma KY, Clark HA. Nucleic acid nanostructures for in vivo applications: the influence of morphology on biological fate. Appl Phys Rev. 2023;10: 011304.
Yu X, Sha L, Liu Q, Zhao Y, Fang H, Cao Y, et al. Recent advances in cell membrane camouflage-based biosensing application. Biosens Bioelectron. 2021;194: 113623.
Chun L, Kim S-E, Cho M, Choe W-S, Nam J, Lee DW, Lee Y. Electrochemical detection of HER2 using single stranded DNA aptamer modified gold nanoparticles electrode. Sens Actuators B. 2013;186:446–50.
Li G, Zeng J, Liu H, Ding P, Liang J, Nie X, et al. A fluorometric aptamer nanoprobe for alpha-fetoprotein by exploiting the FRET between 5-carboxyfluorescein and palladium nanoparticles. Microchim Acta. 2019;186:314.
Zhu N, Li G, Zhou J, Zhang Y, Kang K, Ying B, et al. A light-up fluorescence resonance energy transfer magnetic aptamer-sensor for ultra-sensitive lung cancer exosome detection. J Mater Chem B. 2021;9:2483–93.
Sun D, Lu J, Zhong Y, Yu Y, Wang Y, Zhang B, et al. Sensitive electrochemical aptamer cytosensor for highly specific detection of cancer cells based on the hybrid nanoelectrocatalysts and enzyme for signal amplification. Biosens Bioelectron. 2016;75:301–7.
Wang Y, Zhang Y, Yan T, Fan D, Du B, Ma H, et al. Ultrasensitive electrochemical aptasensor for the detection of thrombin based on dual signal amplification strategy of Au@ GS and DNA-CoPd NPs conjugates. Biosens Bioelectron. 2016;80:640–6.
Wu X, Fu P, Ma W, Xu L, Kuang H, Xu C. SERS-active silver nanoparticle trimers for sub-attomolar detection of alpha fetoprotein. RSC Adv. 2015;5:73395–8.
Wu Z, Li H, Liu Z. An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Sens Actuators B. 2015;206:531–7.
Xu L, Yan W, Ma W, Kuang H, Wu X, Liu L, et al. SERS encoded silver pyramids for attomolar detection of multiplexed disease biomarkers. Adv Mater. 2015;27:1706–11.
Yu T, Dai P-P, Xu J-J, Chen H-Y. Highly sensitive colorimetric cancer cell detection based on dual signal amplification. ACS Appl Mater Interfaces. 2016;8:4434–41.
Zhao S, Ma W, Xu L, Wu X, Kuang H, Wang L, et al. Ultrasensitive SERS detection of VEGF based on a self-assembled Ag ornamented–AU pyramid superstructure. Biosens Bioelectron. 2015;68:593–7.
Zhu D, Li W, Wen H-M, Yu S, Miao Z-Y, Kang A, et al. Silver nanoparticles-enhanced time-resolved fluorescence sensor for VEGF165 based on Mn-doped ZnS quantum dots. Biosens Bioelectron. 2015;74:1053–60.
Tung NT, Tue PT, Thi Ngo Clien T, Ohno Y, Maehashi K, Matsumoto K, et al. Peptide aptamer-modified single-walled carbon nanotube-based transistors for high-performance biosensors. Sci Rep. 2017;7:17881.
Miao X, Li Z, Zhu A, Feng Z, Tian J, Peng X. Ultrasensitive electrochemical detection of protein tyrosine kinase-7 by gold nanoparticles and methylene blue assisted signal amplification. Biosens Bioelectron. 2016;83:39–44.
Ma W, Yin H, Xu L, Wu X, Kuang H, Wang L, et al. Ultrasensitive aptamer-based SERS detection of PSAs by heterogeneous satellite nanoassemblies. Chem Commun. 2014;50:9737–40.
Li Y, Zhang Y, Zhao M, Zhou Q, Wang L, Wang H, et al. A simple aptamer-functionalized gold nanorods based biosensor for the sensitive detection of MCF-7 breast cancer cells. Chem Commun. 2016;52:3959–61.
Tagit O, Hildebrandt N. Fluorescence sensing of circulating diagnostic biomarkers using molecular probes and nanoparticles. ACS Sens. 2017;2:31–45.
Feng J, Wu X, Ma W, Kuang H, Xu L, Xu C. A SERS active bimetallic core–satellite nanostructure for the ultrasensitive detection of Mucin-1. Chem Commun. 2015;51:14761–3.
Shi X, Chen L, Chen S, Sun D. Electrochemical aptasensors for the detection of hepatocellular carcinoma-related biomarkers. New J Chem. 2021;45:15158–69.
Chen Q, Zhang Z, Xie L, Huang C, Lin X, Tang W, et al. A one-step aptasensor for ultrasensitive detection of lung cancer marker homocysteine based on multifunctional carbon nanotubes by square-wave voltammetry. Bioelectrochemistry. 2023;153:108464.
Acknowledgments
The authors acknowledge their respective departments and universities for the support during the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding has been received to conduct this research or prepare this article.
Conflicts of Interest/Competing Interests
Thimmaiah Bargavi Ram, Saravanan Krishnan, Jaison Jeevanandam, Michael K. Danquah, and Sabu Thomas have no conflicts of interest that are directly relevant to the content of this article.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author’ Contributions
First draft: TBR and SK; conceptualization, supervision, final draft, and review: JJ; supervision and expert review of manuscript: MKD and ST.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ram, T.B., Krishnan, S., Jeevanandam, J. et al. Emerging Biohybrids of Aptamer-Based Nano-Biosensing Technologies for Effective Early Cancer Detection. Mol Diagn Ther (2024). https://doi.org/10.1007/s40291-024-00717-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s40291-024-00717-x